Abstract
Context
PAM4 is a monoclonal antibody that shows high specificity for pancreatic ductal adenocarcinoma (PDAC) and its neoplastic precursor lesions. A PAM4-based serum immunoassay is able to detect 71% of early-stage patients and 91% with advanced disease. However, approximately 20% of patients diagnosed with chronic pancreatitis (CP) are also positive for circulating PAM4 antigen. The specificity of the PAM4 antibody is critical to the interpretation of the serum-based and immunohistochemical assays for detection of PDAC.
Objective
To determine whether PAM4 can differentiate PDAC from nonneoplastic lesions of the pancreas.
Design
Tissue microarrays of PDAC (N = 43) and surgical specimens from CP (N = 32) and benign cystic lesions (N=19) were evaluated for expression of the PAM4 biomarker, MUC1, MUC4, CEACAM5/6, and CA19-9.
Results
PAM4 and monoclonal antibodies (MAbs) to MUC1, MUC4, CEACAM5/6, and CA19-9 were each reactive with the majority of PDAC cases; however, PAM4 was the only monoclonal antibody not to react with adjacent, nonneoplastic parenchyma. Although PAM4 labeled 19% (6 of 32) of CP specimens, reactivity was restricted to pancreatic intraepithelial neoplasia associated with CP; inflamed tissues were negative in all cases. In contrast, MUC1, MUC4, CEACAM5/6, and CA19-9 were detected in 90%, 78%, 97%, and 100% of CP, respectively, with reactivity also present in nonneoplastic inflamed tissue.
Conclusions
PAM4 was the only monoclonal antibody able to differentiate PDAC (and pancreatic intraepithelial neoplasia precursor lesions) from benign, nonneoplastic tissues of the pancreas. These results suggest the use of PAM4 for evaluation of tissue specimens, and support its role as an immunoassay for detection of PDAC.
Biomarkers for the early detection and diagnosis of cancer are, for the most part, based upon the identification and quantitation of substances released into a biological fluid, or detectable within tissue specimens derived from the lesion under investigation. For some types of cancer, screening for specific biomarkers has enhanced detection at early stages of tumor growth, when curative procedures may be most effective. However, this has not been the case for pancreatic ductal adenocarcinoma (PDAC). Because of the low frequency of PDAC, screening of the general population is not considered economically feasible, and, further, this type of cancer usually provides no symptoms that might indicate the necessity for medical attention until it has become advanced with metastases.
Nevertheless, there are several current investigations evaluating means for surveillance of patient groups considered at high risk for PDAC, for example, individuals with a family history of PDAC,1–3 patients with chronic pancreatitis (CP),4,5 and those with new-onset diabetes who also meet certain other criteria.6,7 Most of these studies involve the use of imaging procedures to detect small pancreatic masses. Canto et al8 offered surveillance using computed tomography and endoscopic ultrasonography to several groups of individuals considered at high risk for PDAC, including those having had several relatives diagnosed with PDAC and those with Peutz-Jeghers syndrome. If endoscopic ultrasonography was abnormal, endoscopic ultrasonography–fine–needle aspiration and endoscopic retrograde cholangiopancreatography were performed. By use of this protocol, a significant number of early, potentially curable, neoplastic masses were discovered in asymptomatic patients.8 However, the majority of patients examined presented with moderate to severe pancreatitis, a potentially confounding environment for accurate detection and diagnosis by imaging, especially of small neoplastic lesions. Langer et al,9 using an endoscopic ultrasonography/magnetic resonance imaging/magnetic resonance cholangiopancreatography–based screening program for individuals with family background of PDAC, were able to detect several individuals with precursor lesions of PDAC; however, they believed the diagnostic yield of this screening program was low. Even if these imaging procedures prove useful for screening high-risk populations, if a mass or cystic lesion is imaged, the physician still has to determine if it is benign or malignant. In either case, fine-needle aspiration or biopsy has been the method of choice for differential diagnosis, but evaluation of circulating biomarkers would, if available, provide an easier (noninvasive), more objective (quantitative), and more cost-effective means for decision making.
Several reports from our group have demonstrated that use of the PAM4 antibody in a serum-based immunoassay may prove useful for detection of early-stage PDAC with high specificity.10–14 However, approximately 20% of patients with a diagnosis of CP are positive for circulating PAM4 antigen.10 This issue is critical to the interpretation of the serum-based immunoassay, as well as the use of the antibody for immunohistochemical labeling of aspirates and biopsy materials, because the PAM4-positive CP patients represent either an approximate 20% false-positive rate or, perhaps, the discovery of occult neoplasia. The studies reported here support the latter option, and that PAM4 is a useful discriminator for PDAC and its precursor lesions.
MATERIALS AND METHODS
Tissue Specimens
Tissue microarrays of PDAC were created from formalin-fixed, paraffin-embedded tumor specimens from 43 patients who underwent pancreatectomy for PDAC. Two 1-mm cores of PDAC were collected from each case. In addition, for 14 of these cases, adjacent, nonneoplastic pancreatic tissue was also collected. Formalin-fixed, paraffin-embedded surgical specimens were obtained from patients with CP (N=32, with etiologies that included obstruction [13], alcohol [9], autoimmune [2], pancreas divisum [2], groove [2], and unknown [4]), and benign, nonmucinous cystic lesions of the pancreas (N = 19) who underwent pancreatectomy. This Health Insurance Portability and Accountability Act–compliant study was approved by the Vanderbilt University Institutional Review Board.
Immunohistochemical Labeling
Immunohistochemistry was performed as described previously.12 Unstained sections were deparaffinized by routine methods. The tissue sections and microarrays were then heated to 95°C for 20 minutes in a pH 9.0 citrate buffer, Target Retrieval Solution (Dako, Carpinteria, California), allowed to cool to room temperature, and then quenched with 3% H2O2 in methanol for 15 minutes at room temperature. Primary antibodies PAM413 (Immunomedics, Inc, Morris Plains, New Jersey), MA515 (anti-MUC1; Immunomedics), 8G716 (anti-MUC4; Santa Cruz Biotechnology, Santa Cruz, California), MN-15 (reactive with an epitope shared by CEACAM5 and CEACAM6; Immunomedics), CA19-9 (Santa Cruz Biotechnology), and nonbinding, isotype matched control Ag8 (purified in our laboratory from the P3X63-Ag8 murine myeloma cell line, American Type Culture Collection, Manassas, Virginia) were then used at 10 µg/mL with an ABC Vectastain kit (Vector Laboratories, Burlingame, California) for labeling the tissues. The immunohistochemical stains were considered positive when more than 5% of the adenocarcinoma tissue was labeled or more than 1% of the nonmalignant tissue was labeled. This low threshold for positive response in the nonmalignant tissues provided a rigorous test of specificity for application of PAM4 to differential diagnosis of PDAC and CP. Only the appropriate tissue components (eg, adenocarcinoma, pancreatic intraepithelial neoplasia [PanIN], cystic lesions, normal ducts, acinar ductal metaplasia [ADM], etc.) were assessed. Acinar ductal metaplasia represents replacement of acinar cells by cuboidal ductal epithelium. Two types of ADM have been described: one without associated PanIN (isolated ADM) and one with associated PanIN.17
RESULTS
Pancreatic Ductal Adenocarcinoma and Adjacent Nonneoplastic Tissue
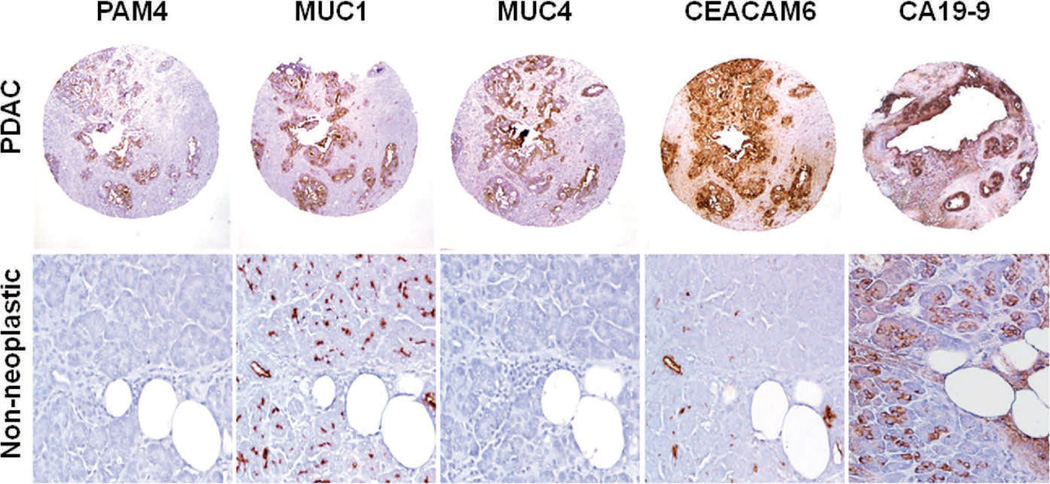
We first examined tissue microarrays consisting of specimens derived from 43 patients with confirmed PDAC. These served as a positive control for monoclonal antibody (MAb) reactivity, as well as providing a means for comparison of immunohistologic staining of malignant and benign tissue specimens. The tissue microarrays contained cores of invasive adenocarcinoma, as well as matched cores from adjacent, nonneoplastic tissue obtained from 14 of the PDAC cases. Immunohistochemical labeling of representative cores of malignant and adjacent, nonneoplastic tissues is presented in Figure 1. As expected, each of the MAbs under investigation provided mostly intense reactivity with the majority of PDAC cores (Table 1); PAM4-reactive antigen was expressed in 34 of 43 cases (79%) with the overwhelming majority of these demonstrating a diffuse labeling pattern (>25% of the PDAC was labeled), whereas MUC1, CEACAM5/6, and CA19-9 were each expressed in at least 90% of cases, again in a mostly diffuse labeling pattern. In contrast, 8G7-defined MUC4 was detected in a considerably lower number of specimens, 29 of 43 (67%), although, as with the other biomarkers, MUC4 was usually evident in a diffuse pattern. Also in Table 1 are data relating biomarker expression with tumor grade. Although trends were observed for PAM4, MUC4, and CEACAM5/6 with decreased expression of the biomarkers in the higher-grade PDAC specimens, statistically significant correlations were not observed. Adjacent, nonneoplastic pancreatic tissues, including acinar cells, isolated ADM, ductal epithelium, and islet cells, did not express the PAM4 antigen (0 of 14 cases; Figure 1); however, MUC4 was expressed in 6 of 14 cases (43%), and MUC1, CEACAM5/6, and CA19-9 each were expressed in 14 of 14 cases (100%).
Figure 1.
Expression of the PAM4 biomarker, MUC1, MUC4, CEACAM5/6, and CA19-9 in tissue cores derived from invasive pancreatic ductal adenocarcinoma (PDAC) and adjacent histologically normal pancreas tissue. Each of the biomarkers was reactive with the overwhelming majority of PDAC cores. The PAM4 antigen was not expressed by any of the nonneoplastic tissue cores within this tissue microarray. On the other hand, MUC1, MUC4, CEACAM5/6, and CA19-9 were identified within acinar cells, small ducts, and isolated acinar ductal metaplasia in 100%, 43%, 100%, and 100% of these nonneoplastic cores, respectively. In the specific case shown, MUC4 was found to be negative (original magnifications ×40 [PDAC cores] and ×200 [nonneoplastic tissue]).
Table 1.
Expression of Biomarkers in Pancreatic Ductal Adenocarcinoma Related to Histologic Grade of Tumor
| PAM4, No. (%) | MUC1, No. (%) | MUC4, No. (%) | CEACAM5/6, No. (%) | CA19-9, No. (%) | |
|---|---|---|---|---|---|
| All grades (N = 43/42)a | |||||
| Total labeled | 34 (79) | 43 (100) | 29 (67) | 38 (90) | 39 (91) |
| Focalb | 8 (19) | 1 (2) | 4 (9) | 3 (7) | 2 (5) |
| Diffuse | 26 (60) | 42 (98) | 25 (58) | 35 (83) | 37 (86) |
| Grade 1 (n = 15/14)a | |||||
| Total labeled | 14 (93) | 15 (100) | 10 (67) | 13 (87) | 14 (93) |
| Focal | 4 (27) | 0 | 1 (7) | 0 | 0 |
| Diffuse | 10 (67) | 15 (100) | 9 (60) | 13 (87) | 14 (93) |
| Grade 2 (n = 20) | |||||
| Total labeled | 14 (70) | 20 (100) | 15 (75) | 19 (95) | 18 (90) |
| Focal | 1 (5) | 0 | 3 (15) | 2 (10) | 0 |
| Diffuse | 13 (65) | 20 (100) | 12 (60) | 17 (85) | 18 (90) |
| Grade 3 (n = 8) | |||||
| Total labeled | 6 (75) | 8 (100) | 4 (50) | 6 (75) | 7 (88) |
| Focal | 3 (38) | 1 (13) | 0 | 1 (13) | 2 (25) |
| Diffuse | 3 (38) | 7 (88) | 4 (50) | 5 (63) | 5 (63) |
| Adjacent normal (n = 14) | 0 | 14 (100) | 6 (43) | 14 (100) | 14 (100) |
All grades, N=43 for all biomarkers except CEACAM5/6, for which N=42; grade 1, n=15 for all biomarkers except CEACAM5/6, for which N=14.
Focal labeling, 5% to 25% of the pancreatic ductal adenocarcinoma tissue labeled with the indicated monoclonal antibody; diffuse, >25% of the pancreatic ductal adenocarcinoma tissue labeled with the indicated monoclonal antibody; total, focal þ diffuse.
Chronic Pancreatitis
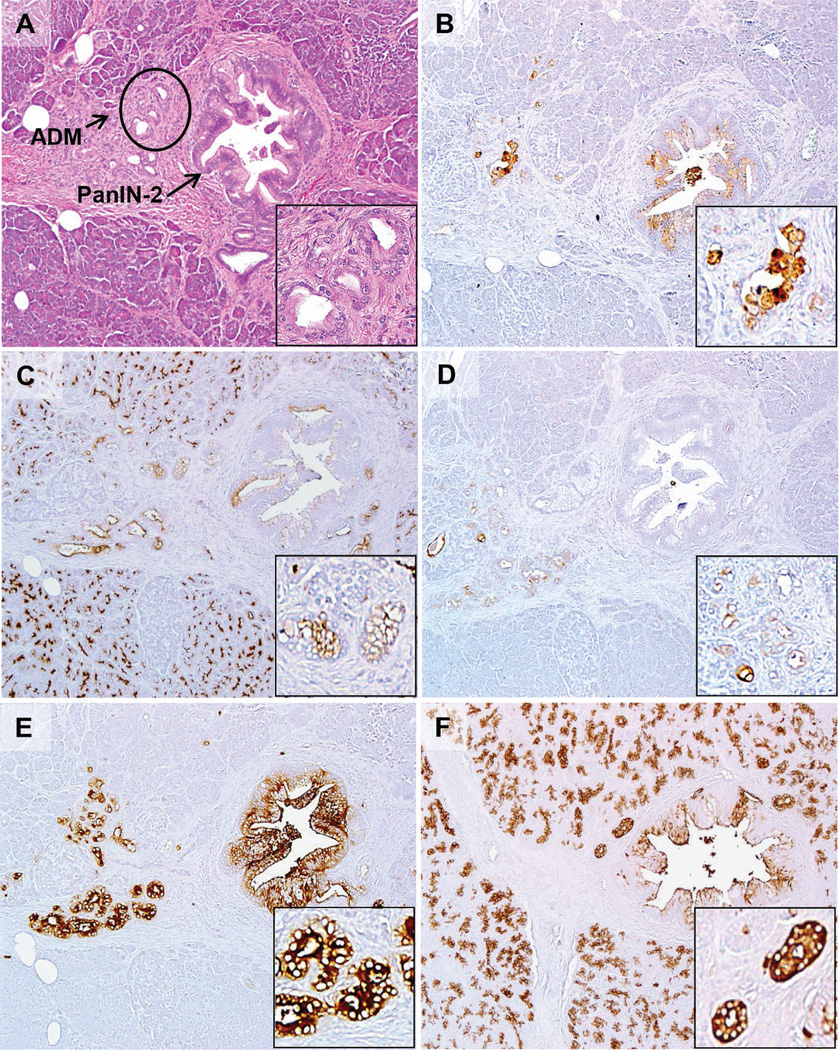
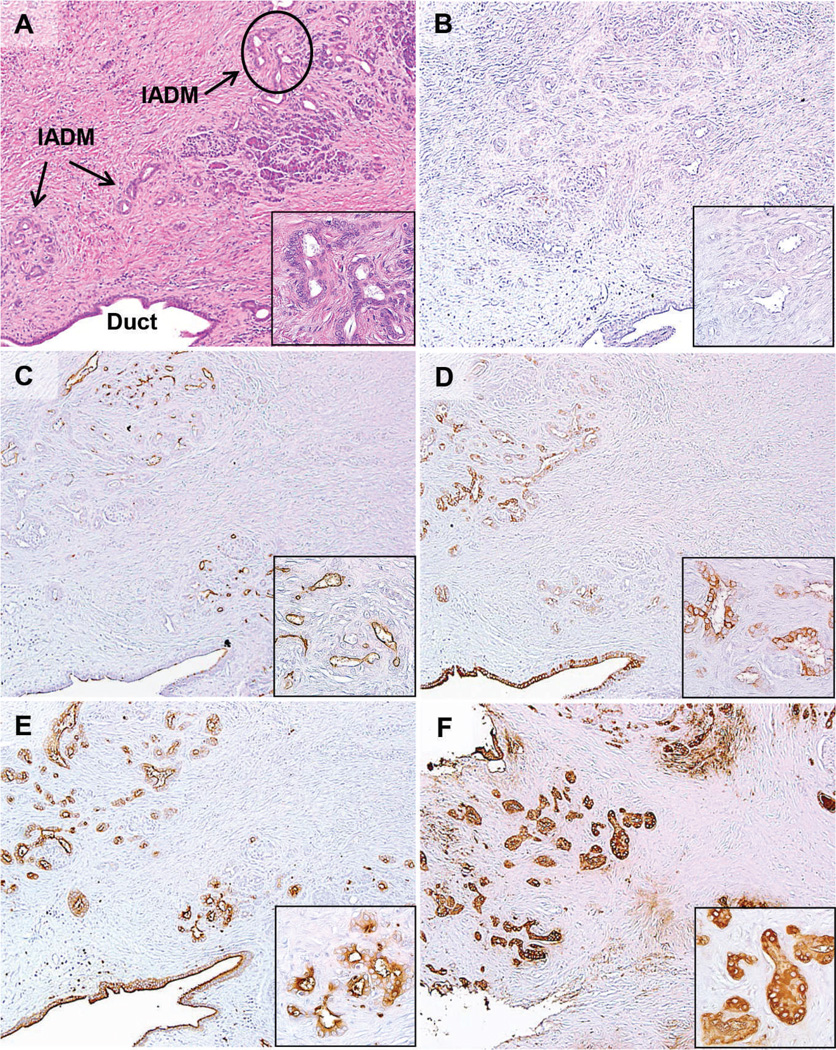
Immunohistochemistry was then performed on sections of resected specimens from 32 patients with CP (Table 2). PAM4 labeled 19% (6 of 32) of the CP specimens; however, reactivity was restricted to the benign, neoplastic PanIN lesions associated with CP, and in 1 case, PanIN-associated ADM (Figure 2, B; the hematoxylin-eosin–stained section is shown in Figure 2, A). Nonneoplastic, inflamed parenchyma was negative in all 32 cases (Figure 3, B; the hematoxylineosin– stained section is shown in Figure 3, A). In contrast, the expression of MUC1 (Figures 2, C, and 3, C), MUC4 (Figures 2, D, and 3, D), CEACAM5/6 (Figures 2, E, and 3, E), and CA19-9 (Figures 2, F, and 3, F) were detected in 90% (27 of 30), 78% (25 of 32), 97% (31 of 32), and 100% (32 of 32), respectively, of CP specimens. In all of these positively labeled tissues, the reactivity was present in nonneoplastic, inflamed pancreatic tissue in addition to the PanINs. Pancreatic intraepithelial neoplasias were identified in 10 of 32 CP cases; 6 of the 10 were positive for both PAM4 and MUC1 biomarkers, with only 3 of these cases demonstrating expression of MUC4. Monoclonal antibodies reactive with CEACAM5/6 and CA19-9 labeled PanINs in all 10 cases and, in addition, anti-CEACAM5/6 labeled background inflammatory cells (granulocytes). A nonbinding control, MAb-Ag8, was evaluated on all tissue specimens with routine negative results (not shown).
Table 2.
Expression of Biomarkers in Benign Pancreatic Disease
| No. | PAM4 | MUC1 | MUC4 | CEACAM5/6 | CA19-9 | |
|---|---|---|---|---|---|---|
| Chronic pancreatitis | 32 | |||||
| PanIN1 | 5 | 2 | 2 | 1 | 5 | 5 |
| PanIN2 | 5 | 4 | 4 | 3 | 5 | 5 |
| Ducts | 32a | 0 | 22 | 25 | 31 | 29 |
| Acinar cells | 32a | 0 | 27 | 8 | 30 | 29 |
| Isolated ADM | 32a | 0 | 24 | 0 | 0 | 26 |
| Benign cysts | 19 | |||||
| SCA | 15 | 1 | 8 | 1 | 2 | 10 |
| Lymphoepithelial | 2 | 0 | 2 | 1 | 2 | 2 |
| Retention | 2 | 0 | 1 | 2 | 2 | 2 |
Abbreviations: ADM, acinar ductal metaplasia; PanIN, pancreatic intraepithelial neoplasia; SCA, serous cystadenoma.
N = 30 for MUC1; N = 29 for CA19-9 ducts and isolated ADM; N = 26 for acinar cells.
Figure 2.
Expression of the PAM4 biomarker, MUC1, MUC4, CEACAM5/6, and CA19-9 in a pancreatic intraepithelial neoplastic (PanIN-2) lesion identified within a case of chronic pancreatitis. A, The hematoxylin-eosin section shows the PanIN-2 arising within a background of chronic pancreatitis with partial loss of acinar cells, some fibrosis, and acinar ductal metaplasia (ADM). Arrows are pointed at PanIN-associated ADM, with the circled ADM shown at higher magnification in the inset. B, PAM4 labeled the PanIN lesion along with focal chronic pancreatitis-associated ADM. C, MUC1 was expressed by the PanIN lesion, as well as associated ADM and acinar cells. D, MUC4 was present within associated ADM, but not the PanIN lesion in this case. E, CEACAM5/6 showed strong labeling of the PanIN lesion, ADM, and inflammatory cells. F, CA19-9 showed moderate labeling of the PanIN lesion, but also labeled the acinar cells, duct, and ADM (original magnifications ×40 [A–F] and ×200 [A–F insets]).
Figure 3.
Expression of PAM4 biomarker, MUC1, MUC4, CEACAM5/6, and CA19-9 in a chronic pancreatitis specimen without pancreatic intraepithelial neoplasia. A, Hematoxylin-eosin shows chronic pancreatitis with extensive fibrosis, distorted pancreatic ducts, acinar cell atrophy, and isolated acinar ductal metaplasia (IADM). No labeling was observed with (B) MAb-PAM4 in any of the tissues within chronic pancreatitis, including IADM (arrows point to IADM with the circled area shown at higher magnification in the inset); however, the other biomarkers, (C) MUC1, (D) MUC4, (E) CEACAM5/6, and (F) CA19-9, were each identified within the isolated ADM, pancreatic ducts, and acinar cells (original magnifications ×40 [A–F] and ×200 [A–F insets]).
Benign, Nonmucinous Cystic Lesions
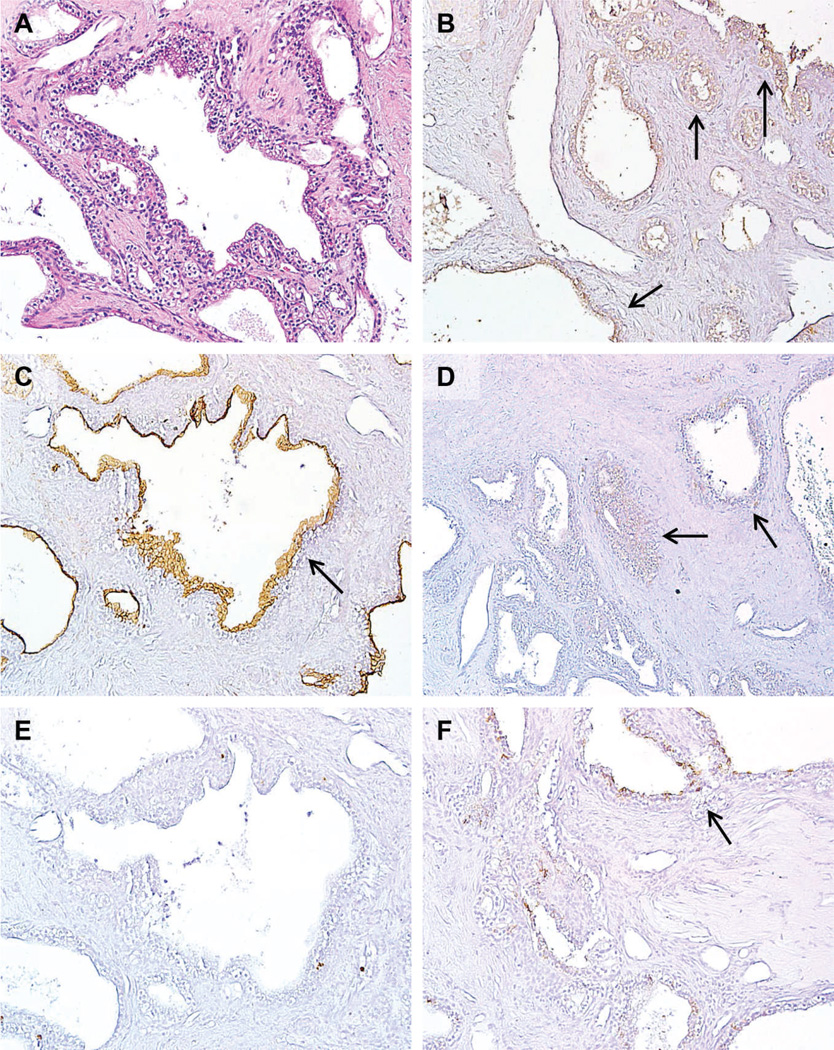
Nineteen benign, nonmucinous cystic lesions of the pancreas, including 15 serous cystadenomas (SCAs) and 4 benign cysts with squamous lining, were examined (Table 2). Serous cystadenomas are composed of uniform cuboidal, glycogen-rich epithelial cells forming small cysts that contain serous fluid (Figure 4, A). PAM4 labeled only 1 of 15 SCAs with a diffuse, weak stain (Figure 4, B). Similarly, MUC4 was expressed in only 1 SCA, again with a diffuse, weak stain (Figure 4, D). On the other hand, MUC1 (Figure 4, C) and CA19-9 (Figure 4, F) were present in more than half of the SCAs (53% and 67%, respectively) with some specimens having an intense stain. Although most of the SCAs did not express CEACAM5/6, very focal CEACAM5/6 labeling was present in 2 of 15 SCAs (13%; Figure 4, E).
Figure 4.
Expression of PAM4 biomarker, MUC1, MUC4, CEACAM5/6, and CA19-9 in a representative case of benign serous cystadenoma (SCA). (A) The hematoxylin-eosin section shows a multilocular cyst lined with cuboidal epithelial cells. This is the only case, of 15 cases, where (B) MAb- PAM4 and (D) MUC4 showed a diffuse, weak labeling (arrows). (C) MUC1 labeling appeared to be significantly greater than the PAM4 biomarker and MUC4, with (E) CEACAM5/6 showing little to no expression in these benign lesions, and (F) CA19-9 showing mostly weak labeling of the cyst linings (original magnifications ×40).
Lymphoepithelial cyst is a rare benign cystic lesion of the pancreas. The walls of the cyst are lined by mature squamous epithelium surrounded by dense lymphoid tissue. Retention cysts are small, dilated pancreatic duct side branches that arise because of obstruction. Squamous metaplasia is frequently associated with retention cysts. We examined the expression of PAM4 antigen in 2 lymphoepithelial cysts and 2 retention cysts with squamous cell lining; PAM4 did not label any of the cysts examined. However, MAbs reactive with MUC1, MUC4, CEACAM5/6, and CA19-9 stained squamous cells in 3, 3, 4, and 4 of the 4 cystic lesions, respectively. Although the immunoreactivity to MUC1 and MUC4 was focal and weak, CEACAM5/6 and CA19-9 labeling was diffuse and strong. Overall, PAM4 antigen, MUC1, MUC4, CEACAM5/6, and CA19-9 were expressed in 1 (5%), 11 (58%), 4 (16%), 6 (32%), and 14 (74%) of 19 benign nonmucinous cysts examined, respectively.
COMMENT
Pancreatic ductal adenocarcinoma is the eighth most common malignancy in the United States. However, it is the fourth leading cause of cancer-related deaths,18 in large measure because of our inability to detect PDAC at early stages of tumor growth. Unfortunately, efforts toward solving this problem face several major challenges. Patients with early pancreatic cancer are generally asymptomatic or present clinically with only vague symptoms not necessarily suspicious of PDAC. Further, distinguishing PDAC from nonneoplastic pancreatic disease, particularly CP, can be difficult and sometimes impossible at both the clinical and radiographic levels.19,20 Thus, the search for biomarkers able to detect and diagnose PDAC is of great interest. At present, the only serum biomarker that is used conventionally for PDAC is mucin-associated carbohydrate antigen CA19-9.21–23 However, serum CA19-9 is frequently elevated in nonneoplastic conditions, such as CP and benign obstructive jaundice, and is also expressed by several nonpancreatic cancers.22,23 Thus, it is of limited value for differential diagnosis, but has proven of clinical value for monitoring disease status.21
With CA19-9 serving as a starting point, the literature is now replete with reports of potential biomarkers for PDAC. For the most part, reverse transcription polymerase chain reaction or polyclonal antibodies and MAbs have been used as a means to identify biomarkers with specificity for malignant neoplasia, but more recently, efforts are being directed toward evaluation of genomic, proteomic, and micro-RNA profiles as a means to differentiate benign and malignant disease of the pancreas. Several of these potential biomarkers, for example, MIC-1, mesothelin, mi-R21/155, and MUC4,24–29 like the CA19-9 marker, are expressed at high frequency in PDAC, and may prove useful in the overall scheme for detection of PDAC, but to date, none has demonstrated sufficient ability to differentiate PDAC from nonneoplastic lesions for true diagnostic accuracy.
In several prior reports, we have shown that PAM4 identifies a biomarker expressed by 90% of PDAC, as well as the precursor lesions PanIN and intraductal papillary mucinous neoplasm, and shows high specificity for PDAC and precursor lesions versus benign, nonneoplastic pancreatic tissues.12,13 Based upon these results, we developed an enzyme immunoassay to detect circulating PAM4 biomarker as a means for the detection and diagnosis of PDAC.10,11,14 Sensitivity of the assay is high (71% and 91%, respectively, for early- and late-stage PDAC); however, approximately 20% of the sera from patients diagnosed with CP were considered positive. Thus, the purpose of the present study was to evaluate critically the specificity of the PAM4 antibody with respect to the discrimination of PDAC and CP, and other benign, nonneoplastic lesions of the pancreas. We included several other potential biomarkers in our studies for comparative purposes: MUC1 (MAb-MA5), MUC4 (MAb-8G7), CEACAM5/6 (MAb-MN-15), and CA19-9.23,30–32
Our results indicate that PAM4 is not reactive with the nonneoplastic tissues from CP patients, but rather with PDAC and its neoplastic precursor lesions, such as PanINs, which are known to develop within the inflamed CP parenchyma. Together with results from a prior study,11 we have evaluated a total of 51 specimens of CP, finding that in no instance was PAM4 reactive with the inflamed parenchyma. On the other hand, each of the other biomarkers investigated, MUC1, MUC4, CEACAM5/6, and CA19-9, as detected by the above listed MAbs, was unable to differentiate PDAC and benign, nonneoplastic tissues. These latter biomarkers were expressed to varying extent in CP-associated PanIN lesions, but also in nonneoplastic ducts and isolated ADM.
However, comparative biomarker evaluations such as performed here and elsewhere suffer from the challenge of comparing biomarker antigen expression versus detection (accessibility?) of specific epitope structures within the biomarker antigen. As an example, the percentage of PDAC cases identified as positive for the MUC4 biomarker is dependent upon the antibody used for detection. Immunohistochemical studies performed with 2 different rabbit polyclonal antibodies have reported MUC4 expression rates of 50% and 77%, respectively, in PDAC.33,34 Another immunohistochemical study reported a MUC4 expression rate of only 30% when using MAb-1G8.35 We used the 8G7, anti-MUC4 MAb in our studies showing that 67% of PDAC cases are positive for expression of this biomarker. However, even when using the identical MAb, in this case 8G7, it appears that other factors come into play; Swartz et al32 and Jhala et al36 reported that 90% of PDAC expressed 8G7-defined MUC4, yet Saitou et al37 reported only 32% of PDAC cases expressed 8G7-defined MUC4.
Although frequency of expression (sensitivity) is an important metric for eventual clinical use of a biomarker, the important observation from the current study is the specificity of the MAb, PAM4, with respect to PDAC, its neoplastic precursors, and nonneoplastic disease. This then provides the significance for true clinical interpretation of the biomarker results. Thus, we hypothesize that CP patients (and perhaps others having disease with high risk for development of PDAC) who are found to have elevated levels of PAM4 antigen in the circulation may harbor occult PDAC, or have significant numbers of precursor lesions producing the PAM4 biomarker.
For the present study, we also evaluated expression of the PAM4 antigen within benign, nonmucinous cystic lesions of the pancreas. With newer radiographic technologies, cystic lesions within the pancreas are being detected with increasing frequency.38–40 Differentiation of cystic lesions with and without malignant potential has become an important issue for decisions on treatment such as surgical resection versus observation alone. Intraductal papillary mucinous neoplasm and mucinous cystic neoplasm are the 2 radiographically detectable precursor lesions for PDAC.41,42 However, current imaging modalities are some-times not sufficiently specific to completely distinguish these lesions from many benign cysts that may have near zero malignant potential.43 For example, there is overlap in imaging appearance between branch intraductal papillary mucinous neoplasms and SCAs, with the latter almost never progressing to invasive carcinoma. Serous cystadenomas account for 10% to 15% of cystic lesions in the pancreas. Accurate differentiation of SCAs from intraductal papillary mucinous neoplasms and mucinous cystic neoplasms, therefore, could avoid excessive surgery. In prior studies, we have shown that approximately 90% of intraductal papillary mucinous neoplasms express the PAM4 antigen.12 Thus, the current findings, in which MAb-PAM4 showed reactivity with only 1 of 19 benign, nonmucinous cystic lesions, suggest the use of this antibody for differentiation of cystic lesions with and without malignant potential, perhaps via immunohistochemical labeling of retrieved fine-needle aspirates and/or biopsy, or via enzyme immunoassay of cystic fluids, as has been reported for detection and quantitation of CEACAM5.44,45 However, it should be pointed out that PAM4 is reactive with normal gastric mucosa,12 which could contaminate fine-needle aspirates of pancreatic tissue and/or fluids when retrieved through the stomach wall. Based upon the results of the current study, further investigation to evaluate larger numbers of these cystic lesions is warranted.
In summary, of all the biomarkers evaluated in this study, only the PAM4 biomarker was able to discriminate PDAC (and the neoplastic PanIN precursor lesions) from benign, nonneoplastic pancreatic tissues, particularly inflamed CP parenchyma. Importantly, the data support the notion that CP patients who are serum positive for expression of PAM4 antigen may have underlying PDAC and/or precursor lesions. Whether or not a positive reaction is of clinical significance is an issue for further investigation. We are now conducting a paired specimen evaluation of presurgical serum and tissue specimens from the same individuals undergoing resection of the pancreas because of CP, in order to determine if a clear biological association exists between the presence of PAM4-positive neoplasia in the tissue and circulating antigen levels. However, this is a difficult project, because pancreatectomy is not the usual treatment for CP.
The potential for prevention of PDAC by detection of PanIN lesions, particularly high-grade, multifocal PanINs, has been considered;46 however, once again, these lesions are mostly asymptomatic and not usually detectable by radiography. Nevertheless, the current data suggest that physicians may want to provide follow-up investigation for the presence of PDAC to individuals with high levels of circulating PAM4 antigen and/or PAM4-positive tissue.
Footnotes
Dr Goldenberg is a shareholder and board member of Immunomedics, Inc. Drs Goldenberg and Gold are inventors of relevant patents (PAM4 antibody). The other authors have no relevant financial interest in the products or companies described in this article.
References
- 1.Brentnall TA, Bronner MP, Byrd DR, Haggitt RC, Kimmey MB. Early diagnosis and treatment of pancreatic dysplasia in patients with a family history of pancreatic cancer. Ann Intern Med. 1999;131(4):247–255. doi: 10.7326/0003-4819-131-4-199908170-00003. [DOI] [PubMed] [Google Scholar]
- 2.Harinck F, Poley JW, Kluijt I, Fockens P, Bruno MJ. Is early diagnosis of pancreatic cancer fiction?: surveillance of individuals at high risk for pancreatic cancer. Dig Dis. 2010;28(4–5):670–678. doi: 10.1159/000320095. [DOI] [PubMed] [Google Scholar]
- 3.Hruban RH, Canto MI, Goggins M, Schulick R, Klein AP. Update on familial pancreatic cancer. Adv Surg. 2010;44(10):293–311. doi: 10.1016/j.yasu.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lowenfels AB, Maisonneuve P, Cavallini G, et al. Pancreatitis and the risk of pancreatic cancer. N Engl J Med. 1993;328(20):1433–1437. doi: 10.1056/NEJM199305203282001. [DOI] [PubMed] [Google Scholar]
- 5.Raimondi S, Lowenfels AB, Morselli-Labate AM, Maisonneuve P, Pezzilli R. Pancreatic cancer in chronic pancreatitis; aetiology, incidence, and early detection. Best Pract Res Clin Gastroenterol. 2010;24(3):349–358. doi: 10.1016/j.bpg.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Chari ST, Leibson CL, Rabe KG, Ransom J, de Andrade M, Petersen GM. Probability of pancreatic cancer following diabetes: a population-based study. Gastroenterology. 2005;129(2):504–511. doi: 10.1053/j.gastro.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pannala R, Basu A, Petersen GM, Chari ST. New-onset diabetes: a potential clue to the early diagnosis of pancreatic cancer. Lancet Oncol. 2009;10(1):88–95. doi: 10.1016/S1470-2045(08)70337-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canto MI, Hruban RH, Fishman EK, et al. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology. 2012;142(4):796–804. doi: 10.1053/j.gastro.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langer P, Kann PH, Fendrich V, et al. Five years of prospective screening of high-risk individuals from families with familial pancreatic cancer. Gut. 2009;58(10):1410–1418. doi: 10.1136/gut.2008.171611. [DOI] [PubMed] [Google Scholar]
- 10.Gold DV, Gaedcke J, Ghadimi BM, et al. Detection of early-stage pancreatic ductal adenocarcinoma (PDAC): sensitivity, specificity, and discriminatory properties of the serum-based PAM4-immunoassay. Cancer. 2013;119(3):522–528. doi: 10.1002/cncr.27762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gold DV, Goggins M, Modrak DE, et al. Detection of early-stage pancreatic adenocarcinoma. Cancer Epidemiol Biomarkers Prev. 2010;19(11):2786–2794. doi: 10.1158/1055-9965.EPI-10-0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gold DV, Karanjawala Z, Modrak DE, Goldenberg DM, Hruban RH. PAM4-reactive MUC1 is a biomarker for early pancreatic adenocarcinoma. Clin Cancer Res. 2007;13(24):7380–7387. doi: 10.1158/1078-0432.CCR-07-1488. [DOI] [PubMed] [Google Scholar]
- 13.Gold DV, Lew K, Maliniak R, Hernandez M, Cardillo T. Characterization of monoclonal antibody PAM4 reactive with a pancreatic cancer mucin. Int J Cancer. 1994;57(2):204–210. doi: 10.1002/ijc.2910570213. [DOI] [PubMed] [Google Scholar]
- 14.Gold DV, Modrak DE, Ying Z, Cardillo TM, Sharkey RM, Goldenberg DM. New MUC1 serum immunoassay differentiates pancreatic cancer from pancreatitis. J Clin Oncol. 2006;24(2):252–258. doi: 10.1200/JCO.2005.02.8282. [DOI] [PubMed] [Google Scholar]
- 15.Ishida M, Major PP, Ura Y, Dion AS. Related glycoproteins from normal secretory and malignant breast cells. Purification and initial comparative characterizations. Tumor Biol. 1989;10(1):12–24. doi: 10.1159/000217589. [DOI] [PubMed] [Google Scholar]
- 16.Moniaux N, Varshney GC, Chauhan SC, et al. Generation and characterization of anti-MUC4 monoclonal antibodies reactive with normal and cancer cells in humans. J Histochem Cytochem. 2004;52(2):253–261. doi: 10.1177/002215540405200213. [DOI] [PubMed] [Google Scholar]
- 17.Shi C, Hong SM, Lim P, et al. KRAS2 mutations in human pancreatic acinar-ductal metaplastic lesions are limited to those with PanIN: implications for the human pancreatic cancer cell of origin. Mol Cancer Res. 2009;7:230–236. doi: 10.1158/1541-7786.MCR-08-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4):212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 19.Oto A, Eltorky MA, Dave A, et al. Mimicks of pancreatic malignancy in patients with chronic pancreatitis: correlation of computed tomography imaging features with histopathologic findings. Curr Probl Diagn Radiol. 2006;35(5):199–205. doi: 10.1067/j.cpradiol.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Taylor B. Carcinoma of the head of the pancreas versus chronic pancreatitis: diagnostic dilemma with significant consequences. World J Surg. 2003;27(11):1249–1257. doi: 10.1007/s00268-003-7245-8. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez JM, Cowgill SM, Al-Saadi S, et al. CA 19-9 velocity predicts disease-free survival and overall survival after pancreatectomy of curative intent. J Gastrointest Surg. 2009;13(2):349–353. doi: 10.1007/s11605-008-0696-3. [DOI] [PubMed] [Google Scholar]
- 22.Itzkowitz SH, Yuan M, Fukushi Y, et al. Immunohistochemical comparison of Lea, monosialosyl Lea (CA 19-9), and disialosyl Lea antigens in human colorectal and pancreatic tissues. Cancer Res. 1988;48(13):3834–3842. [PubMed] [Google Scholar]
- 23.Park HU, Kim JW, Kim GE, et al. Aberrant expression of MUC3 and MUC4 membrane-associated mucins and sialyl Le(x) antigen in pancreatic intraepithelial neoplasia. Pancreas. 2003;26(3):e48–e54. doi: 10.1097/00006676-200304000-00022. [DOI] [PubMed] [Google Scholar]
- 24.Andrianifahanana M, Moniaux N, Schmied BM, et al. Mucin (MUC) gene expression in human pancreatic adenocarcinoma and chronic pancreatitis: a potential role of MUC4 as a tumor marker of diagnostic significance. Clin Cancer Res. 2001;7(12):4033–4040. [PubMed] [Google Scholar]
- 25.Argani P, Iacobuzio-Donahue C, Ryu B, et al. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE) Clin Cancer Res. 2001;7(12):3862–3868. [PubMed] [Google Scholar]
- 26.Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol. 2007;33(3):266–270. doi: 10.1016/j.ejso.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Habbe N, Koorstra JB, Mendell JT, et al. MicroRNA miR-155 is a biomarker of early pancreatic neoplasia. Cancer Biol Ther. 2009;8(4):340–346. doi: 10.4161/cbt.8.4.7338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iacobuzio-Donahue CA, Ashfaq R, Maitra A, et al. Highly expressed genes in pancreatic ductal adenocarcinomas: a comprehensive characterization and comparison of the transcription profiles obtained from three major technologies. Cancer Res. 2003;63(24):8614–8622. [PubMed] [Google Scholar]
- 29.Koopmann J, Rosenzweig CN, Zhang Z, et al. Serum markers in patients with resectable pancreatic adenocarcinoma: macrophage inhibitory cytokine 1 versus CA19-9. Clin Cancer Res. 2006;12(2):442–446. doi: 10.1158/1078-0432.CCR-05-0564. [DOI] [PubMed] [Google Scholar]
- 30.Adsay NV, Merati K, Andea A, et al. The dichotomy in the preinvasive neoplasia to invasive carcinoma sequence in the pancreas: differential expression of MUC1 and MUC2 supports the existence of two separate pathways of carcinogenesis. Mod Pathol. 2002;15(10):1087–1095. doi: 10.1097/01.MP.0000028647.98725.8B. [DOI] [PubMed] [Google Scholar]
- 31.Kim GE, Bae HI, Park HU, et al. Aberrant expression of MUC5AC and MUC6 gastric mucins and sialyl Tn antigen in intraepithelial neoplasms of the pancreas. Gastroenterology. 2002;123(4):1052–1060. doi: 10.1053/gast.2002.36018. [DOI] [PubMed] [Google Scholar]
- 32.Swartz MJ, Batra SK, Varshney GC, et al. MUC4 expression increases progressively in pancreatic intraepithelial neoplasia. Am J Clin Pathol. 2002;117(5):791–796. doi: 10.1309/7Y7N-M1WM-R0YK-M2VA. [DOI] [PubMed] [Google Scholar]
- 33.Bhardwaj A, Marsh WL, Jr, Nash JW, Barbacioru CC, Jones S, Frankel WL. Double immunohistochemical staining with MUC4/p53 is useful in the distinction of pancreatic adenocarcinoma from chronic pancreatitis: a tissue microarray-based study. Arch Pathol Lab Med. 2007;131(4):556–562. doi: 10.5858/2007-131-556-DISWPI. [DOI] [PubMed] [Google Scholar]
- 34.Liu H, Shi J, Anandan V, et al. Reevaluation and identification of the best immunohistochemical panel (pVHL, Maspin, S100P, IMP-3) for ductal adenocarcinoma of the pancreas. Arch Pathol Lab Med. 2012;136(6):601–609. doi: 10.5858/arpa.2011-0326-OA. [DOI] [PubMed] [Google Scholar]
- 35.Westgaard A, Schjlberg AR, Cvancarova M. Differentiation markers in pancreatic head adenocarcinomas: MUC1 and MUC4 expression indicates poor prognosis in pancreatobiliary differentiated tumours. Histopathology. 2009;54(3):337–347. doi: 10.1111/j.1365-2559.2009.03227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jhala N, Jhala D, Vickers SM, et al. Biomarkers in diagnosis of pancreatic carcinoma in fine-needle aspirates. Am J Clin Pathol. 2006;126(4):572–579. doi: 10.1309/cev30be088cbdqd9. [DOI] [PubMed] [Google Scholar]
- 37.Saitou M, Goto M, Horinouchi M, et al. MUC4 expression is a novel prognostic factor in patients with invasive ductal carcinoma of the pancreas. J Clin Pathol. 2005;58(8):845–852. doi: 10.1136/jcp.2004.023572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brugge WR. The incidental pancreatic cyst on abdominal computerized tomography imaging: diagnosis and management. Clin Gastroenterol Hepatol. 2008;6(2):140–144. doi: 10.1016/j.cgh.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 39.Brugge WR, Lewandrowski K, Lee-Lewandrowski E, et al. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126(5):1330–1336. doi: 10.1053/j.gastro.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 40.Fernández-del Castillo C, Targarona J, Thayer SP, Rattner DW, Brugge WR, Warshaw AL. Incidental pancreatic cysts: clinicopathologic characteristics and comparison with symptomatic patients. Arch Surg. 2003;138(4):427–423. doi: 10.1001/archsurg.138.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hong SM, Park JY, Hruban RH, Goggins M. Molecular signatures of pancreatic cancer. Arch Pathol Lab Med. 2011;135(6):716–727. doi: 10.5858/2010-0566-ra.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hruban RH, Takaori K, Klimstra DS, et al. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004;28(8):977–987. doi: 10.1097/01.pas.0000126675.59108.80. [DOI] [PubMed] [Google Scholar]
- 43.Katz DS, Friedel DM, Kho D, Georgiou N, Hines JJ. Relative accuracy of CT and MRI for characterization of cystic pancreatic masses. Am J Roentgenol. 2007;189(3):657–661. doi: 10.2214/AJR.07.2772. [DOI] [PubMed] [Google Scholar]
- 44.Toll AD, Kowalski T, Loren D, Bibbo M. The added value of molecular testing in small pancreatic cysts. JOP. 2010;11(6):582–586. [PubMed] [Google Scholar]
- 45.Mertz H. K-ras mutations correlate with atypical cytology and elevated CEA levels in pancreatic cystic neoplasms. Dig Dis Sci. 2011;56(7):2197–2201. doi: 10.1007/s10620-010-1556-z. [DOI] [PubMed] [Google Scholar]
- 46.Kern S, Hruban R, Hollingsworth MA, et al. A white paper: the product of a pancreas cancer think tank. Cancer Res. 2001;61(12):4923–4932. [PubMed] [Google Scholar]