Abstract
Background: Formocresol, though the center of much controversy is still the most widely used medicament for primary teeth pulpotomy and an intracanal medicament which has undergone a lengthy evolution to shorten the formocresol application time and reduce the concentration of formocresol exposure to the pulp tissue. Hence, the determination of the actual effective dose and concentration of formocresol for clinical application in primary teeth is an important area of research and a thorough clinical, radiographic and histological investigation in human subjects is very much needed. Materials & Methods: The study was conducted on 45 primary molars for the Clinical, Radiographic study and 45 premolars orthodontically indicated for extraction for the Histological study. The samples were randomly and equally divided into 3 groups of 15 each for pulpotomy with full strength formocresol, 1:5 diluted formocresol and 1:25 diluted formocresol respectively. The pulpotomized primary molars were clinically evaluated at 1st, 3rd, 6th and 9th month while the pulpotomized premolars were subjected for histological evaluation after extraction. Results: Obtained by chi-square test revealed that all the pulpotomized primary molars were asymptomatic till the end of the study period; suggesting 100% clinical and radiographic success while histologically, the three concentrations of formocresol showed decreased severity of fixation of the pulp tissue with decreasing concentration of formocresol. Conclusion: It can be inferred that the diluted formulations (1:5 and 1:25) of formocresol are equally efficient when compared to full-strength formocresol and thus, can be recommended for pulpotomy in primary teeth. How to cite the article: Goyal S, Abuwala T, Joshi K, Mehta J, Indushekar KR, Hallikerimath S. The Clinical, Radiographic and Histological evaluation of three different concentrations of Formocresol as a pulpotomy agent. J Int Oral Health 2014;6(2):118-25.
Key words: : Dilute formocresol, formocresol, primary teeth, pulpotomy
Introduction
Formocresol, first introduced by Buckley in 1904, as a pulpotomy medicament 1 has long been considered the “gold standard” to which all other medicaments are compared for primary tooth pulpotomy. 2 Despite years of apparent successful use as a pulpotomy agent, it has become necessary to study and document formocresol because of its toxic, mutagenic and carcinogenic properties. When used judiciously, formocresol is a safe medicament. 3 Hence, keeping this in view, this clinical, radiographic and histological study attempts to identify equally effective diluted formulations of formocresol for vital pulpotomy procedure in children.
Materials and Methods
For the clinical and radiographic study 45 primary molars (maxillary or mandibular first and second primary molars) were selected in 41 healthy children in the age group of 4-8 years. For the histological study, 45 premolars (maxillary or mandibular first and second) from 13 healthy children
in the age group of 12-14 years requiring orthodontic extraction were referred to the department. The samples were equally divided into three groups:
Group A: 15 primary molars and 15 premolars were treated with full strength Formocresol.
Group B: 15 primary molars and 15 premolars were treated with 1:5 diluted Formocresol.
Group C: 15 primary molars and 15 premolars were treated with 1:25 diluted Formocresol.
Pulpotomy medicaments:
Formocresol (TRISOL; Vishal Dentocare Pvt Ltd., Gujarat)
Prepared 1: 5 diluted formocresol
Prepared 1:25 diluted formocresol
For 1:5 dilution: The diluted solution was prepared first by thoroughly mixing 3 ml of glycerine with 1 ml of distilled water. 4 ml of the diluent is then added to the 1 ml of the TRISOL.
For 1:25 dilution: 18 ml of glycerin and 6 ml of distilled water are thoroughly mixed and to which 1 part of TRISOL is added.
The inclusion criteria for the clinical and radiographic study were: 4 , 5
Children within the age group of 4-8 years
Carious or mechanical exposure in vital primary teeth.
Radiographically the carious lesion approximating the pulp.
Complain of pain/discomfort only while eating.
Symptoms suggestive of reversible pulpitis
No clinical and radiographic evidence of pulp degeneration such as excessive bleeding from the root canal, tenderness to percussion, swelling or sinus tract, mobility, internal resorption, interradicular and or periapical bone destruction, advanced physiological root resorption.
Teeth should be restorable after completion of the procedure.
Exclusion Criteria:
History of spontaneous pain and/or swelling
Presence of pathologic mobility and Tenderness on percussion
Presence of pathologic root resorption
Presence of Furcal/ Periapical radiolucency
Patient medically/ physically compromised
For the clinical and radiographic study
The complete treatment procedure was explained to the parents of the children selected for the study and their informed consent was taken before commencement of the procedure. A step by step, one sitting pulpotomy procedure was carried out by the placement of the following pulpotomy medicament:
Group A: A no. 1 foam pellet saturated with TRISOL then twice squeezed to remove excess trisol and placed for 5 minutes.
Similar procedure was followed for Group B and C using 1:5 and 1:25 TRISOL respectively. All the pulpotomized primary molars were restored with Stainless Steel crown in subsequent visits.
Figure 1: Intraoral findings in the case selected for pulpotomy showing caries with 84.
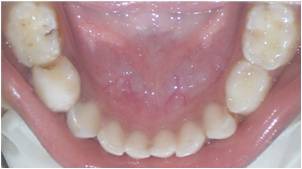
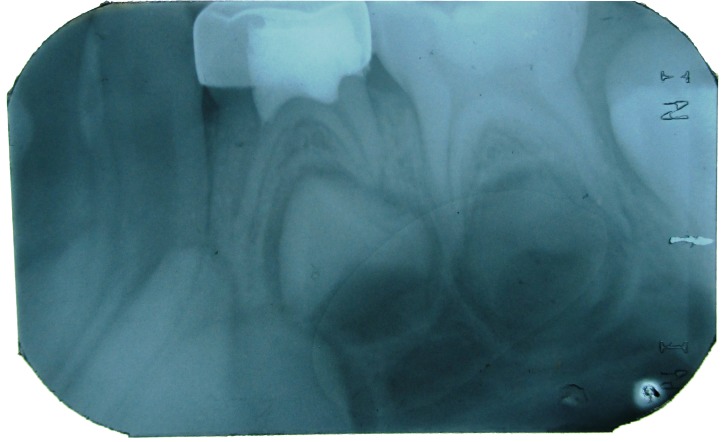
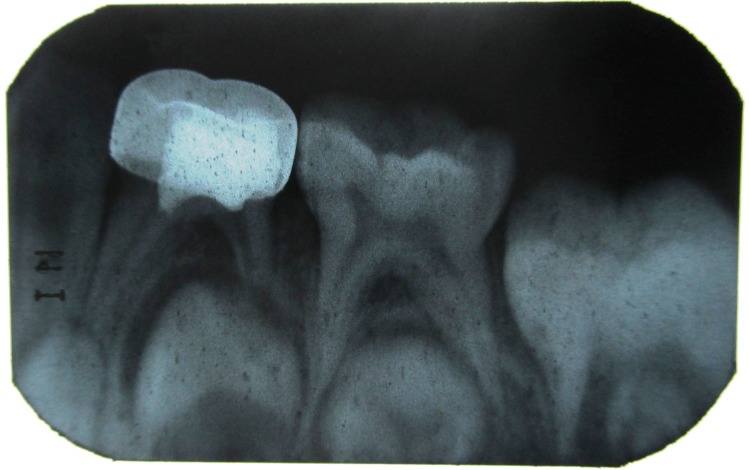
Figure 2: IOPA of 84 showing radiolucency in distal side involving enamel, dentin and approximating the distal pulp horn.
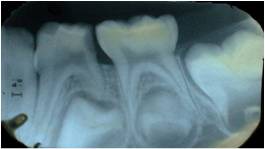
Figure 3: Postoperative photograph showing stainless steel crown with 84 after the pulpotomy procedure.
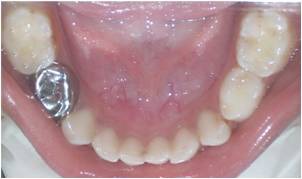
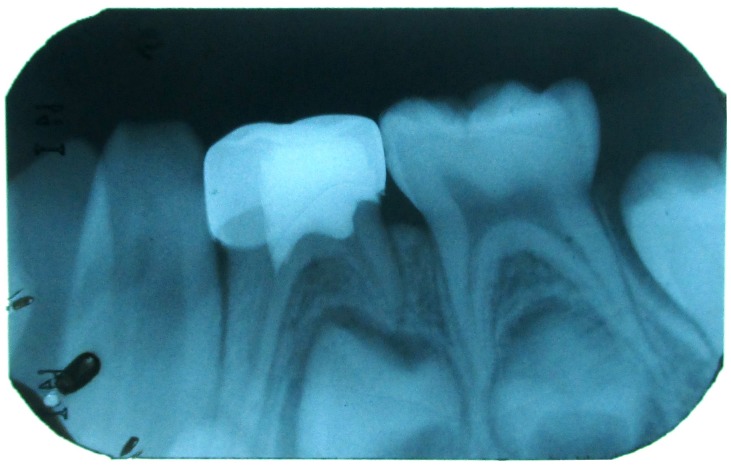
Figure 4: IOPA showing the stainless steel crown restoration and pulpotomy with 84.
The children were recalled for clinical and radiographic evaluation at regular intervals of 1, 3, 6, and 9 months for the following (Figure 1 - 8 ):
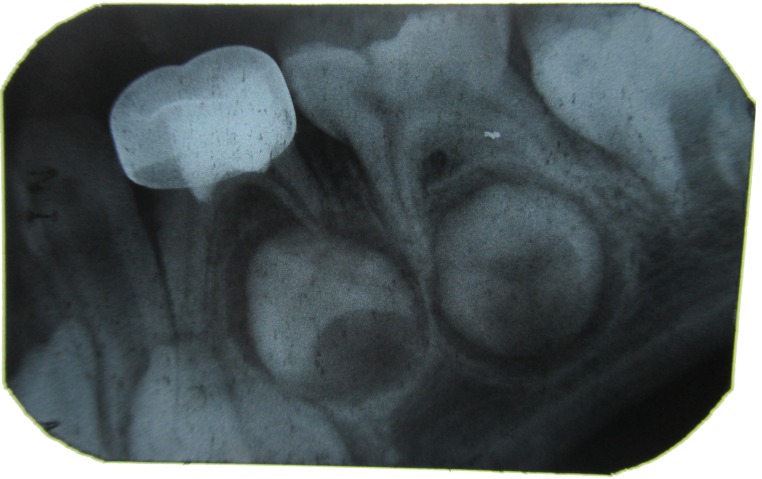
Figure 5: IOPA with 84 after 1 month.
Figure 6: IOPA with 84 after 3 months.
For the Histological study
The above mentioned procedure of pulpotomy was performed for all the children reporting for the extraction of premolars.
Group A: A no. 1 foam pellet saturated with TRISOL which was twice squeezed and placed for 5 minutes on the amputated pulp stumps. In this study a cotton pellet was placed in between the fixed pulp tissue and the temporary restorative material (Cavit) after the application of the medicament to avoid any influence of the temporary restorative material on the pulp. After a preparation time of approximately 15 minutes the pulpotomized premolar was
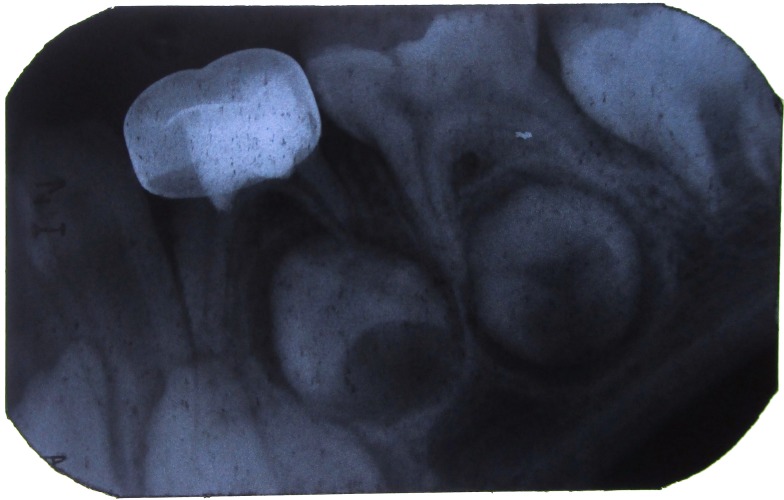
Figure 7: IOPA with 84 after 6 months.
Figure 8: IOPA with 84 after 9 months.
extracted. Similar procedures were done in Group B and Group C using 1:5 and 1:25 formocresol respectively. The extracted teeth were stored in 10% neutral buffered formalin (NBF). After 24 hours the stored specimens were subjected for decalcification. The specimens were hung in a bottle containing 10% HCl for uniform decalcification and the acid was renewed with a freshly prepared 10% HCl
solution every 24 hours. End point of decalcification was judged either by tactile sensation or using a blunt probe.
Method for tissue processing:
The decalcified specimens were kept for 3 hours under running tap water to ensure removal of decalcifying fluids. Following this the steps of tissue dehydration were carried out in ascending grades of 70%, 90%, 100% and 100% alcohol followed by descending grades of 100%, 100%,
90% and 70% alcohol. The specimens were then cleared in xylene and chloroform. They were then embedded in paraffin wax block and sectioned into a 4 μm thickness by a semi-automatic microtome (Leica TM RM 2145, Germany). The sectioned samples were fixed on albumin smeared slides and then stained with hematoxylin and eosin ( Figure 9 ). The prepared slides were observed under Research Microscope (Leica DM 2500) under 4x, 10x and 40x magnifications. The slides were assessed by three different investigators, the inter-observer variability was discussed and common consensus was arrived at. The fibrosis was marked as:
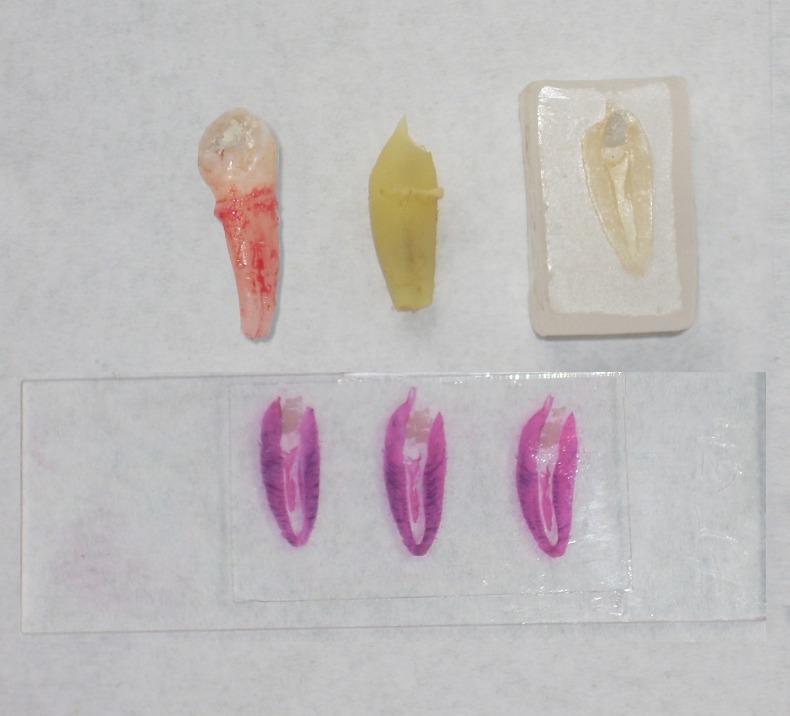
Figure 9: Extracted pulpotomized premolar, decalcified, mounted on paraffin block, sectioned and stained.
+ (mild)
++ (moderate)
+++ (severe/intense)
Statistical analysis
The observed findings of the clinical and radiographic study were noted in a proforma. The treatment was regarded as a failure when one or more of the above mentioned findings were present. The data was subjected to statistical test using chi-square test. Single blinding was followed where the subjects were unaware of their group. The data of the histological study was noted, tabulated, compared and represented as frequency and percentage. Statistical analysis was done using chi-square test.
Results
There were two drop-outs in Group A and C while 1 dropout in Group B at the end of 9 months for the clinical and radiographic study, hence 13 and 14 samples were followed up respectively. There was no difference in the clinical outcomes and radiographic features at the end of 1, 3, 6 and 9 months for all the 3 groups; suggesting 100% clinical and radiographic success (Table 1 and 2 ).
Table 1: Table showing presence/absence of clinical sign and symptoms at the end of 1, 3, 6 and 9 months among different groups.
| Pulpotomy groups | 1 Month | 3 Months | 6 Months | 9 Months | |||||
| Present | Absent | Present | Absent | Present | Absent | Present | Absent | ||
| Group A | n | 0 | 15 | 0 | 15 | 0 | 15 | 0 | 13 |
| % | 0 | 100 | 0 | 100 | 0 | 100 | 0 | 100 | |
| Group B | n | 0 | 15 | 0 | 15 | 0 | 15 | 0 | 14 |
| % | 0 | 100 | 0 | 100 | 0 | 100 | 0 | 100 | |
| Group C | n | 0 | 15 | 0 | 15 | 0 | 15 | 0 | 13 |
| % | 0 | 100 | 0 | 100 | 0 | 100 | 0 | 100 | |
| Total | 0 | 45 | 0 | 45 | 0 | 45 | 0 | 40 | |
Table 2: Table showing presence/absence of radiographic signs at the end of 1, 3, 6 and 9 months among different groups.
| Pulpotomy groups | 1 Month | 3 Months | 6 Months | 9 Months | |||||
| Present | Absent | Present | Absent | Present | Absent | Present | Absent | ||
| Group A | n | 0 | 15 | 0 | 15 | 0 | 15 | 0 | 13 |
| % | 0 | 100 | 0 | 100 | 0 | 100 | 0 | 100 | |
| Group B | n | 0 | 15 | 0 | 15 | 0 | 15 | 0 | 14 |
| % | 0 | 100 | 0 | 100 | 0 | 100 | 0 | 100 | |
| Group C | n | 0 | 15 | 0 | 15 | 0 | 15 | 0 | 13 |
| % | 0 | 100 | 0 | 100 | 0 | 100 | 0 | 100 | |
| Total | 0 | 45 | 0 | 45 | 0 | 45 | 0 | 40 | |
In the histological study, all the teeth belonging to Group A showed severe fibrosis (100%), 93.3% of the samples showed moderate fibrosis in Group B, while 6.67% showed severe fibrosis. 93.3% of the samples showed mild fibrosis in Group C and 6.67% showed moderate fibrosis. The data when tabulated, compared and statistical analysed by the chi-square test the value 82.96 was obtained. When intercomparison was done among the three groups it was seen that the p value < 0.001 showed that there was statistically significant difference in the histological reaction of the pulp to three different concentrations of formocresol. ( Table 3 , Group A vs Group B - p <0.001, Group A vs Group C - p <0.001, Group B vs Group C - p <0.001).
Table 3: Table showing histological comparisons among the three pulpotomy groups.
| Severe Fibrosis | Moderate Fibrosis | Mild Fibrosis | Total | ||
| Group A | N | 15 | 0 | 0 | 15 |
| Full strength Formocresol | % | 100 | 0 | 0 | |
| Group B | N | 1 | 14 | 0 | 15 |
| 1:5 Diluted Formocresol | % | 6.67 | 93.33 | 0 | |
| Group C | N | 0 | 1 | 14 | 15 |
| 1:25 Diluted Formocresol | % | 0 | 6.67 | 93.33 | |
| Total | 16 | 15 | 14 | ||
| p<0.001, Highly Significant | |||||
Discussion
King et al 6 conducted a survey on the concentration of formocresol used by pediatric dentists in Texas for primary tooth pulpotomy, according to which majority of pediatric dentists are not only still using formocresol for primary tooth pulpotomy, but they are also using full strength formocresol, either knowingly or unknowingly which indicates that there is as much confusion among practitioners about formocresol as there is variation in their techniques. Similarly another survey showed 61% of respondents used formocresol for primary tooth vital
pulpotomies in which 28% used undiluted formocresol and 33% used diluted formocresol. The results of this survey suggest that the majority of dentists who used formocresol were not concerned with any adverse effects. 7 Similar findings were also obtained when the author of the present study conducted survey of pedodontic faculty (ISPPD members) and postgraduates of pedodontics attending the ISPPD convention in the year 2013. 8 Keeping this in mind the present study was undertaken in which the clinical and radiographic and the histological efficacy of 3 different concentrations of formocresol (full-strength, 1:5 and 1:25) were evaluated. Though it would have been good if the histological study was carried out on primary molars, ethically this is not possible as the sole aim of pediatric pulp therapy is to preserve the tooth till it exfoliates, hence, premolars were chosen in this study. The initial dilution of formocresol was done in the Department of Pharmacology to ensure accuracy and later the chair-side dilution was done as and when needed as the shelf life of the diluted formocresol is limited 4 ; but the exact time period of expiry is not documented, hence to be on the safer side frequent chair side dilution (once in 3 months) was carried out. Variation in the time of formocresol application from days to minutes has been studied. Microscopic evaluation indicates that the main action of formocresol occurs within the first 5 minutes of application (Emmerson et al; 1959). In the present study medicament was applied for 5 minutes as a standard once only. Hence, the brown-black discoloration of pulp stumps after the medicament is applied may not be considered as the criteria for judgment of fixation as mentioned in various pedodontic textbooks and reapplication may not be necessary. This clinical criterion was confirmed histologically as the fixation of the pulp tissue was observed in all the 3 groups irrespective of the color of the amputated pulp stumps.
The dose of formaldehyde from a single cotton pellet has also been determined. To quote Wesley DJ, Marshall FJ and Rosen S 9 stated that a drop of formocresol weighs approximately 24.7 mg; clinically the dose of formocresol in a no. 4 cotton pellet squeezed dry weighs 4.86 mg (1/5 th drop) which is approximately twice the least effective dose (1/10 th drop) while Milnes 10 has calculated that a no. 4 cotton pellet soaked in full-strength formocresol and then squeezed dry could theoretically deliver a dose of 0.1 to 0.5 mg formocresol to the dental pulp. In the present study the author has calculated the formaldehyde dose from a no. 1 foam pellet twice squeezed dry (Voco, PeleTim, Germany). The mean formaldehyde dose from fullstrength was 2.73 mg, 1.65 mg from 1:5 diluted formocresol and 0.70 mg from 1:25 diluted formocresol.
However, the actual dose reaching the pulp tissue will probably be much lesser, as most of the formocresol will remain in the cotton pellet.
The results of the present study are similar and comparable with that of other human clinical studies using full strength and 1:5 diluted formocresol as mentioned by Morawa et al 11 , Karine et al 12 , Erdem et al 13 and Waterhouse 14 in their detailed review. For 1: 25 diluted formocresol, the author of this studycould not trace any human clinical studies.
In an attempt to reduce the dose of formaldehyde a series of research studies by Loos and Straffon 15 have led to the conclusion that 1:5 diluted Buckley’s formocresol when applied to the tissues achieves the desired cellular response as effectively as the full strength formocresol and also allows a faster recovery of the affected connective tissue cells. The progressive dilutions allowed an earlier recovery. The histological response of the pulp to formocresol has been interpreted in various ways by various researchers. The histological response depends on the duration for which formocresol is in contact with the pulp tissue. 16 , 17 In the present histological study the fixation of the entire pulp tissue up to apical third in all the 3 groups was achieved which shows that there is diffusion of the formaldehyde via the periapical region into the systemic circulation within the first 15 minutes of the pulpotomy procedure. 18 The pulp tissue in all the sections of Group A showed progressive increase in the fibrous component along with decreasing cellularity. Hyalinization of the pulp tissue was
observed; though this feature was not seen in Group B and Group C ( Figure 10 ). Group B showed moderate fibrosis
Figure 10: Pulp tissue treated with full strength formocresol (HandE, 10 X) shows severe fibrosis (hyalinization) of collagen fibers.
and Group C showed mild fibrosis (Figure 11 and 12 ). The sections of a (untreated) premolar showed all the zones of pulp ( Figure 13 ); on the contrary the sections of pulpotomized premolar though showed all the zones but the zones were not well defined, except for the odontoblastic layer. In all the 3 groups the blood vessels appeared engorged and dilated. It was also observed that numerous engorged and dilated blood vessels appeared in Group A as compared to very few in Group C. It is known that vascular dilation can occur from the ‘pumping’ action during tooth extraction or pathologically as a result of pulpal irritation. 19 Hence, with this histological study it can be demonstrated that though the quality of fixation varied with different concentrations of formocresol but the fixation of the pulpal tissue with the diluted formocresol gave clinically similar results as that of full strength formocresol.
Figure 11: Pulp tissue reaction with 1:5 diluted formocresol (Group B) [HandE, 4X] shows fibrocellular stroma with dilated blood vessels.
Figure 12: Pulp tissue reaction to 1:25 diluted formocresol (Group C) [HandE, 4X] shows increased number of fibroblasts (hypercellular stroma) in the stromal tissue.
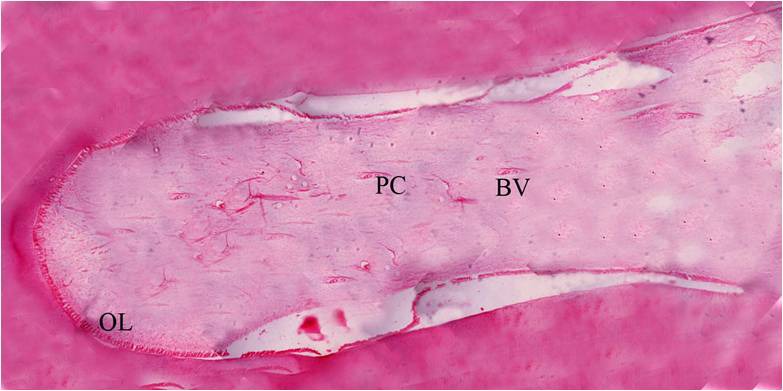
Figure 13: Histology of the decalcified tooth section showing the entire pulp tissue (HandE, 4X).
There can be no doubt that a reparative, biologic approach to pediatric pulp therapy is preferable as compared to the devitalization approach. Various alternatives have been used like glutaraldehyde but it has very limited shelf-life of about 2 weeks, therefore has to be freshly prepared;
whenever it is required. With Calcium Hydroxide Ca (OH)2 more failure is observed due to internal resorption; Ferrous sulphate FeSO 4 is used based on the theory that it might prevent problems encountered with clot formation and thereby minimizes the chance of inflammation and internal resorption. Recently introduced MTA, Lasers, BMPs, Enamel Matrix derivative, Bioactive glass are all more complex to use and/or very expensive, but are yet to be identified as clearly and reproducibly superior to formocresol. 20 Formocresol on the other hand apart from its safety concerns, has many advantages like; it has a longer shelf life of about 2-3 years, easily available, economical, superior clinical and radiographic success which is due to its strong antiseptic and germicidal property, reduced formaldehyde exposure when diluted formulation is used as compared to the full strength formulation. WHO has estimated the daily consumption of formaldehyde approximates to 1.5-14 mg/day (mean 7.8 mg/day), assuming a contribution of 9.4 mg/day from food, 1mg/day from inhalation, and 0.15 mg/day from water, therefore an adult consumes 10.55 mg of formaldehyde per day; it is likely that children are exposed to lower amounts because of lesser food intake. 5 Ranly 9 states about 3000 pulpotomies on a single child are required to cause ill-effects. Hence, a further research and clinical human trials should be directed to know the lowest dose of formaldehyde for a single pulpotomy procedure and its penetration via the periapical region into the systemic circulation. It is unlikely that formocresol, when used in the doses typically employed for the vital pulpotomy procedure, poses any risks to children. 21 However, that the “evidence” for banning this medicament because of safety concerns has been misinterpreted or replaced by better medicament.
Conclusion
All the pulpotomized primary molars were asymptomatic till the end of the study period. There is 100% clinical and radiographic success rate with all the 3 concentrations of formocresol. The proposed brown-black discoloration of amputated pulp stump need not be considered as the
criteria for fixation of the amputated pulp tissue and reapplication is not necessary. The fixation of the pulp tissue in pulpotomized premolars with all the 3 concentrations of formocresol was observed histologically. The degree of fixation decreased with the decrease in the concentration of formocresol. Hence, the diluted formulations are clinically, radiographically and histologically equally efficient when compared to fullstrength formocresol. Research has indicated that a minority of pediatric dentists use dilute formocresol because it is not available commercially, so perhaps it is time for the manufacturers to develop and market dilution of this medicament to replace the “full-strength”
formulations as the effects of the two formulations are equivalent. Pediatric Dentists who wish to continue to use formocresol should apply the lowest dose possible using a standardized size cotton or foam pellet for the least possible time to obtain the desired effect. Hence, it would
be wise to use diluted formocresol to lower the formaldehyde exposure as it is equally efficient and its use in clinical practice is highly recommended.
Clinical significance
Dental professionals have both scientific and moral responsibilities to deliver the best possible health care for the patients. Until a superior alternative is developed or there is definite evidence substantiating the risk of illeffects, there is no reason to discontinue its use. When used judiciously, formocresol is a safe medicament5. The determination of the actual effective dose and concentration for clinical application for pulpotomy in primary teeth is an important area of further research. A thorough clinical, radiographic and histological investigation in human subjects is very much needed with various dilutions of formocresol.
Acknowledgments
The authors would like to acknowledge the ethical committee for their permission and cooperation. The corresponding author is highly grateful to the department of orthodontics and the department of oral pathology for providing their helping hand and constant support.
Footnotes
Source of Support: Nil
Conflict of Interest: None
Contributor Information
Swati Goyal, Department of Pedodontics and Preventive Dentistry, Government Dental College and Hospital, Ahmedabad, Gujarat, India.
Tasnima Abuwala, Department of Conservative Dentistry and Endodontics, Government Dental College and Hospital, Ahmedabad, Gujarat, India.
Keyur Joshi, Department of Pedodontics and Preventive Dentistry, Government Dental College and Hospital, Jamnagar, Gujarat, India.
Jahnvi Mehta, Department of Conservative Dentistry and Endodontics, Government Dental College and Hospital, Jamnagar, Gujarat, India.
K R Indushekar, Department of Pedodontics and Preventive Dentistry, Sudha Rustagi Dental College of Dental Sciences and Research, Bhopani, Faridabad, Haryana, India.
Seema Hallikerimath, Department of Oral Pathology, KLE VK Institute of Dental Sciences, Belgaum, Karnataka, India.
References
- 1.JP Buckley. The chemistry of pulp decomposition, with a rational treatment for this condition & its sequelae. Am Dent J. 1904;3:764. [Google Scholar]
- 2.D Zurn, NS Seale. Light-cured Calcium hydroxide vs Formocresol in Human Primary Molar Pulpotomies: A Randomized Controlled trial. Pediatr Dent. 2008;30(1):34–41. [PubMed] [Google Scholar]
- 3.AR Milnes. Is Formocresol Obsolete? A Fresh Look at the Evidence Concerning Safety Issues. Pediatr Dent. 2008;30(3):237–246. [PubMed] [Google Scholar]
- 4.ME Curzon, JF Roberts, DB Kennedy. Kennedy’s Pediatric Operative Dentistry, 4th ed. Oxford, UK:Butterworth-Hienemann Publication. 1996:59–64. [Google Scholar]
- 5.AB Fuks, G Holan, JM Davis, E Eidelman. Ferric sulfate versus dilute formocresol in pulpotomized primary molars: long term follow up. Pediatr Dent. 1997;19(5):327–330. [PubMed] [Google Scholar]
- 6.SR King, AG McWhorter, NS Seale. Concentration of formocresol used by pediatric dentists in primary tooth pulpotomy. Pediatr Dent. 2002;24(2):157–159. [PubMed] [Google Scholar]
- 7.RK Yoon, S Chussid, MJ Davis, KC Bruckman. Preferred treatment methods for primary tooth vital pulpotomies. N Y State Dent J. 2008;74(2):47–49. [PubMed] [Google Scholar]
- 8.S Goyal, KR Indushekar. Use of formocresol by the Pediatric Dentists across India- A questionnaire survey. J Evol Med Dent Sci. 2013;2(32):5943–5951. [Google Scholar]
- 9.DJ Wesley, FJ Marshall, S Rosen. The quantitation of formocresol as a root canal medicament. Oral Surg. 1970;29(4):610–612. doi: 10.1016/0030-4220(70)90472-x. [DOI] [PubMed] [Google Scholar]
- 10.AR Milnes. Persuasive Evidence that Formocresol Use in Pediatric Dentistry Is Safe. J Can Dent Assoc. 2006;72(3):247–248. [PubMed] [Google Scholar]
- 11.AP Morawa, LH Straffon, SS Han, RE Corpon. Clinical Evaluation of Pulpotomies using Dilute Formocresol. J Dent Child. 1975;42:360–363. [PubMed] [Google Scholar]
- 12.KC Huth, N Hajek-Al-Khatar, P Wolf, N Ilie, R Hickel, E Paschos. Long- term Effectiveness of four Pulpotomy techniques: 3- year randomised controlled trial. Clin Oral Investig. 2012;16(4):1243–1250. doi: 10.1007/s00784-011-0602-3. [DOI] [PubMed] [Google Scholar]
- 13.AP Erdem, Y Guven, B Balli, B Ilhan, E Sepet, I Ulukapi, O Aktoren. Success rates of mineral trioxide aggregate, ferric sulfate and formocresolpulpotomies: a 24- month study. Pediatr Dent. 2011;33(2):165–170. [PubMed] [Google Scholar]
- 14.PJ Waterhouse. Formocresol and alternative primary molar pulpotomy medicaments: a review. Endod Dent Traumatol. 1995;11:157–162. doi: 10.1111/j.1600-9657.1995.tb00479.x. [DOI] [PubMed] [Google Scholar]
- 15.PJ Loos, SS Han. An enzyme histochemical study of the effect of various concentrations of formocresol on connective tissues. Oral Surg. 1971;31(4):571–585. doi: 10.1016/0030-4220(71)90354-9. [DOI] [PubMed] [Google Scholar]
- 16.JE Berger. Pulp Tissue Reaction to Formocresol and Zinc Oxide –Eugenol. ASDC J Dent Child. 1965;32:13–28. [PubMed] [Google Scholar]
- 17.RE Stewart, TK Barber, KC Troutman, SH Wei. Pediatric Dentistry Scientific foundations and clinical practice. St. Louis:CV Mosby Company. 1982:918–932. [Google Scholar]
- 18.M Rusmah, ZH Rahim. Diffusion of buffered glutaraldehyde and formocresol from pulptomized primary teeth. ASDC J Dent Child. 1992;59(2):108–110. [PubMed] [Google Scholar]
- 19.WG Schafer, MK Hine, BM Levy, CE Tomich. A Textbook of Oral pathology, 4th ed. Philadelphia:WB Saunders. 2001 [Google Scholar]
- 20.O Cortes, J Fernandez, JR Boj, C Canalda. Effect of Formaldehyde on Rat Liver in Doses Used in Pulpotomies. J Clin Pediatr Dent. 2007;31(3):181–184. doi: 10.17796/jcpd.31.3.j87172840r7q3x24. [DOI] [PubMed] [Google Scholar]
- 21.J Kahl, J Easton, G Johnson, J Zuk, S Wilson, J Galinkin. Formocresol Blood levels in Children receiving Dental Treatment under General Anaesthesia. . Pediatr Dent. 2008;30(5):393–399. [PubMed] [Google Scholar]



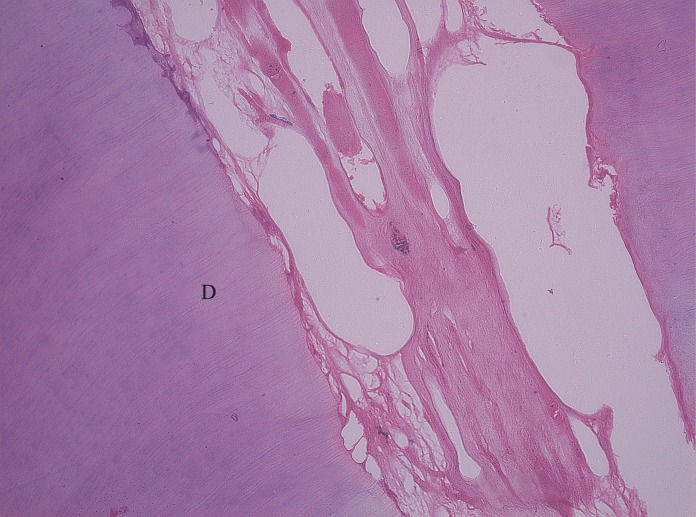
![Figure 11: Pulp tissue reaction with 1:5 diluted
formocresol (Group B) [HandE, 4X] shows fibrocellular stroma with
dilated blood vessels.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/6bd8/4037803/364398b825f2/jioh-06-02-118-g011.jpg)
![Figure 12: Pulp tissue reaction to 1:25 diluted formocresol
(Group C) [HandE, 4X] shows increased number of fibroblasts
(hypercellular stroma) in the stromal tissue.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/6bd8/4037803/8d54295dd22c/jioh-06-02-118-g012.jpg)