Abstract
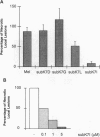
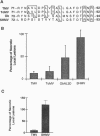
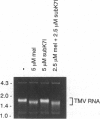
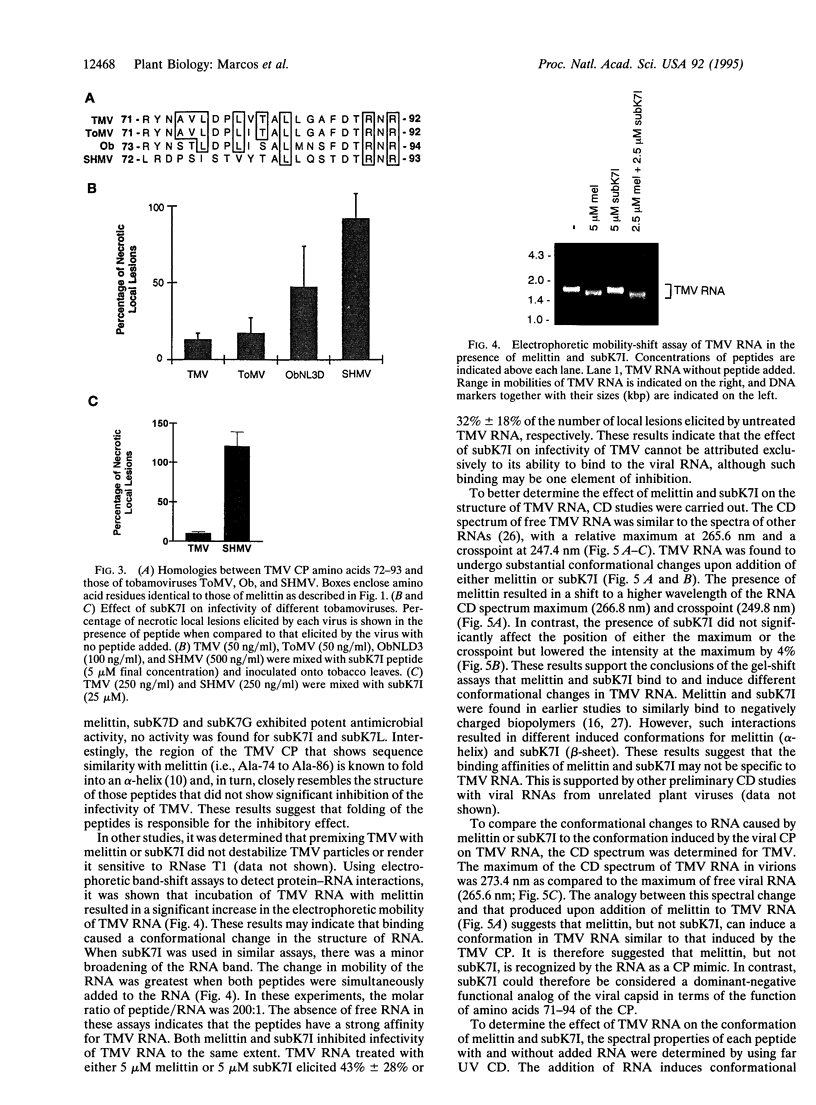
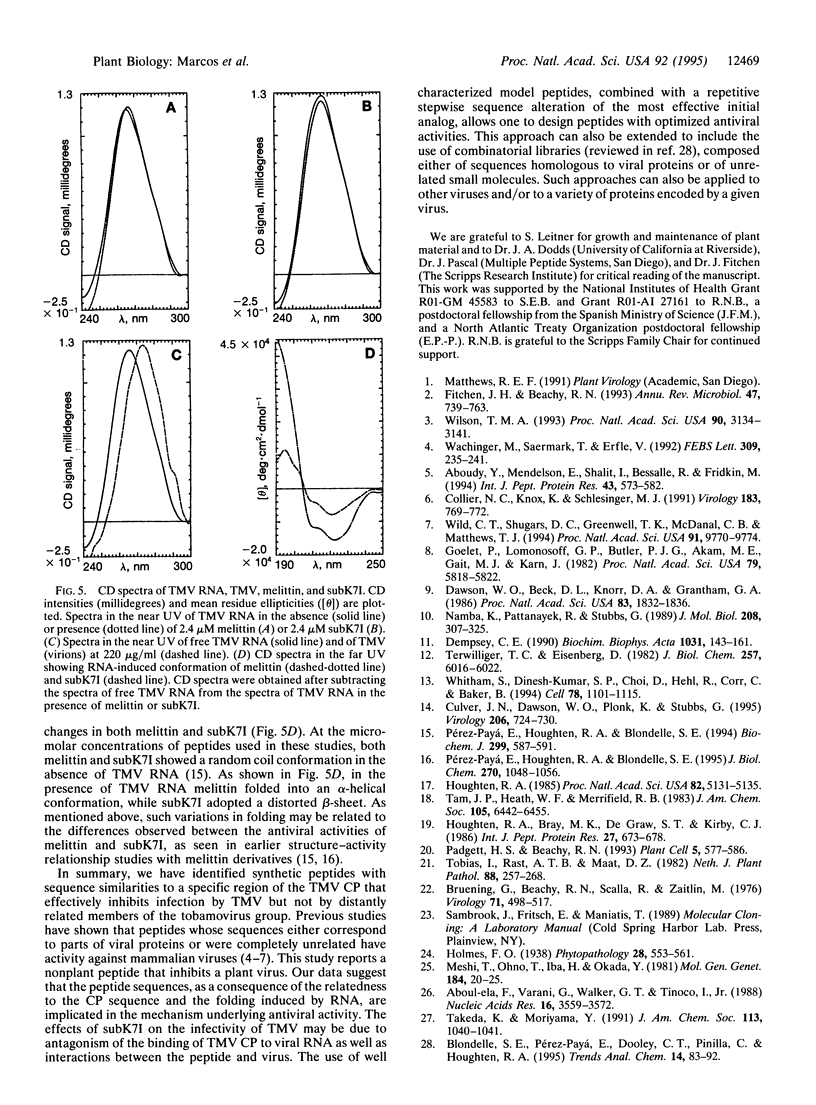
An approach that enables identification of specific synthetic peptide inhibitors of plant viral infection is reported. Synthetic analogs of melittin that have sequence and structural similarities to an essential domain of tobacco mosaic virus coat protein were found to possess highly specific antiviral activity. This approach involves modification of residues located at positions analogous to those that are critical for virus assembly. The degree of inhibition found correlates well with sequence similarities between the viral capsid protein and the melittin analogs studied as well as with the induced conformational changes that result upon interaction of the peptides and ribonucleic acid.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboudy Y., Mendelson E., Shalit I., Bessalle R., Fridkin M. Activity of two synthetic amphiphilic peptides and magainin-2 against herpes simplex virus types 1 and 2. Int J Pept Protein Res. 1994 Jun;43(6):573–582. doi: 10.1111/j.1399-3011.1994.tb00559.x. [DOI] [PubMed] [Google Scholar]
- Aboul-ela F., Varani G., Walker G. T., Tinoco I., Jr The TFIIIA recognition fragment d(GGATGGGAG).d(CTCCCATCC) is B-form in solution. Nucleic Acids Res. 1988 Apr 25;16(8):3559–3572. doi: 10.1093/nar/16.8.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruening G., Beachy R. N., Scalla R., Zaitlin M. In vitro and in vivo translation of the ribonucleic acids of a cowpea strain of tobacco mosaic virus. Virology. 1976 Jun;71(2):498–517. doi: 10.1016/0042-6822(76)90377-9. [DOI] [PubMed] [Google Scholar]
- Collier N. C., Knox K., Schlesinger M. J. Inhibition of influenza virus formation by a peptide that corresponds to sequences in the cytoplasmic domain of the hemagglutinin. Virology. 1991 Aug;183(2):769–772. doi: 10.1016/0042-6822(91)91008-5. [DOI] [PubMed] [Google Scholar]
- Culver J. N., Dawson W. O., Plonk K., Stubbs G. Site-directed mutagenesis confirms the involvement of carboxylate groups in the disassembly of tobacco mosaic virus. Virology. 1995 Jan 10;206(1):724–730. doi: 10.1016/s0042-6822(95)80096-4. [DOI] [PubMed] [Google Scholar]
- Dawson W. O., Beck D. L., Knorr D. A., Grantham G. L. cDNA cloning of the complete genome of tobacco mosaic virus and production of infectious transcripts. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1832–1836. doi: 10.1073/pnas.83.6.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey C. E. The actions of melittin on membranes. Biochim Biophys Acta. 1990 May 7;1031(2):143–161. doi: 10.1016/0304-4157(90)90006-x. [DOI] [PubMed] [Google Scholar]
- Fitchen J. H., Beachy R. N. Genetically engineered protection against viruses in transgenic plants. Annu Rev Microbiol. 1993;47:739–763. doi: 10.1146/annurev.mi.47.100193.003515. [DOI] [PubMed] [Google Scholar]
- Goelet P., Lomonossoff G. P., Butler P. J., Akam M. E., Gait M. J., Karn J. Nucleotide sequence of tobacco mosaic virus RNA. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5818–5822. doi: 10.1073/pnas.79.19.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghten R. A., Bray M. K., Degraw S. T., Kirby C. J. Simplified procedure for carrying out simultaneous multiple hydrogen fluoride cleavages of protected peptide resins. Int J Pept Protein Res. 1986 Jun;27(6):673–678. doi: 10.1111/j.1399-3011.1986.tb01064.x. [DOI] [PubMed] [Google Scholar]
- Houghten R. A. General method for the rapid solid-phase synthesis of large numbers of peptides: specificity of antigen-antibody interaction at the level of individual amino acids. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5131–5135. doi: 10.1073/pnas.82.15.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshi T., Ohno T., Iba H., Okada Y. Nucleotide sequence of a cloned cDNA copy of TMV (cowpea strain) RNA, including the assembly origin, the coat protein cistron, and the 3' non-coding region. Mol Gen Genet. 1981;184(1):20–25. doi: 10.1007/BF00271189. [DOI] [PubMed] [Google Scholar]
- Namba K., Pattanayek R., Stubbs G. Visualization of protein-nucleic acid interactions in a virus. Refined structure of intact tobacco mosaic virus at 2.9 A resolution by X-ray fiber diffraction. J Mol Biol. 1989 Jul 20;208(2):307–325. doi: 10.1016/0022-2836(89)90391-4. [DOI] [PubMed] [Google Scholar]
- Padgett H. S., Beachy R. N. Analysis of a tobacco mosaic virus strain capable of overcoming N gene-mediated resistance. Plant Cell. 1993 May;5(5):577–586. doi: 10.1105/tpc.5.5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Payá E., Houghten R. A., Blondelle S. E. Determination of the secondary structure of selected melittin analogues with different haemolytic activities. Biochem J. 1994 Apr 15;299(Pt 2):587–591. doi: 10.1042/bj2990587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Payá E., Houghten R. A., Blondelle S. E. The role of amphipathicity in the folding, self-association and biological activity of multiple subunit small proteins. J Biol Chem. 1995 Jan 20;270(3):1048–1056. doi: 10.1074/jbc.270.3.1048. [DOI] [PubMed] [Google Scholar]
- Terwilliger T. C., Eisenberg D. The structure of melittin. II. Interpretation of the structure. J Biol Chem. 1982 Jun 10;257(11):6016–6022. [PubMed] [Google Scholar]
- Wachinger M., Saermark T., Erfle V. Influence of amphipathic peptides on the HIV-1 production in persistently infected T lymphoma cells. FEBS Lett. 1992 Sep 14;309(3):235–241. doi: 10.1016/0014-5793(92)80780-k. [DOI] [PubMed] [Google Scholar]
- Whitham S., Dinesh-Kumar S. P., Choi D., Hehl R., Corr C., Baker B. The product of the tobacco mosaic virus resistance gene N: similarity to toll and the interleukin-1 receptor. Cell. 1994 Sep 23;78(6):1101–1115. doi: 10.1016/0092-8674(94)90283-6. [DOI] [PubMed] [Google Scholar]
- Wild C. T., Shugars D. C., Greenwell T. K., McDanal C. B., Matthews T. J. Peptides corresponding to a predictive alpha-helical domain of human immunodeficiency virus type 1 gp41 are potent inhibitors of virus infection. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):9770–9774. doi: 10.1073/pnas.91.21.9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson T. M. Strategies to protect crop plants against viruses: pathogen-derived resistance blossoms. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3134–3141. doi: 10.1073/pnas.90.8.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]