Abstract
Chronic challenge of renin–angiotensin causes recruitment of renin-producing cells in the kidney along the media layer of afferent arterioles and hypertrophy of cells in the juxtaglomerular apparatus. This study aimed to define the role of nitric oxide (NO) with regard to the recruitment pattern of renin-producing cells and to the possible pathways along which NO could act. We considered the hypothesis that endothelium-derived NO acts via NO-sensitive guanylate cyclase. Mice were treated with low-salt diet in combination with the angiotensin I–converting enzyme inhibitor enalapril for 3 weeks, which led to a 13-fold increase in renin expression associated with marked recruitment of renin cells in afferent arterioles and hypertrophy of the juxtaglomerular apparatus in wild-type mice. In wild-type mice additionally treated with the nonselective NO synthase inhibitor L-NAME, the recruitment of renin-expressing cells along the afferent arterioles was absent and juxtaglomerular hypertrophy was diminished. An almost identical attenuation of renin cell recruitment as with L-NAME treatment in wild-type mice was found in mice lacking the endothelial isoform of NO synthase. Treatment of mice lacking NO-sensitive guanylate cyclase in renin-expressing cells and preglomerular smooth muscle cells with low-salt diet in combination with the angiotensin I–converting enzyme inhibitor enalapril for 3 weeks produced juxtaglomerular hypertrophy like in wild-type mice, but no recruitment in afferent arterioles. These findings suggest that endothelium-derived NO and concomitant formation of cGMP in preglomerular renin cell precursors supports recruitment of renin-expressing cells along preglomerular vessels, but not in the juxtaglomerular apparatus.
Keywords: guanylate cyclase, juxtaglomerular apparatus, nitric oxide, recruitment, renin
Chronic challenges of the renin–angiotensin system lead to an enhancement of renin gene expression accompanied by hypertrophy of juxtaglomerular cells1 and by metaplastic transformation of preglomerular vascular smooth muscle cells into renin-producing cells.2 The cellular mechanisms triggering the recruitment of renin-producing cells are not well understood. It is a common observation in this context that systemic application of nitric oxide synthase (NOS) inhibitors attenuates any stimulation of renin gene expression, regardless of the underlying challenge of the renin system.3 It has been speculated therefore that NO might play a fundamental role for the production of renin. In fact, renin-producing cells and its potential precursor cells are surrounded by cells expressing different NOS isoforms. Thus, endothelial cells express NOS-3, and macula densa cells express NOS-1.4,5 A special role of vascular NOS-1 has previously been hypothesized in a state of strong renin cell expression by rennin–angiotensin–aldosterone system (RAAS) inhibition.6,7 NO activates NO-sensitive guanylate cyclase (NO-GC) that is present in renin-expressing cells and in preglomerular smooth muscle cells.8 Cyclic GMP generated by NO-GC can exert a variety of cellular effects, including inhibition of cAMP-phohodiesterase-39 that is present in renin-producing cells and in preglomerular smooth muscle cells.10 Inhibition of phohodiesterase-3 increases intracellular cAMP levels, and thus enhances the cAMP signaling pathway, which is regulatory for renin secretion and fundamental for renin gene transcription,11–13 including renin cell recruitment.14–16 Although there is agreement about a direct stimulatory effect of NO on renin secretion that is mediated by the cAMP pathway,17 a direct effect of NO on the metaplastic transformation of vascular smooth muscle cells into renin-producing cells has not yet been established. It is relevant in this context that systemic inhibition of NOS does not only interfere with NO signaling in renin-producing cells and its potential precursors, but also markedly increases blood pressure.18–20 The blood pressure, in particular the renal perfusion pressure, is known as a strong negative regulator of renin cell recruitment.21–23 To obtain more information about understanding the mechanisms of renin cell recruitment, our study focused on 2 main goals, namely, first, to identify the origin of NO relevant for renin cell recruitment and, second, to distinguish between indirect (via blood pressure) and direct (via NO-GC) effects of NO on renin cell recruitment.
For this purpose, we used an experimental maneuver, which produced a strong recruitment of renin-producing cells, namely, a combination treatment of low-salt (LS) diet with an angiotensin I–converting enzyme inhibitor. We applied this maneuver to mice with and without systemic NOS inhibition, to mice lacking endothelial NOS (eNOS) and to mice lacking NO-GC in preglomerular smooth muscle cells and renin-expressing cells. Our results suggest that the effects of NO on renin cell recruitment are, in part, directly mediated at the level of the renin-expressing cells and, in part, indirectly mediated by changes of blood pressure.
Methods
Animals
The eNOS−/− mice were generated and provided by Gödecke et al.24 Wild-type mice (wt) were obtained from the F2 generations of eNOS−/− and C57/Bl6 breedings. Renin cell-specific NO-GC−/− mice were derived from crossings of mice bearing a heterozygous insertion of Cre recombinase in the Ren1d gene locus (Ren1dCre/+) on a 129SVxC57/Bl6 background2 and mice carrying a floxed exon 10 of NO-GC-β1 subunit (NO-GCfl/fl).25 Genotyping was performed by PCR on DNA isolated from tail biopsies (NO-GC: loxP-β1-U1 5′-AAGATGCTGAAGGGAAGGATGC-3′; loxP-β1-L1 5 ′-CAGCCCAAAGAAACAAGAAGAAAG-3′; del-β1-L1 5′-GATGTGGGATTGTTTCTGAGGA-3′; Ren-Cre: 653 Ren1d 5′-GAAGGAGAGCAAAAGGTAAGAG-3′, 400 Ren1d 5′-GTAGTAGAAGGGGGAGTTGTG-3′; 468 Cre 5′-TTGGTGTACGGTCAGTAAATTGGAC-3′). For studies with Ren1d+/Cre–NO-GCfl/fl animals considered as Ren-GC−/−, Ren1d+/+NO-GCfl/fl were considered as controls. All experiments were performed on male 8- to 12-week-old mice and age-matched controls. Animals were kept on standard rodent chow with free access to tap water. All experiments were conducted according to the National Institutes of Health guidelines for care and use of animals in research. The experiments were approved by the local government.
Treatment Regimen
Ten experimental groups were analyzed.
Group 1
wt (C57/Bl6 or Ren1d+/+–NO-GCfl/fl) mice on normal chow without additional treatment.
Group 2
wt mice on normal chow receiving the NOS-inhibitor, N-nitro-L-arginine methyl ester (L-NAME, Sigma Aldrich; 0.5 mg/mL in the tap water), for 3 weeks.
Group 3
wt mice receiving LS diet (0.02% wt/wt NaCl; Ssniff, Germany)+enalapril (Ena, 10 mg/kg×d−1; Sigma-Aldrich) for 3 weeks.
Group 4
wt mice receiving LS diet+enalapril and L-NAME for 3 weeks.
Group 5
eNOS−/− mice on normal chow without additional treatment.
Group 6
eNOS−/− mice receiving L-NAME for 3 weeks.
Group 7
eNOS−/− mice receiving LS diet+enalapril for 3 weeks.
Group 8
eNOS−/− mice receiving LS diet+enalapril and L-NAME for 3 weeks.
Group 9
Ren1d+/Cre–NO-GCfl/fl mice normal chow without additional treatment.
Group 10
Ren1d+/Cre–NO-GCfl/fl mice receiving LS diet+enalapril for 3 weeks.
Blood Pressure Measurement
Systolic blood pressure of conscious mice was determined by tail cuff manometry (TSE Systems, Germany). After a training period of 7 consecutive days, in which mice were placed into the holding device for 15 minutes per day, the values of 4 days were considered for evaluation.
Immunohistochemistry and 3D Reconstruction
5-μm serial slices of perfusion-fixed paraffin-embedded kidneys were stained for renin and α-smooth muscle actin, as described previously.26 For NO-GC immunostainings, a polyclonal antiguanylyl-cyclase β1 antibody (ER-19; Sigma Aldrich) was used. After digitalization of 80 to 100 serial slices (Axiovert 200+AxioCam MRm, Zeiss), data for α-smooth muscle actin and renin immunoreactivity were imported into Amira 5.4.2 software (VSG, Germany) for subsequent generation of aligned greyscale-stacks of α-smooth muscle actin and renin. In a labeling step, kidney structures and renin-positive areas were rebuilt from the gray values of each data set.
Volume Calculations
Renin volume per glomerulus was calculated from of 3D kidney reconstructions of 3 animals per experimental group by Amira 5.4.2. as volume pixels (voxels). The number of renin-labeled voxels adjacent to each individual glomerulus and its the respective afferent arteriole up to 30 μm proximal from the vascular pole was taken as a single value.
Isolated Perfused Mouse Kidney
Mouse kidneys were perfused ex situ with a modified Krebs-Henseleit solution27 supplemented with 6 g/100 mL BSA and human red blood cells (10% hematocrit) at a constant pressure of 90 mm Hg, as described previously.28 After a control perfusion period, sodium nitroprusside (Sigma-Aldrich) at a final concentration of 30 μmol/L was added to the perfusion medium. Every 2 minutes, venous perfusate samples were taken for analysis of renin-secretion rate and perfusate flow was recorded. Renin activity was measured as the generation of angiotensin I in presence of plasma from bilaterally nephrectomized rats, serving as excess of renin substrate. Angiotensin I was determined by radioimmunoassay (RENCTK; DiaSorin, Germany).
Sample Collection
Mice were anesthetized with an intraperitoneal injection of 12 mg/kg xylazine (Serumwerk, Germany) and 80 mg/kg ketamine (Bela-Pharm, Germany). After ligation of the left renal artery, the kidney was excised, frozen in liquid N2, and stored for mRNA analysis. For immunohisto-chemistry and 3D reconstruction, the right kidney was perfusion fixed in situ with a paraformaldehyde solution (3% wt/vol in PBS).
mRNA Analysis
Total RNA was isolated from frozen kidneys and cDNA synthesis, renin and GAPDH mRNA was conducted as described previously.29
Statistical Analysis
Values are denoted as means±SE. Differences between experimental groups were analyzed by ANOVA and Bonferroni adjustment for multiple comparisons. P<0.05 was considered statistically significant.
Results
Renin Cell Distribution in WT Kidneys
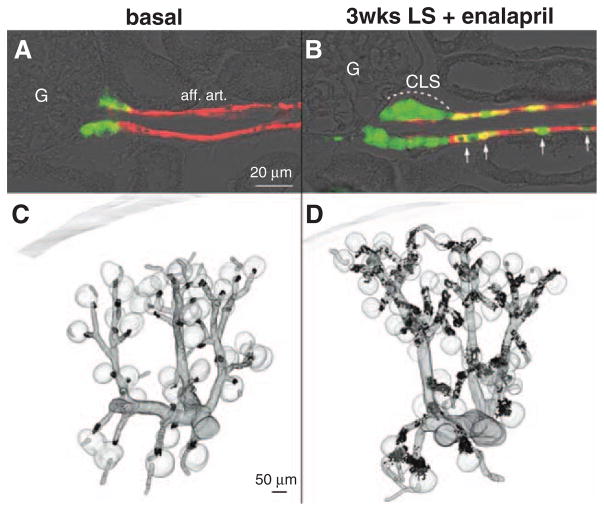
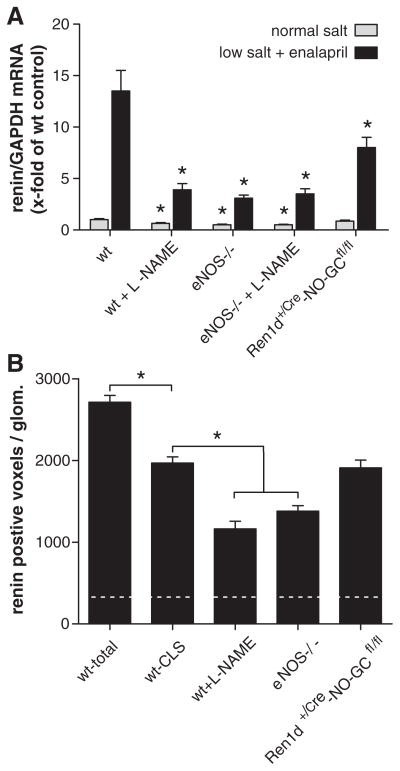
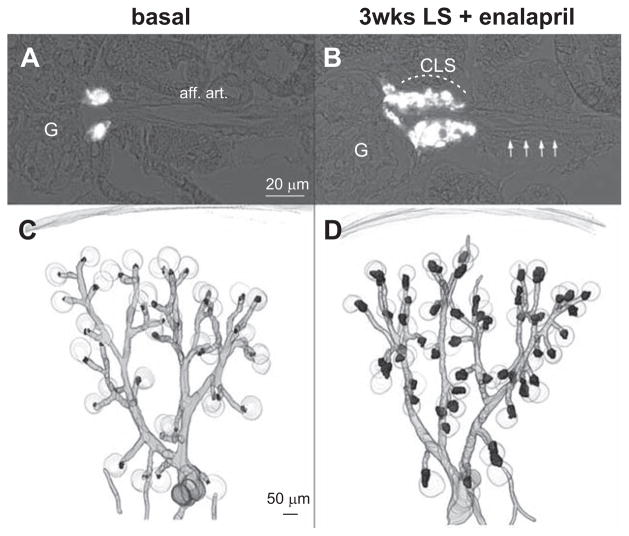
In wt mice kept on normal-salt diet, renin-expressing cells were confined to the juxtaglomerular poles of glomeruli (Figure 1A and 1C). Virtually every juxtaglomerular region contained a few renin-expressing cells that formed an extension of the media layer of afferent arterioles apparatus to the branching of the glomerular capillaries (Figure 1A and 1C). Feeding wt mice a combination of LS diet together with the angiotensin I–converting enzyme inhibitor enalapril for 3 weeks led to a marked increase of renin-expressing cells (Figure 1B and 1D). Additional renin-expressing cells appeared along the media layer of afferent arterioles, and within the periglomerular perivascular cells, which formed cuff-like appositional layers of renin-expressing cells at the glomerular vascular pole, leading to the impression of juxtaglomerular hypertrophy (Figure 1B and 1D). In parallel, also kidney renin mRNA levels increased 13-fold relative to wt on normal-salt diet (Figure 2A). To distinguish between recruitment along afferent arterioles and juxtaglomerular hypertrophy, we calculated renin-immunoreactive volumes per glomerulus from the 3D-reconstructions. Renin immunoreactivity within the first 30 μm from the glomerular vascular pole was considered as juxtaglomerular renin expression (juxtaglomerular hypertrophy). Figure 2B shows that stimulation of renin expression in wt animals led to a 9-fold increase of renin-immunoreactive volume. About two third of this increase occurred in the juxtaglomerular area leading to juxtaglomerular hypertrophy; the remaining one third was a result of vascular recruitment.
Figure 1.
Immunohistochemistry for renin (green) and α-smooth muscle actin (α-SMA, red) in kidney sections of wild-type (wt) mice before (A) and after treatment with low-salt (LS) diet+enalapril for 3 weeks (B). aff. art. indicates afferent arteriole; CLS, renin cells forming cuff-like structures; and G, glomeruli. Arrows indicate recruited renin-expressing cells in the afferent arteriole. 3D-reconstruction of α-SMA (gray) and of renin-immunoreactive areas (black) in kidneys of wt mice before (C) and after receiving a LS diet+enalapril for 3 weeks (D).
Figure 2.
A, Kidney renin mRNA abundance before and after 3 weeks of treatment with low-salt (LS) diet+enalapril in wild-type (wt) mice, in wt mice treated with L-NAME, in mice lacking endothelial isoform of nitric oxide synthase (eNOS−/−), in eNOS−/− treated with L-NAME and Ren1d+/Cre–NO-sensitive guanylate cyclase (NO-GC)fl/fl mice (n=5/group). For Ren1d+/Cre–NO-GCfl/fl mice, Ren1d+/+–NO-GCfl/fl mice were considered as wt controls. Renin mRNA levels were not different between C57Bl6 and Ren1d+/+–NO-GCfl/fl mice. Data are means±SE of 5 animals in each group. Asterisks indicate P<0.05 between respective wt controls. B, Renin-immunoreactive volume (voxels) per glomerulus calculated from 3D-reconstructions. Dotted horizontal line reflects renin-immunoreactive volume per glomerulus of untreated wt mice. Wt-total indicates total (juxtaglomerular and afferent arteriolar) immunoreactive volumes per glomerulus in wt mice after treatment with LS/enalapril. Wt cuff-like structures (CLS) indicates juxtaglomerular renin-immunoreactive volume within the first 30 μm from the glomerular vascular pole, considered as measure of juxtaglomerular hypertrophy. For wt mice treated with L-NAME, eNOS−/− mice, and Ren1d+/+–NO-GCfl/fl mice on LS/ enalapril only, renin-immunoreactive volumes within the first 30 μm from the glomerular vascular pole are shown, which, however, were identical to the respective total renin-immunoreactive volumes, because there was no renin expression beyond 30 μm from the glomerular vascular poles in these mice. Data are means±SEM of 60 glomeruli analyzed from 3 kidneys per group. *P<0.05 vs wt CLS.
Renin Cell Distribution in Kidneys of WT Mice With Systemic NOS Inhibition
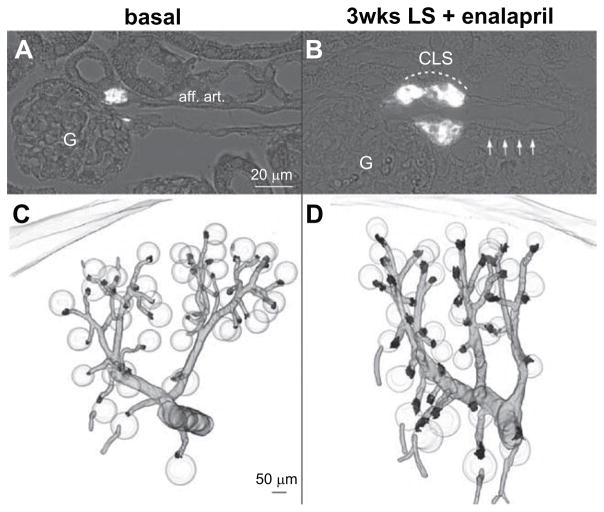
Based on our previous finding that systemic inhibition of NO-formation attenuates the increase of renin mRNA in response to LS/Enal,30 We now examined the effect of the nonselective NOS inhibitor L-NAME on the renin cell recruitment pattern. 3D-reconstruction of renin-expressing cells in combination with the preglomerular vascular tree revealed that recruitment of renin cells along the preglomerular vessels was lacking (Figure 3). Moreover, juxtaglomerular hypertrophy was attenuated, but not abolished, by treatment with L-NAME (Figures 2B and 3). This attenuation of renin cell recruitment induced by L-NAME was also reflected by renin mRNA levels, which were markedly lower in animals receiving L-NAME, in addition to the LS/Enal treatment (Figure 2A). In wt mice receiving L-NAME on normal salt, renin mRNA levels were moderately reduced by ≈40% (Figure 2A).
Figure 3.
Renin immunohistochemistry (white) in kidney sections of L-NAME treated wild-type (wt) mice before (A) and after treatment with low-salt (LS) diet+enalapril for 3 weeks (B). aff. art. indicates afferent arteriole; CLS, renin cells forming cuff-like structures; and G, glomeruli. Arrows indicate afferent arteriole. 3D-reconstruction of α-smooth muscle actin (α-SMA; grey) and of renin-immunoreactive areas (black) in kidneys of L-NAME treated wt before (C) and after treatment with LS diet+enalapril for 3 weeks (D).
Renin Cell Distribution in Kidneys of Mice Lacking eNOS
Because treatment with L-NAME can also induce side effects that are not specifically related to the availability of NO,31 we examined in as far the effects of L-NAME on renin cell recruitment can be mimicked by the absence of NOS, in particular, by their endothelial isoform (eNOS). We therefore analyzed renin cell recruitment in mice lacking endothelial NOS (eNOS−/−). In eNOS−/− mice, renin cells were located at typical juxtaglomerular position (Figure 4A and 4C), but basal renin mRNA expression was reduced by 50% (Figure 2A). LS diet in combination with enalapril induced no recruitment of renin cells along afferent arterioles in eNOS−/− mice (Figure 4B and 4D). Juxtaglomerular hypertrophy developed, but to a clearly lesser extent, as in wt mice (Figures 2B, 4B, and 4D). In line, the increase of renin mRNA was markedly attenuated in eNOS−/− mice (Figure 2). To address a possible additional role of also other NOS, such as inducible or neuronal NOS, for renin cell recruitment, we analyzed renin cell expression in eNOS−/− mice that were treated with the nonselective NOS inhibitor L-NAME on top. L-NAME treatment of eNOS−/− mice, however, did not produce an additional effect on renin mRNA levels (Figure 2A) nor on renin cell recruitment (Figure S1 in the online-only Data Supplement), neither under basal conditions nor during stimulation with LS diet in combination with enalapril.
Figure 4.
Renin Immunohistochemistry (white) in kidney sections of untreated mice lacking endothelial isoform of nitric oxide synthase (eNOS−/−) (A) and eNOS−/− treated with low-salt (LS) diet+enalapril for 3 weeks (B). aff. art. indicates afferent arteriole; CLS, renin cells forming cuff-like structures; and G, glomeruli. Arrows indicate afferent arteriole. 3D-reconstruction of α-smooth muscle actin (α-SMA; grey) and of renin-immunoreactive areas (black) in kidneys of eNOS−/− before (C) and after receiving a LS diet+enalapril for 3 weeks (D).
Renin Cell Distribution in Kidneys of Mice Lacking Soluble Guanylate Cyclase in Renin-Producing Cells
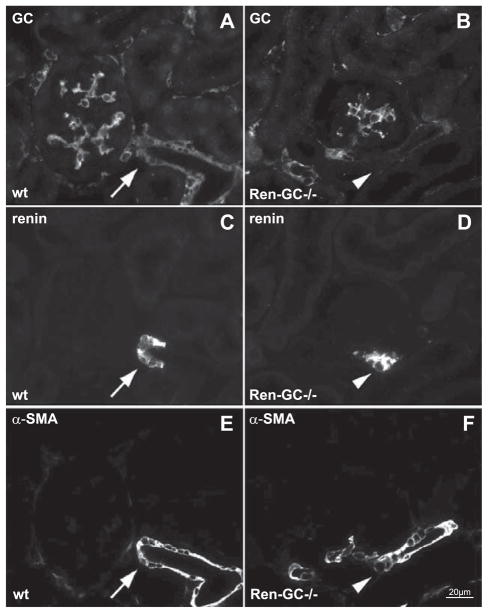
Assuming a relevant role of endothelium-derived NO for renin cell recruitment led to the question about signaling pathways along which NO could exert its effect on renin expression. A well-documented pathway of NO action is the activation of NO-GC in its target cells.32 Within the kidney, NO-GC expression as indicated by NO-GC-β1 immunoreactivity was found in mesangial cells, renin-producing cells, vascular smooth muscle cells, and interstitial cells (Figure 5A). We therefore aimed to study renin cell recruitment in mice lacking NO-GC in renin-expressing cells and in renin cell precursors. For this purpose, we generated mice lacking NO-GC in renin-expressing cells, and their descendents with the Cre–lox recombination system (Ren1d+/Cre-NO-GCfl/fl mice). In Ren1d+/Cre–NO-GCfl/fl mice, immunoreactivity for the NO-GC subunit had disappeared from renin-expressing cells and from most smooth muscle cells of the preglomerular vessels, but was maintained in mesangial and interstitial cells (Figure 5B). The disappearance of NO-GC from preglomerular smooth muscle cells of Ren1d+/Cre–NO-GCfl/fl mice is in accordance with renin cell lineage studies, demonstrating that preglomerular smooth muscle cells are descendents of renin-expressing cells.2
Figure 5.
Coimmunostaining in Ren1d+/+–NO-sensitive guanylate cyclase (NO-GC)fl/fl (wild-type [wt]; A,C,E) and Ren1d+/Cre–NO-GCfl/fl (Ren-GC−/−; B,D,F) kidney sections for guanylate cyclase (GC; A,B), renin (C,D), and α-smooth muscle actin (α-SMA; E,F). Arrows indicate colocalization of GC–renin–α-SMA. Arrowheads indicate absence of colocalization.
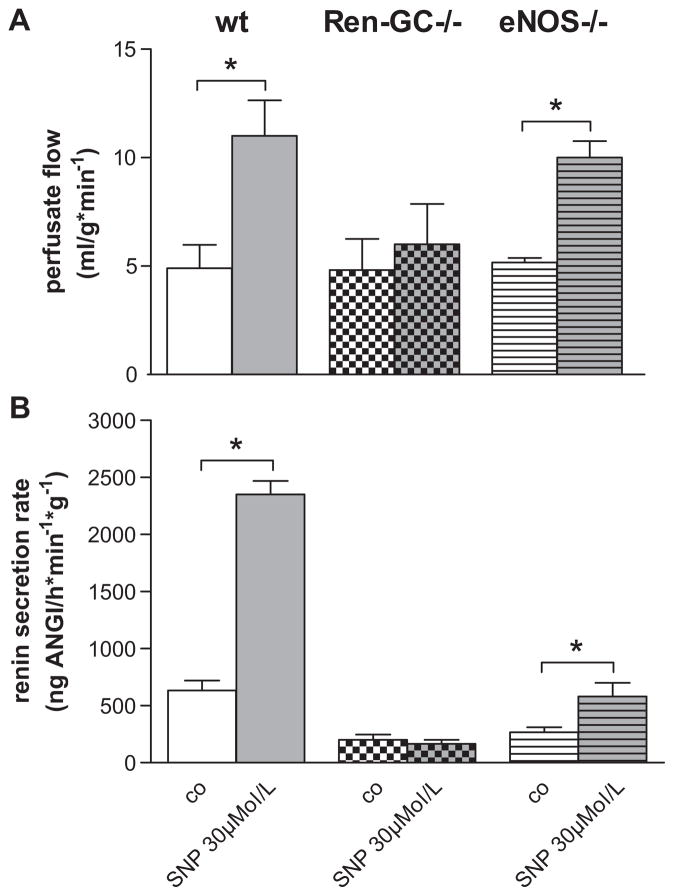
The functional deletion of NO-GC was further confirmed by experiments with isolated perfused kidneys showing that the NO-donor sodium nitroprusside failed to increase perfusate flow and renin secretion in Ren1d+/Cre–NO-GCfl/fl kidneys, in contrast to Ren1d+/+–NO-GCfl/fl (wt) and to eNOS-deficient kidneys (Figure 6).
Figure 6.
Effect of sodium nitroprusside (SNP) on perfusate flow (A) and renin secretion (B) in kidneys from Ren1d+/+–NO-sensitive guanylate cyclase (NO-GC)fl/fl (wild-type [wt]), Ren1d+/Cre–NO-GCfl/fl (Ren-GC−/−), and mice lacking endothelial isoform of nitric oxide synthase (eNOS−/−). Kidneys were perfused with isoproterenol (10 nmol/L) at a constant pressure of 90 mm Hg. Data are means±SE of 3 mice in each group. *P<0.05.
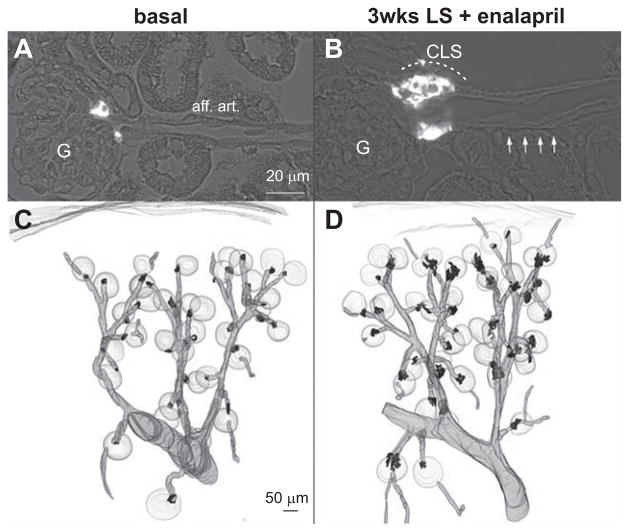
In Ren1d+/Cre–NO-GCfl/fl kidneys, renin cells were located at typical juxtaglomerular position, but basal renin expression was slightly reduced by 20% (Figure 2). LS diet in combination with enalapril induced no recruitment of renin cells along afferent arterioles in Ren1d+/Cre–NO-GCfl/fl mice (Figure 7). Juxtaglomerular hypertrophy, however, was developed to a very similar extent as in wt mice (Figures 2B and 7). In accordance, renin mRNA levels were reduced by 30% in comparison with wt mice (Figure 2).
Figure 7.
Renin immunohistochemistry (white) in kidney sections of untreated Ren1d+/Cre–NO-sensitive guanylate cyclase (NO-GC)fl/ fl mice (A) and Ren1d+/Cre–NO-GCfl/fl mice treated with low-salt (LS) diet+enalapril for 3 weeks (B). aff. art. indicates afferent arteriole; CLS, renin cells forming cuff-like structures; and G, glomeruli. Arrows indicate afferent arteriole. 3D-reconstruction of α-smooth muscle actin (α-SMA; grey) and of renin-immunoreactive areas (black) in kidneys of mice lacking endothelial isoform of nitric oxide synthase (eNOS−/−) before (C) and after receiving a LS diet+enalapril for 3 weeks (D).
Blood Pressure
Because the blood pressure is considered as an important factor influencing renin cell recruitment,21,33,34 we measured systolic blood pressure values of animals enrolled in this study (Table). It turned out that wt mice treated with L-NAME and eNOS−/− mice were clearly hypertensive with average systolic blood pressure values around 160 mm Hg (versus 125 mm Hg in untreated wt mice). L-NAME treatment of eNOS−/− mice did not further increase blood pressure. Ren1d+/Cre–NO-GCfl/fl mice had normal blood pressure. LS diet in combination with enalapril treatment for 3 weeks lowered blood pressure to around 85 to 90 mm Hg in wt and in Ren1d+/Cre–NO-GCfl/fl mice, and to around 115 to 120 mm Hg in wt mice treated with L-NAME and in eNOS−/− mice. L-NAME treatment of eNOS−/− mice exerted no effect on blood pressure.
Table.
Systolic Blood Pressure Before and After 3 Weeks of Treatment With LS Diet+Enalapril in wt, in wt Treated With L-NAME, in eNOS−/−, in eNOS−/− Treated With L-NAME, and in Ren1d+/Cre–NO-GCfl/fl Mice
| Experimental Group | Systolic Blood Pressure (mm Hg)
|
|
|---|---|---|
| Basal | Low Salt+Enalapril | |
| Wt | 125±6 | 86±4 |
| Wt+L-NAME | 161±9* | 115±7* |
| eNOS−/− | 164±11* | 114±6* |
| eNOS−/−+L-NAME | 162±8* | 120±1* |
| Ren1d+/Cre–NO-GCfl/fl | 130±5 | 88±4 |
eNOS indicates endothelial isoform of nitric oxide synthase; LS, low salt; NO-GC, nitric oxide-sensitive guanylate cyclase; and wt, wild-type.
Data are means±SE of 5 mice in each group. Asterisks indicate P<0.05 vs respective wt controls.
Discussion
This study aimed to define the role of NO for the hypertrophy of the juxtaglomerular apparatus and for recruitment of renin-expressing cells along the muscular media layer of afferent arterioles in the kidney, in situations of a chronically challenged renin–angiotensin–aldosterone system.
Our results show, and thus confirm previous reports,3,30 that inhibition of NO-formation attenuates the compensatory increase in renin expression induced by blockade of the RAAS, suggesting that the availability of NO is relevant for this process. Going beyond that, our data now demonstrate that the availability of NO is required for the recruitment of additional renin-producing cells, in particular, for the metaplastic transformation of preglomerular vascular smooth muscle cells into renin-producing cells, and to a lesser extent, also for transformation of juxtaglomerular cells into renin producers. Our data also strongly suggest that it is eNOS-derived NO that is relevant in this context, because the effects of systemic NOS blockade and of eNOS deletion on renin cell recruitment could not be distinguished on the level of renin cell distribution, nor on the level of renin mRNA. Moreover, systemic NOS-blockade exerted no additional effect on renin cell recruitment in the absence of eNOS. Therefore, we conclude that it is eNOS-derived rather than NO from other sources that is relevant for renin cell recruitment. Although extraendothelial localizations of eNOS have meanwhile been identified, the endothelium is considered as the main expression site of eNOS.4,35 Assuming that it is NO of endothelial origin that is important for renin cell recruitment, both indirect and direct effects of NO on renin-producing cells and their precursors might explain the effects of NO. The indirect effect of endothelial NO on renin cell recruitment would be related to the relevance of endothelial NO for systemic vascular resistance and blood pressure. It is well known that inhibition of endothelial NO formation induces hypertension,24,36–39 as also seen in this study. Hypertension per se would be expected to lower renin expression and to reduce the number of renin-producing cells in the kidney. In a previous rat study, we have found that inhibition of NO synthesis with L-NAME increases blood pressure and counteracts the stimulation of renin expression by RAAS-blockade. In that study, we also found that there was no clear correlation of blood pressure increase and inhibition of renin expression on the level of individual animals,40 suggesting that mechanisms other than blood pressure changes might also contribute to the effect of NO on renin cell recruitment. One obvious mechanism in this context would be a direct effect of NO on renin cells and their precursors.
The most common signaling pathway of endothelial NO in blood vessels is the activation of NO-GC.32,41 In confirmation of a previous report, we found NO-GC protein in renin-producing, mesangial and vascular smooth muscle cells.8 Our data now show that in mice lacking NO-GC in renin-producing and vascular smooth muscle cells, recruitment of renin cells in afferent arterioles is absent like in eNOS-deficient mice, whereas blood pressure is not different from wt mice. These findings suggest that the intravascular eNOS–NO–NO-GC pathway is important for the metaplastic transformation of vascular smooth muscle cells into renin-producing cells.
Although it cannot be definitively ruled out that the influence of intrarenal NO-GC on renin cell recruitment could be indirectly mediated by its effect on renal vascular resistance and, in consequence, on renal perfusion, we consider this possibility less likely for 2 main reasons. First, in situations in which intrarenal perfusion pressure and perfusion rate change, pressure but not perfusion determines the function of renin-producing cells.23 At constant perfusion pressure, there is no obvious link between perfusion rate and the function of renin-producing cells.42,43 Second, there would exist a plausible explanation for a direct role of NO–NO-GC for renin cell recruitment. We and others have previously obtained pharmacological and functional evidence that the NO–NO-GC pathway stimulates renin secretion and decreases renovascular resistance through the cAMP signaling pathway by inhibition of phosphodiesterase-3.9,10,44 Activation of the cAMP signaling pathway is, in fact, also considered to be essential for the transformation of vascular smooth muscles cells into renin-producing cells,14–16 thus providing an explanation for the role of NO-GC in renin cell recruitment. Whether the role of NO–NO-GC in renin cell recruitment is a direct regulatory one, by upregulating and downregulating cAMP levels, or more a sensitizing one, by stabilizing cAMP levels thus supporting the activity of regulatory adenylate cyclase activators, cannot be directly answered from this study. Available evidence, however, suggests that renin and renal eNOS expression are inversely regulated by the RAAS activity. Thus, infusion of angiotensin II that suppresses renin expression upregulates eNOS expression,45 whereas animals lacking angiotensinogen, which have elevated renin expression, show reduced eNOS expression.46 Therefore, we speculate that the role of NO-GC in renin cell recruitment is a sensitizing rather than a regulatory one.
It is, of note, that juxtaglomerular hypertrophy was not altered in the absence of NO-GC, whereas it was attenuated in eNOS-deficient mice. If it is the activation of the cAMP signaling pathway that causes transformation of juxtaglomerular precursors into renin-producing cells, then it does not require stabilization of cAMP levels by NO-GC cGMP-induced phohodiesterase-3 inhibition. This could indicate that there is already strong activation of adenylate cyclase in these cells. A likely adenylate cyclase activator candidate in this context could be prostaglandin E2 that is produced by the macula densa cells, regulated by their cyclooxygenase-2 activity.47–49 LS diet in combination with angiotensin I–converting enzyme inhibitor treatment causes an almost 10-fold increase of COX-2 expression in the macula densa, suggesting strong juxtaglomerular prostanoid formation in this situation.50 In line, stimulation of renin expression by RAAS inhibition is attenuated in COX-2–deficient mice51,52 and in wt mice treated with selective COX-2 inhibitors.53 Such a role of macula densa-derived prostanoids for juxtaglomerular renin cell recruitment induced by RAAS inhibition could also explain the attenuated juxtaglomerular hypertrophy seen in eNOS-deficient mice, because the stimulation of COX-2 expression by RAAS inhibition is clearly attenuated in states of hypertension.50
Perspectives
The recruitment of renin-expressing cells in response to chronic challenges of the renin–angiotensin system comprises 2 distinct components, namely metaplastic transformation of smooth muscle cells of preglomerular vessels and induction of renin expression in perivascular cells in the juxtaglomerular area. Metaplastic transformation of vascular smooth muscle appears to be dependent on the local availability of endothelium-derived NO and cGMP levels in renin precursor cells. Recruitment of renin-expressing cells in the juxtaglomerular area is likely dependent on the renal perfusion pressure in a more NO-independent manner. As endothelial production of NO can be compromised in states of disease, such a deficit of NO may become relevant for the correct adaptation of the renin–angiotensin system to demand.
Novelty and Significance.
What Is New?
The novel finding of our study is that the endothelial nitric oxide (NO)–guanylate cyclase pathway plays a role in recruitment of renin-producing cells in the kidney.
What Is Relevant?
The number of renin-producing cells, in turn, is a determinant of the activity of the renin–angiotensin–aldosterone system. Metaplastic transformation of vascular smooth muscle appears to be dependent on the local availability of endothelium-derived NO and cGMP levels in renin precursor cells.
Summary
As a summary, we suggest that the recruitment of renin-expressing cells in the kidney comprises 2 distinct components, namely metaplastic transformation of smooth muscle cells of preglomerular vessels and induction of renin expression in perivascular cells in the juxtaglomerular area.
Acknowledgments
We thank Gerda Treuner, Anna M’Bangui, Marcela Loza-Hilares, Michaela Kümmel, and Robert Götz for expert technical assistance and Prof Gödecke (University of Düsseldorf) for providing eNOS−/− animals.
Sources of Funding
This work was financially supported by the Deutsche Forschungsgemeinschaft SFB 699 and FR 1725/1-3.
Footnotes
The online-only Data Supplement is available with this article at http://hyper.ahajournals.org/lookup/suppl/doi:10.1161/HYPERTENSIONAHA.111.00221/-/DC1.
Disclosures
None.
References
- 1.Owen RA, Molon-Noblot S, Hubert MF, Kindt MV, Keenan KP, RS The morphology of juxtaglomerular cell hyperplasia and hypertrophy in normotensive rats and monkeys given an angiotensin II receptor antagonist. Toxicol Pathol. 1995;23:606–619. doi: 10.1177/019262339502300319. [DOI] [PubMed] [Google Scholar]
- 2.Sequeira López ML, Pentz ES, Nomasa T, Smithies O, Gomez RA. Renin cells are precursors for multiple cell types that switch to the renin phenotype when homeostasis is threatened. Dev Cell. 2004;6:719–728. doi: 10.1016/s1534-5807(04)00134-0. [DOI] [PubMed] [Google Scholar]
- 3.Tharaux PL, Dussaule JC, Pauti MD, Vassitch Y, Ardaillou R, Chatziantoniou C. Activation of renin synthesis is dependent on intact nitric oxide production. Kidney Int. 1997;51:1780–1787. doi: 10.1038/ki.1997.245. [DOI] [PubMed] [Google Scholar]
- 4.Bachmann S, Bosse HM, Mundel P. Topography of nitric oxide synthesis by localizing constitutive NO synthases in mammalian kidney. Am J Physiol. 1995;268(5 Pt 2):F885–F898. doi: 10.1152/ajprenal.1995.268.5.F885. [DOI] [PubMed] [Google Scholar]
- 5.Kone BC, Baylis C. Biosynthesis and homeostatic roles of nitric oxide in the normal kidney. Am J Physiol. 1997;272(5 Pt 2):F561–F578. doi: 10.1152/ajprenal.1997.272.5.F561. [DOI] [PubMed] [Google Scholar]
- 6.Park S, Harrison-Bernard LM. Augmented renal vascular nNOS and renin protein expression in angiotensin type 1 receptor null mice. J Histochem Cytochem. 2008;56:401–414. doi: 10.1369/jhc.2007.950220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pieruzzi F, Munforti C, Di Blasio A, Busca G, Dadone V, Zanchetti A, Golin R. Neuronal nitric oxide synthase and renin stimulation by sodium deprivation are dependent on the renal nerves. J Hypertens. 2002;20:2039–2045. doi: 10.1097/00004872-200210000-00024. [DOI] [PubMed] [Google Scholar]
- 8.Theilig F, Bostanjoglo M, Pavenstädt H, Grupp C, Holland G, Slosarek I, Gressner AM, Russwurm M, Koesling D, Bachmann S. Cellular distribution and function of soluble guanylyl cyclase in rat kidney and liver. J Am Soc Nephrol. 2001;12:2209–2220. doi: 10.1681/ASN.V12112209. [DOI] [PubMed] [Google Scholar]
- 9.Kurtz A, Götz KH, Hamann M, Wagner C. Stimulation of renin secretion by nitric oxide is mediated by phosphodiesterase 3. Proc Natl Acad Sci USA. 1998;95:4743–4747. doi: 10.1073/pnas.95.8.4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friis UG, Jensen BL, Sethi S, Andreasen D, Hansen PB, Skøtt O. Control of renin secretion from rat juxtaglomerular cells by cAMP-specific phosphodiesterases. Circ Res. 2002;90:996–1003. doi: 10.1161/01.res.0000017622.25365.71. [DOI] [PubMed] [Google Scholar]
- 11.Germain S, Konoshita T, Fuchs S, Philippe J, Corvol P, Pinet F. Regulation of human renin gene transcription by cAMP. Clin Exp Hypertens. 1997;19:543–550. doi: 10.3109/10641969709083168. [DOI] [PubMed] [Google Scholar]
- 12.Klar J, Sandner P, Müller MW, Kurtz A. Cyclic AMP stimulates renin gene transcription in juxtaglomerular cells. Pflugers Arch. 2002;444:335–344. doi: 10.1007/s00424-002-0818-9. [DOI] [PubMed] [Google Scholar]
- 13.Ying L, Morris BJ, Sigmund CD. Transactivation of the human renin promoter by the cyclic AMP/protein kinase A pathway is mediated by both cAMP-responsive element binding protein-1 (CREB)-dependent and CREB-independent mechanisms in Calu-6 cells. J Biol Chem. 1997;272:2412–2420. doi: 10.1074/jbc.272.4.2412. [DOI] [PubMed] [Google Scholar]
- 14.Neubauer B, Machura K, Chen M, Weinstein LS, Oppermann M, Sequeira-Lopez ML, Gomez RA, Schnermann J, Castrop H, Kurtz A, Wagner C. Development of vascular renin expression in the kidney critically depends on the cyclic AMP pathway. Am J Physiol Renal Physiol. 2009;296:F1006–F1012. doi: 10.1152/ajprenal.90448.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brunskill EW, Sequeira-Lopez ML, Pentz ES, Lin E, Yu J, Aronow BJ, Potter SS, Gomez RA. Genes that confer the identity of the renin cell. J Am Soc Nephrol. 2011;22:2213–2225. doi: 10.1681/ASN.2011040401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pentz ES, Lopez ML, Cordaillat M, Gomez RA. Identity of the renin cell is mediated by cAMP and chromatin remodeling: an in vitro model for studying cell recruitment and plasticity. Am J Physiol Heart Circ Physiol. 2008;294:H699–H707. doi: 10.1152/ajpheart.01152.2007. [DOI] [PubMed] [Google Scholar]
- 17.Beierwaltes WH. cGMP stimulates renin secretion in vivo by inhibiting phosphodiesterase-3. AmJPhysiolRenalPhysiol. 2006;290:F1376–F1381. doi: 10.1152/ajprenal.00209.2005. [DOI] [PubMed] [Google Scholar]
- 18.Lahera V, Salom MG, Miranda-Guardiola F, Moncada S, Romero JC. Effects of NG-nitro-L-arginine methyl ester on renal function and blood pressure. Am J Physiol. 1991;261(6 Pt 2):F1033–F1037. doi: 10.1152/ajprenal.1991.261.6.F1033. [DOI] [PubMed] [Google Scholar]
- 19.Mattson DL, Lu S, Nakanishi K, Papanek PE, Cowley AW., Jr Effect of chronic renal medullary nitric oxide inhibition on blood pressure. Am J Physiol. 1994;266(5 Pt 2):H1918–H1926. doi: 10.1152/ajpheart.1994.266.5.H1918. [DOI] [PubMed] [Google Scholar]
- 20.Rudd MA, Trolliet M, Hope S, Scribner AW, Daumerie G, Toolan G, Cloutier T, Loscalzo J. Salt-induced hypertension in Dahl salt-resistant and salt-sensitive rats with NOS II inhibition. Am J Physiol. 1999;277(2 Pt 2):H732–H739. doi: 10.1152/ajpheart.1999.277.2.H732. [DOI] [PubMed] [Google Scholar]
- 21.Machura K, Neubauer B, Steppan D, Kettl R, Groβ A, Kurtz A. Role of blood pressure in mediating the influence of salt intake on renin expression in the kidney. Am J Physiol Renal Physiol. 2012;302:F1278–F1285. doi: 10.1152/ajprenal.00688.2011. [DOI] [PubMed] [Google Scholar]
- 22.Hackenthal E, Paul M, Ganten D, Taugner R. Morphology, physiology, and molecular biology of renin secretion. Physiol Rev. 1990;70:1067–1116. doi: 10.1152/physrev.1990.70.4.1067. [DOI] [PubMed] [Google Scholar]
- 23.Nafz B, Berthold H, Ehmke H, Hackenthal E, Kirchheim HR, Persson PB. Flow versus pressure in the control of renin release in conscious dogs. Am J Physiol. 1997;273(2 Pt 2):F200–F205. doi: 10.1152/ajprenal.1997.273.2.F200. [DOI] [PubMed] [Google Scholar]
- 24.Gödecke A, Decking UK, Ding Z, Hirchenhain J, Bidmon HJ, Gödecke S, Schrader J. Coronary hemodynamics in endothelial NO synthase knockout mice. Circ Res. 1998;82:186–194. doi: 10.1161/01.res.82.2.186. [DOI] [PubMed] [Google Scholar]
- 25.Friebe A, Mergia E, Dangel O, Lange A, Koesling D. Fatal gastrointestinal obstruction and hypertension in mice lacking nitric oxide-sensitive guanylyl cyclase. Proc Natl Acad Sci USA. 2007;104:7699–7704. doi: 10.1073/pnas.0609778104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Machura K, Steppan D, Neubauer B, Alenina N, Coffman TM, Facemire CS, Hilgers KF, Eckardt KU, Wagner C, Kurtz A. Developmental renin expression in mice with a defective renin-angiotensin system. Am J Physiol Renal Physiol. 2009;297:F1371–F1380. doi: 10.1152/ajprenal.00378.2009. [DOI] [PubMed] [Google Scholar]
- 27.Schweda F, Wagner C, Krämer BK, Schnermann J, Kurtz A. Preserved macula densa-dependent renin secretion in A1 adenosine receptor knockout mice. Am J Physiol Renal Physiol. 2003;284:F770–F777. doi: 10.1152/ajprenal.00280.2002. [DOI] [PubMed] [Google Scholar]
- 28.Schweda F, Segerer F, Castrop H, Schnermann J, Kurtz A. Blood pressure-dependent inhibition of renin secretion requires A1 adenosine receptors. Hypertension. 2005;46:780–786. doi: 10.1161/01.HYP.0000183963.07801.65. [DOI] [PubMed] [Google Scholar]
- 29.Neubauer B, Machura K, Schnermann J, Wagner C. Renin expression in large renal vessels during fetal development depends on functional beta1/beta2-adrenergic receptors. Am J Physiol Renal Physiol. 2011;301:F71–F77. doi: 10.1152/ajprenal.00443.2010. [DOI] [PubMed] [Google Scholar]
- 30.Castrop H, Kammerl M, Mann B, Jensen BL, Krämer BK, Kurtz A. Cyclooxygenase 2 and neuronal nitric oxide synthase expression in the renal cortex are not interdependent in states of salt deficiency. Pflugers Arch. 2000;441:235–240. doi: 10.1007/s004240000438. [DOI] [PubMed] [Google Scholar]
- 31.Kadekaro M, Terrell ML, Liu H, Gestl S, Bui V, Summy-Long JY. Effects of L-NAME on cerebral metabolic, vasopressin, oxytocin, and blood pressure responses in hemorrhaged rats. Am J Physiol. 1998;274(4 Pt 2):R1070–R1077. doi: 10.1152/ajpregu.1998.274.4.R1070. [DOI] [PubMed] [Google Scholar]
- 32.Bellamy TC, Wood J, Garthwaite J. On the activation of soluble guanylyl cyclase by nitric oxide. Proc Natl Acad Sci USA. 2002;99:507–510. doi: 10.1073/pnas.012368499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tufro-McReddie A, Chevalier RL, Everett AD, Gomez RA. Decreased perfusion pressure modulates renin and ANG II type 1 receptor gene expression in the rat kidney. Am J Physiol. 1993;264(4 Pt 2):R696–R702. doi: 10.1152/ajpregu.1993.264.4.R696. [DOI] [PubMed] [Google Scholar]
- 34.Moosavi SM, Johns EJ. Effect of renal perfusion pressure on renal function, renin release and renin and angiotensinogen gene expression in rats. J Physiol (Lond) 1999;520(Pt 1):261–269. doi: 10.1111/j.1469-7793.1999.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ujiie K, Yuen J, Hogarth L, Danziger R, Star RA. Localization and regulation of endothelial NO synthase mRNA expression in rat kidney. Am J Physiol. 1994;267(2 Pt 2):F296–F302. doi: 10.1152/ajprenal.1994.267.2.F296. [DOI] [PubMed] [Google Scholar]
- 36.Shesely EG, Maeda N, Kim HS, Desai KM, Krege JH, Laubach VE, Sherman PA, Sessa WC, Smithies O. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 1996;93:13176–13181. doi: 10.1073/pnas.93.23.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ribeiro MO, Antunes E, de Nucci G, Lovisolo SM, Zatz R. Chronic inhibition of nitric oxide synthesis. A new model of arterial hypertension. Hypertension. 1992;20:298–303. doi: 10.1161/01.hyp.20.3.298. [DOI] [PubMed] [Google Scholar]
- 38.Baylis C, Mitruka B, Deng A. Chronic blockade of nitric oxide synthesis in the rat produces systemic hypertension and glomerular damage. J Clin Invest. 1992;90:278–281. doi: 10.1172/JCI115849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 40.Schricker K, Hegyi I, Hamann M, Kaissling B, Kurtz A. Tonic stimulation of renin gene expression by nitric oxide is counteracted by tonic inhibition through angiotensin II. Proc Natl Acad Sci USA. 1995;92:8006–8010. doi: 10.1073/pnas.92.17.8006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Friebe A, Koesling D. Regulation of nitric oxide-sensitive guanylyl cyclase. Circ Res. 2003;93:96–105. doi: 10.1161/01.RES.0000082524.34487.31. [DOI] [PubMed] [Google Scholar]
- 42.Scholz H, Kurtz A. Disparate effects of calcium channel blockers on pressure dependence of renin secretion and flow in the isolated perfused rat kidney. Pflugers Arch. 1992;421:155–162. doi: 10.1007/BF00374822. [DOI] [PubMed] [Google Scholar]
- 43.Schweda F, Krämer BK, Kurtz A. Differential roles of the sodium-calcium exchanger in renin secretion and renal vascular resistance. Pflugers Arch. 2001;442:693–699. doi: 10.1007/s004240100591. [DOI] [PubMed] [Google Scholar]
- 44.Reid IA. Role of phosphodiesterase isoenzymes in the control of renin secretion: effects of selective enzyme inhibitors. Curr Pharm Des. 1999;5:725–735. [PubMed] [Google Scholar]
- 45.Bae EH, Ma SK, Lee J, Kim SW. Altered regulation of renal nitric oxide and atrial natriuretic peptide systems in angiotensin II-induced hypertension. Regul Pept. 2011;170:31–37. doi: 10.1016/j.regpep.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 46.Kihara M, Sato K, Hashimoto T, Imai N, Toya Y, Umemura S. Expression of endothelial nitric oxide synthase is suppressed in the renal vasculature of angiotensinogen-gene knockout mice. Cell Tissue Res. 2006;323:313–320. doi: 10.1007/s00441-005-0058-3. [DOI] [PubMed] [Google Scholar]
- 47.Friis UG, Stubbe J, Uhrenholt TR, Svenningsen P, Nüsing RM, Skøtt O, Jensen BL. Prostaglandin E2 EP2 and EP4 receptor activation mediates cAMP-dependent hyperpolarization and exocytosis of renin in juxtaglomerular cells. Am J Physiol Renal Physiol. 2005;289:F989–F997. doi: 10.1152/ajprenal.00201.2005. [DOI] [PubMed] [Google Scholar]
- 48.Jensen BL, Schmid C, Kurtz A. Prostaglandins stimulate renin secretion and renin mRNA in mouse renal juxtaglomerular cells. Am J Physiol. 1996;271(3 Pt 2):F659–F669. doi: 10.1152/ajprenal.1996.271.3.F659. [DOI] [PubMed] [Google Scholar]
- 49.Peti-Peterdi J, Harris RC. Macula densa sensing and signaling mechanisms of renin release. J Am Soc Nephrol. 2010;21:1093–1096. doi: 10.1681/ASN.2009070759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Castrop H, Klar J, Wagner C, Hocherl K, Kurtz A. General inhibition of renocortical cyclooxygenase-2 expression by the renin-angiotensin system. Am J Physiol Renal Physiol. 2003;284:F518–F524. doi: 10.1152/ajprenal.00338.2002. [DOI] [PubMed] [Google Scholar]
- 51.Cheng HF, Wang JL, Zhang MZ, Wang SW, McKanna JA, Harris RC. Genetic deletion of COX-2 prevents increased renin expression in response to ACE inhibition. Am J Physiol Renal Physiol. 2001;280:F449–F456. doi: 10.1152/ajprenal.2001.280.3.F449. [DOI] [PubMed] [Google Scholar]
- 52.Schnermann J, Briggs JP. Synthesis and secretion of renin in mice with induced genetic mutations. Kidney Int. 2012;81:529–538. doi: 10.1038/ki.2011.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matzdorf C, Kurtz A, Höcherl K. COX-2 activity determines the level of renin expression but is dispensable for acute upregulation of renin expression in rat kidneys. Am J Physiol Renal Physiol. 2007;292:F1782–F1790. doi: 10.1152/ajprenal.00513.2006. [DOI] [PubMed] [Google Scholar]