Abstract
The ubiquitous distribution of lysosomes and their heterogeneous protein composition reflects the versatility of these organelles in maintaining cell homeostasis and their importance in tissue differentiation and remodeling. In lysosomes, the degradation of complex, macromolecular substrates requires the synergistic action of multiple hydrolases that usually work in a stepwise fashion. This catalytic machinery explains the existence of lysosomal enzyme complexes that can be dynamically assembled and disassembled to efficiently and quickly adapt to the pool of substrates to be processed or degraded, adding extra tiers to the regulation of the individual protein components. An example of such a complex is the one composed of three hydrolases that are ubiquitously but differentially expressed: the serine carboxypeptidase, protective protein/cathepsin A (PPCA), the sialidase, neuraminidase-1 (NEU1), and the glycosidase β-galactosidase (β-GAL). Next to this ‘core’ complex, the existence of sub-complexes, which may contain additional components, and function at the cell surface or extracellularly, suggests as yet unexplored functions of these enzymes. Here we review how studies of basic biological processes in the mouse models of three lysosomal storage disorders, galactosialidosis, sialidosis, and GM1-gangliosidosis, revealed new and unexpected roles for the three respective affected enzymes, Ppca, Neu1, and β-Gal, that go beyond their canonical degradative activities. These findings have broadened our perspective on their functions and may pave the way for the development of new therapies for these lysosomal storage disorders.
Keywords: Protective protein/cathepsin A (PPCA), Neuraminidase 1 (NEU1), β-Galactosidase (β-GAL), Lysosomal exocytosis, Sialic acid, Elastin-binding protein (EBP)
Introduction
Multienzyme complexes allow for a tighter regulation and more rapid and efficient response to changes in equilibrium between substrate supply and demand than would the individual enzymes. Examples of such complexes are those formed by lysosomal hydrolases that are responsible for the coordinated degradation of glycosphingolipids, glycosaminoglycans, oligosaccharides, and glycoproteins. While not all lysosomal enzymes require accessory proteins or co-factors to recognize and hydrolyze their target substrates, several of them work exclusively in complexes of two or more proteins. In addition, auxiliary, non-catalytic proteins may be part of such complexes and function as activators, cofactors, stabilizers, substrate sensors, or substrate binders. Enzyme complexes act in specific metabolic/catabolic pathways, where each enzyme takes the product of the previous enzyme as substrate, and may be regulated by the levels of the substrate and/or the demand of the end product. An example of such complexes is the one composed of the two glycosidases, neuraminidase 1 (NEU1) and β-galactosidase (β-GAL), and of the serine carboxypeptidase, protective protein/cathepsin A (PPCA) [1]. The dynamic and regulated interplay among these three enzymes, either in complex or alone, favors the acquisition of an optimal composition to meet the metabolic or catabolic needs of individual cell types in different tissues and organs.
Hydrolytic composition of a prototypical lysosomal multienzyme complex
Evidence for the existence of this high-molecular-weight (>1,000 kDa) lysosomal multienzyme complex (LMC) came from genetic and biochemical studies dating back to the early 1980s [1, 2]. These and later studies established the three-enzyme composition of the LMC and identified PPCA as the scaffold without which the complex is not formed [3, 4]. Although the enzyme N-acetylgalactosamine-6-sulfate sulfatase (GALNS) was found to be part of this complex in one study [5], it is still unclear whether its activity and function depend on its interaction with any of the other partners. Therefore, in this review we will focus solely on the core components of the LMC, PPCA, NEU1, and β-GAL, as well as a catalytically inactive shorter variant of the latter enzyme, the elastin-binding protein (EBP) (see Table 1).
Table 1.
Components of the multienzyme complex
| Enzyme/protein | Disease | Gene locus | Substrates | Pathways |
|---|---|---|---|---|
| PPCA | Galactosialidosis | 20q13.1 |
Bioactive peptidesa LAMP2A |
Blood pressure regulation Chaperone-mediated autophagy |
| NEU1 |
Sialidosis Galactosialidosisb Colon and adenocarcinoma |
6p21.3 | Sialoglycoproteinsc on LM, cell surface, and ECM |
Lysosomal exocytosis Cell proliferation cancer migration/invasion Elastogenesis |
| β-GAL |
GM1-gangliosidosis Galactosialidosisb |
3p21.33 | GM1-ganglioside |
UPR Mitochondrial apoptosis |
| EBP | GM1-gangliosidosis | 3p21.33 | Tropoelastin | Elastogenesis |
| GALNS |
Morquio disease type A Galactosialidosisb |
16q24.3 | Keratin/chondroitin sulfate | Unknown |
aBioactive peptides included endothelin I, angiotensin I, substance P, bradykinin, oxytocin, and other tachykinins
bSecondary deficiency
cSialoglycoproteins include LAMP1, LAMP2, PDGF-R, IGF-1R, MUC1, TLR-2, -3, and -4, and microfibrillar proteins
NEU1 and β-GAL are the first two enzymes in the sequential removal of sugar nucleotides from O-linked- or complex N-linked glycoconjugates starting at their terminal, non-reducing end. NEU1 is active toward sialoglycoconjugates, primarily sialoglycoproteins and sialoglycopeptides, and β-GAL has a preference for GM1-ganglioside (GM1) and keratan sulfate. Further degradation of the oligosaccharide side chains requires the orderly action of β-N-acetylhexosaminidase, β-mannosidase, α-mannosidase, and α-fucosidase. However, there is no experimental evidence for the association of these enzymes with the LMC. We speculate that the cathepsin A activity of PPCA targets the protein side of glycoprotein substrates while in complex with the other two glycosidases.
At least two forms of the LMC can be purified from cells or tissue. The first sub-complex of about 680 kDa contains mainly cathepsin A and β-GAL activities and is devoid of NEU1 activity [6]. The second, much larger complex of about 1.3 MDa includes all measurable NEU1 activity but has low cathepsin A and β-GAL activities [7]. NEU1 remains catalytically active only when associated with PPCA and β-GAL in this large complex. Association of NEU1 and β-GAL with the precursor form of PPCA in an early biosynthetic compartment assures the regulated trafficking of the two glycosidases to the lysosome, as well as their intralysosomal activation and stable conformation [6, 8].
Mammalian PPCA is a member of the serine protease family of enzymes and in this capacity exerts carboxypeptidase, deamidase and esterase activity on selected bioactive peptides, including angiotensin I, substance P, oxytocin, and endothelin I [9–12]. Its one-chain precursor is a zymogen that is cleaved at acidic pH into a two-chain mature and fully active enzyme [4, 7]. The 3D structure of the PPCA zymogen revealed an unusual mechanism of enzyme activation: a two-step proteolysis and removal of an internal excision peptide converts the 54-kDa one-chain precursor into a 32/20-kDa two-chain form, held together by disulfide bridges, thereby uncovering the catalytic pocket already poised for catalysis [13, 14].
Maturation of the β-GAL 86-kDa precursor requires proteolytic processing at its carboxy terminus, releasing a 20-kDa peptide that remains attached to the mature enzyme [6]. In contrast, the 46-kDa NEU1 polypeptide is apparently not proteolytically processed, but only modified at its glycan site en route to the lysosome. The catalytic activation of NEU1 depends on its oligomerization, a process that is mediated by PPCA [7, 15]. Early in biosynthesis PPCA binds to NEU1 to form a heterodimeric complex that prevents the premature oligomerization of the latter enzyme [15]. In complex with PPCA, NEU1 is then transported to the lysosomal compartment via the mannose-6-phospate receptor pathway. Once in lysosomes, PPCA promotes the oligomerization and catalytic activation of NEU1 [7]. Similarly, β-GAL is also routed to the lysosomes in complex with PPCA [6]. In lysosomes, the stable assembly of the three-enzyme complex takes place. Interestingly, the catalytic activity of PPCA does not appear to be necessary for the activation of NEU1 or β-GAL [16]. However, in the absence of PPCA the LMC fails to form [7, 8]. These findings unequivocally defined PPCA as a molecular chaperone for the subcellular localization and activation of NEU1 and β-GAL [7, 8].
Aside from their known degradative capacity, the extent of which is not yet fully understood, recent studies have uncovered additional, new functions of these enzymes in complex or alone, as well as of the substrates they target.
Lysosomal disorders due to deficiency of enzymes within the LMC
The enzymes in the LMC are ubiquitously but differentially expressed in tissues and cells. Their individual expression pattern does not always concur, which hints at their independent roles in metabolic/catabolic pathways even outside the LMC. Their lack of redundancy is exemplified by the occurrence of three distinct lysosomal storage disorders (LSDs): galactosialidosis (GS) or PPCA deficiency with a secondary combined deficiency of NEU1 and β-GAL (OMIM #256540) [17], sialidosis or NEU1 deficiency (OMIM #256550) [18], and GM1-gangliosidosis (GM1) or β-GAL deficiency (OMIM #230500, #23060, #230600) [19, 20]. Each disease is inherited as an autosomal recessive trait and is characterized by a broad spectrum of clinical phenotypes, ranging from early infantile severe forms to late infantile, juvenile/adult cases mostly associated with equally dismal prognosis. All three diseases are systemic, involving visceral organs, bone, cartilage, muscle, and invariably the nervous system. Clinical manifestations often correlate with the type of mutation, level of residual enzyme activity, and extent and type of accumulated substrates [20, 21].
Galactosialidosis, sialidosis
GS and sialidosis belong to the glycoproteinosis subgroup of LSDs [17, 18, 22]. Patients with GS develop clinical manifestations characteristic of these disorders and are usually classified in three clinical forms that are distinguished by the age of onset of the symptoms and the extent and type of phenotypic abnormalities. The early infantile form of GS is associated with hydrops foetalis, inguinal hernia, coarse face, macular cherry-red spot, dysostosis multiplex, enlargement of the spleen, liver, and occasionally the heart, kidney involvement, and early death. Short statures, dysostosis multiplex, heart valve problems, hepatosplenomegaly, coarse facial features, and minor or absent neurological signs characterize the late infantile types [17]. Most reported patients have the juvenile/adult form of the disease and are mainly of Japanese origin. Symptoms include ataxia, myoclonus, angiokeratomas, seizures, progressive cognitive disability, neurologic deterioration, absence of visceromegaly, and long survival [17].
The absence of functional PPCA leads to a near-complete secondary deficiency of NEU1, which explains why GS and sialidosis patients have similar multisystemic disease features. In contrast to NEU1, β-GAL is only partially affected in GS patients, offering an explanation for the limited overlap in clinical characteristics between GS and GM1 diseases (see below) [17]. Patients with sialidosis are usually classified in two main subtypes [18]. Type I (normomorphic) sialidosis is also referred to as cherry-red spot myoclonus syndrome and is the less severe form of the disease. Symptoms include gait disturbance, progressively reduced visual capacity, tremor, ataxia, seizures, and no neurological signs. Type II (dysmorphic) patients instead have a very severe disease and are further divided into congenital and infantile/juvenile forms. Congenital sialidosis is a fulminant condition that develops before birth; the patients are stillborn or die soon after birth [18]. Signs and symptoms of infantile/juvenile sialidosis resemble those diagnosed in the severe forms of GS and include cherry-red spots and myoclonus, coarse facies, hepatosplenomegaly, dysostosis multiplex, vertebral deformities, and severe mental retardation [17, 18].
The biochemical hallmark of both GS and sialidosis is the progressive accumulation of sialylated glycoproteins/glycopeptides and oligosaccharides in lysosomes of many cell types, as well as excretion of sialyloligosaccharides in body fluids, hence the classification of these diseases as glycoproteinoses [17, 18].
Mouse models of GS and sialidosis
Mice homozygous for null mutations at the Ppca and Neu1 loci develop phenotypes closely resembling patients with the severe, early onset forms of the corresponding diseases [23, 24]. Ppca −/− mice maintain the biochemical features, which are diagnostic of GS, namely severe secondary deficiency of Neu1 associated with sialyloligosacchariduria [24]. However, β-Gal activity is only marginally affected in the mouse model and only in certain tissues, indicating less dependency of the murine enzyme on a functional Ppca. The widespread pathologic manifestations of Ppca −/− mice also resemble those in patients with early onset GS and are at least in part similar to the ones found in Neu1 −/− mice [23, 24]; common symptoms include progressive nephropathy, time-dependent splenomegaly, heart involvement, and shortened lifespan, although Ppca −/− mice generally live longer (≤9–10 months) than the Neu1 −/− mice (≤5–6 months) [23, 24]. However, unique to the Ppca −/− mice is the occurrence early in life of a severe and progressive ataxia that is associated with gradual and regional loss of cerebellar Purkinje cells [24, 25]. It is noteworthy that Ppca mRNA expression is markedly variable among murine tissues and that expression levels do not always correlate with the extent of lysosomal storage, particularly in the brain [25]. Thus, loss of Purkinje cells in Ppca −/− mice may reflect a requirement for the catalytic rather that the protective function of PPCA and/or the presence of cell-specific substrates for both Neu1 and Ppca in these neuronal cell types. Although no specific neuronal targets for Ppca have been identified as yet that may explain the loss of Purkinje cells, as discussed above, substrates for this enzyme have been identified in other human cells and tissues. PPCA from platelets [26], endothelial cells [10], or heart [12] has esterase and deamidase activities toward several bioactive peptides that regulate blood circulation and blood pressure. Therefore, cardiomyopathy and arterial hypertension, conditions that are quite common in patients with GS [17], could result from the loss of PPCA catalytic activity rather than that of NEU1 and/or β-GAL. A cardiovascular role of Ppca was confirmed in a knockin mouse model that introduced an amino acid substitution abrogating the cathepsin A activity of PPCA but not the formation of the LMC, nor the activities of the other associated enzymes [27]. However, in these mice, defective cathepsin A activity was insufficient to induce the loss of Purkinje cells seen in the Ppca knockout mice [23, 27]. This indicates that the dual lack of cathepsin A and Neu1 activities may indeed be synergistic to the loss of these neurons.
Neu1 −/− mice develop a multisystemic, severe phenotype closely resembling that of patients with type-II sialidosis [23]. Homozygous-null mice have reduced or undetectable Neu1 activity in most tissues compared to that of wild-type littermates and extensive oligosacchariduria; low levels of residual enzyme activity in some of the tissues likely results from the expression of other mammalian sialidases, NEU2, NEU3, and NEU4 [28–31]. Heterozygous Neu1 −/− mice show intermediate levels of enzyme activity but are phenotypically normal. Shortly after birth, mutant mice exhibit severe nephropathy, splenomegaly, kyphosis, and progressive edema of the subcutaneous tissues, limbs, penis, forehead, and eyelids. Phenotypic abnormalities that appear specific for the sialidosis rather than GS mouse model include progressive deformity of the spine, age-dependent splenic extramedullary hematopoiesis (EMH), and lack of early degeneration of cerebellar Purkinje cells [23]. At the end of their lifespan, Neu1 −/− mice appear weak, lethargic, and debilitated; they suffer from dyspnea, loss of weight, enlarged kidneys, gait abnormalities, and tremor [23].
The differences and similarities identified in these two mouse models should help to better understand the pathophysiology of GS and sialidosis in children, which could in principle translate to more tailored therapy and more focused clinical care for these diseases.
GM1-Gangliosidosis
GM1 is a bona fide glycosphingolipid storage disease, characterized by generalized CNS involvement with mental and motor decline, progressive neurodegeneration, and early death [19]. This condition is usually classified into three major types based on the age of onset of the first symptoms, but in fact given the overlapping of clinical features, GM1 presents with a continuum of the disease spectrum. Type I or infantile GM1 is the most severe form. Patients show signs and symptoms of the disease at ~6 months of age, which begin with muscle weakness and an exaggerated startle response. As the disease progresses, they develop hepatosplenomegaly, dysmorphism, generalized skeletal dysplasia, and corneal clouding with macular cherry-red spots. Most patients die within the first 2 years of life [19]. The late infantile/juvenile form of GM1, also known as type II, has an onset of symptoms between 18 months (late infantile form) to 5 years (juvenile form). Type II patients experience developmental regression and shortened lifespan, but no cherry-red spots, facial abnormalities, or enlarged organs. The third type of GM1 is known as the adult or chronic form, and it represents the mildest end of the disease spectrum. The characteristic features of this type include dystonia and abnormalities of the vertebrae. Life expectancy varies [19].
The primary biochemical and pathologic hallmarks of GM1 are the accumulation of GM1 in the brain and spinal cord, and the formation of membranous cytoplasmic bodies in neurons [32].
Mouse model of GM1
The mouse model of GM1 (β-Gal −/− mice) closely resembles the early onset, severe form of the disease [32]. These mice develop a profound CNS condition characterized by tremors, ataxia, and abnormal gait that culminate with paralysis of the hind limbs. In contrast to the sialidosis and GS mouse models, β-Gal −/− mice have only marginal systemic involvement, but display massive and progressive accumulation of GM1 throughout the brain and the spinal cord, which is associated with the gradual loss of motor functions and widespread CNS inflammation [32, 33]. These features are also characteristic of the human disease [19].
The mouse models of GS, GM1, and sialidosis have led to the discovery of unexpected functions of the respective enzymes and their substrates in normal cell physiology. They have also proven extremely useful for studying molecular mechanisms of disease pathogenesis and for the implementation of various therapeutic modalities, including gene therapy [24, 34–43]. The success of the preclinical studies in the GS mice has set the basis for a future clinical trial for this disease.
The LMC and its components in tissue and cell homeostasis
PPCA regulates chaperone-mediated autophagy
A serendipitous finding gave the first indication of an in vivo physiological role of the cathepsin A activity of PPCA. The enzyme was found to co-purify with the lysosome-associated membrane protein 2a (LAMP2a) from lysosomal preparations of rat liver [44]. LAMP2a is one of three isoforms of LAMP2 that are generated through alternative splicing of its mRNA. They are highly homologous, differing only in the composition of their transmembrane domain and short carboxy-terminal cytoplasmic tail; their heavily glycosylated, luminal domains are identical [45]. Deletion of the Lamp2 gene impairs macroautophagy and results in the accumulation of autophagic vacuoles in most tissues [46]. The LAMP2a is the only isoform that serves as a receptor for chaperone-mediated autophagy (CMA) [47]. CMA is activated in response to cellular stress (e.g., nutrient deprivation and exposure to toxins) and promotes lysosomal internalization and degradation of cytosolic protein substrates [48]. These substrates are equipped with a targeting motif that is recognized by the cytosolic heat shock protein Hsp70 [49], which in turn binds to the short cytosolic tail of LAMP2a. This binding causes the substrate to unfold and translocate across the lysosomal membrane (LM), and eventually be released into the lysosomal matrix and degraded [47, 49]. CMA is activated when the turnover rate of LAMP2a decreases, indicating that LAMP2a regulates this process at the LM [47, 50]. Human and mouse GS fibroblasts showed increased levels of CMA, regardless of nutritional status. Thus, even under normal nutritional conditions, Ppca-deficient cells are actively undergoing CMA [44]. In addition, the rate of LAMP2a degradation decreased in Ppca-deficient cells, indicating that under non-stress conditions PPCA prevents the activation of CMA by targeting LAMP2a for degradation [44]. It is still unclear, however, whether this function of PPCA catalytic activity requires its interaction with NEU1 and β-GAL.
NEU1 is a negative regulator of lysosomal exocytosis
Lysosomal exocytosis is a Ca2+-regulated physiological process that entails the active recruitment of a specific pool of lysosomes by the cytoskeletal network, followed by their docking at the PM and finally their fusion with the PM, which results in the release of their luminal contents into the extracellular space [51–54]. This tightly regulated process that is present in virtually all cell types plays a fundamental role in both physiologic and pathologic processes including the repair and replenishment of damaged PM, the removal of pathogenic bacteria, the release of human immunodeficiency virus from infected cells, and the remodeling of the extracellular matrix (ECM). Several gene defects have been associated with impaired exocytosis of the so-called secretory lysosomes in cytotoxic T lymphocytes, platelets, and melanocytes [55]. Characterization of these genetic defects has enabled the identification of proteins, which are required for the movement of lysosomes along the microtubule network and for their fusion with the PM [51, 53].
With the purpose of unraveling the causes of the EMH phenotype in Neu1 −/− mice, we discovered a new role of NEU1 as a negative regulator of lysosomal exocytosis [54]. The previously unknown substrate of Neu1 that affects a specific step in this process is the lysosome-associated membrane protein 1 or LAMP1. This common marker of the lysosomal compartment has a heavily glycosylated and sialylated N-terminal luminal portion, a transmembrane domain, and a 12-amino-acid cytoplasmic tail. LAMP1 plays a pivotal role in the docking of lysosomes at the PM, although the underlying molecular mechanism is still not fully elucidated [54, 56]. In the absence of NEU1 catalytic activity, impaired removal of the sialic acids from LAMP1 changes the turnover rate of this protein and in turn increases the number of lysosomes poised to dock at the PM ready to fuse with the PM and engage in lysosomal exocytosis upon Ca2+ influx (Fig. 1) [54]. Thus, NEU1, by controlling the sialic acid content of LAMP1, negatively regulates lysosomal exocytosis in multiple tissues and cell types [54, 57, 58]. This conclusion is further supported by the observation that silencing LAMP1 expression in NEU1-deficient cells normalizes the number of lysosomes docked at the PM, which correlates with decreased levels of lysosomal exocytosis.
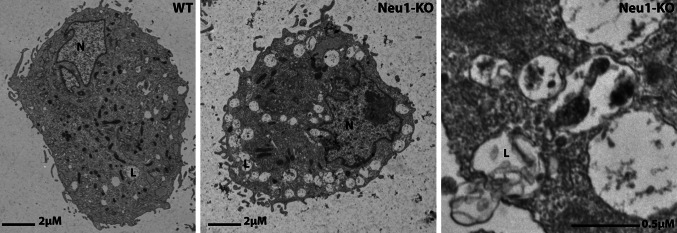
Fig. 1.
Electron microscopy of Neu1 +/+ and Neu1 −/− macrophages shows lysosomes in Neu1 −/− cells that have fused with the PM and are being exocytosed (arrows). Adapted from a research originally published in Developmental Cell [54]. PM plasma membrane
These findings strongly support a role for LAMP1 in the process of anchoring a selected pool of lysosomes to the cytoskeleton and in the migration of these lysosomes to the periphery of the cell [54]. It is tempting to speculate that in all cells, secretory and non-secretory alike, pools of lysosomes exist that vary in their sialic acid profile of LAMP1 and that this in turn is dictated by the level of NEU1 activity in these pools of lysosomes. By this distinction, secretory lysosomes would have low NEU1 activity and in turn hypersialylated LAMP1, which would prime them to be selected for exocytosis. It has long been known that NEU1 activity may be regulated by modulating the composition of the LMC, because the enzyme is exclusively stable and catalytically active when it is in complex with PPCA [7]. Thus, dissociation of NEU1 from PPCA would be an immediate way to reduce the level of NEU1 activity in distinct pools of lysosomes, which consequentially would become poised for lysosomal exocytosis. Given the high degree of similarity between the luminal portions of LAMP1 and LAMP2, and the role that PPCA plays in CMA by binding and targeting the latter protein, we hypothesize that PPCA may also be required for binding to LAMP1, thereby giving NEU1 the opportunity to desialylate this protein.
NEU1 deficiency and excessive lysosomal exocytosis drive the pathogenesis of sialidosis
Studying the molecular bases of pathogenesis in the sialidosis mouse model, we found that the uncontrolled release of lysosomal luminal content from Neu1 −/− cells of different tissues and organs is responsible for at least four disease phenotypes [54, 57, 58, 100]. However, this may constitute only the tip of the iceberg because other disease symptoms present with features that could be explained by deregulated lysosomal exocytosis.
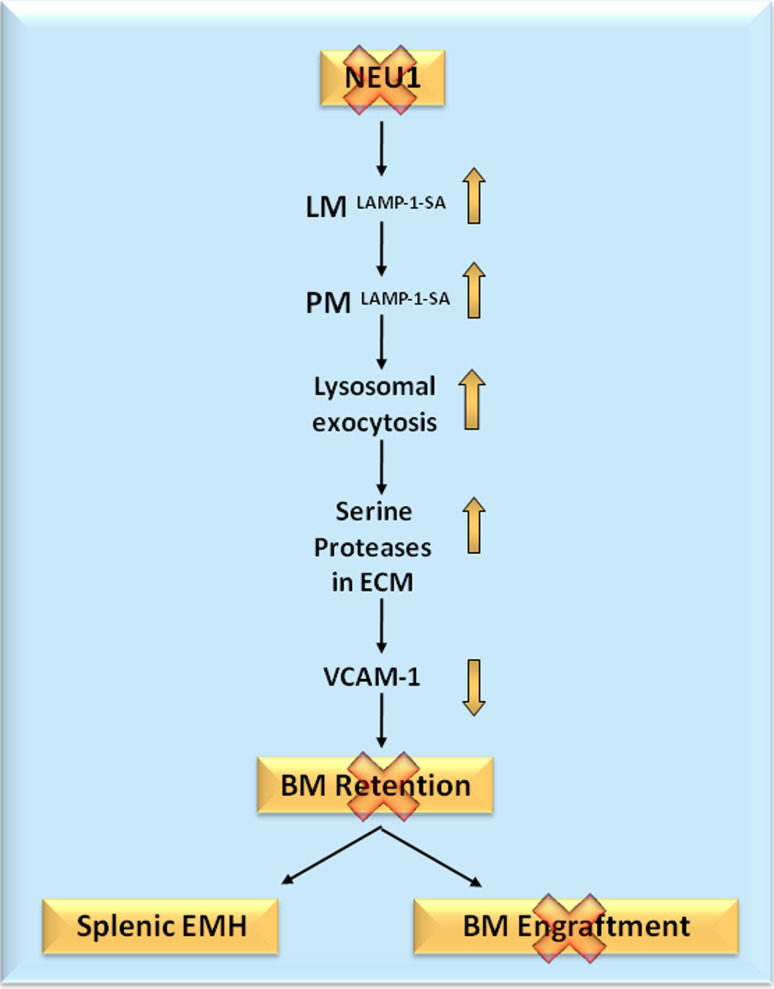
In the bone niche, excessive exocytic release of active lysosomal hydrolases, including neutrophil elastase and cathepsin G, causes premature processing and degradation of vascular cell adhesion molecule 1 (VCAM1) on the surface of stromal cells, leading to impaired retention of hematopoietic progenitor cells in the bone niche, early onset EMH, and failure to engraft donor bone marrow (BM) (Fig. 2) [54]. It is likely that hepato-splenomegaly, a prominent pathologic manifestation in children with the severe form of sialidosis, is due to EMH caused by excessive lysosomal exocytosis and abnormal degradation of VCAM1.
Fig. 2.
Schematic representation of the events that lead to loss of BM progenitors, splenic EMH, and loss of BM engraftment in sialidosis mice. BM bone marrow, EMH extramedullary hematopoiesis, LM lysosomal membrane, PM plasma membrane, SA hypersialylation, VCAM1 vascular cell adhesion molecule 1
As is the case for sialidosis patients, Neu1-null mice display profound hearing loss involving both conductive and sensorineural components [58]. Similarly to what is seen in the BM, morphologic changes in the cochleae of Neu1-null mice are associated with oversialylation of Lamp1. The increased expression and apical localization of Lamp1 in marginal cells of the stria vascularis were indicative of exacerbation of lysosomal exocytosis into the endolymph. This provokes, a reduction in the endolymphatic potential, which likely contributes to loss of hearing [58].
Severe neuromuscular defects have been documented in patients with type II sialidosis, although the cause of this phenotype has remained unknown [18]. However, a comprehensive analysis of the skeletal muscle abnormalities in Neu1-null mice has provided supportive evidence that this disease phenotype could be the result of excessive lysosomal exocytosis [57]. Dramatic expansion of connective tissue in the epimysium and perimysium spaces appeared to be associated with the proliferation and infiltration of fibroblast-like cells [57]. Fibroblasts isolated from Neu1-null skeletal muscle have increased levels of lysosomal exocytosis and in turn enhanced proliferation and migration. Muscle fibers adjacent to the expanded connective tissue become infiltrated by connective tissue fibroblasts, which results in their progressive cytosolic fragmentation and degeneration without the apparent occurrence of necrosis or inflammation. The infiltrated spaces within individual muscle fibers are strongly positive for lysosomal markers and proteases, such as LAMP1, cathepsin B, and metalloproteinases, which might facilitate the aberrant infiltration of muscle fibers [57].
In a recently published report, we provide experimental evidence of the link between Neu1 deficiency-exacerbated lysosomal exocytosis and the spontaneous occurrence of an Alzheimer’s disease (AD)-like amyloidogenic process in the hippocampal region of the Neu1-null mice [100]. This phenotype is associated with the accumulation and amyloidogenic processing of an oversialylated amyloid precursor protein (APP) in lysosomes, followed by the extracellular release of toxic Aβ peptides by excessive lysosomal exocytosis [100]. These findings are relevant because they uncover a new pathway for the secretion of Aβ and identify NEU1 as a potential therapeutic target for AD [100].
Role of NEU1/EBP/PPCA complex in elastogenesis
Besides the LMC containing PPCA, NEU1, and β-GAL, another related enzyme complex, named the cell surface elastin receptor or CSER, has been identified at the PM of a variety of human cells. This complex plays an essential role in the process of elastogenesis and has three major constituents: PPCA, NEU1, and elastin-binding protein (EBP) [59]. The latter protein belongs to a family of S-type, Ca2+-independent, β-galactoside-specific lectins [60]. EBP is the product of alternative splicing of the β-GAL pre-mRNA that results in the deletion of two non-contiguous protein-encoding exons and in the shifting of the reading frame of the intermediate exon [61]. EBP shares most of its amino acid sequence with the β-GAL enzyme but is catalytically inactive and does not localize to lysosomes [61]. In addition, this protein includes a unique 32-amino-acid motif with high affinity for the VGVAPG hydrophobic domain of elastin [62]. EBP functions as a chaperone for tropoelastin, protecting it from premature self-aggregation and degradation by elastases, and thereby facilitates the extracellular deposition of elastic fibers onto the microfibrillar scaffold. In fact, EBP was shown to colocalize with tropoelastin both intracellularly and at the cell surface, suggesting that the two proteins already associate in an early biosynthetic compartment and as such are routed to the cell surface [63].
The process of elastin fiber deposition appears to require the concerted action of both EBP and NEU1, since both proteins are present at the PM of aortic smooth muscle cells (ASMC) and treatment with sialidase inhibitors represses the assembly of elastic fibers in both cultured cells and in chick embryos [63]. In contrast, addition of an exogenous sialidase to ASMC cultures results in the hydrolysis of terminal sialic acid residues from the microfibrillar scaffold and matrix glycoconjugates, thereby exposing galactosugars for which EBP has high affinity. Binding of terminally exposed galactose to the galectin site of EBP promotes its dissociation from tropoelastin, and subsequent assembly into elastic fibers. Most relevantly, fibroblasts from both sialidosis and GS patients have impaired elastogenesis [63]. In addition, it was recently reported that a 41-year-old patient with the late infantile form of GS developed emphysema, a condition that is linked to a defect in primary elastic fiber assembly [64]. These authors suggest that surviving patients with GS should routinely be checked for their pulmonary function.
Curiously, the splicing mechanism that gives rise to the EBP transcript is not conserved in the mouse β-gal gene, and a murine EBP-homolog is yet to be identified. However, in mice Neu1 was shown to be involved in elastic fiber formation because Neu1-deficient mice have a tight-skin phenotype similar to that of homozygous tight-skin (Tsk) mice [65], indicative of defective deposition of elastic fibers. In addition, the lungs of Neu1-deficient mice have an emphysematous appearance. Concomitantly, fibulin-5, an extracellular basement membrane protein required for the polymerization of elastin, and fibrillin-2, an ECM protein involved in elastic fiber assembly, are present in reduced amounts, and their localization is diffuse and disorganized in Neu1-null lungs [65]. Elastin lamellae appear thinner and straighter than in wild-type tissue and are separated by large spaces abnormally filled with sialic acid–containing material. These findings suggest that also in mouse tissues Neu1 activity is essential for the correct deposition and assembly of elastic fibers.
It is currently unknown how the NEU1 is transported to the cell surface. It could be routed directly via the biosynthetic pathway to the PM in complex with PPCA and EBP, or could originate from the LMC. Because neither PPCA nor NEU1 is an integral membrane protein, their membrane association would require at least one or more as yet unidentified membrane-associated partners. It is conceivable that in cells that deposit elastic fibers, e.g., fibroblasts, an LM-associated complex of PPCA and NEU1 is relocated to the cell surface via exocytic fusion of the LM with the PM. If this would be the case, this protein complex would need to recruit EBP/tropoelastin to initiate the process of elastin deposition and assembly.
NEU1 at the cell surface modulates cell proliferation
The glycocalix of epithelia as well as the cell surface of virtually all cell types is abundantly decorated with sialoglycoproteins and sialoglycolipids. Sialoglycoproteins are in general poor substrates for the plasma membrane sialidase NEU3, which instead is mostly active toward sialoglycolipids [66]. Thus, desialylation of cell surface glycoproteins, including mitogenic receptors, is now considered a task of NEU1, acting at the PM. Several recent studies have shown that removal of sialic acids from cell surface receptors can influence cell proliferation by controlling the activation of downstream signaling pathways [59, 67]. For example, ASMCs treated with a sialidase inhibitor or an antibody that blocks NEU1 activity have increased proliferative capacity in the presence of fetal bovine serum, while addition of Clostridium perfringens sialidase reverts this phenotype [59]. Having established that NEU1 participates with EBP in the proper formation of elastin, which in turn can sequester mitogenic growth factors, the same authors went on to demonstrate the indirect involvement of NEU1 in cell proliferation via desialylation of growth factor receptors. Indeed, treatment of ASMCs with an exogenous sialidase was shown to abolish the mitogenic responses to growth factors, such as PDGF-BB and IGF-2, through removal of the sialic acids from their respective cognate receptors PDGF-R and IGF-1R [59]. Similarly, in rat skeletal L6 myoblasts, alterations of the sialic acid content of the insulin receptor (IR) by mouse-derived Neu1 or C. perfringens sialidase were shown to modulate the proliferative capacity of these cells in response to insulin signaling [67, 68]. In this respect, a compelling observation was that ASMCs exposed to therapeutic concentrations of insulin have de novo formation of elastic fibers, independently of their rate of proliferation. These results were confirmed in fibroblasts from patients with sialidosis, which appear to proliferate faster, respond more strongly to recombinant growth factors than normal fibroblasts, and have impaired insulin-induced phosphorylation of downstream signaling proteins [59, 68]. The latter observation suggests an interesting link between NEU1/EBP-dependent elastogenesis and vascular pathologies associated with diabetes, many of which are linked to elastic fiber disease [69].
Collectively, these findings support the notion that downregulation or lack of NEU1, likely in complex with PPCA at the cell surface, promotes cell proliferation and regulates mitogenic signaling pathways similarly to what has been observed with fibroblasts isolated from Neu1-deficient skeletal muscle [57].
Role of NEU1 in the immune response
Removal of sialic acids from glycoconjugates at the cell surface of immune cells has been implicated in numerous processes during development of both innate and adaptive immune responses. These range from regulated differentiation of monocytes into macrophages or dendritic cells [70, 71] to bacterial lipopolysaccharide (LPS)-induced production of cytokines in differentiated dendritic cells and peripheral blood mononuclear cells (PBMCs) [71, 72]. During monocyte differentiation into dendritic cells, both NEU1 and NEU3 were found to be upregulated, but it is NEU3 in this case that controls cytokine production [71]. However, differentiation of monocytes into macrophages requires NEU1 activity; the enzyme is mobilized at the cell surface together with PPCA, but both proteins are first targeted to lysosomes and then sorted in a LAMP1-, MHC class II-positive compartment to the PM [70]. Activation of T lymphocytes by exposure to anti-CD-3 or anti-CD-28 IgG also induces an increase in NEU1 mRNA and protein levels, and the enzyme is again found at the PM in tight association with PPCA. In addition, inhibition of sialidase activity results in reduced expression of IFN-gamma in activated T lymphocytes, indicating that NEU1, likely in complex with PPCA, contributes to hyposialylation of cell surface receptors and in turn activation of T lymphocytes [73].
Similarly, pretreatment of PBMCs with an exogenous sialidase increases their cytokine response to LPS through a mechanism that involves activation of the TLR4, CD14, and MD2, three components of the LPS receptor complex [72]. Overexpression of NEU1 increases TLR4-mediated NF-kB activation in response to LPS, while inhibition of NEU1 suppresses its activation. These authors conclude that sialic acids on TLR4 modulate the LPS response by facilitating clustering of the homodimers. The activity of NEU1 mobilized at the cell surface during cell activation seems to play a role in this process. In fact, in another study NEU1 was shown to form a complex with TLR-2, -3, and -4 receptors on the cell-surface of naïve and activated macrophages [74, 75]. Because of their high number of N-glycosylation sites, the inner concave surface of these TLRs, predicted to be involved in ligand binding, is masked by glycans, and ligand binding activates NEU1 by a yet unknown mechanism. These authors hypothesize that NEU1 hydrolyzes α-2,3 sialyl residues, sialic acid residues of TLRs, which in turn unmasks its ectodomain and allows TLR dimerization and activation [75]. Subsequently, TLR dimerization and activation mediate MyD88/TLR4 complex formation and activation of the transcription factor NF-κB, as seen in macrophages treated with the endotoxin LPS. LPS binding to TLR4 apparently induces the interaction of NEU1 with metalloproteinase-9 (MMP9), which in turn stimulates downstream guanine protein-coupled receptor (GPCR) signaling [76]. This NEU1-MMP9 complex was shown to bind to TLR4 on the cell surface of naïve macrophages, but the inhibition of MMP9 and GPCR Gαi-signaling proteins blocked LPS-induced NEU1 activity and downstream NF-κB activation.
Lillehoj et al. [77] recently demonstrated that in human primary small airway epithelial cells NEU1 associates with epidermal growth factor receptor (EGFR) and MUC1, which appear to be natural substrates of the enzyme. Depletion of NEU1 increases EGF-stimulated EGFR Tyr-1068 autophosphorylation and activation, and decreases MUC1-mediated Pseudomonas aeruginosa adhesion and flagellin-stimulated ERK1/2 activation. In contrast, overexpression of NEU1 reduces EGFR activation and increases MUC1-dependent bacterial adhesion and signaling. Thus, human airway epithelia express NEU1 at the cell surface; at this site, the enzyme regulates EGFR- and MUC1-dependent signaling and bacterial adhesion, therefore providing an extra tier of regulation for the airway epithelia in response to harmful stimuli.
Loss of NEU1 promotes invasion and metastasis of cancer cells
It is known that cancer cells not only undergo dramatic changes in their gene expression profiles, but also in the level of glycosylation and particularly sialylation of their cell surface glycoproteins and lipids [78, 79]. Such changes in sialic acid content could result from either altered expression of sialyltransferases [80] or modulation of the expression levels and activity of NEU1 and other mammalian sialidases. It has already been demonstrated that an increase in the sialylation of cell surface glycoconjugates correlates with malignant transformation and with an increase in metastatic potential and invasiveness.
Notably, the expression of NEU1 was found to be downregulated in highly metastatic colon adenocarcinoma cells compared to that in tumor cells with low metastatic potential [81]. On the other hand, overexpression of NEU1 in human colon adenocarcinoma HT-29 cells leads to the suppression of cell migration and invasion, while knockdown of NEU1 has the opposite effect [82]. Mice that were transsplenically injected with NEU1-overexpressing HT-29 cells showed less liver metastasis in vivo than mice injected with the parental HT-29 cells [82]. In these carcinoma cells, NEU1 overexpression results in hyposialylation and decreased phosphorylation of β4-integrin, and attenuation of the focal adhesion kinase and ERK1/2 pathways. In addition, matrix metalloproteinases, which are induced in cancer cells with high metastatic potential, were found to be profoundly downregulated. In contrast to what was observed for NEU1, NEU3 expression is 3- to 100-fold higher in colon carcinomas than in other cancer cell lines, which leads to increased levels of anti-apoptotic Bcl-XL and decreased amounts of proapoptotic caspase-3 [83]. Together these results indicate that NEU1 and NEU3 both have the intrinsic capacity to modulate the invasion and metastatic potential of carcinoma cells; however, the two enzymes seem to target different substrates and signaling pathways, resulting in opposite downstream effects.
β-Gal deficiency and neuronal apoptosis
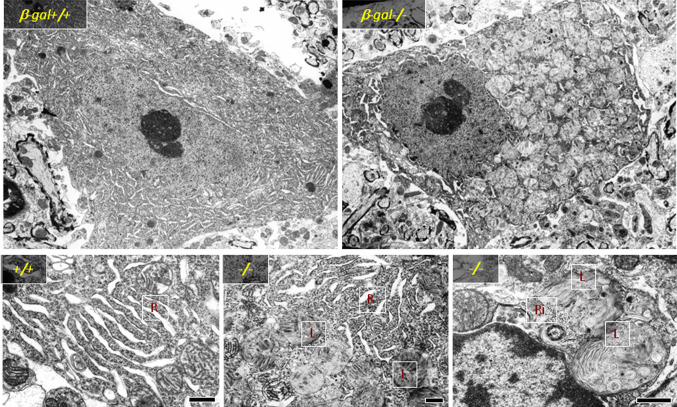
It is now well documented that the build up of GM1 in the brain of β-Gal −/− mice triggers the activation of molecular effectors that lead to neuronal cell death, the process responsible for the progressive neurodegeneration characteristic of this mouse model as well as of patients affected by the disease. The brain and spinal cord of β-Gal −/− mice undergo profound morphological changes associated with lysosomal distension and accumulation of GM1 as the disease progresses [32]. At the histopathological level, neurons appear filled with distended vacuoles, containing membranous material and remnants of the ER and ribosomes (Fig. 3) [32]. In addition, an increased number of apoptotic cells was detected throughout the central nervous system as early as at 1 month of age [84], but their distribution pattern was random rather than involving specific neuronal nuclei. These features were indicative of activation of an ER stress response or unfolded protein response (UPR). This process, which cells use primarily to survive under disparate stress conditions, entails a cascade of molecular events that are set to ensure and maintain the homeostasis of the ER. Although the UPR has traditionally been associated with stress conditions because of the accumulation of unfolded or misfolded proteins, it is becoming increasingly apparent that it can be engaged by other stimuli. Under ER stress, activation of the UPR reduces the accumulation of unfolded proteins by promoting the expansion of the ER membrane, the upregulation of ER-resident chaperones and folding catalysts, such as BiP, and inhibiting protein synthesis [85–87]. However, prolonged ER stress can cause irreversible damage, triggering an apoptotic program that includes the induction of the transcription factor CHOP [85], activation of c-Jun N-terminal kinases (JNK) [88], and cleavage of the ER-specific cysteine protease caspase-12 [89].
Fig. 3.
Electron microscopy of spinal cord neurons from β-gal +/+ and β-gal −/− mice (3 months of age) shows numerous enlarged lysosomes (L) in β-gal −/− neurons, which are filled with membranous material, ribosomes (R) and ER. Scale bars: upper panel 1 μm; lower panels 0.5 μm. Adapted from a study originally published in Molecular Cell [84]
In β-Gal −/− brain and spinal cord, upregulation and activation of the UPR molecular effectors responsible for cell survival (e.g., BiP) are accompanied by increased levels of the transcription factor ATF6, the proapoptotic mediator CHOP, and the phosphorylated form of the kinase JNK2, as well as by proteolytic activation of caspase-12 [84]. The combined induction of these effectors culminates in neuronal apoptosis and can be fully recapitulated in primary mouse embryonic fibroblasts (MEFs) or neurospheres isolated from β-Gal −/− mice, and in wild-type MEFs and neurospheres exogenously loaded with GM1. Also in these cultures apoptosis is preceded by the activation of the aforementioned UPR effectors at both the mRNA and protein levels. Most notably, activation of the UPR was found to be directly downstream of GM1 accumulation at the ER membrane that subsequently provokes depletion of the ER Ca2+ stores. In line with these findings is the observation that UPR-mediated apoptosis does not occur in mice deficient for both β-Gal and β-4-N-acetylgalactosaminyltransferase (β-Gal −/− /GalNAcT −/−) that have both defective synthesis and degradation of GM1 and do not accumulate this ganglioside [84].
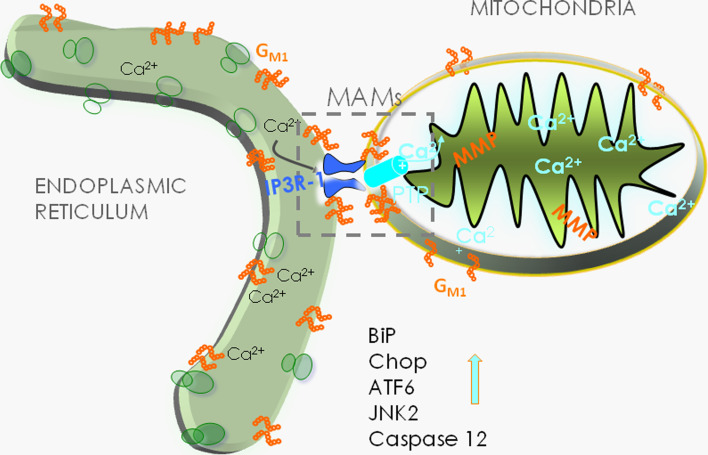
The finding that GM1 accumulation at the ER membrane triggers the release of Ca2+ from the ER led to the hypothesis that the latter event could directly influence the concentration of Ca2+ in the mitochondria and engage these organelles in the apoptotic process. ER and mitochondrial membranes juxtapose at specific contact sites, known as the mitochondria-associated ER membranes or MAMs. These microdomains represent highly dynamic signaling and metabolic platforms that are implicated in fundamental cellular processes, such as lipid biosynthesis and transport, energy metabolism, Ca2+ signaling and buffering, cell survival, and apoptosis [90–93]. A plethora of specific ER and mitochondrial proteins colocalize at the MAMs. These include the inositol trisphosphate receptor (IP3R) at the ER face of the MAMs, the molecular chaperone glucose-regulated-protein 75 (grp75), which bridges the IP3R-1 with voltage-dependent anion channel 1 (VDAC1) at the outer mitochondrial membrane, Sigma-1 receptor, calreticulin, mitofusin 2, and phosphofurin acidic cluster sorting protein 2 [94–97]. Due to the intrinsic ability of GM1 to bind Ca2+ [98, 99], we hypothesized that, if this ganglioside were present in these specialized microdomains, it could influence the Ca2+ conductance and buffer capacity of the MAMs. Ultrastructural analyses of crude β-Gal −/− mitochondrial preparations revealed numerous juxtaposed ER vesicles, which were indicative of increased contact sites between ER and mitochondrial membranes. In addition, MAMs purified from β-gal −/− brains showed increased levels of GM1 that were even higher in the glycosphingolipid-enriched microdomains (GEMs) extracted from the β-gal −/− MAMs. This implied that GM1 preferentially segregates in these sub-microdomains [93], an idea that was supported by the detection of small amounts of this ganglioside also in β-gal +/+ GEMs. These studies also identified the GEMs as structural components of the MAMs that function as specialized subdomains for Ca2+ transfer from the ER to the mitochondria and hence are strongly influenced by GM1 levels. The abnormal accumulation of this ganglioside in the GEMs is accompanied by increased levels of IP3R-1, VDAC1, and GRP75, which potentiate the activity of the Ca2+ megachannel composed by these proteins. Furthermore, GM1 was shown to physically interact with the phosphorylated and active form of IP3R-1 (P-IP3R1), a phenomenon that can drastically affect the properties and topology of the receptor and alter the dynamics of the lipid microenvironment, favoring the segregation/clustering of an active IP3R-1 along with VDAC1 and GRP75. The net result of these events is an increase of Ca2+ into the mitochondrial matrix, which ultimately has an impact on the bioenergetic activities of the organelle and activates the mitochondrial leg of the intrinsic apoptotic pathway (Fig. 4) [93]. Signs of mitochondrial dysfunction include mitochondria depolarization, opening of the permeability transition pore (PTP), and mitochondrial membrane permeabilization, which together contribute to mitochondria-mediated apoptosis downstream of GM1 accumulation in the GEMs [93]. This pathogenic cascade occurring in the mitochondria of β-Gal −/− neurons emphasizes the importance of the MAM/GEM microdomains in maintaining normal ER homeostasis and mitochondrial bioenergetic activity. In line with this conclusion is the observation that treatment of β-Gal −/− cells with methyl β-cyclodextrin, a circular oligosaccharide that efficiently extracts GM1 from the MAMs, rescues the opening of the PTP, dissipation of the potential, and apoptosis [93].
Fig. 4.
Schematic rendering of the molecular events in β-gal −/− neurons that occur downstream of GM1 accumulation at the MAMs, leading to ER stress and mitochondria-mediated neuronal apoptosis. Adapted from a study originally published in Molecular Cell [93]
Taken together, these studies demonstrate that β-gal deficiency and impaired degradation of GM1 trigger both UPR- and mitochondria-mediated apoptosis through the loss of cellular Ca2+ homeostasis. The fact that these pathways are not altered in the brains of Ppca −/− or Neu1 −/− mice further supports the notion that downstream events associated with the primary enzyme deficiency and consequent substrate accumulation are controlled by different molecular effectors in these three LSDs.
Perspectives
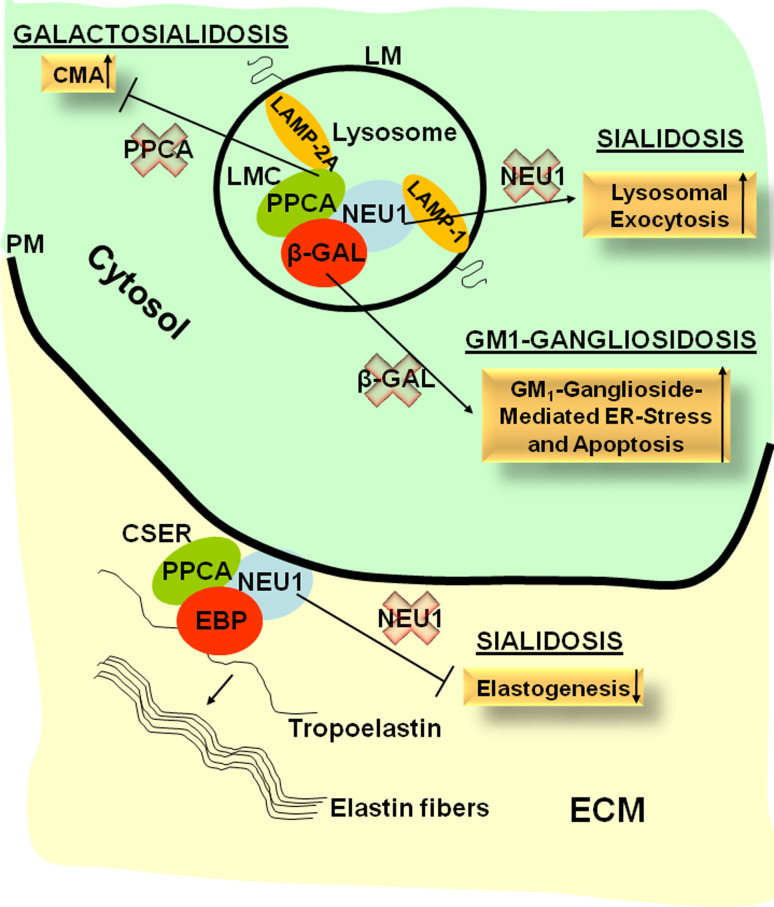
In this review, we emphasized the concept that lysosomal enzymes, often considered housekeeping proteins, requiring little or no regulation, have in fact specific functions that go beyond their canonical degradative capacity and are involved in processes that greatly influence cellular homeostasis and/or the extracellular environment (Fig. 5). The PPCA-containing LMC comprises multiple enzymes, including NEU1, β-GAL, and possibly GALNS. These enzymes may not all be part of the LMC at the same time, but rather the composition of the LMC may vary in different cell types or metabolic conditions and be dictated by the presence of specific substrates or certain physiological stimuli. At the PM, a similar enzyme/protein complex, CSER, is formed in which β-GAL is replaced by its shorter, catalytically inactive isoform, EBP. This complex is essential for the formation and deposition of elastic fibers. Although NEU1 and β-GAL function in a multienzyme complex, their dependency on PPCA may vary. In addition, the deficiency of each of these enzymes impacts on very different aspects of cellular physiology, as evidenced by the multiple phenotypes characteristic of the respective LSDs. A better understanding of the disease pathogenesis and the various pathways that are affected because of the impairment of single or multiple enzymes will be crucial for the development of effective therapy. Therapeutic supply of the defective enzymes by enzyme replacement or gene therapy may be more effective when used in combination with drugs that target the affected cellular processes downstream of the enzyme deficiency, such as lysosomal exocytosis in sialidosis patients, or the synthesis of complex lipids in GM1 patients.
Fig. 5.
Schematic representation of the pathways that involve the components of the LMC and the CSER and that become deregulated in case of single or combined enzyme deficiencies in sialidosis, GM1, and GS. CMA chaperone-mediated autophagy, CSER cell surface elastin receptor, EBP elastin-binding protein, ECM extracellular matrix, LM lysosomal membrane, LMC lysosomal multienzyme complex, PM plasma membrane
Acknowledgments
We thank Dr. Angela McArthur for editing this manuscript. A.d’A. holds the Jewelers for Children Endowed Chair in Genetics and Gene Therapy. Work from d’Azzo’s laboratory that is included in this review was supported by the National Institutes of Health (NIH) grants GM60905 and DK52025, the Assisi Foundation of Memphis, the American Lebanese Syrian Associated Charities (ALSAC) and the National Tay-Sachs & Allied Disease Association (NTSAD).
Abbreviations
- ASMC
Aortic smooth muscle cell
- BiP
Binding immunoglobulin protein
- β-GAL
β-Galactosidase
- BM
Bone marrow
- CHOP
CCAAT/enhancer-binding protein homologous protein
- CMA
Chaperone-mediated autophagy
- EBP
Elastin-binding protein
- ECM
Extracellular matrix
- EMH
Extramedullary hematopoiesis
- ER
Endoplasmic reticulum
- GALNS
N-acetylgalactosamine-6-sulfate sulfatase
- GEMs
Glysosphingolipid-enriched microdomains
- GM1
GM1-gangliosidosis
- GPCR
Guanine protein-coupled receptor
- GS
Galactosialidosis
- Hsp
Heat shock protein
- IGF-1R
Insulin-like growth factor 1 receptor
- IP3R
Inositol trisphosphate receptor
- JNK
c-Jun N-terminal kinases
- LAMP
Lysosomal membrane-associated protein
- LM
Lysosomal membrane
- LMC
Lysosomal multienzyme complex
- LPS
Lipopolysaccharide
- LSD
Lysosomal storage disorder
- MAMs
Mitochondria-associated ER membranes
- MAPK/ERK
Mitogen-activated protein kinase
- MAP2K/MEK
Mitogen-activated protein kinase kinase
- MPP
Metalloproteinase
- MyD88
Myeloid differentiation primary response gene (88)
- NEU1
Neuraminidase-1
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- PDGF-R
Platelet-derived growth factor receptor
- PI3K
Phosphoinositide 3-kinase
- PLCγ
Phospholipase C gamma
- PM
Plasma membrane
- PPCA
Protective protein/cathepsin A
- Ras
Rapidly accelerated fibrosarcoma
- TUNEL
Terminal deoxynucleotidyl transferase dUTP nick end labeling
- UPR
Unfolded protein response
- VCAM1
Vascular cell adhesion molecule 1
References
- 1.D’Azzo A, Hoogeveen A, Reuser AJ, Robinson D, Galjaard H. Molecular defect in combined beta-galactosidase and neuraminidase deficiency in man. Proc Natl Acad Sci USA. 1982;79(15):4535–4539. doi: 10.1073/pnas.79.15.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verheijen F, Brossmer R, Galjaard H. Purification of acid beta-galactosidase and acid neuraminidase from bovine testis: evidence for an enzyme complex. Biochem Biophys Res Commun. 1982;108(2):868–875. doi: 10.1016/0006-291X(82)90911-1. [DOI] [PubMed] [Google Scholar]
- 3.Galjart NJ, Gillemans N, Harris A, van der Horst GT, Verheijen FW, Galjaard H, d’Azzo A. Expression of cDNA encoding the human “protective protein” associated with lysosomal beta-galactosidase and neuraminidase: homology to yeast proteases. Cell. 1988;54(6):755–764. doi: 10.1016/S0092-8674(88)90999-3. [DOI] [PubMed] [Google Scholar]
- 4.Bonten EJ, Galjart NJ, Willemsen R, Usmany M, Vlak JM, d’Azzo A. Lysosomal protective protein/cathepsin A. Role of the “linker” domain in catalytic activation. J Biol Chem. 1995;270(44):26441–26445. doi: 10.1074/jbc.270.44.26441. [DOI] [PubMed] [Google Scholar]
- 5.Pshezhetsky AV, Potier M. Association of N-acetylgalactosamine-6-sulfate sulfatase with the multienzyme lysosomal complex of beta-galactosidase, cathepsin A, and neuraminidase. Possible implication for intralysosomal catabolism of keratan sulfate. J Biol Chem. 1996;271(45):28359–28365. doi: 10.1074/jbc.271.45.28359. [DOI] [PubMed] [Google Scholar]
- 6.van der Spoel A, Bonten E, d’Azzo A. Processing of lysosomal beta-galactosidase. The C-terminal precursor fragment is an essential domain of the mature enzyme. J Biol Chem. 2000;275(14):10035–10040. doi: 10.1074/jbc.275.14.10035. [DOI] [PubMed] [Google Scholar]
- 7.Bonten EJ, d’Azzo A. Lysosomal neuraminidase. Catalytic activation in insect cells is controlled by the protective protein/cathepsin A. J Biol Chem. 2000;275(48):37657–37663. doi: 10.1074/jbc.M007380200. [DOI] [PubMed] [Google Scholar]
- 8.van der Spoel A, Bonten E, d’Azzo A. Transport of human lysosomal neuraminidase to mature lysosomes requires protective protein/cathepsin A. EMBO J. 1998;17(6):1588–1597. doi: 10.1093/emboj/17.6.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galjart NJ, Gillemans N, Harris A, van der Horst GTJ, Verheijen FW, Galjaard H, d’Azzo A. Expression of cDNA encoding the human protective protein associated with lysosomal beta-galactosidase and neuraminidase: homology to yeast proteases. Cell. 1988;54:755–764. doi: 10.1016/S0092-8674(88)90999-3. [DOI] [PubMed] [Google Scholar]
- 10.Jackman HL, Morris PW, Deddish PA, Skidgel RA, Erdos EG. Inactivation of endothelin I by deamidase (lysosomal protective protein) J Biol Chem. 1992;267(5):2872–2875. [PubMed] [Google Scholar]
- 11.Hanna WL, Turbov JM, Jackman HL, Tan F, Froelich CJ. Dominant chymotrypsin-like esterase activity in human lymphocyte granules is mediated by the serine carboxypeptidase called cathepsin A-like protective protein. J Immunol. 1994;153(10):4663–4672. [PubMed] [Google Scholar]
- 12.Jackman HL, Massad MG, Sekosan M, Tan F, Brovkovych V, Marcic BM, Erdos EG. Angiotensin 1–9 and 1–7 release in human heart: role of cathepsin A. Hypertension. 2002;39(5):976–981. doi: 10.1161/01.HYP.0000017283.67962.02. [DOI] [PubMed] [Google Scholar]
- 13.Rudenko G, Bonten E, d’Azzo A, Hol WG. Structure determination of the human protective protein: twofold averaging reveals the three-dimensional structure of a domain which was entirely absent in the initial model. Acta Crystallogr D Biol Crystallogr. 1996;52(Pt 5):923–936. doi: 10.1107/S0907444996004702. [DOI] [PubMed] [Google Scholar]
- 14.Rudenko G, Bonten E, d’Azzo A, Hol WG. Three-dimensional structure of the human ‘protective protein’: structure of the precursor form suggests a complex activation mechanism. Structure. 1995;3(11):1249–1259. doi: 10.1016/S0969-2126(01)00260-X. [DOI] [PubMed] [Google Scholar]
- 15.Bonten EJ, Campos Y, Zaitsev V, Nourse A, Waddell B, Lewis W, Taylor G, d’Azzo A. Heterodimerization of the sialidase NEU1 with the chaperone protective protein/cathepsin A prevents its premature oligomerization. J Biol Chem. 2009;284(41):28430–28441. doi: 10.1074/jbc.M109.031419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galjart NJ, Morreau H, Willemsen R, Gillemans N, Bonten EJ, d’Azzo A. Human lysosomal protective protein has cathepsin A-like activity distinct from its protective function. J Biol Chem. 1991;266(22):14754–14762. [PubMed] [Google Scholar]
- 17.d’Azzo A, Andria G, Strisciuglio P, Galjaard H. Galactosialidosis. In: Scriver C, Beaudet A, Sly W, Valle D, editors. The metabolic and molecular bases of inherited disease. 8. New York: McGraw-Hill Publishing Co.; 2001. pp. 3811–3826. [Google Scholar]
- 18.Thomas GH. Disorders of glycoprotein degradation and structure: α-mannosidosis, β-mannosidosis, fucosidosis, and sialidosis. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited disease. 8. New York: McGraw Hill Inc.; 2001. pp. 3507–3534. [Google Scholar]
- 19.Suzuki YAO, Namba E. β-Galactosidase deficiency (β-Galactosialidosis): GM1 gangliosidosis and morquio B disease. In: Scriver C, Beaudet A, Sly W, Valle D, editors. The metabolic and molecular bases of inherited disease. New York: McGraw-Hill Publishing Co; 2001. pp. 3775–3810. [Google Scholar]
- 20.Caciotti A, Garman SC, Rivera-Colon Y, Procopio E, Catarzi S, Ferri L, Guido C, Martelli P, Parini R, Antuzzi D, Battini R, Sibilio M, Simonati A, Fontana E, Salviati A, Akinci G, Cereda C, Dionisi-Vici C, Deodato F, d’Amico A, d’Azzo A, Bertini E, Filocamo M, Scarpa M, di Rocco M, Tifft CJ, Ciani F, Gasperini S, Pasquini E, Guerrini R, Donati MA, Morrone A. GM1 gangliosidosis and Morquio B disease: an update on genetic alterations and clinical findings. Biochim Biophys Acta. 2011;1812(7):782–790. doi: 10.1016/j.bbadis.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caciotti A, Catarzi S, Tonin R, Lugli L, Perez CR, Michelakakis H, Mavridou I, Donati MA, Guerrini R, d’Azzo A, Morrone A. Galactosialidosis: review and analysis of CTSA gene mutations. Orphanet J Rare Dis. 2013;8:114. doi: 10.1186/1750-1172-8-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.d’Azzo A, Bonten E. Molecular mechanisms of pathogenesis in a glycosphingolipid and a glycoprotein storage disease. Biochem Soc Trans. 2010;38(6):1453–1457. doi: 10.1042/BST0381453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Geest N, Bonten E, Mann L, de Sousa-Hitzler J, Hahn C, d’Azzo A. Systemic and neurologic abnormalities distinguish the lysosomal disorders sialidosis and galactosialidosis in mice. Hum Mol Genet. 2002;11(12):1455–1464. doi: 10.1093/hmg/11.12.1455. [DOI] [PubMed] [Google Scholar]
- 24.Zhou XY, Morreau H, Rottier R, Davis D, Bonten E, Gillemans N, Wenger D, Grosveld FG, Doherty P, Suzuki K, Grosveld GC, d’Azzo A. Mouse model for the lysosomal disorder galactosialidosis and correction of the phenotype with over-expressing erythroid precursor cells. Genes Dev. 1995;9:2623–2634. doi: 10.1101/gad.9.21.2623. [DOI] [PubMed] [Google Scholar]
- 25.Rottier RJ, Hahn CN, Mann LW, del Pilar Martin M, Smeyne RJ, Suzuki K, d’Azzo A. Lack of PPCA expression only partially coincides with lysosomal storage in galactosialidosis mice: indirect evidence for spatial requirement of the catalytic rather than the protective function of PPCA. Hum Mol Genet. 1998;7(11):1787–1794. doi: 10.1093/hmg/7.11.1787. [DOI] [PubMed] [Google Scholar]
- 26.Jackman HL, Tan FL, Tamei H, Beurling-Harbury C, Li XY, Skidgel RA, Erdos EG. A peptidase in human platelets that deamidates tachykinins. Probable identity with the lysosomal “protective protein”. J Biol Chem. 1990;265(19):11265–11272. [PubMed] [Google Scholar]
- 27.Seyrantepe V, Hinek A, Peng J, Fedjaev M, Ernest S, Kadota Y, Canuel M, Itoh K, Morales CR, Lavoie J, Tremblay J, Pshezhetsky AV. Enzymatic activity of lysosomal carboxypeptidase (cathepsin) A is required for proper elastic fiber formation and inactivation of endothelin-1. Circulation. 2008;117(15):1973–1981. doi: 10.1161/CIRCULATIONAHA.107.733212. [DOI] [PubMed] [Google Scholar]
- 28.Monti E, Bassi MT, Bresciani R, Civini S, Croci GL, Papini N, Riboni M, Zanchetti G, Ballabio A, Preti A, Tettamanti G, Venerando B, Borsani G. Molecular cloning and characterization of NEU4, the fourth member of the human sialidase gene family. Genomics. 2004;83(3):445–453. doi: 10.1016/j.ygeno.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 29.Monti E, Bassi MT, Papini N, Riboni M, Manzoni M, Venerando B, Croci G, Preti A, Ballabio A, Tettamanti G, Borsani G. Identification and expression of NEU3, a novel human sialidase associated to the plasma membrane. Biochem J. 2000;349(Pt 1):343–351. doi: 10.1042/0264-6021:3490343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monti E, Bonten E, D’Azzo A, Bresciani R, Venerando B, Borsani G, Schauer R, Tettamanti G. Sialidases in vertebrates: a family of enzymes tailored for several cell functions. Adv Carbohydr Chem Biochem. 2010;64:403–479. doi: 10.1016/S0065-2318(10)64007-3. [DOI] [PubMed] [Google Scholar]
- 31.Monti E, Preti A, Rossi E, Ballabio A, Borsani G. Cloning and characterization of NEU2, a human gene homologous to rodent soluble sialidases. Genomics. 1999;57(1):137–143. doi: 10.1006/geno.1999.5749. [DOI] [PubMed] [Google Scholar]
- 32.Hahn CN, del Pilar Martin M, Schroder M, Vanier MT, Hara Y, Suzuki K, d’Azzo A. Generalized CNS disease and massive GM1-ganglioside accumulation in mice defective in lysosomal acid beta-galactosidase. Hum Mol Genet. 1997;6(2):205–211. doi: 10.1093/hmg/6.2.205. [DOI] [PubMed] [Google Scholar]
- 33.Jeyakumar M, Thomas R, Elliot-Smith E, Smith DA, van der Spoel AC, d’Azzo A, Perry VH, Butters TD, Dwek RA, Platt FM. Central nervous system inflammation is a hallmark of pathogenesis in mouse models of GM1 and GM2 gangliosidosis. Brain. 2003;126(Pt 4):974–987. doi: 10.1093/brain/awg089. [DOI] [PubMed] [Google Scholar]
- 34.Wang D, Bonten EJ, Yogalingam G, Mann L, d’Azzo A. Short-term, high dose enzyme replacement therapy in sialidosis mice. Mol Genet Metab. 2005;85(3):181–189. doi: 10.1016/j.ymgme.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 35.Baek RC, Broekman ML, Leroy SG, Tierney LA, Sandberg MA, d’Azzo A, Seyfried TN, Sena-Esteves M. AAV-mediated gene delivery in adult GM1-gangliosidosis mice corrects lysosomal storage in CNS and improves survival. PLoS ONE. 2010;5(10):e13468. doi: 10.1371/journal.pone.0013468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonten EJ, Wang D, Toy JN, Mann L, Mignardot A, Yogalingam G, d’Azzo A. Targeting macrophages with baculovirus-produced lysosomal enzymes: implications for enzyme replacement therapy of the glycoprotein storage disorder galactosialidosis. FASEB J. 2004;18(9):971–973. doi: 10.1096/fj.03-0941fje. [DOI] [PubMed] [Google Scholar]
- 37.Bonten EJ, Yogalingam G, Hu H, Gomero E, van de Vlekkert D, d’Azzo A. Chaperone-mediated gene therapy with recombinant AAV-PPCA in a new mouse model of type I sialidosis. Biochim Biophys Acta. 2013;1832(10):1784–1792. doi: 10.1016/j.bbadis.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elliot-Smith E, Speak AO, Lloyd-Evans E, Smith DA, van der Spoel AC, Jeyakumar M, Butters TD, Dwek RA, d’Azzo A, Platt FM. Beneficial effects of substrate reduction therapy in a mouse model of GM1 gangliosidosis. Mol Genet Metab. 2008;94(2):204–211. doi: 10.1016/j.ymgme.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 39.Hu H, Gomero E, Bonten E, Gray JT, Allay J, Wu Y, Wu J, Calabrese C, Nienhuis A, d’Azzo A. Preclinical dose-finding study with a liver-tropic, recombinant AAV-2/8 vector in the mouse model of galactosialidosis. Mol Ther. 2012;20(2):267–274. doi: 10.1038/mt.2011.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kasperzyk JL, d’Azzo A, Platt FM, Alroy J, Seyfried TN. Substrate reduction reduces gangliosides in postnatal cerebrum-brainstem and cerebellum in GM1 gangliosidosis mice. J Lipid Res. 2005;46(4):744–751. doi: 10.1194/jlr.M400411-JLR200. [DOI] [PubMed] [Google Scholar]
- 41.Kasperzyk JL, El-Abbadi MM, Hauser EC, d’Azzo A, Platt FM, Seyfried TN. N-butyldeoxygalactonojirimycin reduces neonatal brain ganglioside content in a mouse model of GM1 gangliosidosis. J Neurochem. 2004;89(3):645–653. doi: 10.1046/j.1471-4159.2004.02381.x. [DOI] [PubMed] [Google Scholar]
- 42.Leimig T, Mann L, Martin Mdel P, Bonten E, Persons D, Knowles J, Allay JA, Cunningham J, Nienhuis AW, Smeyne R, d’Azzo A. Functional amelioration of murine galactosialidosis by genetically modified bone marrow hematopoietic progenitor cells. Blood. 2002;99(9):3169–3178. doi: 10.1182/blood.V99.9.3169. [DOI] [PubMed] [Google Scholar]
- 43.Sano R, Tessitore A, Ingrassia A, d’Azzo A. Chemokine-induced recruitment of genetically modified bone marrow cells into the CNS of GM1-gangliosidosis mice corrects neuronal pathology. Blood. 2005;106(7):2259–2268. doi: 10.1182/blood-2005-03-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cuervo AM, Mann L, Bonten EJ, d’Azzo A, Dice JF. Cathepsin A regulates chaperone-mediated autophagy through cleavage of the lysosomal receptor. EMBO J. 2003;22(1):47–59. doi: 10.1093/emboj/cdg002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lichter-Konecki U, Moter SE, Krawisz BR, Schlotter M, Hipke C, Konecki DS. Expression patterns of murine lysosome-associated membrane protein 2 (Lamp-2) transcripts during morphogenesis. Differentiation. 1999;65(1):43–58. doi: 10.1046/j.1432-0436.1999.6510043.x. [DOI] [PubMed] [Google Scholar]
- 46.Tanaka Y, Guhde G, Suter A, Eskelinen EL, Hartmann D, Lullmann-Rauch R, Janssen PM, Blanz J, von Figura K, Saftig P. Accumulation of autophagic vacuoles and cardiomyopathy in LAMP-2-deficient mice. Nature. 2000;406(6798):902–906. doi: 10.1038/35022595. [DOI] [PubMed] [Google Scholar]
- 47.Cuervo AM, Dice JF. A receptor for the selective uptake and degradation of proteins by lysosomes. Science. 1996;273(5274):501–503. doi: 10.1126/science.273.5274.501. [DOI] [PubMed] [Google Scholar]
- 48.Arias E, Cuervo AM. Chaperone-mediated autophagy in protein quality control. Curr Opin Cell Biol. 2011;23(2):184–189. doi: 10.1016/j.ceb.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaushik S, Cuervo AM. Chaperone-mediated autophagy: a unique way to enter the lysosome world. Trends Cell Biol. 2012;22(8):407–417. doi: 10.1016/j.tcb.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cuervo AM, Dice JF. Regulation of lamp2a levels in the lysosomal membrane. Traffic. 2000;1(7):570–583. doi: 10.1034/j.1600-0854.2000.010707.x. [DOI] [PubMed] [Google Scholar]
- 51.Bossi G, Griffiths GM. CTL secretory lysosomes: biogenesis and secretion of a harmful organelle. Semin Immunol. 2005;17(1):87–94. doi: 10.1016/j.smim.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 52.Andrews NW. Regulated secretion of conventional lysosomes. Trends Cell Biol. 2000;10(8):316–321. doi: 10.1016/S0962-8924(00)01794-3. [DOI] [PubMed] [Google Scholar]
- 53.Bossi G, Booth S, Clark R, Davis EG, Liesner R, Richards K, Starcevic M, Stinchcombe J, Trambas C, Dell’Angelica EC, Griffiths GM. Normal lytic granule secretion by cytotoxic T lymphocytes deficient in BLOC-1, -2 and -3 and myosins Va, VIIa and XV. Traffic. 2005;6(3):243–251. doi: 10.1111/j.1600-0854.2005.00264.x. [DOI] [PubMed] [Google Scholar]
- 54.Yogalingam G, Bonten EJ, van de Vlekkert D, Hu H, Moshiach S, Connell SA, d’Azzo A. Neuraminidase 1 is a negative regulator of lysosomal exocytosis. Dev Cell. 2008;15(1):74–86. doi: 10.1016/j.devcel.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stinchcombe J, Bossi G, Griffiths GM. Linking albinism and immunity: the secrets of secretory lysosomes. Science. 2004;305(5680):55–59. doi: 10.1126/science.1095291. [DOI] [PubMed] [Google Scholar]
- 56.Kima PE, Burleigh B, Andrews NW. Surface-targeted lysosomal membrane glycoprotein-1 (Lamp-1) enhances lysosome exocytosis and cell invasion by Trypanosoma cruzi. Cell Microbiol. 2000;2(6):477–486. doi: 10.1046/j.1462-5822.2000.00071.x. [DOI] [PubMed] [Google Scholar]
- 57.Zanoteli E, van de Vlekkert D, Bonten EJ, Hu H, Mann L, Gomero EM, Harris AJ, Ghersi G, d’Azzo A. Muscle degeneration in neuraminidase 1-deficient mice results from infiltration of the muscle fibers by expanded connective tissue. Biochim Biophys Acta. 2010;1802(7–8):659–672. doi: 10.1016/j.bbadis.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu X, Steigelman KA, Bonten E, Hu H, He W, Ren T, Zuo J, d’Azzo A. Vacuolization and alterations of lysosomal membrane proteins in cochlear marginal cells contribute to hearing loss in neuraminidase 1-deficient mice. Biochim Biophys Acta. 2010;1802(2):259–268. doi: 10.1016/j.bbadis.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hinek A, Bodnaruk TD, Bunda S, Wang Y, Liu K. Neuraminidase-1, a subunit of the cell surface elastin receptor, desialylates and functionally inactivates adjacent receptors interacting with the mitogenic growth factors PDGF-BB and IGF-2. Am J Pathol. 2008;173(4):1042–1056. doi: 10.2353/ajpath.2008.071081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hinek A, Rabinovitch M, Keeley F, Okamura-Oho Y, Callahan J. The 67-kD elastin/laminin-binding protein is related to an enzymatically inactive, alternatively spliced form of beta-galactosidase. J Clin Invest. 1993;91(3):1198–1205. doi: 10.1172/JCI116280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morreau H, Galjart NJ, Gillemans N, Willemsen R, van der Horst GT, d’Azzo A. Alternative splicing of beta-galactosidase mRNA generates the classic lysosomal enzyme and a beta-galactosidase-related protein. J Biol Chem. 1989;264(34):20655–20663. [PubMed] [Google Scholar]
- 62.Blanchevoye C, Floquet N, Scandolera A, Baud S, Maurice P, Bocquet O, Blaise S, Ghoneim C, Cantarelli B, Delacoux F, Dauchez M, Efremov RG, Martiny L, Duca L, Debelle L. Interaction between the elastin peptide VGVAPG and human elastin binding protein. J Biol Chem. 2013;288(2):1317–1328. doi: 10.1074/jbc.M112.419929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hinek A, Pshezhetsky AV, von Itzstein M, Starcher B. Lysosomal sialidase (neuraminidase-1) is targeted to the cell surface in a multiprotein complex that facilitates elastic fiber assembly. J Biol Chem. 2006;281(6):3698–3710. doi: 10.1074/jbc.M508736200. [DOI] [PubMed] [Google Scholar]
- 64.Lehman A, Mattman A, Sin D, Pare P, Zong Z, d’Azzo A, Campos Y, Sirrs S, Hinek A. Emphysema in an adult with galactosialidosis linked to a defect in primary elastic fiber assembly. Mol Genet Metab. 2012;106(1):99–103. doi: 10.1016/j.ymgme.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 65.Starcher B, d’Azzo A, Keller PW, Rao GK, Nadarajah D, Hinek A. Neuraminidase-1 is required for the normal assembly of elastic fibers. Am J Physiol Lung Cell Mol Physiol. 2008;295(4):L637–L647. doi: 10.1152/ajplung.90346.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miyagi T, Wada T, Iwamatsu A, Hata K, Yoshikawa Y, Tokuyama S, Sawada M. Molecular cloning and characterization of a plasma membrane-associated sialidase specific for gangliosides. J Biol Chem. 1999;274(8):5004–5011. doi: 10.1074/jbc.274.8.5004. [DOI] [PubMed] [Google Scholar]
- 67.Arabkhari M, Bunda S, Wang Y, Wang A, Pshezhetsky AV, Hinek A. Desialylation of insulin receptors and IGF-1 receptors by neuraminidase-1 controls the net proliferative response of L6 myoblasts to insulin. Glycobiology. 2010;20(5):603–616. doi: 10.1093/glycob/cwq010. [DOI] [PubMed] [Google Scholar]
- 68.Dridi L, Seyrantepe V, Fougerat A, Pan X, Bonneil E, Thibault P, Moreau A, Mitchell GA, Heveker N, Cairo CW, Issad T, Hinek A, Pshezhetsky AV. Positive regulation of insulin signaling by neuraminidase 1. Diabetes. 2013;62(7):2338–2346. doi: 10.2337/db12-1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shi J, Wang A, Sen S, Wang Y, Kim HJ, Mitts TF, Hinek A. Insulin induces production of new elastin in cultures of human aortic smooth muscle cells. Am J pathol. 2012;180(2):715–726. doi: 10.1016/j.ajpath.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 70.Liang F, Seyrantepe V, Landry K, Ahmad R, Ahmad A, Stamatos NM, Pshezhetsky AV. Monocyte differentiation up-regulates the expression of the lysosomal sialidase, Neu1, and triggers its targeting to the plasma membrane via major histocompatibility complex class II-positive compartments. J Biol Chem. 2006;281(37):27526–27538. doi: 10.1074/jbc.M605633200. [DOI] [PubMed] [Google Scholar]
- 71.Stamatos NM, Carubelli I, van de Vlekkert D, Bonten EJ, Papini N, Feng C, Venerando B, d’Azzo A, Cross AS, Wang LX, Gomatos PJ. LPS-induced cytokine production in human dendritic cells is regulated by sialidase activity. J Leukoc Biol. 2010;88(6):1227–1239. doi: 10.1189/jlb.1209776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Feng C, Stamatos NM, Dragan AI, Medvedev A, Whitford M, Zhang L, Song C, Rallabhandi P, Cole L, Nhu QM, Vogel SN, Geddes CD, Cross AS. Sialyl residues modulate LPS-mediated signaling through the Toll-like receptor 4 complex. PLoS ONE. 2012;7(4):e32359. doi: 10.1371/journal.pone.0032359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nan X, Carubelli I, Stamatos NM. Sialidase expression in activated human T lymphocytes influences production of IFN-gamma. J Leukoc Biol. 2007;81(1):284–296. doi: 10.1189/jlb.1105692. [DOI] [PubMed] [Google Scholar]
- 74.Amith SR, Jayanth P, Franchuk S, Siddiqui S, Seyrantepe V, Gee K, Basta S, Beyaert R, Pshezhetsky AV, Szewczuk MR. Dependence of pathogen molecule-induced toll-like receptor activation and cell function on Neu1 sialidase. Glycoconj J. 2009;26(9):1197–1212. doi: 10.1007/s10719-009-9239-8. [DOI] [PubMed] [Google Scholar]
- 75.Amith SR, Jayanth P, Franchuk S, Finlay T, Seyrantepe V, Beyaert R, Pshezhetsky AV, Szewczuk MR. Neu1 desialylation of sialyl alpha-2,3-linked beta-galactosyl residues of TOLL-like receptor 4 is essential for receptor activation and cellular signaling. Cell Signal. 2010;22(2):314–324. doi: 10.1016/j.cellsig.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 76.Abdulkhalek S, Amith SR, Franchuk SL, Jayanth P, Guo M, Finlay T, Gilmour A, Guzzo C, Gee K, Beyaert R, Szewczuk MR. Neu1 sialidase and matrix metalloproteinase-9 cross-talk is essential for Toll-like receptor activation and cellular signaling. J Biol Chem. 2011;286(42):36532–36549. doi: 10.1074/jbc.M111.237578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lillehoj EP, Hyun SW, Feng C, Zhang L, Liu A, Guang W, Nguyen C, Luzina IG, Atamas SP, Passaniti A, Twaddell WS, Puche AC, Wang LX, Cross AS, Goldblum SE. NEU1 sialidase expressed in human airway epithelia regulates epidermal growth factor receptor (EGFR) and MUC1 protein signaling. J Biol Chem. 2012;287(11):8214–8231. doi: 10.1074/jbc.M111.292888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dall’Olio F, Malagolini N, Trinchera M, Chiricolo M. Mechanisms of cancer-associated glycosylation changes. Front Biosci (Landmark Ed) 2012;17:670–699. doi: 10.2741/3951. [DOI] [PubMed] [Google Scholar]
- 79.Adamczyk B, Tharmalingam T, Rudd PM. Glycans as cancer biomarkers. Biochim Biophys Acta. 2011;1820(9):134. doi: 10.1016/j.bbagen.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 80.Harduin-Lepers A, Krzewinski-Recchi MA, Colomb F, Foulquier F, Groux-Degroote S, Delannoy P. Sialyltransferases functions in cancers. Front Biosci (Elite Ed) 2012;4:499–515. doi: 10.2741/E396. [DOI] [PubMed] [Google Scholar]
- 81.Miyagi T, Wada T, Yamaguchi K, Shiozaki K, Sato I, Kakugawa Y, Yamanami H, Fujiya T. Human sialidase as a cancer marker. Proteomics. 2008;8(16):3303–3311. doi: 10.1002/pmic.200800248. [DOI] [PubMed] [Google Scholar]
- 82.Uemura T, Shiozaki K, Yamaguchi K, Miyazaki S, Satomi S, Kato K, Sakuraba H, Miyagi T. Contribution of sialidase NEU1 to suppression of metastasis of human colon cancer cells through desialylation of integrin beta4. Oncogene. 2009;28(9):1218–1229. doi: 10.1038/onc.2008.471. [DOI] [PubMed] [Google Scholar]
- 83.Wada T, Hata K, Yamaguchi K, Shiozaki K, Koseki K, Moriya S, Miyagi T. A crucial role of plasma membrane-associated sialidase in the survival of human cancer cells. Oncogene. 2007;26(17):2483–2490. doi: 10.1038/sj.onc.1210341. [DOI] [PubMed] [Google Scholar]
- 84.Tessitore A, del Pilar Martin M, Sano R, Ma Y, Mann L, Ingrassia A, Laywell ED, Steindler DA, Hendershot LM, d’Azzo A. GM1-ganglioside-mediated activation of the unfolded protein response causes neuronal death in a neurodegenerative gangliosidosis. Mol Cell. 2004;15(5):753–766. doi: 10.1016/j.molcel.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 85.Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13(10):1211–1233. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- 86.Mori K. Tripartite management of unfolded proteins in the endoplasmic reticulum. Cell. 2000;101(5):451–454. doi: 10.1016/S0092-8674(00)80855-7. [DOI] [PubMed] [Google Scholar]
- 87.Ma Y, Hendershot LM. The unfolding tale of the unfolded protein response. Cell. 2001;107(7):827–830. doi: 10.1016/S0092-8674(01)00623-7. [DOI] [PubMed] [Google Scholar]
- 88.Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287(5453):664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 89.Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403(6765):98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 90.Vance JE. Phospholipid synthesis in a membrane fraction associated with mitochondria. J Biol Chem. 1990;265(13):7248–7256. [PubMed] [Google Scholar]
- 91.Ardail D, Popa I, Bodennec J, Louisot P, Schmitt D, Portoukalian J. The mitochondria-associated endoplasmic-reticulum subcompartment (MAM fraction) of rat liver contains highly active sphingolipid-specific glycosyltransferases. Biochem J. 2003;371:1013–1019. doi: 10.1042/BJ20021834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bionda C, Portoukalian J, Schmitt D, Rodriguez-Lafrasse C, Ardail D. Subcellular compartmentalization of ceramide metabolism: MAM (mitochondria-associated membrane) and/or mitochondria? Biochem J. 2004;382:527–533. doi: 10.1042/BJ20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sano R, Annunziata I, Patterson A, Moshiach S, Gomero E, Opferman J, Forte M, d’Azzo A. GM1-ganglioside accumulation at the mitochondria-associated ER membranes links ER stress to Ca(2+)-dependent mitochondrial apoptosis. Mol Cell. 2009;36(3):500–511. doi: 10.1016/j.molcel.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456(7222):605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 95.Szabadkai G, Bianchi K, Varnai P, De Stefani D, Wieckowski MR, Cavagna D, Nagy AI, Balla T, Rizzuto R. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J Cell Biol. 2006;175:901–911. doi: 10.1083/jcb.200608073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell. 2007;131(3):596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 97.Rizzuto R, Brini M, Murgia M, Pozzan T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993;262:744–747. doi: 10.1126/science.8235595. [DOI] [PubMed] [Google Scholar]
- 98.Ledeen RW, Wu G. Ganglioside function in calcium homeostasis and signaling. Neurochem Res. 2002;27(7–8):637–647. doi: 10.1023/A:1020224016830. [DOI] [PubMed] [Google Scholar]
- 99.Ledeen RW, Wu G. GM1 ganglioside: another nuclear lipid that modulates nuclear calcium. GM1 potentiates the nuclear sodium-calcium exchanger. Can J Physiol Pharmacol. 2006;84:393–402. doi: 10.1139/y05-133. [DOI] [PubMed] [Google Scholar]
- 100.Annunziata I, Patterson A, Helton D, Hu H, Moshiach S, Gomero E, Nixon R, d’Azzo A. Lysosomal NEU1 deficiency affects amyloid precursor protein levels and amyloid-β secretion via deregulated lysosomal exocytosis. Nature Commun. 2013 doi: 10.1038/ncomms3734. [DOI] [PMC free article] [PubMed] [Google Scholar]