Summary
Infective endocarditis is a life threatening disease caused by a bacterial infection of the endocardial surfaces of the heart. The oral pathogen, Streptococcus gordonii is amongst the most common pathogens isolated from infective endocarditis patients. Previously we identified a novel cell wall protein expressed on S. gordonii called platelet adherence protein A (PadA) that specifically interacts with platelet GPIIb/IIIa. The interaction between PadA and GPIIb/IIIa resulted in firm platelet adhesion, dense granule secretion and platelet spreading on immobilised S. gordonii. This study set out to identify specific motifs on the PadA protein that interacts with platelet GPIIb/IIIa. Proteomic analysis of the PadA protein identified two short amino acid motifs which have been previously shown to be important for fibrinogen binding to GPIIb/IIIa and contributing to the generation of outside-in signalling. Site directed mutagenesis on the PadA protein in which 454AGD was substituted to AAA, and the 383RGT was substituted to AAA suggests the RGT motif has no role in supporting platelet adhesion however plays a role in dense granule secretion and platelet spreading. In contrast to this the AGD motif has no role to play in supporting firm platelet adhesion or dense granule secretion however plays a role in platelet spreading. These results suggest that multiple sites on S. gordonii PadA interact with GPIIb/IIIa to mediate a number of platelet responses that likely contribute to the thrombotic complications of Infective Endocarditis.
Keywords: Bacterial surface protein, platelet adhesion, aggregation, spreading, dense granule release, shear adhesion
Introduction
Infective Endocarditis (IE) is a microbial infection characterised by the presence of septic vegetations on the surface of the endocardium[1]. It most commonly occurs on heart valves, however may also affect the inner lining of the cardiac chambers[2]. IE typically develops on endocardium that has been damaged by congenital defects, previous disease or trauma. As a result, these sites have the ability to generate turbulent blood flow which in turn can cause endothelial surface damage leading to exposure of underlying matrix proteins[3]. Once exposed this highly thrombogenic surface leads to rapid platelet deposition and the formation of a fibrin network. Circulating bacteria from a transient bacteremia may then lead to this sterile platelet fibrin nidus which allows a secondary accumulation of platelets that encase the bacteria[4]. Current treatment regimes consist of prolonged combination antibiotic therapy, frequently combined with valve replacement surgery[5]. Prolonged antibiotic use is often less than successful with 40% of patients relapsing within 2 months of finishing clinically effective therapy. Surgery is a risky alternative, but is necessary in up to 47% of patients[6, 7]. In many cases surgery is not preferable due to risks associated with cardiac failure, further spread of infection leading to persistent sepsis associated with surgical removal of an infected thrombus, or life threatening embolisation[8-11].
The number of cases of IE that are caused by viridians-group streptococci of which Streptococcus gordonii is a prominent member varies between 6.8-45% [7, 12]. S. gordonii is a component of the normal oral microbiota that plays a key role in the initiation of dental plaque formation on oral cavity surfaces [13]. Chronic oral disease leading to inflammation and tissue destruction provides a route of entry for S. gordonii into the circulation, leading to transient bacteremia. Once in the bloodstream S. gordonii is well known for its ability to colonise damaged heart valves and interact with circulating platelets. S. gordonii is among the bacteria most frequently identified as being the primary etiological agents of subacute infective endocarditis[14].
Platelet adhesion to immobilised S. gordonii is thought to be an initial step leading to thrombus formation on heart valves[1] and is thought to involve binding to the major platelet receptor GPIIb/IIIa[15]. GPIIb/IIIa is the integrin expressed on platelets that mediates a range of platelet functions including adhesion, aggregation and stable thrombus formation[16]. These events occur as a direct result of both inside out and outside in signalling[17]. Engagement of fibrinogen with the extracellular domain of GPIIb/IIIa is necessary for platelet aggregation and results in conformational change, the consequence of inside out signalling. As platelets aggregate, GPIIb/IIIa clusters and trigger an outside in signal that stabilises the aggregate by amplification through dense granule secretion, induces platelet spreading and finally initiates clot retraction[18]. Typically GPIIb/IIIa recognises its ligand via a short amino acid residue sequence, Arg-Gly-Asp (RGD) found on the alpha chain of fibrinogen (Aα 95-97 and Aα572-575) and on vWf (698-700 and 2507-2509)[16, 18]. Fibrinogen also contains an additional GPIIb/IIIa recognition motif, Ala-Gly-Asp (AGD) on the gamma chain (γ408-410)[19].
Previously we demonstrated that upon firm adhesion to immobilized S. gordonii, platelets receive a signal that leads to dense granule secretion and extension of filopodia and lamellipodia with controlled orientation of stress fibers leading to full platelet spreading[20]. Spreading on immobilised S. gordonii is a critical event that allows the platelet to withstand the shear forces experienced in the vasculature. As part of the characterization process of these platelet responses we identified a large protein (approximately 3,500 amino acid residues) expressed on S. gordonii designated platelet adherence protein A (PadA). We have shown that disruption of the padA gene significantly reduced platelet adhesion and abolished both dense granule secretion and the signal that leads to spreading[15, 20]. We also demonstrated that PadA specifically bound to platelets in a GPIIb/IIIa dependent manner. These results suggested that PadA binding to GPIIb/IIIa might be responsible for transducing outside in signals to the platelet to mediate dense granule release and spreading.
This study was undertaken to determine the mechanism through which PadA is capable of transducing signals into the platelet leading to adhesion, dense granule release or platelet spreading. These findings may have important implications for understanding the molecular mechanisms through which the platelet response in patients contributes to the development of a thrombus in patients with IE.
Materials and Methods
Materials
Brain heart infusion and M17 media were purchased from Oxoid (Basingstoke, UK). DNA polymerase Phusion® was from FinnEnzymes (Loughborough, UK). The platelet agonist thrombin receptor-activating peptide (TRAP) was obtained from the Peptide Synthesis Laboratory, RCSI, Ireland. Chronolume® was from Stago BNL. Vectashield mounting medium was from Vector Laboratories, CA, USA. All other laboratory reagents were purchased from Sigma-Aldrich (Dublin, Ireland).
Bacterial strains and plasmids
Bacterial strains utilized in this study were Streptococcus gordonii DL1 (Challis), S. gordonii UB1890 ΔpadA (Petersen et al., 2010), Escherichia coli JM109 (New England BioLabs), E. coli BL21 (Novagen) and plasmid pET46 (Novagen). Streptococci were routinely cultivated in BHY medium (Brian Heart Infusion Broth containing 0.5% Yeast Extract) at 37 °C without shaking, and E. coli strains were cultivated in Luria-Bertani (LB) medium at 37 °C with shaking (200 r.p.m).
Generation of recombinant PadA fragments
The DNA encoding NH2-terminal PadA fragment II was ligated into pET46 to generate pET46-padA2 as previously described (Petersen et al., 2010). The regions encoding potential platelet interactive motifs AGD and RGT were subjected to alanine-substitution mutagenesis. Primers rgtF 5′GCAGAAACCGGTAACAAGGTTAAAATCTCTGTTCCAGATAGTGTCTTCAAT (AgeI) and rgtR 5′GCAGAAACCGGTCTGTGTGATTGCAGCAGCGATTTCCTTGCTAGG G (AgeI), with base mismatches to padA in boldface, were utilized in inverse PCR-amplification of pET46-padA2 to convert RGT to AAA (see Figure 1). Likewise, primers agdF 5′GCAGAAGCGGCCGCCTTCGAAACACGACAAATCGGTGGT (NotI) and agdR 5′GCAGAAGCGGCCGCTTTAAATTTACCAGTCAATTCTGGAACTGT (NotI), with base mismatches in boldface, were utilized in inverse PCR-amplification of pET46-padA2 to convert AGD to AAA (see Figure 1). Following PCR-amplification with high fidelity DNA Polymerase Phusion, PCR products were digested with either AgeI or Not1, in combination with DpnI to remove methylated template DNA, ligated at 16°C for 16 h, and transformed into competent cells of E.coli JM109. Plasmids were extracted from transformants, digested with AgeI or NotI as appropriate, and screened by agarose gel electrophoresis. Plasmids carrying mutagenized padA2 fragments were confirmed by DNA sequencing analyses and then transformed into competent cells of E. coli BL21.
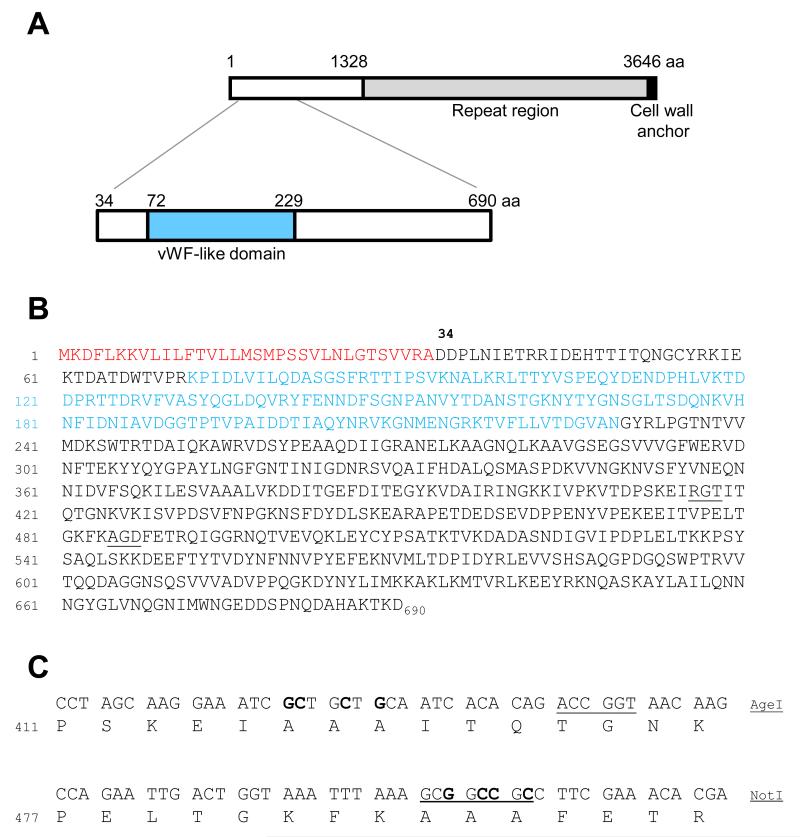
Figure 1. Diagrammatic representation and primary sequence of the PadA polypeptide and the Fragment II region, with subsequent engineered mutations.
(A) The PadA polypeptide (3646 amino acid residues) comprises a leader peptide (33 aa), an N terminal region (1295 aa), a region of amino acid residue repeat blocks and a cell wall anchor region. The N terminal Fragment II recombinant polypeptide (657 aa) is highlighted and contains within it a vWf-like domain. (B) The primary sequence of Fragment II shows the leader peptide (33 aa) highlighted in red. The vWf-like domain (72-229 aa) is shown in blue. Two potential integrin recognition motifs are underlined (RGT and AGD). (C) Site Directed Mutagenesis strategy used to introduce alanine substitutions in the RGT and AGD motifs of the Fragment II region. Nucleotides in bold show nucleotide base substitutions and underlined nucleotides indicate engineered restriction enzyme sites for AgeI and NotI enzymes.
Protein purification
E.coli BL21 cells carrying plasmid pET46-padA2, which incorporates N-terminal 6xHis tag onto the expressed polypeptide, were grown for 16 h in LB medium with ampicillin (100 μg/ml) and inoculated (0.5 ml) into fresh LB medium (50 ml) containing ampicillin. The culture was incubated at 37°C with shaking to early exponential phase (OD600 = 0.5) and protein expression was induced with 1 mM IPTG (isopropyl-β-D-thiogalactopyranoside). After 1 h incubation at 37°C, followed by 14 h at 20 °C, cells were harvested by centrifugation (5000 × g, 5 min) and lysed in Bugbuster (Novagen) for 1 h at 22 °C with gentle rocking. The suspension was then centrifuged (7000 × g, 4°C, 30 min) and the supernatant (soluble fraction) subjected to immobilised metal ion affinity chromatography (IMAC). Sepharose (IMAC Sepharose™ 6 Fast Flow) was rinsed thoroughly with dH2O and charged with 0.5 M CoCl2. The charged sepharose was added to a plastic syringe column (5 ml) together the Bugbuster soluble fraction, and the flow through was collected. Proteins bound non-specifically to the column were eluted by washing with buffer A (50 mM HEPES pH7.4, containing 10% glycerol, 0.5 M NaCl, and 0.1% Triton X-100) and buffer B (buffer A containing 5 mM imidazole) and 1 ml fractions were collected. Affinity-bound proteins were then eluted in buffer C (buffer A containing 150 mM imidazole). All collected fractions were checked for protein purity by SDS-PAGE, with gels either stained with Coomassie Blue, or electroblotted onto nitrocellulose and probed with anti-tetra-His antibody (Qiagen), followed by detection with HRP-conjugated anti-mouse antibody (Dako) at 1:1000 dilution. Fractions containing pure recombinant polypeptides were pooled, dialyzed into dH2O supplemented with Protease Inhibitor Cocktail (Sigma), and then freeze-dried.
Human blood samples
Whole blood was collected using a 19 gauge butterfly needle from the anticubital fossa of healthy subjects who had abstained from taking any non-steroidal anti-inflammatory drugs (NSAIDS) in the previous 10 days. Informed consent was obtained from all subjects and study approval was obtained from the Royal college of Surgeons in Ireland Research Ethics Committee (REC679b).
Preparation of platelets from whole blood
For aggregation and dense granule release studies a total of 9 volumes of blood were added to 1 volume of 3.2 % (w/v) sodium citrate. Whole blood was subsequently centrifuged at 150 × g for 10 min. The top layer consisting of platelet rich plasma (PRP) was removed and used for platelet aggregation and dense granule release studies. For platelet adhesion and platelet spreading studies a total of 8.5 volumes of blood were added to 1.5 volumes of acid-citrate-dextrose (ACD). Following preparation of PRP mthe pH of the platelets was adjusted to 6.5 using ACD. Prostaglandin E1 (1 μM) was added to the platelets to prevent activation during centrifugation. PRP was centrifuged at 650 × g for 10 min. The supernatant was carefully removed and discarded. One ml of modified HEPES tyrodes buffer (JNL; 6 mM D-glucose, 130 mM NaCl2, 9 mM NaCl2, 10 mM Na Citrate, 10 mM Tris, 3 mM KCl, 0.8 mM KH2PO4, and 0.9 mM MgCl2; pH 7.4) was layered on top of the platelet pellet and removed. Once resuspended the platelets were passed through a packed Sepharose 2B column. Gel filtered platelets (GFP) were collected and diluted to obtain a platelet count of 4×108 platelets/ml.
Granule secretion
Dense granule secretion was measured by luminometry using a luciferin/luciferase assay. Samples (90 μl) taken from the adhered platelets were removed and added to a 96 well luciferase plate. Chrono-lume®, a luciferin luciferase conjugant was added to each well to measure ADP/ATP release from dense granules. The luminescence was read within 2 min of the aggregation peak being reached on a microtiter plate reader (Wallac Victor 2; Perkin Elmer). Luminescence for recombinant protein fragments were compared to that of S. gordonii . Granule secretion was also measured by flow cytometry. Platelets were incubated with TRAP, S. gordonii or fragments for 10 min. CD62p-PE (P-selectin; alpha granule), CD41a (GPIIb; alpha granule), CD63 (granulophysin; dense granule) or appropriately labelled isotype control was added to the platelets. After 20 min incubation the samples were diluted with 1 ml of PBS and analysed on a FACSCalibur flow cytometer (Becton Dickinson, UK) on the Fl-2 channel. Data were analysed using CellQuest software (Becton Dickinson, UK).
Static platelet adhesion
Static platelet adhesion was measured as described previously (Kerrigan et al., 2007). Briefly, 96 well plates were coated with bacteria (1 × 109 cells/ml), fibrinogen (100 μg/ml), recombinant protein fragments (100 μg/ml) or BSA (1% w/v). Gel filtered platelets were adjusted to a concentration of 4 × 108 platelets/ml and were added to each well and allowed to adhere to immobilised substrate for 45 min at 37 °C. Following a gentle wash to remove non-adhered platelets, lysis buffer containing a substrate for acid phosphatase was then added to the wells and incubated for 20 min at 37 °C. The resultant colour (Absorbance) was then measured at 405 nm in a microtiter plate reader (Wallac Victor , Perkin-Elmer, Cambridge, UK.)
Shear induced platelet adhesion
Platelet interactions with immobilized bacteria under shear were assessed using a parallel flow chamber (Glycotech, Maryland, USA) as described previously[21]. Briefly 2 ml purified protein or bacteria (OD at 600nm = 1.6) were allowed to adhere to glass coverslips (Bellco Glass, New Jersey, USA) and incubated for 2 h at 37 °C in a humidity chamber. Uniform coating of bacteria was confirmed by differential interference contrast microscopy. Coverslips were washed 3 times with equal volumes of PBS and blocked for 1 h at 37 °C with 1% (w/v) BSA. Blood was incubated for 10 min at RT with the fluorescent dye DiOC6 (1 μM) and then perfused at 50 s-1 over the coated surfaces for 300 sec at 37 °C. Images were captured in real-time at the centre of the chamber, downstream of the flow entrance, using a fluorescent microscope (Axio Observer.Z1 Microscope, Plan-Apochromat 63x/1.40 Oil DIC objective lens and a GFP filter) and processed using Zeiss Axiovision 4.7 software.
Platelet spreading on immobilised bacteria
Poly-L-lysine coated glass slides were coated with S. gordonii (1 × 109 cells/ml) or PadA fragment (native or mutated; 100 μg/ml)) for 16 h at 4™C. Slides were then blocked with 1% BSA for 2 h at 37™C and finally washed with TBS to remove any unbound BSA. Gel filtered platelets (5 × 106 platelets/ml) were allowed to spread on either S. gordonii or PadA fragments for 45 min at 37™C. After gently rinsing 3 times with modified HEPES-Tyrodes buffer, spread platelets were fixed with 3.7% paraformaldehyde for 10 min at room temperature and permeabilised in ice cold acetone for 5 min. Platelets were stained using Alexa 546 phalloidin for 20 min at room temperature in the dark. Samples were mounted in Vectashield mounting medium and images were acquired (63X) using a Zeiss LSM 510 confocal microscope in DIC (differential interference contrast) or using an argon laser at 488 nm.
Statistical analysis
Statistics were performed using InStat statistical software (GraphPad software, SD, USA). Data shown are the means plus or minus standard error of the mean and comparisons between mean values were performed using the Student paired or unpaired t-test.
Results
Platelet adhesion to S. gordonii PadA under shear conditions
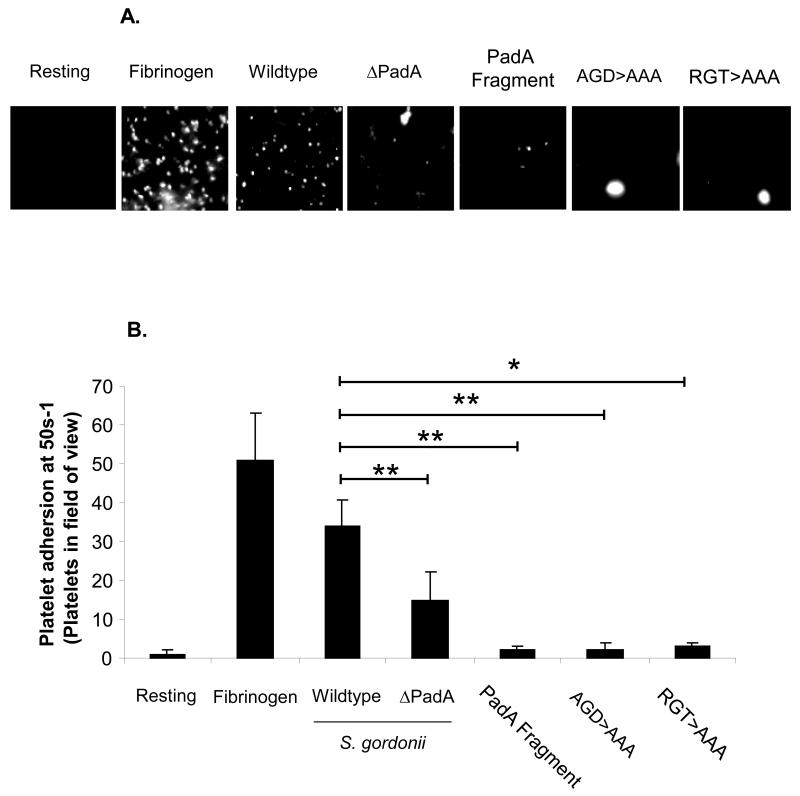
Previous studies have demonstrated that under shear conditions platelets may differentially interact with immobilised substrates. Our group have demonstrated that platelets interact with immobilised S. gordonii, Streptococcus sanguinis or Staphylococcus aureus in unique manners that otherwise would not be detected in static systems[21-23]. Therefore we investigated the ability of platelets to interact with PadA under shear conditions. Under low shear conditions (50 s−1) single platelets adhered to immobilised fibrinogen (Figure 2B). Consistent with previous observations platelets rolled on immobilised S. gordonii followed by firm adhesion (Figure 2A and 2B). Platelets maintained their ability to roll on the isogenic mutant strain, which was defective in PadA expression, however firm adhesion was significantly reduced (Figure 2A and 2B, P<0.05, compared to the wildtype S. gordonii strain). These results are consistent with previous observation that platelets roll on immobilised bacteria via a GPIb – Hsa interaction. Firm adhesion occurs following GPIIbIIIa engagement with PadA In the absence of PadA platelets can roll however cannot firmly adhere. To further characterise the site-specific molecular interaction between PadA and platelets we purified a recombinant PadA fragment consisting of 690 amino acid residues (Figure 1) that we previously identified to be important in platelet recognition[15]. This fragment contains two short amino acid motifs (AGD and RG(T)) that have been previously shown to be important for binding of fibrinogen to the major platelet receptor GPIIb/IIIa[24]. We therefore investigated platelet adhesion under low shear to either immobilised purified PadA fragment or mutant fragments of PadA in which 454AGD was substituted to AAA, or 383RGT was substituted to AAA. Platelets perfused over the native fragment or the mutant fragments under low shear failed to adhere (Figure 2A and 2B, P<0.05 native fragment vs wild type S. gordonii ; P<0.05 AGD>AAA vs wild type S. gordonii ; P<0.01 RGT>AAA vs wild type S. gordonii ) suggesting that the PadA protein cannot support platelet adhesion under shear conditions.
Figure 2. Platelet adhesion to Streptococcus gordonii PadA under shear conditions.
Platelets were perfused over immobilised BSA, Fibrinogen, wildtype S. gordonii, or purified PadA at 50s-1. (A) Images are representative fields taken from 1 of 3 independent experiments that yielded similar results. (B) The number of platelets adhered was randomly selected from 5 areas on the slide. Images were acquired using x63 oil immersion lens using an argon laser at 488 nm. *P<0.01, **P<0.05, n=3-5.
Platelet adhesion to S. gordonii PadA under static conditions
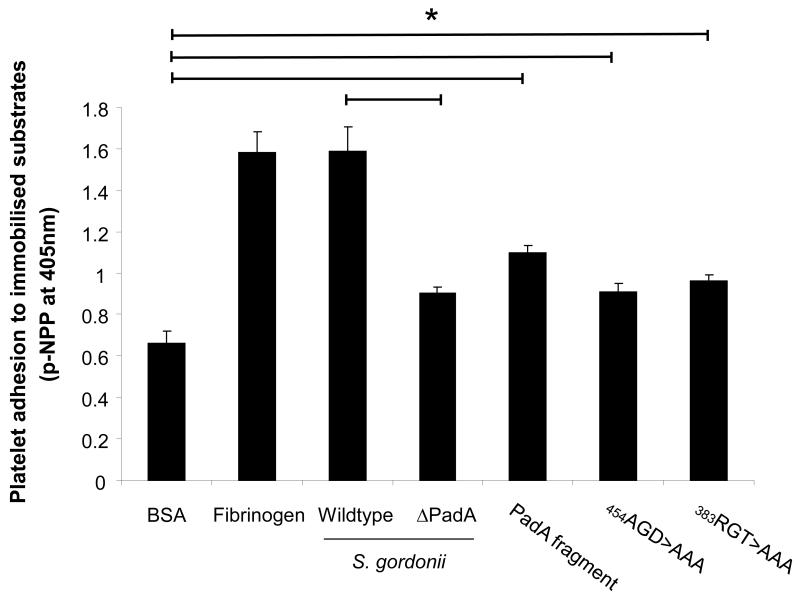
It is possible that platelets cannot interact with PadA under shear as they are moving too fast to slow down to allow the firm adhesion between GPIIb/IIIa and PadA. We therefore investigated platelet adhesion to immobilised S. gordonii under static conditions. Consistent with previous observations S. gordonii supported platelet adhesion under static conditions (Figure 3). Platelet adhesion was significantly reduced in a strain defective in expression of PadA (Figure 3, P<0.05 wildtype versus PadA defective strain). The purified PadA fragment, and the mutant fragments 454AGD to AAA, and the 383RGT to AAA supported platelet adhesion under static conditions, significantly more than platelet adhesion to BSA (Figure 3, P<0.05). There was no significant difference in adhesion between the PadA fragment and the mutant fragment 454AGD to AAA, and the 383RGT to AAA (Figure 3, P=NS). These results suggest that platelet adhesion to PadA under static conditions does not involve the common GPIIb/IIIa recognition sites AGD or RGT.
Figure 3. Platelet adhesion to Streptococcus gordonii PadA under static conditions.
Platelets (2×108 platelets/ml) were allowed to adhere to immobilised BSA, Fibrinogen, wildtype S. gordonii, or purified PadA for 45 minutes. Wells were washed, and adhered platelets were lysed using 0.1% Triton X-100. As a measure of the number of platelets bound, alkaline phosphatase activity was determined by the change in absorbance at 405 nm. *P<0.05, n=5
S. gordonii induced granule secretion
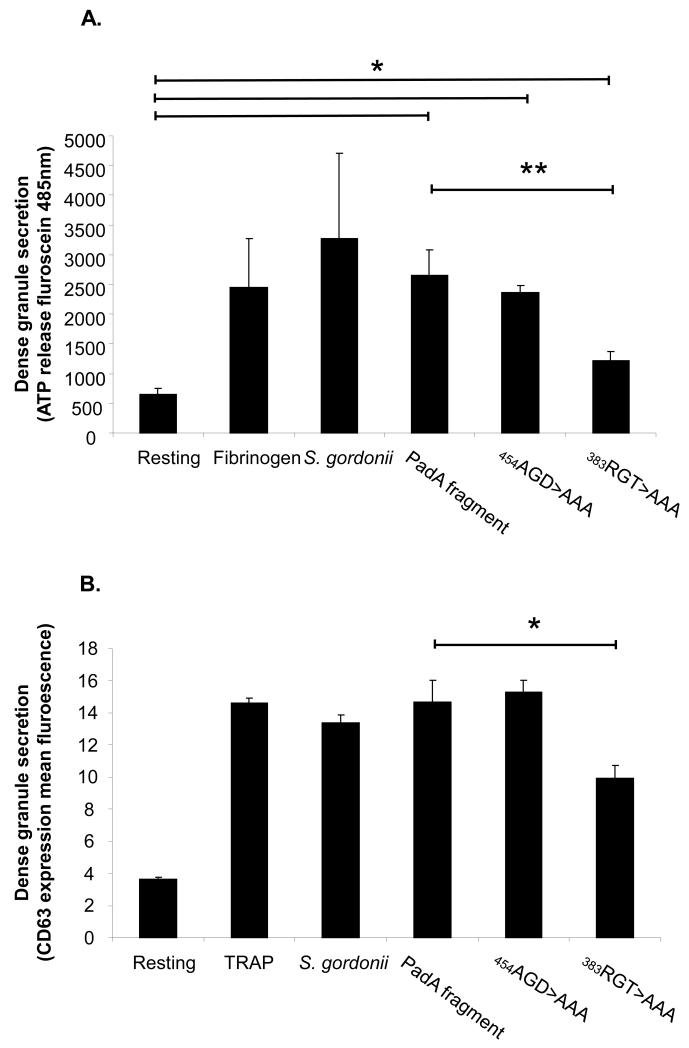
Platelet adhesion to S. gordonii causes release of ADP from platelet dense granules. ATP also exists in the dense granules and is often used as a measure of dense granule release. Therefore, we next determined whether the specific sites on PadA (AGD or RGT) played any role in inducing dense granule release. Platelets were therefore allowed adhere to immobilised S. gordonii or the PadA native or mutated fragments and ATP release measured in a luminescence assay. As shown in Figure 4A, platelets that adhered to S. gordonii exhibited secretion of ATP similar to that induced by platelet adhesion to fibrinogen (P=NS). Platelet adhesion to recombinant PadA fragment resulted in granule secretion, highlighting the importance of PadA in triggering the outside in signal that results in platelet secretion. Interestingly, substituting 383RGT with AAA significantly reduced platelet secretion (Figure 4A, P<0.005, compared to the native PadA fragment), while, substitution of 454AGT for AAA failed to have any significant effect on secretion (Figure 4A, P<NS, compared to the native PadA fragment). Platelets secretion was significantly increased following adhesion to all of the PadA fragments, native and mutant (454AGD to AAA, and the 383RGT to AAA) (Figure 4A, P<0.05). We also measured another marker of dense granule secretion, CD63 expression on the surface of platelets following addition of the S. gordonii and the fragments. There was no statistical difference between TRAP, S. gordonii, PadA fragment or substitution of 454AGT for AAA on the PadA fragment on CD63 expression on the platelet (Figure 4B, P<NS). However consistent with above substituting 383RGT with AAA significantly reduced platelet section of CD63 (Figure 4B, P<0.05, compared to the native PadA fragment).
Figure 4. Platelet dense granule secretion following adhesion to Streptococcus gordonii PadA.
(A) Samples were taken from the platelets adhered to immobilized substrates and transferred to a 96 well microtitre plate. The extent of dense granule secretion from the platelets was measured by addition of a chronolume luciferin/luciferase mix. Luminescence was read at 485nm in a microtitre plate reader. (B) Platelets were incubated with S. gordonii for fragments for 10 min. CD63 or appropriately labelled isotype control was added to the platelets. After 20 min incubation the samples were diluted with 1 ml of PBS and analysed on a FACSCalibur flow cytometer. *P<0.05, **P<0.005, n=3-5
P-selectin exists in the alpha granules and is commonly used as a measure of alpha granule release. S. gordonii were mixed with platelets and P-selectin expression was analysed by flow cytometry. S. gordonii failed to induce P-selectin expression in platelets (Resting; 2.6±0.2 fluorescent units compared to S. gordonii activated platelets; 3.2±0.1 fluorescent units, P=NS, n=7). In control experiments TRAP induced significantly more p-selectin expression in platelets than S. gordonii (TRAP; 35.7±3.5 fluorescent units compared to S. gordonii 3.2±0.1 fluorescent units, P<0.0001, n=3-7). GPIIbIIIa is also found in alpha granules and is also expressed on the platelet surface following platelet activation. GPIIbIIIa expression was measured by CD41a binding and assessed by flow cytometry. Consistent with the above findings S. gordonii also failed to increase CD41a binding in platelets (Resting; 63.4±5.6 fluorescent units compared to S. gordonii activated platelets; 61.3±1.2 fluorescent units, P=NS, n=3. In control experiments TRAP induced significantly more CD41a expression on the platelet surface than S. gordonii (TRAP; 86.7±3.1 fluorescent units compared to S. gordonii 61.3±1.2 fluorescent units, P<0.001, n=3).
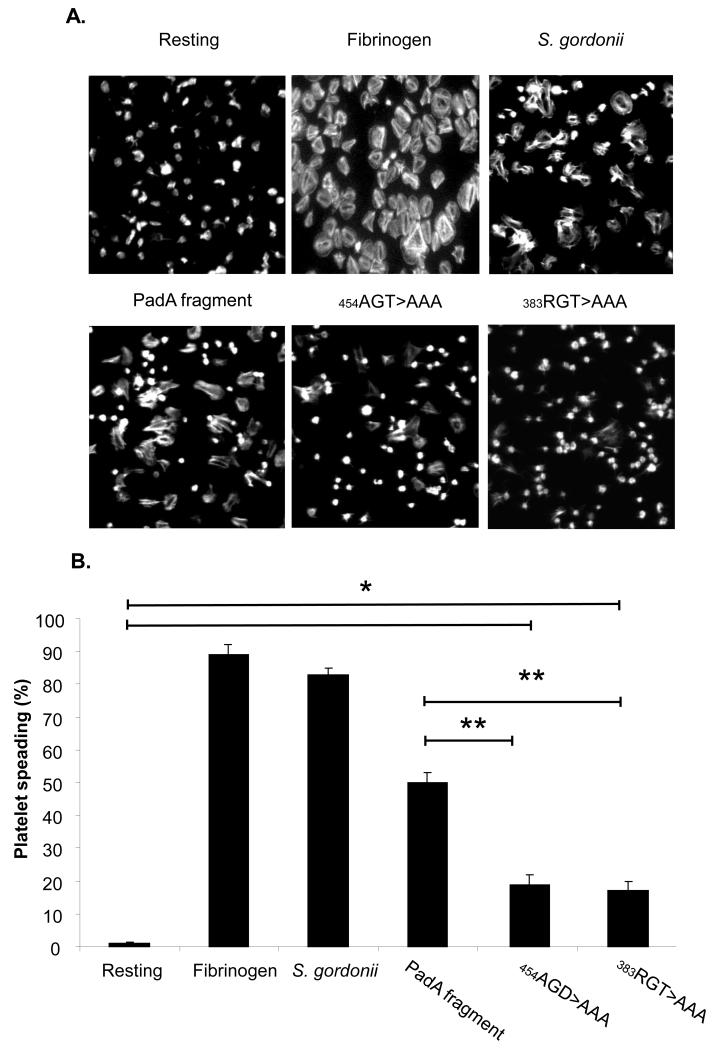
Platelet spreading on S. gordonii PadA
Platelet adhesion to S. gordonii results in an outside in signal leading to dense granule secretion of ADP which plays a key role in platelet spreading. Previously we demonstrated that platelets were capable of spreading on immobilised S. gordonii PadA. Our next approach was to investigate the specific nature of the interaction between platelet GPIIb/IIIa and PadA by using the site-specific recombinant mutants of PadA. Platelets were allowed adhere to BSA, fibrinogen, S. gordonii, or recombinant fragments of PadA (native or mutant) and spreading was evaluated by immunofluorescence microscopy. To quantify the number of platelets spread, we randomly selected five areas on the slide. The percentage of platelets that spread was calculated by dividing the total number of platelets in the field of view by the number of spread platelets. Platelets failed to spread on BSA, but spread on immobilised fibrinogen and S. gordonii as defined by evidence of pseudopod and lamellipod formation (Figure 5A and 5B). Platelets also spread on the purified PadA fragment and the mutant fragments, (454AGD to AAA, and the 383RGT to AAA), compared to the BSA control (figure 5A and 5B, P<0.01). Platelet spreading was significantly reduced on the mutant fragments of PadA (454AGD to AAA, and the 383RGT to AAA) (Figure 5A and 5B, P<0.005, compared to the native PadA fragment). These results suggest that the common GPIIb/IIIa recognition motifs AGD and RG(T) are involved in platelet spreading on S. gordonii PadA.
Figure 5. Platelet spreading on immobilized Streptococcus gordonii PadA.
Plasma-free platelets were allowed to adhere to BSA, fibrinogen, or S gordonii for 45 minutes. (A) Images were acquired (x63) using an argon laser at 488 nm. (B) The number of platelets spread was randomly selected from 5 areas on the slide. *P<0.01, **P<0.005, n=3
Discussion
S. gordonii is a component of the natural microbiota within the human oral cavity. It plays a key role in the development of oral microbial communities [13]. Excessive growth of bacteria can lead to tissue destruction, inflammation and bleeding of the gums which provides a route of entry for the bacteria into the circulation. Once inside the circulation, S. gordonii can interact with circulating platelets and cause unwanted thrombus formation[25]. Thrombus formation triggered by S. gordonii plays a critical role in the development of IE, characterised by vegetative growth on the surface of the heart valves[1]. If untreated this is often fatal and, even with aggressive therapy, mortality rates can be as high as 20-40%[7].
Significant progress has been made in the last number of years characterising the molecular mechanism through which S. gordonii interacts with platelets. Recently we demonstrated that PadA surface protein could bind to platelets in a GPIIb/IIIa dependent manner causing dense granule secretion and full platelet spreading[20]. Disruption of the padA gene reduced binding, but ablated dense granule secretion and full platelet spreading suggesting that the signal that leads to dense granule secretion and platelet spreading is a result of a signal transduced through GPIIb/IIIa[20]. In the present study, we performed site directed mutagenesis on the recombinant PadA protein that expresses the well characterised GPIIb/IIIa recognition motifs RG(T) and AGD to test if these sites were important for outside in signals, that result in dense granule secretion and platelet spreading following S. gordonii binding. Our results provide evidence that neither RGT nor AGD play any role in supporting either static or shear induced platelet adhesion. Although RGT does appear to play a key role in dense granule release, both the RGT and the AGD sites contributed to platelet spreading suggesting that there may be specific sequences on PadA that mediate specific platelet functions.
GPIIb/IIIa binds multiple ligands, including the extracellular matrix proteins, fibrinogen, fibronectin, vitronectin and vonWillebrand factor (vWf)[26]. Outside in signalling through GPIIb/IIIa on a vascular matrix of fibrinogen or vWf is mediated by specific recognition motifs, RGD and QAGDV[27]. Protein analysis of S. gordonii PadA identified both of these GPIIb/IIIa interactive sequences; Arg-Gly-Thr (RGT: 383-385 aa) and Ala-GLy-Asp (AGD: 454-456 aa) however replacing these motifs with AAA failed to have any effect on either static adhesion or shear induced adhesion. Previously we demonstrated that platelets rolled along immobilised S. gordonii in a manner similar to platelet rolling on vWf at sites of vascular injury[23]. Platelet rolling on S. gordonii eventually resulted in firm platelet adhesion. S. gordonii expresses a protein named Hsa which is capable of binding platelet glycoprotein Ibα (GPIbα) and is believed to be responsible for mediating platelet rolling[28]. Firm adhesion is complete when platelet GPIIb/IIIa interacts with S. gordonii PadA[15]. Disruption of the hsa gene in S. gordonii ablated both platelet rolling and firm adhesion, because the platelets do not slow down long enough to allow GPIIb/IIIa to interact with or bind to PadA. Therefore it is perhaps not surprising that deletion of RGT or AGD appeared to make no difference to platelet binding under shear forces.
Another way to investigate the role of these motifs in binding GPIIb/IIIa is to study platelet adhesion under static conditions. Interestingly, platelet adhesion to the RGT or AGD mutant fragments was similar to that of the native PadA fragment suggesting that neither of these motifs play a role in platelet adhesion. These results are consistent with previous studies investigating the interaction of the RGD and AGDV motifs on fibrinogen with GPIIb/IIIa which showed that neither the substitution of RGD sequence with RGE [27, 29, 30] nor the disruption of the last four amino acid residues of the gamma chain of fibrinogen (AGDV) had any affect on platelet binding to immobilised fibrinogen [24, 31]. Collectively these results agree with previous findings that neither the RGT nor the AGD motifs are critical in supporting platelet adhesion, and that other or indeed multiple motifs on fibrinogen may be involved in binding GPIIb/IIIa.
Platelet spreading is a consequence of an outside-in signal generated through engagement of GPIIb/IIIa[32, 33] by its ligands. This is a critical event to allow the platelet withstand the shear forces experienced in the vasculature. Platelets spread on immobilised S. gordonii to a similar degree as platelet spreading on fibrinogen[20]. Platelets also spread on a purified PadA fragment providing evidence that S. gordonii may have evolved in such a way that mimics plasma fibrinogen. Interestingly however substitution of either RGT or AGD on the PadA fragment with AAA significantly reduced platelet spreading, suggesting that both of these motifs play a role in platelet spreading. Consistent with this, early reports have demonstrated that deletion of the RGD motif [34] or using a fragment of fibrinogen that lacks the gamma chain (AGDV) of fibrinogen [31] significantly reduced platelet spreading, highlighting the importance of these motifs in platelet spreading.
Release of potentiating amounts of ADP into the local platelet milieu facilitates cytoskeletal rearrangements necessary for platelet spreading[17]. Previously we demonstrated that platelets adhered to immobilised S. gordonii or the PadA fragment triggered ADP secretion[20] suggesting that S. gordonii binding to platelets provides an outside in signal that results in dense granule secretion. Replacing the AGD motif on PadA with AAA failed to have any effect on ADP secretion, however replacing the RGT with AAA significantly reduced ADP secretion. Consistent with this, CD63 (granulophysin), another marker of dense granule secretion was also reduced when the RGT was replaced with AAA, while the AGD mutation was unaffected. Collectively these results suggest that the RGD motif on PadA is an important motif in providing the outside in signal leading to dense granule secretion. Of particular interest is that regardless of the amount of dense granule secreted ADP in the local environment if the AGD motif is missing the platelets will be unable to spread efficiently. We have not been able to demonstrate that S. gordonii is capable of secreting alpha granules upon activating platelets as measured by P-selectin or an increase in GPIIbIIIa expression. These results suggest that S. gordonii may have the ability to differentially secrete granules in platelets (alpha versus dense). The concept of differential release of platelet granules was first shown by Italiano et al. who demonstrated that platelet alpha granule proteins were organised into separate and distinct granules, and that platelets had the capability of differentially releasing specific subpopulation of the alpha granules[35]. Our work presented here build on these initial observations by suggesting that platelets are not only capable of differential secretion of subpopulations of alpha granules but also capable of differential secretion of alpha and dense granules. Critically, it might be advantageous for bacteria to not induce alpha granule secretion in platelets, as these granules contain anti-microbial peptides.
The work presented here extends previous knowledge in S. gordonii platelet interactions by identifying two motifs on PadA that are similar to the known binding motifs in fibrinogen that recognise GPIIb/IIIa. Of particular interest is the role these motifs play in platelet function. It appears that the RGT motif has no role in supporting platelet adhesion however plays a role in dense granule secretion and platelet spreading. In contrast to this, AGD has no role in supporting platelet adhesion or dense granule secretion, however plays a role in platelet spreading. These results provide evidence for the first time that there are critical motifs expressed on PadA that lead to or mediated different responses in the platelet. A greater understanding into how the different motifs trigger different platelet responses is currently under way. The fact that none of the platelet functions examined were completely absent following RGT or AGD substitution by AAA suggests two possibilities; first that the motifs are compensating for each other, or second, that other motifs on PadA exist that also play key roles. The latter is most likely true as these results mimic GPIIb/IIIa binding to fibrinogen where deletion of the RGD or the AGD either separately or together on fibrinogen failed to abolish platelet functions such as firm adhesion, dense granule secretion, clot retraction or spreading. It is not particularly surprising that additional recognition motifs exist, due to conformation changes that occur within GPIIb/IIIa or fibrinogen. Other potential GPIIb/IIIa binding sites have been proposed including fibrinogen γ316-322 and γ370-381[36, 37] however further structural analysis will need to be done in order to assess if PadA contain corresponding sequences. Ultimately, such advances in our knowledge may lead to the development of novel approaches for prevention and treatment of IE.
What is known about this topic
Streptococcus gordonii is a commensal of the oral cavity and is well known for its ability to interact with platelets and may be an important contributor for the initiation of Infective Endocarditis.
Streptococcus gordonii expresses a large protein called platelet adhesion protein A (PadA) which is capable of binding directly to platelet GPIIb/IIIa.
Streptococcus gordonii PadA binding to platelet GPIIb/IIIa results in the generation of outside-in signals that initiate a series of cytosolic changes that promote firm adhesion, platelet spreading and platelet secretion.
What this paper adds
Streptococccus gordonii PadA contains two short amino acid residue motifs [AGD and RG(T)], both of which are expressed on fibrinogen and play a key role in binding to GPIIbIIIa.
Site directed mutagenesis on the PadA protein in which 454AGD motif was substituted to AAA, and the 383RGT motif was substituted to AAA suggests the RGT motif has no role in supporting platelet adhesion however plays a role in dense granule secretion and platelet spreading. In contrast to this the AGD motif has no role to play in supporting firm platelet adhesion or dense granule secretion however plays a role in platelet spreading.
Our results suggest that RGT and the AGD motifs found on S. gordonii PadA bind to platelet GPIIbIIIa and mediate specific functions that lead to enhanced platelet deposition on the heart valves of Infective Endocarditis patients.
Acknowledgements
This study was supported by Wellcome Trust Clinical Training Fellowships #084979 and #097285, Health Research of Ireland (RP/2006/211) and the Irish Research Council for Science, Engineering and Technology (RS/2009/1177). We thank Lindsay Dutton and Jane Brittan for excellent technical assistance, and Ang Nobbs for helpful discussions.
Footnotes
Conflicts of interest: None declared
References
- 1.Moreillon P, Que YA. Infective endocarditis. Lancet. 2004;363(9403):139–49. doi: 10.1016/S0140-6736(03)15266-X. [DOI] [PubMed] [Google Scholar]
- 2.Durack DT. Prevention of infective endocarditis. N Engl J Med. 1995;332(1):38–44. doi: 10.1056/NEJM199501053320107. [DOI] [PubMed] [Google Scholar]
- 3.Ruggeri ZM. Platelet adhesion under flow. Microcirculation. 2009;16(1):58–83. doi: 10.1080/10739680802651477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moreillon P, Que YA, Bayer AS. Pathogenesis of streptococcal and staphylococcal endocarditis. Infect Dis Clin North Am. 2002;16(2):297–318. doi: 10.1016/s0891-5520(01)00009-5. [DOI] [PubMed] [Google Scholar]
- 5.Prendergast BD, Tornos P. Surgery for infective endocarditis: who and when? Circulation. 2010;121(9):1141–52. doi: 10.1161/CIRCULATIONAHA.108.773598. [DOI] [PubMed] [Google Scholar]
- 6.Castillo JC, et al. Long term outcome of infective endocarditis in patients who were not drug addicts: a 10 year study. Heart. 2000;83(5):525–30. doi: 10.1136/heart.83.5.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murdoch DR, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med. 2009;169(5):463–73. doi: 10.1001/archinternmed.2008.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jault F, et al. Active native valve endocarditis: determinants of operative death and late mortality. Ann Thorac Surg. 1997;63(6):1737–41. doi: 10.1016/s0003-4975(97)00117-3. [DOI] [PubMed] [Google Scholar]
- 9.Remadi JP, et al. Predictors of death and impact of surgery in Staphylococcus aureus infective endocarditis. Ann Thorac Surg. 2007;83(4):1295–302. doi: 10.1016/j.athoracsur.2006.09.093. [DOI] [PubMed] [Google Scholar]
- 10.Thuny F, et al. Risk of embolism and death in infective endocarditis: prognostic value of echocardiography: a prospective multicenter study. Circulation. 2005;112(1):69–75. doi: 10.1161/CIRCULATIONAHA.104.493155. [DOI] [PubMed] [Google Scholar]
- 11.Heiro M, et al. Neurologic manifestations of infective endocarditis: a 17-year experience in a teaching hospital in Finland. Arch Intern Med. 2000;160(18):2781–7. doi: 10.1001/archinte.160.18.2781. [DOI] [PubMed] [Google Scholar]
- 12.Tleyjeh IM, et al. A systematic review of population-based studies of infective endocarditis. Chest. 2007;132(3):1025–35. doi: 10.1378/chest.06-2048. [DOI] [PubMed] [Google Scholar]
- 13.Nobbs AH, Lamont RJ, Jenkinson HF. Streptococcus adherence and colonization. Microbiol Mol Biol Rev. 2009;73(3):407–50. doi: 10.1128/MMBR.00014-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Douglas CW, et al. Identity of viridans streptococci isolated from cases of infective endocarditis. J Med Microbiol. 1993;39(3):179–82. doi: 10.1099/00222615-39-3-179. [DOI] [PubMed] [Google Scholar]
- 15.Petersen HJ, et al. Human platelets recognize a novel surface protein, PadA, on Streptococcus gordonii through a unique interaction involving fibrinogen receptor GPIIbIIIa. Infect Immun. 2010;78(1):413–22. doi: 10.1128/IAI.00664-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cox D, Brennan M, Moran N. Integrins as therapeutic targets: lessons and opportunities. Nat Rev Drug Discov. 2010;9(10):804–20. doi: 10.1038/nrd3266. [DOI] [PubMed] [Google Scholar]
- 17.Shattil SJ, Ginsberg MH, Brugge JS. Adhesive signaling in platelets. Curr Opin Cell Biol. 1994;6(5):695–704. doi: 10.1016/0955-0674(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 18.Clemetson KJ. Platelets and primary haemostasis. Thromb Res. 2012;129(3):220–4. doi: 10.1016/j.thromres.2011.11.036. [DOI] [PubMed] [Google Scholar]
- 19.Kloczewiak M, Timmons S, Hawiger J. Localization of a site interacting with human platelet receptor on carboxy-terminal segment of human fibrinogen gamma chain. Biochem Biophys Res Commun. 1982;107(1):181–7. doi: 10.1016/0006-291x(82)91686-2. [DOI] [PubMed] [Google Scholar]
- 20.Keane C, et al. Mechanism of outside-in {alpha}IIb{beta}3-mediated activation of human platelets by the colonizing Bacterium, Streptococcus gordonii. Arterioscler Thromb Vasc Biol. 2010;30(12):2408–15. doi: 10.1161/ATVBAHA.110.216515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerrigan SW, et al. Molecular basis for Staphylococcus aureus-mediated platelet aggregate formation under arterial shear in vitro. Arterioscler Thromb Vasc Biol. 2008;28(2):335–40. doi: 10.1161/ATVBAHA.107.152058. [DOI] [PubMed] [Google Scholar]
- 22.Plummer C, et al. A serine-rich glycoprotein of Streptococcus sanguis mediates adhesion to platelets via GPIb. Br J Haematol. 2005;129(1):101–9. doi: 10.1111/j.1365-2141.2005.05421.x. [DOI] [PubMed] [Google Scholar]
- 23.Kerrigan SW, et al. Role of Streptococcus gordonii surface proteins SspA/SspB and Hsa in platelet function. Infect Immun. 2007;75(12):5740–7. doi: 10.1128/IAI.00909-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Q, et al. Role of the gamma chain Ala-Gly-Asp-Val and Aalpha chain Arg-Gly-Asp-Ser sites of fibrinogen in coaggregation of platelets and fibrinogen-coated beads. Biochim Biophys Acta. 1998;1385(1):33–42. doi: 10.1016/s0167-4838(98)00039-9. [DOI] [PubMed] [Google Scholar]
- 25.Kerrigan SW, Cox D. Platelet-bacterial interactions. Cell Mol Life Sci. 2010;67(4):513–23. doi: 10.1007/s00018-009-0207-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plow EF, et al. Ligand binding to integrins. J Biol Chem. 2000;275(29):21785–8. doi: 10.1074/jbc.R000003200. [DOI] [PubMed] [Google Scholar]
- 27.Farrell DH, Thiagarajan P. Binding of recombinant fibrinogen mutants to platelets. J Biol Chem. 1994;269(1):226–31. [PubMed] [Google Scholar]
- 28.Bensing BA, Lopez JA, Sullam PM. The Streptococcus gordonii surface proteins GspB and Hsa mediate binding to sialylated carbohydrate epitopes on the platelet membrane glycoprotein Ibalpha. Infect Immun. 2004;72(11):6528–37. doi: 10.1128/IAI.72.11.6528-6537.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farrell DH, et al. Role of fibrinogen alpha and gamma chain sites in platelet aggregation. Proc Natl Acad Sci U S A. 1992;89(22):10729–32. doi: 10.1073/pnas.89.22.10729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaidi TN, et al. Adhesion of platelets to surface-bound fibrinogen under flow. Blood. 1996;88(8):2967–72. [PubMed] [Google Scholar]
- 31.Savage B, Bottini E, Ruggeri ZM. Interaction of integrin alpha IIb beta 3 with multiple fibrinogen domains during platelet adhesion. J Biol Chem. 1995;270(48):28812–7. doi: 10.1074/jbc.270.48.28812. [DOI] [PubMed] [Google Scholar]
- 32.Phillips DR, et al. Integrin tyrosine phosphorylation in platelet signaling. Curr Opin Cell Biol. 2001;13(5):546–54. doi: 10.1016/s0955-0674(00)00250-7. [DOI] [PubMed] [Google Scholar]
- 33.Obergfell A, et al. Coordinate interactions of Csk, Src, and Syk kinases with [alpha]IIb[beta]3 initiate integrin signaling to the cytoskeleton. J Cell Biol. 2002;157(2):265–75. doi: 10.1083/jcb.200112113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Savage B, Ruggeri ZM. Selective recognition of adhesive sites in surface-bound fibrinogen by glycoprotein IIb-IIIa on nonactivated platelets. J Biol Chem. 1991;266(17):11227–33. [PubMed] [Google Scholar]
- 35.Italiano JE, Jr., et al. Angiogenesis is regulated by a novel mechanism: pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood. 2008;111(3):1227–33. doi: 10.1182/blood-2007-09-113837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Remijn JA, et al. Mutations on fibrinogen (gamma 316-322) are associated with reduction in platelet adhesion under flow conditions. Ann N Y Acad Sci. 2001;936:444–8. doi: 10.1111/j.1749-6632.2001.tb03528.x. [DOI] [PubMed] [Google Scholar]
- 37.Podolnikova NP, et al. A cluster of basic amino acid residues in the gamma370-381 sequence of fibrinogen comprises a binding site for platelet integrin alpha(IIb)beta3 (glycoprotein IIb/IIIa) Biochemistry. 2005;44(51):16920–30. doi: 10.1021/bi051581d. [DOI] [PubMed] [Google Scholar]