Abstract
Purpose
This is a retrospective analysis of the impact of moderate dysplasia at the resection margin for early stage cancer of the oral tongue.
Materials and Methods
Patients with T1-2N0 oral tongue cancer treated with surgery alone at Fox Chase Cancer Center (FCCC) from 1990 – 2010 were reviewed. Tumor and margin characteristics were abstracted from the pathology report.
Overall survival (OS), disease-free survival (DFS) and local control (LC) were calculated using the Kaplan Meier method. Predictors of LC, OS and DFS were analyzed.
Results
126 patients met the inclusion criteria. Dysplasia was present at the final margin in 36% of the cases (severe: 9%, moderate: 15%, mild: 12%).
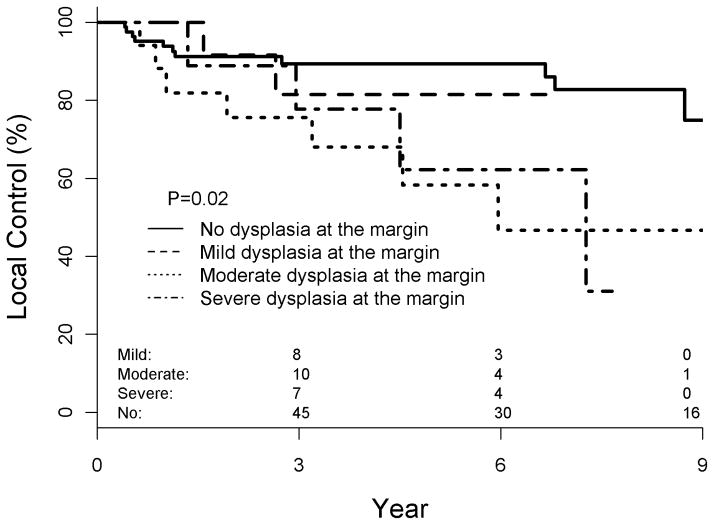
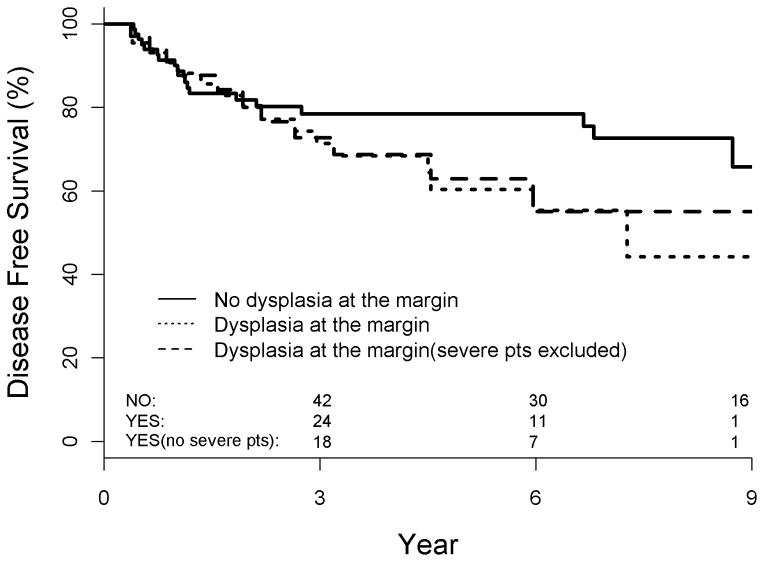
Median follow-up was 52 months. 3 and 5-year actuarial LC for the entire cohort was 77 and 73%, respectively. Actuarial 5-year LC and DFS were significantly worse for patients with moderate or severe dysplasia at the margin vs. none or mild dysplasia at the margin (49 v 82%, p = 0.005 and 49 v 80%, p = 0.008, respectively); 3-year comparisons were not significant. When analyzed separately, the detrimental local effect of moderate dysplasia at the margin persisted (p = 0.02) and the effect of severe dysplasia at the margin was approaching significance (p = 0.1). Mild dysplasia at the margin did not significantly impair LC or DFS.
Multivariate analysis demonstrated worse LC (HR: 2.99, p=0.006) and DFS (HR: 2.84, p=0.008) associated with severe or moderate dysplasia at the margin.
Conclusions
Both severe and moderate dysplasia at the margin appear to be correlated with inferior LC and DFS. Additional therapy may be justified, despite added morbidity.
Keywords: Oral tongue cancer, dysplasia, margin
Introduction
Pathologic margin is an important prognostic factor for relapse-free survival in head and neck cancer managed with primary surgery1–4. Given the prognostic significance of microscopic clearance of tumor, a ‘margin’ of grossly normal tissue is typically resected beyond the macroscopic tumor at the time of resection. Microscopic evaluation of the margin informs decisions regarding the application of postoperative therapy. Presence of tumor at a margin is an accepted indication for postoperative chemoradiation5,6. With a clear margin, in the absence of other risk factors, no additional treatment is needed7. What represents a “close margin” (defined as invasive carcinoma within a certain distance from the specimen limit) is institution-specific, ranging from 1 – 5 mm8–10. Despite the differences between centers in the definition of a “close” margin, retrospective analyses suggest that a close margin heralds increased locoregional failure11,12. While there are considerable data characterizing the influence of invasive carcinoma at the margin, the impact of dysplasia at the margin is less clear.
The concept of oral field cancerization was first promulgated in the 1950s13. It describes a situation in which, though clinical cancer is found in a seemingly discrete location (e.g. oral tongue, floor of mouth), the entire epithelial surface of the upper aerodigestive tract harbors multiple genetic abnormalities and potentially (pre)malignant lesions. The field effect has been attributed to multiple risk factors, most notably tobacco14 and ethanol15. Premalignant changes are typically widespread and their relationship to invasive cancer may be uncertain. Additionally excising an area of dysplasia adjacent to an invasive cancer in the course of an extirpative procedure can affect quality of life—larger resections generally result in worse functional outcome16. In an effort to maximize the therapeutic ratio, historic multidisciplinary guidelines recommended that severe dysplasia (carcinoma in-situ) and invasive carcinoma at the margin be considered a ‘positive margin’17 warranting the morbidity of additional resection, while both moderate and mild dysplasia at the margin did not warrant further therapy.
The prognostic importance of severe dysplasia at the margin appears significant, although the true impact is difficult to estimate because such cases are typically reported as ‘positive margin’ in retrospective reports. The prognostic importance of moderate and mild dysplasia at the margin is relatively unknown, because dedicated reports describing the outcomes of these patients are rare and management techniques range from re-excision for any dysplasia 18 to close observation19. Current CAP (College of American Pathologists) guidelines have recently been amended to recommend that both severe and moderate dysplasia at resection margins of oral cavity cancers be characterized as “positive” margins20.
At this institution moderate dysplasia at the margin has not been considered an involved margin justifying further therapy (e.g. additional resection or postoperative radiotherapy). Hence, it is possible to perform a retrospective analysis of patients with early oral tongue cancer managed with surgery alone to evaluate the impact of dysplasia at the resection margin.
Materials and Methods
We retrospectively identified 126 consecutive patients with early stage squamous cell carcinoma of the oral tongue who were treated with primary surgical resection alone from 1990 – 2010. Inclusion criteria were American Joint Committee on Cancer (AJCC) T1-2, N0 squamous cell carcinoma of the oral tongue with definitive surgical resection performed at Fox Chase Cancer Center. Patients who received postoperative radiation for accepted indications8 were considered representative of a higher risk population and were excluded. Neck dissection was performed according to guidelines of acceptable surgical practice21. Patient demographics, tumor characteristics and treatment related outcomes were abstracted from the relevant medical records in accordance with a Fox Chase Cancer Center Institutional Review Board-approved protocol and the Health Insurance Portability and Accountability Act.
All patients underwent primary tongue surgery as definitive management of their cancer. Patients presenting from an outside institution after a diagnostic excisional biopsy were submitted to additional wide local resection/partial glossectomy to confirm adequate surgical clearance of tumor. In the instance of prior excisional biopsy, the margins from the institution’s definitive resection were considered the final margins.
In a typical case a glossectomy specimen was excised with clinically appropriate margins and sent to the Pathology laboratory for analysis. Additional specimens were taken from the patient’s clinically uninvolved tissue and sent for intraoperative frozen section analysis. Margins deemed close or involved (by the pathologist in discussion with the surgeon) were additionally excised. This process was repeated until any cancer or severe dysplasia was cleared (most commonly with a single additional excision) or anatomic constraints prevented further resection. Although the decision to re-resect a margin was based on frozen section analysis at the time of resection, it was the final, permanent section of the separately submitted margin that was interpreted as the true margin for this retrospective analysis. In instances when no re-excision of a margin was performed, the permanent margin of the oriented primary specimen was interpreted as the true margin.
Dysplasia was reported as either mild, moderate, or severe according to the World Health Organization criteria22. Severe dysplasia at an initial frozen-section margin was additionally excised, while severe dysplasia appearing on a final margin was recommended additional resection as a separate surgical procedure. During the study period moderate and/or mild dysplasia at the margin was not re-resected when assigned on frozen section analysis and was not considered an indication for postoperative therapy when it appeared on permanent sections.
Tumor and margin characteristics were abstracted from the original surgical pathology analysis. A local recurrence was defined as one involving the tongue/floor of mouth – ‘elsewhere’ oral cavity tumors were interpreted as second tumors rather than as recurrences. Neck nodes above the supraclavicular fossa were interpreted as regional recurrences. All other recurrences were interpreted as distant. As there is no consensus threshold that separates true local recurrence from second primary tumors, we evaluated oral tongue cancers occurring both ≥ 3 and ≥ 5 years after initial resection as second primary tumors in separate analyses.
Overall survival (OS), disease-free survival (DFS) and local control (LC) were calculated using the Kaplan Meier method. The impact of any grade dysplasia at the margin was investigated as was the impact of either moderate or severe dysplasia (together) at the margin. Univariate and multivariate analyses were performed to evaluate potential predictors of OS, DFS and LC. Covariates investigated included: age, tobacco pack years, pathologic T stage, histologic grade, skeletal muscle involvement (intrinsic musculature of the tongue – a surrogate for depth of invasion), dysplasia at margin, tumor differentiation and neck dissection.
The endpoints of the study were Overall survival (OS), disease-free survival (DFS) and local control (LC) respectively. Patient demographic characteristics and tumor staging factors were assessed in regard of associations with the endpoints. Univariate analysis was performed using Kaplan-Meier estimation and log-rank test. Multivariable analysis (MVA) was done via a multiple Cox regression. Both analyses were performed on the three endpoints respectively. A p-value <0.05 was considered significant. All analyses were carried out with SAS 9.2.
Results
A total of 126 patients with 131 tumors met the inclusion criteria. Patient, tumor and treatment characteristics are detailed in Table 1. The median patient age was 62 years (range: 20–98), 76% had a history of tobacco use including 24% who were actively using tobacco at diagnosis. Seventy-one percent of the primary surgical procedures were performed at our institution, while the remaining 29% were re-operations after excisional biopsies performed elsewhere. A neck dissection was undertaken on 51% of patients, and was defined as “supraomohyoid” in 90% of these patients. Pathologic evaluation revealed that 81% of our cohort had pT1 cancers, and that all close or positive margins were resected to tumor-free margins. Dysplasia was present at a final margin in 36% of the cases (severe: 9%, moderate: 15%, mild: 12%) and was identified only on permanent pathologic section in 50% of cases.
Table 1.
Clinical/pathologic characteristics
| Characteristic | Number of Patients (%) |
|---|---|
|
| |
| Age (years) | |
| <60 | 61 (47) |
| >60 | 70 (53) |
|
| |
| Sex | |
| Male | 73 (56) |
| Female | 58 (44) |
|
| |
| Tobacco use | |
| Current | 32 (24) |
| History | 68 (52) |
| Never | 31 (24) |
|
| |
| Clinical Tumor Stage | |
| cT1 | 86 (66) |
| cT2 | 29 (22) |
| cTx | 16 (12) |
|
| |
| Pathologic Tumor Stage | |
| pT1 | 105 (80) |
| pT2 | 23 (18) |
| pTx | 3 (2) |
|
| |
| Neck Dissection | |
| None | 64 (49) |
| Supraomohyoid (SOH) | 60 (46) |
| Modified Radical (MRND) | 7 (5) |
|
| |
| Histologic Grade | |
| Well | 22 (19) |
| Moderate | 68 (57) |
| Poor | 21 (18) |
| n/a | 8 (6) |
|
| |
| Dysplasia at Final Margin | |
| No | 83 (63) |
| Yes | 48 (37) |
|
| |
| Grade of Dysplasia at Final Margin | |
| Mild | 15 (31) |
| Moderate | 21 (44) |
| Severe | 12 (25) |
|
| |
| Skeletal (Intrinsic) Muscle Invasion | |
| No | 91 (69) |
| Yes | 40 (31) |
With a median follow-up of 52 months (range: 1–250), the 3- and 5-year actuarial LC, DFS and OS for the entire cohort was 77% and 76%, and 84% and 73%, 72% and 74%, respectively. Overall, 27 patients developed recurrent disease within 3 years of initial surgery, including 16 local recurrences (12%), 10 regional recurrences (8%) and 1 distant failure (1%). Re-evaluation of outcomes within 5 years of the original operation increased local recurrences only (n = 19, 15%). The local control (80 v 71%, p = 0.43) and disease free survival (78 v 71%, p = 0.53) detriments of dysplasia at the margin were not significant at 3 years after complete resection but were approaching significance by 5 years of follow-up (LC: 80 v 60%, p = 0.12 and DFS: 78 v 60%, p = 0.17). Given the worsening LC and DFS as follow-up lengthened of patients with dysplasia at the margin the data were interrogated in terms of a 5-year determinant of true local failures.
We elected to initially evaluate moderate dysplasia and severe dysplasia at the margin together secondary to seemingly similar clinical behavior of the two variables (Figure 1). This conclusively demonstrated worse local control for moderate/severe dysplasia at the margin (82% v 49%, p = 0.005). Similar to local control data, the importance of either moderate or severe dysplasia at the margin influenced DFS (80% v 49%, p =0.008). We then evaluated each degree of dysplasia at the margin separately. This demonstrated that, despite similar rates at five years, only moderate dysplasia at the margin (49% local control, p = 0.02) was still significant while severe dysplasia at the margin was approaching significance (54% local control, p = 0.1). This was thought to be secondary to less instances of severe dysplasia at the margin (n = 12) than moderate (n = 21). Other factors that approached significance were neck dissection performed (p = 0.16) and poor histologic grade (p = 0.09). Five year overall survival was 74% for the entire population and not influenced by the presence of dysplasia (Table 2) at the margin (p = 0.6).
Figure 1.
Locoregional control as a function of dysplasia at the final surgical margin
Table 2.
5-year actuarial outcomes
| LC | DFS | OS | |
|---|---|---|---|
| Overall | 73% | 72% | 74% |
| No dysplasia at the margin | 80% | 78% | 72% |
| Any dysplasia at the margin | 60% | 60% | 77% |
| Severe dysplasia at the margin | 54% | 54% | 74% |
| Moderate dysplasia at the margin | 49% | 49% | 77% |
| Mild dysplasia at the margin | 81% | 81% | 76% |
Two multivariate analyses (MVA) of locoregional control and disease free survival were performed; one that evaluated moderate and severe dysplasia together and another that evaluated all degrees of dysplasia separately. When evaluated together, the MVA demonstrated that moderate and severe dysplasia at the margin (p = 0.007), an observed neck (p = 0.04) and poorly differentiated histology (p = 0.009) were predictive of locoregional recurrence (Table 3). Evaluation of disease-free survival with a similar MVA demonstrated that only moderate or severe dysplasia at the margin (p = 0.008) and poorly differentiated histology were significant (Table 4). A second MVA was then run that evaluated all degrees of dysplasia at the margin separately. This demonstrated that only moderate dysplasia at the margin (p = 0.036), poor histologic grade (p = 0.0078), and an observed neck (p = 0.04) were predictive of local recurrence (Table 3). Evaluation of DFS with a similar MVA demonstrated that both moderate dysplasia at the margin (p = 0.036) and poorly differentiated histology (p = 0.011) were associated with recurrence.
Table 3.
Multivariate analysis of potentially predictive factors of local control
| Variable | Comparison | Hazard Ratio | p- value | Hazard Ratio | p- value |
|---|---|---|---|---|---|
| Dysplasia at margin | Moderate/Severe v Mild/None | 2.99 (1.35–6.61) | 0.0067 | ||
| Dysplasia at margin | Severe v None | 2.94 (0.91–9.49) | 0.071 | ||
| Dysplasia at margin | Moderate v None | 2.72 (1.07–6.89) | 0.036 | ||
| Dysplasia at margin | Mild v None | 0.62 (0.13–2.77) | 0.53 | ||
| Histologic Grade | Poor v Well/Mod/Unk | 3.47 (1.36–8.83) | 0.0090 | 3.63 (1.40–9.37) | 0.0078 |
| Pack years | Continuous | 0.99 (0.98–1.01) | 0.72 | 0.99 (0.98–1.01) | 0.73 |
| Neck Dissection | No vs yes | 2.59 (1.03–6.49) | 0.043 | 2.61 (1.05–6.55) | 0.040 |
| Age | Continuous | 1.00 (0.98–1.03) | 0.96 | 1.00 (0.98–1.03) | 0.97 |
| Skeletal Muscle | Yes v No | 1.80 (0.82–3.94) | 0.14 | 1.80 (0.82–3.94) | 0.14 |
| pT | 2 v 1 | 1.76 (0.59–5.23) | 0.31 | 1.70 (0.58–5.02) | 0.34 |
Table 4.
Multivariate analysis of potentially predictive factors of disease-free survival
| Variable | Comparison | Hazard Ratio | p- value | Hazard Ratio | p- value |
|---|---|---|---|---|---|
| Dysplasia at margin | Moderate/Severe v Mild/None | 2.84 (1.30–6.19) | 0.0086 | ||
| Dysplasia at margin | Severe v None | 2.57 (0.81–8.16) | 0.11 | ||
| Dysplasia at margin | Moderate v None | 2.66 (1.07–6.64) | 0.036 | ||
| Dysplasia at margin | Mild v None | 0.57 (0.13–2.54) | 0.46 | ||
| Histologic Grade | Poor v Well/Mod/Unk | 3.21 (1.27–8.08) | 0.013 | 3.34 (1.31–8.53) | 0.011 |
| Pack years | Continuous | 0.99 (0.97–1.01) | 0.86 | 0.99 (0.99–1.01) | 0.90 |
| Neck Dissection | No vs yes | 2.18 (0.91–5.23) | 0.082 | 2.20 (0.92–5.25) | 0.077 |
| Age | Continuous | 1.01 (0.98–1.03) | 0.66 | 1.01 (0.91–1.03) | 0.65 |
| Skeletal Muscle | Yes v No | 1.97 (0.92–4.22) | 0.08 | 1.98 (0.92–4.23) | 0.080 |
| pT | 2 v 1 | 1.46 (0.51–4.24) | 0.48 | 1.42 (0.49–4.09) | 0.51 |
Discussion
The pathologic evaluation of oral epithelial dysplasia, dependent upon the thickness of the involved epithelium, is subject to significant intra- and inter-observer differences in the grading of specimens. Institution of guidelines 22 has improved intra-observer grading 23, but an undefined amount of subjectivity persists. Progression to invasive cancer is best characterized for severe dysplasia and carcinoma in situ 24, and is thought to be mediated by p53 mutations 25.
When dysplasia is encountered at the margin of a head and neck cancer resection, it has been common to extrapolate from the experience of dysplasia unassociated with invasive cancer to determine the need for further therapy. As a consequence, at Fox Chase Cancer Center, mild dysplasia at the margin is observed. All patients are counseled to discontinue exposure to potential environmental carcinogens (ethanol, tobacco, and mouth rinses containing alcohol). Similarly, severe dysplasia at the margin is re-resected until cleared during the initial operative procedure. In settings where severe dysplasia cannot be cleared, it is treated as a surrogate of residual disease and postoperative radiotherapy is typically recommended 8.
Moderate dysplasia at the margin has not been widely interpreted as an indicator for additional therapy and has often been approached expectantly. Recently, CAP guidelines were updated to recommend that both moderate and severe dysplasia at the margin be considered “positive” margins 20. This study evaluates the consequences of an expectant approach (observation) to moderate dysplasia at the margin. It relied upon dysplasia grading performed by multiple staff pathologists and fellows over a 20-year time period that was abstracted from the medical chart. There was no re-grading of material. Hence, these results reflect everyday practice at a National Cancer Institute designated Comprehensive Cancer Center. It suggests that moderate or severe dysplasia present at the final margin raises risk of local recurrence and worsens disease-free survival. A small number of patients (n = 12) with severe dysplasia at the margin were recommended to receive further therapy (either re-resection or postoperative radiotherapy) and for a variety of reasons never received the proposed treatment. In addition to analyzing moderate and severe dysplasia together, we analyzed all degrees of dysplasia separately in an attempt to determine if the patients with severe dysplasia were influencing the results (as they did not receive recommended therapy). This demonstrated that moderate dysplasia at the margin was independently indicative of increased local recurrence (p = 0.02) and worse disease-free survival (p = 0.03). The effect of severe dysplasia was not significant for either local control (p = 0.12) or disease free survival (p = 0.16), despite a similar hazard ratio, likely secondary to limited patient numbers. As expected, mild dysplasia was not associated with worse local control (p = 0.63) or disease-free survival (p = 0.57).
To investigate potentially prognostic clinical and pathologic variables a multivariate analysis was undertaken. This evaluation is unique because all patients included were considered at ‘low risk’ and received no postoperative therapy. One might expect that no factors are independently associated with failure in such a cohort. However, three factors were found to be significant for locoregional failure - moderate or severe dysplasia at the margin, poor histologic grade, and no neck dissection performed. A disease-free survival MVA similarly demonstrated the significance of moderate dysplasia at the margin and poorly differentiated histology. A second MVA was performed to investigate the effect of both moderate and severe dysplasia at the margin separately. This demonstrated findings similar to when moderate and severe dysplasia were analyzed together, seeming to confirm that both moderate and severe dysplasia at the margin herald an increased risk of recurrence.
As the dominant mode of recurrence was local (63% of recurrences), it is difficult to interpret the significance of a local control detriment of a clinically observed neck, as this variable would not be expected to influence local (tongue) failure, and is perhaps reflection of limited power in a relatively small data set. Nonetheless, it reinforces the importance of appropriate use of a neck dissection in seemingly ‘low-risk’ oral tongue cancers. Poor differentiation is a known risk factor for oral tongue treatment failure26. Oral tongue cancer depth of invasion is an accepted indication for elective therapy to the neck 21,27,28. Given the retrospective nature of this analysis, we did not have depth of invasion data for all tumors. Skeletal muscle invasion, a surrogate for depth of invasion, demonstrated a detriment to DFS that was approaching significance, further suggesting that deeper tumors seem to display a higher propensity for failure, absent postoperative treatment. Finally, this analysis is the first to suggest that moderate dysplasia at the margin is suggestive of not just worse locoregional control, but also of worse disease free survival.
Conclusions
The presence of moderate or severe dysplasia at the margin is strongly correlated with inferior local control and potentially with worse disease-free survival. In the absence of well-tolerated postoperative therapy with reversible toxicity additional excision in an effort to clear moderate dysplasia at the margin is justified, despite added morbidity. Whether postoperative radiotherapy or chemoprevention can improve the poor prognosis of patients with persistent dysplasia at the margin warrants prospective investigation.
Figure 2.
Disease-free survival as a function of dysplasia at the final surgical margin
Acknowledgments
This publication was supported by grant number P30 CA006927 from the National Cancer Institute, NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Abstract presented at the scientific session of the 54th annual meeting of the American Society for Radiation Oncology in Boston, MA, October 28-31; 2012.
Conflict of Interest Statement: None.
References
- 1.Ganly I, Patel S, Shah J. Early stage squamous cell cancer of the oral tongue--clinicopathologic features affecting outcome. Cancer. 2012 Jan 1;118(1):101–111. doi: 10.1002/cncr.26229. [DOI] [PubMed] [Google Scholar]
- 2.Loree TR, Strong EW. Significance of positive margins in oral cavity squamous carcinoma. American journal of surgery. 1990 Oct;160(4):410–414. doi: 10.1016/s0002-9610(05)80555-0. [DOI] [PubMed] [Google Scholar]
- 3.Rogers SN, Brown JS, Woolgar JA, et al. Survival following primary surgery for oral cancer. Oral oncology. 2009 Mar;45(3):201–211. doi: 10.1016/j.oraloncology.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Sutton DN, Brown JS, Rogers SN, Vaughan ED, Woolgar JA. The prognostic implications of the surgical margin in oral squamous cell carcinoma. International journal of oral and maxillofacial surgery. 2003 Feb;32(1):30–34. doi: 10.1054/ijom.2002.0313. [DOI] [PubMed] [Google Scholar]
- 5.Cooper JS, Zhang Q, Pajak TF, et al. Long-term Follow-up of the RTOG 9501/Intergroup Phase III Trial: Postoperative Concurrent Radiation Therapy and Chemotherapy in High-Risk Squamous Cell Carcinoma of the Head and Neck. International journal of radiation oncology, biology, physics. 2012 Dec 1;84(5):1198–1205. doi: 10.1016/j.ijrobp.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernier J, Cooper JS, Pajak TF, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501) Head & neck. 2005 Oct;27(10):843–850. doi: 10.1002/hed.20279. [DOI] [PubMed] [Google Scholar]
- 7.Beadle BM, et al. Outcomes and Risk Factors in Patients Treated with Surgery and Postoperative Radiation Therapy Alone: A Subset Update of a Randomized Trial. International Journal of Radiation, Oncology, Biology, and Physics. 2011 Oct 1;81(2):S104–S105. 2011. [Google Scholar]
- 8.Hinerman RW, Mendenhall WM, Morris CG, Amdur RJ, Werning JW, Villaret DB. Postoperative irradiation for squamous cell carcinoma of the oral cavity: 35-year experience. Head & neck. 2004 Nov;26(11):984–994. doi: 10.1002/hed.20091. [DOI] [PubMed] [Google Scholar]
- 9.Daly ME, Le QT, Kozak MM, et al. Intensity-modulated radiotherapy for oral cavity squamous cell carcinoma: patterns of failure and predictors of local control. International journal of radiation oncology, biology, physics. 2011 Aug 1;80(5):1412–1422. doi: 10.1016/j.ijrobp.2010.04.031. [DOI] [PubMed] [Google Scholar]
- 10.Meier JD, Oliver DA, Varvares MA. Surgical margin determination in head and neck oncology: current clinical practice. The results of an International American Head and Neck Society Member Survey. Head & neck. 2005 Nov;27(11):952–958. doi: 10.1002/hed.20269. [DOI] [PubMed] [Google Scholar]
- 11.Pfreundner L, Willner J, Marx A, Hoppe F, Beckmann G, Flentje M. The influence of the radicality of resection and dose of postoperative radiation therapy on local control and survival in carcinomas of the upper aerodigestive tract. International journal of radiation oncology, biology, physics. 2000 Jul 15;47(5):1287–1297. doi: 10.1016/s0360-3016(00)00514-9. [DOI] [PubMed] [Google Scholar]
- 12.Langendijk JA, Slotman BJ, van der Waal I, Doornaert P, Berkof J, Leemans CR. Risk-group definition by recursive partitioning analysis of patients with squamous cell head and neck carcinoma treated with surgery and postoperative radiotherapy. Cancer. 2005 Oct 1;104(7):1408–1417. doi: 10.1002/cncr.21340. [DOI] [PubMed] [Google Scholar]
- 13.Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953 Sep;6(5):963–968. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 14.Lewin F, Norell SE, Johansson H, et al. Smoking tobacco, oral snuff, and alcohol in the etiology of squamous cell carcinoma of the head and neck: a population-based case-referent study in Sweden. Cancer. 1998 Apr 1;82(7):1367–1375. doi: 10.1002/(sici)1097-0142(19980401)82:7<1367::aid-cncr21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 15.Hashibe M, Brennan P, Benhamou S, et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Journal of the National Cancer Institute. 2007 May 16;99(10):777–789. doi: 10.1093/jnci/djk179. [DOI] [PubMed] [Google Scholar]
- 16.Thomas L, Moore EJ, Olsen KD, Kasperbauer JL. Long-term quality of life in young adults treated for oral cavity squamous cell cancer. The Annals of otology, rhinology, and laryngology. 2012 Jun;121(6):395–401. doi: 10.1177/000348941212100606. [DOI] [PubMed] [Google Scholar]
- 17.Pfister DG, Ang KK, Brizel DM, et al. Head and neck cancers. Journal of the National Comprehensive Cancer Network: JNCCN. 2011 Jun 1;9(6):596–650. doi: 10.6004/jnccn.2011.0053. [DOI] [PubMed] [Google Scholar]
- 18.Weijers M, Snow GB, Bezemer PD, van der Wal JE, van der Waal I. The clinical relevance of epithelial dysplasia in the surgical margins of tongue and floor of mouth squamous cell carcinoma: an analysis of 37 patients. Journal of oral pathology & medicine: official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. 2002 Jan;31(1):11–15. doi: 10.1034/j.1600-0714.2002.310103.x. [DOI] [PubMed] [Google Scholar]
- 19.Kurita H, Nakanishi Y, Nishizawa R, et al. Impact of different surgical margin conditions on local recurrence of oral squamous cell carcinoma. Oral oncology. 2010 Nov;46(11):814–817. doi: 10.1016/j.oraloncology.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 20.Richardson MS, et al. Pathologists CoA., editor. Protocol for the Examination of Specimens with Carcinomas of the Lip and Oral Cavity. LipOralCavity 3.1.0.2. www.cap.org/cancerprotocols2011.
- 21.Fukano H, Matsuura H, Hasegawa Y, Nakamura S. Depth of invasion as a predictive factor for cervical lymph node metastasis in tongue carcinoma. Head & neck. 1997 May;19(3):205–210. doi: 10.1002/(sici)1097-0347(199705)19:3<205::aid-hed7>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 22.Gale N, et al. Tumors of the Oral Cavity and the Oropharynx, Epithelial Precursor Lesions. In: Barnes L, et al., editors. World Health Organization Classification of Tumors: Pathology and Genetics: Head and Neck Tumors. Switzerland: WHO Press; 2005. pp. 164–208. [Google Scholar]
- 23.Kujan O, Khattab A, Oliver RJ, Roberts SA, Thakker N, Sloan P. Why oral histopathology suffers inter-observer variability on grading oral epithelial dysplasia: an attempt to understand the sources of variation. Oral oncology. 2007 Mar;43(3):224–231. doi: 10.1016/j.oraloncology.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 24.Hintz BL, Kagan AR, Nussbaum H, Rao AR, Chan PY, Miles J. A ‘watchful waiting’ policy for in situ carcinoma of the vocal cords. Archives of otolaryngology. 1981 Dec;107(12):746–751. doi: 10.1001/archotol.1981.00790480022006. [DOI] [PubMed] [Google Scholar]
- 25.Boyle JO, Hakim J, Koch W, et al. The incidence of p53 mutations increases with progression of head and neck cancer. Cancer research. 1993 Oct 1;53(19):4477–4480. [PubMed] [Google Scholar]
- 26.Beenken SW, Krontiras H, Maddox WA, Peters GE, Soong S, Urist MM. T1 and T2 squamous cell carcinoma of the oral tongue: prognostic factors and the role of elective lymph node dissection. Head & neck. 1999 Mar;21(2):124–130. doi: 10.1002/(sici)1097-0347(199903)21:2<124::aid-hed5>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 27.Matsuura K, Hirokawa Y, Fujita M, Akagi Y, Ito K. Treatment results of stage I and II oral tongue cancer with interstitial brachytherapy: maximum tumor thickness is prognostic of nodal metastasis. International journal of radiation oncology, biology, physics. 1998 Feb 1;40(3):535–539. doi: 10.1016/s0360-3016(97)00811-0. [DOI] [PubMed] [Google Scholar]
- 28.Spiro RH, Spiro JD, Strong EW. Surgical approach to squamous carcinoma confined to the tongue and the floor of the mouth. Head & neck surgery. 1986 Sep-Oct;9(1):27–31. doi: 10.1002/hed.2890090106. [DOI] [PubMed] [Google Scholar]