Abstract
Background : The aim of the current study is to examine the effect of systemically administered BP-Pamidronate, on Orthodontic Tooth Movement (OTM) along with osteoclastic quantification in New Zealand white rabbits. Materials & Methods : Twenty rabbits used in the study, were equally divided into 2 groups ; Group-1 as Control & Group-2 as Experimental. A sentalloy NITI closed coil spring (GAC International, USA) of 100 gram force, ligated between the lower first molar and the anterior most incisors of the rabbit has served as orthodontic force element. The BP- Pamidronate was administered at the dosage of 1.5 mg/kg body intra-peritonially, on the 1st, 7th and 14th day of the experiment. On the 21st day both group of animals were sacrificed, mandibles were dissected. The formed diastema between the 1st and 2nd molar was measured on the dissected mandibles using standard metric scale, which is considered as the OTM in the mesial direction. Next, the alveolar bone regions along with intact mesial surfaces were processed for histological investigation (osteoclastic count). Results : The student ‘t’ test has been done to compare the mean values of molar tooth movement and osteoclastic count. Parameter :1 molar tooth movement has shown a significant difference between the control (3.750 ± 0.548 mm) and the experimental group (3.050 ± 0.556 mm) with calculated ‘p’ value (p-value <0.05) is significant at 0.0110 level. Parameter : 2 osteoclastic count has shown a significant difference between the control (13.335000 ± 0.735856 per square mm.) and the experimental group (11.426900 ± 1.49369 per square mm) calculated ‘p’ value (p-value <0.05) is significant at 0.003 level. Conclusion : The molar tooth movement and the osteoclastic count were significantly reduced in BP – Pamidronate administered animals than non-drug recipients. How to cite the article: Venkataramana V, Chidambaram S, Reddy BV, Goud EV, Arafath M, Krishnan S. Impact of Bisphosphonate on Orthodontic tooth movement and olsteoclastic count: An Animal Study. J Int Oral Health 2014;6(2):1-8.
Key words: : Bisphosphonate (BP)–Pamidronate, dissected mandibles, Orthodontic Tooth Movement (OTM), osteoclastic count, systemic administration
Introduction
Orthodontic tooth movement (OTM) is a phenomenon that results from constant application of force on a tooth structure. In response to the OF on a tooth structure a series of biological changes takes place in the periodontal ligament (PDL) and alveolar bone with the influence of various cellular and molecular reactions . Till date the exact cellular mechanism involved during OTM is not explained with clarity. It is well documented that remodelling of the alveolar bone is due to inflammatory response in the PDL. Osteoclasts (resorption) and osteoblasts (opposition) are responsible for remodelling process. During OTM various extracellular and intracellular signaling molecules,
chemical mediators and cytokines are responsible for inflammatory reactions. 1 However, it is clear that osteoclastic activity will direct the OTM and be responsible for final treatment outcome. Certain drugs interfere with bone resorption by affecting osteoclastic activity.
Any pharmacological agents / hormones / nutritional supplements administered into human body through various routes, enters the circulation and reaches tissues of PDL, and interrupts the cellular events during OTM, and these agents may exhibit positive effect (promotion of OTM) or negative effect (inhibition of OTM). PTH hormones, 2 prostaglandins 3 (PGs), cytokines, 4 corticosteroids on long term consumption, 5 calcitriol on local administration 3 are considered as promoters of OTM; NSAIDs 6 - 8 [acetyl salicylic acid, diclofenac, indometacin, ibuprofen, rofecoxib acid], leukotriene antagonists (montelukast and zafirlukast), 9 bisphosphonates 10 - 20 etc., are considered as inhibitors of OTM.
Bisphosphonates (BPs)
BPs are a class of drugs prescribed for various skeletal disorders / osteopenic conditions (Paget's disease, osteoporosis, malignancy metastasis to the skeleton, multiple myeloma, osteogenesis inperfecta etc.,) associated with excessive bone resorption. 21 - 22 BPs are synthetic analogues of inorganic pyrophosphates . In pyrophosphate structure oxygen is bonded with phosphates (P-O-P), whereas the oxygen molecule is replaced with carbon atom (P-C-P) in bisphosphonate structure. The P-C-P bondage gives resistant to enzymatic degradation and strong affinity to calcium hydroxy appetite of the bone structure. 23 BPs are classified into two sub types, based on the presence of Nitrogen atom; they are Nitrogenous BPs - N-BPs [ Zoledronate, Alendronate, Pamidronate, Risedronate, Ibandronate etc.,] and Non-Nitrogenous BPs - Non-N BPs [Clodronate, Etidronate, Tiludronate etc.,]. The presence of Nitrogen gives more potency to N-BPs. NBPs acts on osteoclasts by inhibiting protein synthesis and induction of apoptosis by the production isoprenoid compounds (farneyl / pyrophosphate and geranyl / genanyl /pyrophosphate) in mevalonate pathway. 24
In clinical dentistry, patients under BP regime should be tackled carefully when considering for dental procedures (extraction, implant placement, periodontal surgery etc.,) and also orthodontic treatment (extraction therapy, excessive force application etc.,) because, they may be at the risk to develop 'BP related osteonecrosis of the jaw BRONJ, 25 due to the anti-vascular activity of BPs. In such patients conservative procedures like non extraction orthodontic treatment, mild force application, avoiding orthognathic surgeries etc., are advocated. In clinical orthodontics, few cases were reported with impeded OTM, increase relapse tendency, delayed duration of treatment who are currently using BPs. 26
In few animal studies authors have demonstrated the negative impacts of BPs i.e., Inhibition of OTM, depleted osteoclastic count, decreased relapse tendency etc., conversely, some others suggested that these negative impacts can also be utilized in a positive way to prevent selective tooth movement on local administration, which was called as "Pharmacological Anchorage Method". 17 - 20
The purpose of the current animal study is to evaluate the effect of intra peritoneal administration of BPPamidronate on OTM and alveolar bone resorption.
Materials and Methods
Rabbit as Experimental Model:
This animal experimental study was approved by the Institutional Animal Ethical Committee (IAEC) at the medical college, Annamalai University, India. The approved code number 16/99/CPSEA-54. Sixteen weeks old male NewZealand rabbits (Oryctologus cuniculus)
weighing between 3.5 and 4 kg weight (mean weight 3.75 kgs) were selected for this study. All animals were bred at animal house of Annamalai University. Animals were divided into 2 groups i.e., Group 1 served as Control and Group 2 served as experimental. All of them were accommodated in separate metallic cages and maintained under constant room temperature 25 0 c with 40-60 % humidity. They were fed with standard diet in the form of pellets and water. The experiment duration was 21 days (3 weeks).
Experiment Procedure:
Anesthesia
In this study, the anesthetic protocol was pursued under the guidance of veterinarian staff of the animal house.
Total twenty animals (10-control Group 1 and 10-experimental Group 2) received orthodontic appliance on the first day of the experiment under anesthesia. Food was discontinued eight hours prior to the anesthesia. To decrease the vagal tonus, atropine sulphate (0.2 mg/kg
dosage) injection was given to each animal, later ketamine (50 mg/kg body weight) and diazepam (5.0 mg/kg body weight) injections were given. All these drugs were administered through Intra Muscular route- I.M. Animals under anesthesia were installed with orthodontic
appliance.
Preparation of Orthodontic Force Device ( Figure 1 )
Figure 1: Rabbit with appliance.
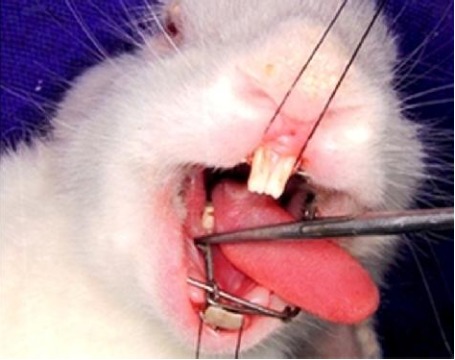
The appliance design in this experiment was closely followed the previous studies. 27 under anesthesia, rabbit's mouth was opened wide using custom made mouth prop, prepared with 19 guage SS wire, in order to get good access to fabricate intraoral appliance preparation easier. In the mandibular arch, grooves were made around the first molar and around the incisors closer to the cervical margins with the help of inverted cone bar in a slow speed hand piece. The grooved areas were etched with 37% phosphoric acid for 30 seconds and rinsed for 15 seconds. The isolation was achieved with chip syringe and suction. Later using a thin artery forceps a ligature wire (0.009"S.S) was passed interdentally between first and second molar and circumferentially wrapped around the first molar till it fits
into the grooved portion; similarly, in the anterior region the ligature wire was wrapped in the figure of eight manner passing between two incisors and fitted into the grooved portion. A NITI sentalloy closed coil spring (GAC International, USA) was used as a force element; it was tied to the ligature wire near first mandibular molar and stretched anteriorly till the 100gm force exertion, measured with the help of electronic force gauge (LT Lutron model 5000A Taiwan) and tied around the incisors. To prevent the appliance displacement, the
grooved portions along with the wrapped ligature wires, were dried and cured with light cure composite materials (3M Transbond XT, Monrovia, California, USA.) around the molar and incisors.
Drug Administration
In this study, the experimental (Group 2) animals following appliance installation, received the drug through intraperitonial route. 12 The rabbit was restrained with the exposure of the upper abdominal portion and head pointed downward. The syringe was loaded with 1.5 mg/ 1 ml BPPamidronate [Trade name – Biodronate 30mg vial, prepared by United Biotech Co., New Delhi, India]. The injection site was wiped with the surgical spirit near the lower right quadrant of the abdominal cavity and the needle was inserted, towards upper direction with 15-20 degree angulation. Optimal care was taken, in order to avoid the puncture of abdominal organs. All animals in Group 2 received the drug in three intervals i.e., 1 st day, 7 th day and 14 th day.
Parameters Undertaken:
Measurement of molar tooth movement
On the 21 st day, animals were anaesthetized as described earlier and sacrificed with 2 ml potassium chloride intra cardiac injection. 28 The mandible of each animal was removed, dissected from the attaching soft tissue and OTM was assessed as a diastema was formed between the first molar and the second molar due to mesial drift of the first molar ( Figure 2 ). The diastema was measured manually with standard metric scale from the mesioocclusal margin of the second molar to the disto-occlusal margin of the first molar. In both groups the measured values of molar tooth movement in the mesial direction are given in Table-1
Figure 2: Dissected mandible with appliance, diastema is visible in between 1st and 2nd molar.
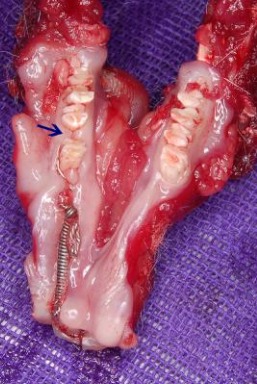
Table 1:Molar tooth movement values measured on dissected mandibles in (millimeters).
| Animals | GROUP – 1 (mm) | GROUP – 2 (mm) |
| 1. | 3.1 | 2.0 |
| 2. | 3.0 | 2.5 |
| 3. | 3.9 | 3.0 |
| 4. | 2.9 | 2.6 |
| 5. | 4.1 | 3.5 |
| 6. | 4.0 | 3.2 |
| 7. | 4.4 | 3.5 |
| 8. | 4.3 | 3.9 |
| 9. | 4.0 | 3.2 |
| 10. | 3.8 | 3.1 |
Histological study
After measuring the molar tooth movement on the dissected mandibles, the bony sections were separated from the mesial aspect of the first molar with intact alveolar bone and stored in 10% formalin; later samples were decalcified with 9% formic acid for an approximate period of one month. The progression of decalcification was observed with micro x-ray.
After decalcification the specimens including mandibular first molar along with alveolar bone (mesial aspect = compression side) were processed and hemi sectioned at coronal, middle third and apical third at root level. Each section was again serially sectioned at 4 to 6 μm in the coronal plane. The sections were mounted on glass microscope slides and stained with hematoxylin and eosin. The Trinocular compound microscope was used with x100 magnification to examine the osteoclastic count. The special attention was made during osteoclastic count in the alveolar bone at two levels/fields i.e., coronal and middle third levels of the roots of the mandibular first molar tooth. The values obtained from these two fields (coronal and middle third) were averaged. Field count was calculated and osteoclast numbers were expressed as per square millimeter. The multinuclear osteoclasts on the stained sections were seen with ruffled border (Figure 3 and 4 ), which were counted by two oral pathologists twice at different times using light microscope ( Table 2 ). The method error was calculated by using Dahlberg’s equation. 18
Figure 3: Control group - [Magnification x100, Hematoxylin and Eosin stain] illustration of multiple osteoclasts (arrow) in the mesial alveolar bony portion of the mandibular molar in Control group.
Figure 4: Experimental group - [Magnification x100, Hematoxylin and Eosin stain] illustration of reduced osteoclasts (arrow) in the mesial alveolar bony portion of the mandibular molar in Experimental group.
Table 2: The significant reduction in the amount of molar tooth movement in Experimental animals (Gr-2) than controls (Gr-1) at *p- 0.0110 level (p-value <0.05).
| Measurements on dissected mandibles | N | Mean | Std. Deviation | t- value | *p- value |
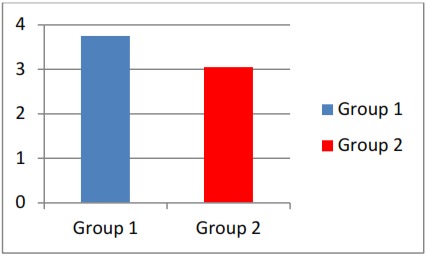
| GROUP - 1 | 10 | 3.750 | 0.548 | 2.8342 | 0.0110(Significant) |
| GROUP - 2 | 10 | 3.050 | 0.556 |
Method error = √Σd2/2n
Where, d=difference between the two measurements and n=number of samples.
Statistical Analysis
The results were reported as mean ± standard deviation. The student (t) test was used to calculate the difference between two means. If the 'p' value is less than 0.05 (p<0.05), it is considered as significant difference between two groups.
Results
The experimental group i.e. drug administered animals (Group 2) have shown significant decreased tooth movement and depleted osteoclastic count when compared to non drug administered controls (Group 1). The amount of molar tooth movement achieved from both
groups, measured on dissected mandibles is given in Table 1 . The mean values of molar tooth movement in both groups were statistically evaluated ( Table 2 , Graph 1 ) and observed the significant difference at 0.0110 level (p < 0.005) between control and experimental groups, which indicates decreased amount of OTM in drug received group.
Graph 1: Comparision of molar tooth movement between two groups.
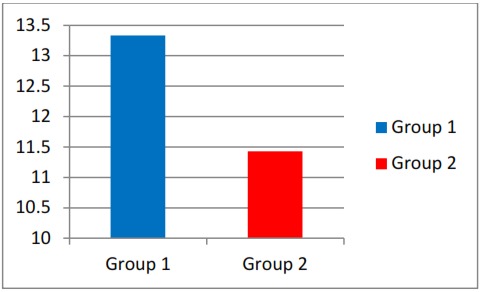
The osteoclastic count values ( Table 3 ) obtained from the mesial aspect of mandibular 1 st molars is given in Table 3 . The mean values of osteoclastic count in both groups were statistically evaluated ( Table 4 , Graph 2 ) and found the significant difference at 0.003 level (p < 0.005) between control and experimental groups, which indicates decreased amount of OTM in drug received group.
Table 3: Osteoclastic count in the compression site - mesial to the molar (per sq mm).
| Animals | GROUP - 1 | GROUP - 2 |
| 1. | 13.764 | 10.8225 |
| 2. | 13.055 | 12.3725 |
| 3. | 14.208 | 10.007 |
| 4. | 13.243 | 9.0065 |
| 5. | 13.726 | 11.4675 |
| 6. | 13.352 | 12.2465 |
| 7. | 11.547 | 12.4125 |
| 8. | 13.92 | 12.1165 |
| 9. | 13.004 | 11.6165 |
| 10. | 13.531 | 12.201 |
Table 4: The significant reduction in the osteoclastic count in Bp administered rabbits (Gr-2) than controls (Gr-1) at *p- 0.0003 level (p-value <0.05).
| Ost.Cl.Count | N | Mean | Std. Deviation | t- value | *p- value |
| GROUP - 1 | 10 | 13.335000 | 0.735856 | 4.4213 | *0.003 (Significant) |
| GROUP - 2 | 10 | 11.426900 | 1.49369 |
Graph 2: Comparision of osteoclatic count between two groups in the mesial aspect of molar.
Discussion
In the present animal study, we evaluated the magnitude of molar tooth movement in the mesial direction along with osteoclastic quantification. The data in this experiment has suggested that the BP – Pamidronate administered animal
group has shown decreased OTM, along with depleted osteoclastic count when compared to non drug administered group.
The reasons for choosing rabbits in this study – easy maintenance, feeding and appliance installation was least cumbersome. To avoid estrous cycle dependant variation during experimental tooth movement only male rabbits were used. The NiTi closed coil springs were used as a force element. 28
In the current world, the orthodontic specialty practice is not only bound to the younger and healthy individuals. In the literature, few clinical and radiographic case studies of orthodontic patients along with BP therapy were reported with widen PDL spaces, sclerotic zones around the teeth, hyper mineralized alveolar bone etc., all these changes might cause delayed treatment time, decelerated tooth movement, difficulty in extraction space closure, poor root parallelism, tooth mobility etc., these deleterious effects might have occurred be due to the expression of the antiresorptive activity / anti-osteoclastic activity in the jawbones of BP users, who underwent orthodontic
treatment. 26 In order to substantiate the findings of the previous case reports, in the current study, we have evaluated the outcome of OTM and anti-osteoclastic effect in rabbits treated with BP.
In the current animal study, the OTM was initiated by the NiTi closed coil spring with constant force of 100gm; thus the alveolar bone resorption (osteoclastic activity) would've evoked in the mesial (pressure side) aspect of the mandibular first molar. But, in the BP administered animals, the anti-osteoclastic activity (anti-resorptive activity) would be due to increased osteoclastic apoptosis thereby programmed cell death, inhibition of osteoclastic recruitment and restriction of osteoblast-mediated osteoclastic resorption 29 , 30 might have occurred in the mesial aspect of mandibular molar. Thus, the tooth movement would've inhibited, which was apparently evident on the dissected mandibles.
In the current animal study, BP-Pamidronate was administered systemically and its effect on OTM was evaluated; which is similar to previous BP systemic administered animal studies 10 - 16 – [Shetty et al (2007), Keles et al (2007), Shiefi and Aggachi et al (2009), Jeremy et al (2009), Yuji Fujimura et al (2009), hoi.J et al (2009), Sirisoontorn et al (2012) and Megami Heshimato et al (2013)].
In a short term study the effects of systemically administered BP during experimental tooth movement in New Zealand rabbits by Shetty et al, 10 and noticed significant retention (prevention of relapse /reduced tooth movement) was encountered. In our study also significant inhibition of tooth movement was noticed in BP received animals.
In a BP administered mice model, the inhibited tooth movement and reduced osteoclastic count was encountered by Keles et al., 11 They also explained that the mice, which received BP have shown minimal apoptosis of osteoclasts and reduced tooth movement under constant orthodontic force. Similarly, in the current study also reduced osteoclastic count and decelerated tooth movement was noticed.
The effect of BP (pamidronate) in intra peritoneal administered rats, was investigated by Siefi and Aghaeei, 12 and examined the inhibition of tooth movement on dissected upper jaw and resorptive lacunae were counted and found no increasing in the rate of root resorption. In our experiment, inhibited tooth movement was recorded on dissected mandibles and osteolastic quantification has been done in the alveolar bone mesial to the rabbit's molar after intra peritoneal injection.
Jermy et al, 13 in their experiment BP (Alendronate) in Sprague Dawley ras, and reported an inhibition of tooth movement based on die-stone models of rats jaws / teeth, which may be considered as indirect method. In our study, the direct measurements were taken on dissected mandibles of rabbits.
Choi J et al, 14 demonstrated the decreased OTM and increased duration of the treatment based on the histo morphometric analysis, done in rats after BP-clodronate administration. In the current study also, similar reduction in the OTM was demonstrated in BP received rabbits.
The effect of BP- Zolendronate in overectomized Wister rats was studied by Sirisoontorn et al 15 and found impeded tooth movement and Orthodontically Induced Root Resorption (OIRR). Similiarly, based on two parameters our study also demonstrated alveolar bone resorption (osteoclastic count) and inhibited tooth movement in BPtreated animals.
Megumi Hashimoto et al, 16 have noticed the significant reduction of OTM in rats, administered with BP (Zolendronate) and also stated that there is a significant negative correlation between the bone mass and OTM. In the current study, the reduction in the osteoclastic count (anti resorptive action) was noticed in the mesial aspect of molars in BP administered rabbits; thus to some extent the intact part of the alveolar bone might have aided in tooth movement inhibition.
Apart from above studies, some authors have investigated the effects of local administered BPs during OTM in animals. 18 - 21 In all studies decelerated tooth movement or reduced relapse were noticed with the histological evidence (osteoclastic quantification). The authors also suggest that the local administered BPs may be utilized to inhibit the specific tooth / teeth movement and aid to generate "Pharmacological Anchorage effect". 18 But, unfortunately till now this concept is limited to animal models only. 17 , 19 , 20
Conclusion
This study was performed to elucidate the effect of systemically administered BP-Pamidronate on osteoclastic activity during OTM in rabbits. In this study, BP was administered in three intervals on the 1 st , 7 th and 21 st day. In BP administered animals, the molar tooth movement was inhibited significantly and lesser amount of osteoclasts appeared along the alveolar bone surfaces towards the PDL, could be due to structural impairment and resorptive activity of osteoclasts.
The clinical implication of this study is mainly to consider the patients under orthodontic therapy along with BP medication, the orthodontist must be cautious and also the risk possibilities like delayed OTM, compromised treatment, discontinuation of treatment etc., should be explained to the patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None
Contributor Information
V Venkataramana, Department of Orthodontics & Dento-facial Orthopaedics, Panineeya Mahavidhyalaya Institute of Dental Sciences, Kamalanagar, Dilshuknagar, Hyderabad, Andhra Pradesh, India.
S Chidambaram, Department of Orthodontics & Dento-facial Orthopaedics, Rajah Muthiah College of Dental Sciences, Annamalai University, Chidambaram, Tamil Nadu, India.
B Vishnuvardhan Reddy, Department of Orthodontics, G Pulla Reddy Dental College & Hospital, G Pulla Reddy Nagar, Kurnool, Andhra Pradesh, India.
E V Soma Shekara Goud, Department of Oral & Maxillofacial Pathology, Chandra Dental College, Uttar Pradesh, India.
Mohammed Arafath, Department of Orthodontics & Dentofacial Orthopedics, Rajah Muthiah College of Dental Sciences, Annamalai University, Chidambaram, Tamil Nadu, India.
Santhana Krishnan, Division of Orthodontics & Dento-facial Orthopedics, Rajah Muthiah College of Dental Sciences, Annamalai University, Chidambaram, Tamil Nadu, India.
References
- 1.V Krishnan, Z Davidovitch. Cellular, molecular, and tissue-level reactions to orthodontic force. Am J Orthod Dentofacial Orthop. 2006;129(4):469–469. doi: 10.1016/j.ajodo.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 2.S Soma, M Iwamoto, Y Higuchi, K Kurisu. Effects of continuous infusion of PTH on experimental tooth movement in rats. J Bone Miner Res. 1999;14:546–554. doi: 10.1359/jbmr.1999.14.4.546. [DOI] [PubMed] [Google Scholar]
- 3.S Kale, I Kocadereli, P Atilla, E Asan. Comparison of the effects of 1,25 dihydroxycholecalciferol and prostaglandin E2 on orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 2004;125:607–614. doi: 10.1016/j.ajodo.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 4.LR Iwasaki, LD Crouch, A Tutor, S Gibson, N Hukmani, DB Marx, JC Nickel. Tooth movement and cytokines in gingival crevicular fluid and whole blood in growing and adult subjects Am J Orthod Dentofacial Orthop. 2005;128:483–491. doi: 10.1016/j.ajodo.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 5.MB Ashcraft, KA Southard, EA Tolley. The effect of corticosteroid- induced osteoporosis on orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 1992;102:310–319. doi: 10.1016/0889-5406(92)70046-D. [DOI] [PubMed] [Google Scholar]
- 6.OR Arias, MC Marquez-Orozco. Aspirin, acetaminophen, and ibuprofen: their effects on orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 2006;130:364–370. doi: 10.1016/j.ajodo.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 7.F de Carlos, J Cobo, B Diaz-Esnal, J Arguelles, M Vijande, M Costales. Orthodontic tooth movement after inhibition ofcyclooxygenase-2. Am J Orthod Dentofacial Orthop. 2006;129:402–406. doi: 10.1016/j.ajodo.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 8.AB Chumbley, OC Tuncay. The effect of indometacin (an aspirin-like drug) on the rate of orthodontic tooth movement. Am J Orthod. 1986;89:312–314. doi: 10.1016/0002-9416(86)90053-9. [DOI] [PubMed] [Google Scholar]
- 9.AH Mohammed, DN Tatakis, R Dziak. Leukotrienes in orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 1989;95:231–237. doi: 10.1016/0889-5406(89)90053-x. [DOI] [PubMed] [Google Scholar]
- 10.S Shetty, S Mogra, S Shetty. The effect of the short term administration of a Bisphosphonate upon dental relapse. J Indian Orthod Soc. 2006;39:198–203. [Google Scholar]
- 11.A Keles, B Grunes, C DiFuria, E Gagari, V Srinivasan, MA Darendeliler, al et. Inhibition of tooth movement by osteoprotegerin v/s pamidronate under conditions of constant orthodontic force. Eur J Oral Sci. 2007;115:131–136. doi: 10.1111/j.1600-0722.2007.00433.x. [DOI] [PubMed] [Google Scholar]
- 12.M Seifi, NA Pour. Effect of Pamidronate on tooth movement and root resorption in rat. J Dent Sch. 2009;27(2):67–71. [Google Scholar]
- 13.JC Karras, JR Miller, JS Hodges, JP Beyer, BE Larson. Effect of alendronate on orthodontic tooth movement in rats. Am J Orthod Dentofacial Orthop. 2009;136:843–847. doi: 10.1016/j.ajodo.2007.11.035. [DOI] [PubMed] [Google Scholar]
- 14.J Choi, SH Baek, JI Lee, YI Chang. Effects of clodronate on early alveolar bone remodeling and root resorption related to orthodonticforces: a histomorphometric analysis. Am J Orthod Dentofacial Orthop. 2010;138(5):548–548. doi: 10.1016/j.ajodo.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 15.I Sirisoontorn, H Hotokezaka, M Hashimoto, C Gonzales, S Luppanapornlarp, MA Darendeliler, N Yoshida. Orthodontic tooth movement and root resorption in ovariectomized rats treated by systemic administration of zoledronic acid. Am J Orthod Dentofacial Orthop. 2012;141(5):563–573. doi: 10.1016/j.ajodo.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 16.M Hashimoto, H Hotokezaka, I Sirisoontorn, T Nakano, K Arita, M Tanaka, N Yoshida. The effect of bone morphometric changes on orthodontic tooth movement in an osteoporotic animal model. Angle Orthod. 2013;83(5):766–773. doi: 10.2319/111312-869.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.K Igarashi, H Mitani, H Adachi, H Shinoda. Anchorage and retentive effects of a bisphosphonate (AHBuBP) on tooth movement in rats. Am J Orthod Dentofacial Orthop. 1994;106:279–289. doi: 10.1016/S0889-5406(94)70048-6. [DOI] [PubMed] [Google Scholar]
- 18.H Adachi, K Igarashi, H Mitani, J Shinoda. Effects of Topical Administration of a Bisphosphonate (Risedronate) on Orthodontic Tooth movement. J Dent Res. 1994;73:1478–1486. doi: 10.1177/00220345940730081301. [DOI] [PubMed] [Google Scholar]
- 19.L Liu, I Kaoru, N Haruyama, S Saeki, H Shinoda, H Mitani. Effects of local administration of clodronate on orthodontic tooth movement and root resorption in rats. Eur J Orthod. 2004;26:469–473. doi: 10.1093/ejo/26.5.469. [DOI] [PubMed] [Google Scholar]
- 20.V Venkataramana, K Rajasigamani, N Madhavan, SN Reddy, K Karthik, N Kumaran. Inhibitory effect of Bisphosphonate (Pamidronate) on orthodontic tooth movement in New Zealand albino rabbits. J Int Dent Med Res. 2012;5(3):136–142. [Google Scholar]
- 21.JG Seedor, HA Quartuccio, DD Thompson. The bisphosphonate alendronate (MK-217) inhibits bone loss due to ovariectomy in rats. J Bone Miner Res. 1991;6:339–346. doi: 10.1002/jbmr.5650060405. [DOI] [PubMed] [Google Scholar]
- 22.A Licata. Discovery, clinical development and therapeutic uses of bisphosphonates. Ann Pharmacother. 2005;39:668–677. doi: 10.1345/aph.1E357. [DOI] [PubMed] [Google Scholar]
- 23.GA Rodan, AA Reszka. Bisphosphonate Mechanism of Action. Curr Mol Med. 2002;2:571–577. doi: 10.2174/1566524023362104. [DOI] [PubMed] [Google Scholar]
- 24.AA Reszka, GA Rodan. Nitrogen-containing bisphosphonate mechanism of action. Mini Rev Med Chem. 2004;4:711–719. [PubMed] [Google Scholar]
- 25.Affairs ADA Council on Scientific. Dental management of patients receiving oral bisphosphonate therapy: Expert panel recommendations. J Clin Endocrinol Metab. 2005;90:1897–1899. [Google Scholar]
- 26.JJ Zahrowski. Bisphosphonate treatment: an orthodontic concern calling for a proactive approach. Am J Orthod Dentofacial Orthop. 2007;131:311–320. doi: 10.1016/j.ajodo.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 27.J Yu, W Lee, JH Park, M Bayome, Y Kim, Y Kook. Histologic effects of intentional-socket-assisted orthodontic movement in rabbits. Korean J Orthod. 2012;42(4):207–217. doi: 10.4041/kjod.2012.42.4.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MA Matos, U Tannuri, R Guarniero. The effect of zoledronate during bone healing. J Orthop Traumatol. 2010;11(1):7–12. doi: 10.1007/s10195-010-0083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.RG Russell, MJ Rogers, JC Frith, SP Luckman, FP Coxon, HL Benford, PI Croucher, C Shipman, HA Fleisch. The pharmacology og bisphosphonates and new insights into their mechanisms of action. J Bone Miner Res. 1999;14 Suppl2:53–65. doi: 10.1002/jbmr.5650140212. [DOI] [PubMed] [Google Scholar]
- 30.MJ Rogers, DJ Watts, RG Russell. Overview of bisphosphonates. Cancer. 1997;80 Suppl8:1652–1660. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1652::aid-cncr15>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]



![Figure 3: Control group - [Magnification x100, Hematoxylin
and Eosin stain] illustration of multiple osteoclasts (arrow) in
the mesial alveolar bony portion of the mandibular molar in
Control group.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/6e4a/4037805/d59e6350ca57/jioh-06-02-001-g003.jpg)
![Figure 4: Experimental group - [Magnification x100,
Hematoxylin and Eosin stain] illustration of reduced osteoclasts
(arrow) in the mesial alveolar bony portion of the mandibular
molar in Experimental group.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/6e4a/4037805/b69831ffd20c/jioh-06-02-001-g004.jpg)