Abstract
Before starting a smoking cessation treatment, 51 smokers took part in a study aimed at investigating brain mechanisms associated with attention allocation. ERPs to acoustic startle probes were recorded from 129 sensors during the presentation of neutral, pleasant, unpleasant, and cigarette-related pictures. Results indicated that the amplitude of the startle probe P3 component was reduced for pleasant, unpleasant, and cigarette-related conditions relative to neutral. Surface Laplacian estimates showed that sources of electrocortical activity under frontal and parietal sensors contributed to the modulation of this effect. For smokers, cigarette-related stimuli, like intrinsically motivating ones, capture attentional resources and therefore reduce the ability to process competing stimuli. The depletion of attentional resources in the presence of cigarette-related cues may contribute to the high relapse rate observed during smoking quit attempts.
Keywords: Smoking, Attention, ERP, P3, Startle, Emotion
Introduction
Many theories of drug addiction attribute great importance to the role of drug-related cues with respect to inducing craving and triggering relapse in addicted individuals (Baker, Piper, McCarthy, Majeskie, & Fiore, 2004; Everitt, Dickinson, & Robbins, 2001; Hyman, 2005; Robinson & Berridge, 2001; Robinson & Berridge, 2003). In particular, the incentive-sensitization theory (Robinson & Berridge, 2001; Robinson & Berridge, 2003) posits that addictive drugs alter neural systems in the mesolimbic cortex that mediate the attribution of incentive salience to stimuli. Under normal circumstances, these brain systems direct appetitive and defensive reactions aimed at promoting the survival of the individual. Visual cues depicting motivationally relevant situations engage these systems allowing the individual to orient attentional resources and enhance processing of potential sources of reward or threat (Lang, Bradley, & Cuthbert, 1997). The neural sensitization induced by drugs of abuse is posited to lead to excessive attribution of incentive salience to otherwise neutral drug-related cues. Increased salience allows drug-related cues to “grab” attention, and it is associated with the “wanting” aspect of drug dependence. This hypothesis is supported by findings that presenting drug-related stimuli to drug-dependent individuals increases activation in brain circuits normally involved in learning, memory, motivation, reward, and attention; namely the hippocampus, the amygdala, the ventral striatum, the prefrontal cortex, and the anterior cingulate cortex (Berridge & Robinson, 1998; David et al., 2005; David et al., 2007; Di Chiara, 1999; Hyman, 2005; Robbins & Everitt, 1996). Orienting attentional resources towards drug-related stimuli is thought to limit the ability to consider alternatives to the drug seeking behavior and to increase the probability of relapse during quitting attempts (Curtin, McCarthy, Piper, & Baker, 2006). Several studies used a modified Stroop test and found that compared to abstinent smokers (Johnsen, Thayer, Laberg, & Asbjornsen, 1997) and non-smokers (Munafo, Mogg, Roberts, Bradley, & Murphy, 2003), current smokers showed slower reaction time to cigarette-related words, indicating that current smokers may have an attentional bias towards cigarette-related words. Furthermore, short-term smoke deprivation increased current smokers’ attentional bias, hence their reaction times, to cigarette-related words (Mogg & Bradley, 2002; Waters & Feyerabend, 2000). Not only do current smokers show an attentional bias towards cigarette-related texts, they pay more attention to cigarette-related pictures than to neutral pictures. In two studies that used a visual probe task, current smokers reacted significantly faster to a probe when the probe replaced a cigarette-related picture than when the probe replaced a neutral picture (Mogg & Bradley, 2002; Waters, Shiffman, Bradley, & Mogg, 2003). These findings suggest cigarette-related cues are motivationally salient for and capture the attention of current smokers.
To investigate the neural mechanisms associated with the distribution of attentional resources when cigarette-related cues are present, we recorded, from a dense sensor array (129 channels), the event-related potentials (ERPs) to startle probes when smokers looked at pleasant, unpleasant, cigarette-related, and neutral pictures. The startle probe is a sudden burst of intense noise that elicits a reflexive startle response modulated by hedonic valence (Cinciripini et al., 2006; Lang, Bradley, & Cuthbert, 1990), and an ERP modulated by emotional arousal. Several studies have shown that the P3 component (a positive-going waveform in the ERPs) evoked by startle probes is reduced when viewing motivationally relevant pleasant or unpleasant vs. neutral images (Bradley, Codispoti, & Lang, 2006; Cuthbert, Schupp, Bradley, McManis, & Lang, 1998; Keil et al., 2007; Schupp, Cuthbert, Bradley, Birbaumer, & Lang, 1997). The reduction of the P3 amplitude has been attributed to the diminished attentional resources available to process the startle probe. When a motivationally relevant stimulus is presented, it captures the person’s attention and reduces the attentional resources available to process the competing startle probe stimulus, in turn reducing the P3 (Lang et al., 1997). Source estimation analyses attributed the modulation of the startle probe P3 to activity localized in frontocentral and temporoparietal areas (Keil et al., 2007).
Cigarette-related cues are hypothesized to capture the attentional resources of smokers by exploiting the same mechanisms that allow motivationally relevant stimuli to receive prioritized processing. Hence, measuring the startle probe P3 amplitude during picture viewing might provide information about how the brain of a smoker prioritizes allocation of attentional resources in the presence of cigarette-related cues.
In this study, we collected the EEG from a dense-electrode array net (129 channels) in a group of smokers interested in quitting, but prior to the onset of treatment. Smokers were asked to view cigarette-related, pleasant, unpleasant, and neutral images during which startle probes were delivered. We measured the amplitude of the P3 evoked by the startle probes as a function of picture category. This provides an index of the attentional resources available to process the probe during the presentation of the visual stimuli.
We hypothesized that cigarette-related cues, like other motivationally relevant stimuli, would reduce the attentional resources available to process the startle probe, as indicated by a significant reduction of the P3 amplitude relative to the condition when neutral stimuli were presented. Such findings would be consistent with the notion that cigarette-related cues acquire motivational significance to a smoker on a level similar to that observed for emotional stimuli, suggesting that, for a smoker, the presence of cigarette-related cues in the environment can alter ongoing cognitive function associated with information processing. We also hypothesized that the reduction of the P3 amplitude observed during the presentation of cigarette-related images would be mediated by the same cortical sources, as measured by the surface Laplacian estimates, that modulate the probe P3 amplitude when intrinsically motivating images (i.e., pleasant and unpleasant) are presented.
Materials and Methods
Participants
Participants for this study were recruited via local (Houston metropolitan area) radio and newspapers ads asking for volunteers who wanted to quit smoking and were willing to participate in a smoking cessation medication clinical trial. Following screening for basic inclusion/exclusion criteria, eligible participants completed the baseline laboratory session of the study as described in this manuscript. This session was conducted prior to any treatment randomization or intervention. Two additional laboratory sessions were conducted after treatment randomization at post-quit follow-up points. The data from these laboratory sessions, as well as the outcome of the clinical trial, will be the subject of other papers. In this study, only the results of the baseline session are discussed.
A total of 87 participants were screened for this study, with 56 meeting basic inclusion/exclusion criteria. Inclusion criteria were as follows: Being 18–65 years old, smoking 10 or more cigarettes per day, producing an expired CO greater than 10 ppm at the baseline visit, being fluent in English, and having a working telephone. Exclusion criteria included currently taking psychotropic medication, having a current psychiatric disorder, having a current substance abuse disorder except smoking, being involved in any current smoking cessation activities, having contraindications for bupropion (e.g., history of seizure disorder) or nortriptyline (e.g., alcohol dependence), or having an uncontrolled medical illness. Because of poor recording quality or technical errors, laboratory data for five participants were discarded, yielding a total of 51 participants in this study (33 men, 18 women). All participants provided informed consent and the research was approved by the University of Texas M. D. Anderson Cancer Center IRB.
Material and design
Three equivalent picture sets were created using pictures selected from the IAPS (Lang, Bradley, & Cuthbert, 2005) and from smoking-related picture collections previously used in our laboratory (Carter et al., 2006) and others (Gilbert & Rabinovich, 1999). Each set included 4 categories (pleasant, unpleasant, neutral, and cigarette-related) with each category consisting of 9 slides (36 total slides). Pleasant and Unpleasant slides were matched on emotional arousal1. Participants viewed one set during each session and the order of presentation of the sets was randomized across participants. During the slide presentation, images were presented in pseudo random sequences with no more than two pictures of the same valence presented consecutively. Each picture was presented for 6 s and was followed by a random intertrial interval of 10–16 s. During each session, 6 of the 9 slides of each picture type were probed by presenting a burst of 100 dB white noise for 50 ms between 3 and 5 s after picture onset. Probes were also presented in 1/3 of the intertrial intervals to reduce predictability. Stimuli were presented with a Pentium 4 computer using Psychology Tools’ E-prime software (Pittsburgh, PA) on a plasma monitor placed approximately 1.5 m from the participant's eyes. The images subtended approximately a 24° horizontal viewing angle.
Procedure
Participants were instructed to smoke normally prior to the laboratory session so as to be in a non-deprived state. Upon arrival at the laboratory the participants completed the Fagerström Test for Nicotine Dependence (Heatherton, Kozlowski, Frecker, & Fagerström, 1991) and provided an expired carbon monoxide (CO) sample (other data not reported here were also collected). After completing the questionnaires, participants were escorted into the laboratory and sensors were applied. The slide presentation started after a 15 minute resting period.
During the slide presentation sequence, EEG was recorded using a 129-channel Geodesic Sensor Net and amplified with an AC-coupled high input impedance (200 MΩ) amplifier (Geodesic EEG System 200; Electrical Geodesics Inc., Eugene, OR) and referenced to Cz. Sampling rate was 250 Hz and data were filtered online using 0.1 Hz high-pass and 100 Hz low-pass filters. Scalp impedance of each sensor was kept below 50 KΩ, as suggested by the manufacturer.
Data Analyses and Reduction
Following data collection, a 30-Hz low pass filter was applied off line, data were visually inspected and channels consistently contaminated during the recording were interpolated using spherical splines. No participant had more than 5 channels interpolated. After this first step the EEG data were transformed to the average reference (necessary for accurate topographic mapping and topographic waveform plots). Eye blinks were then corrected using a spatial filtering method as implemented in BESA 5.1.8.10 (MEGIS Software GmbH, Gräfelfing, Germany) and the data were segmented in 900 ms segments starting 100 ms before the onset of the startle probe. Baseline was defined as the 100 ms interval preceding the startle probe. Using the segmented data, artifacts contaminating sensors within specific trials were identified. A sensor was marked as bad if amplitude was above 200 or below −200 µV, if the absolute difference between two data points was above 100 µV, or if the difference between two contiguous data points was above 25 µV. If, within each segment, more than 10 % of the sensors were contaminated, the segment was rejected. Overall fewer than 5% of the segments were rejected.
At the end of this process average event related potentials (ERP) were calculated at each scalp site for each condition (i.e. cigarette-related, pleasant, unpleasant, and neutral pictures). To identify latencies of components where differences among conditions were maximal, the global field power (Hamburger & vd Burgt, 1991) was calculated for each condition for each subject and the significance of the differences between each of the emotional conditions (i.e., pleasant, unpleasant, and cigarette-related) and the neutral condition was calculated at each data point. A 100-ms window was centered on the peak of the component isolated from the global field power calculations. Within this window the mean voltage at each sensor was determined for each condition.
The presence of significant differences between each of the motivationally significant conditions (pleasant, unpleasant, cigarette-related) and neutral was calculated on the whole voltage topographies using a randomization procedure (Maris, 2004). This procedure provides a means for adjusting p-values of multiple comparisons from the 129 electrode configuration. The randomization procedure involves two steps: (a) computing a statistic (in this case an F statistic) for each sensor, and (b) evaluating its p-value under the randomization distribution. The randomization distribution is built by first randomly assigning to different data vectors the data matrix obtained for each participant within each experimental condition. The statistic of interest is then calculated for each sensor and the highest value is stored. This process is repeated 10000 times to form a distribution for the F statistic associated with the hypothesis of interest. Following the construction of these randomly generated data vectors, the F-statistic is computed at each sensor using the actual data as obtained from the experiment. If the value of the F-statistic obtained during actual hypothesis testing exceeds the F-value marking the upper 5% of the distribution obtained from the random iterations, it is considered significant at the .05 level.
To improve the spatial resolution of the EEG, the surface Laplacian method was applied to the voltage data. The surface Laplacian is an estimate of current density entering or exiting the scalp through the skull and isolates the aspects of the EEG attributable to sources that are spatially localized in superficial areas of the cortex (Srinivasan, 2005). The statistical significance of the differences among conditions was tested on the whole topographies using the randomization procedure described above.
Results
Participant Characteristics
As can be seen in Table 1, the participants in this study were primarily white men in their 40’s who smoked about a pack a day for an average of 25 years. They were moderate to high in nicotine dependence as measured by the Fagerström Test for Nicotine Dependence. Expired CO values all averaged over 10, indicating that all participants were current smokers.
Table 1.
Baseline demographic and smoking characteristics of the sample (n=51).
| Characteristic/Measure | Percentage | (n) |
|---|---|---|
| Men | 64.7 | (33) |
| White, non-hispanic | 80.4 | (41) |
| Right-handed | 76.5 | (39) |
| Mean | (SD) | |
| Age | 44.4 | (9.87) |
| BMI | 29.3 | (7.01) |
| Cigs/day | 21.3 | (11.41) |
| CO | 23.2 | (8.54) |
| FTND | 4.9 | (2.21) |
| Years of Smoking | 25.0 | (10.96) |
Note: Handedness was measured by the Chapman Handedness Questionnaire and Cigs/day by the Fagerström Test for Nicotine Dependence (FTND).
Scalp Potential
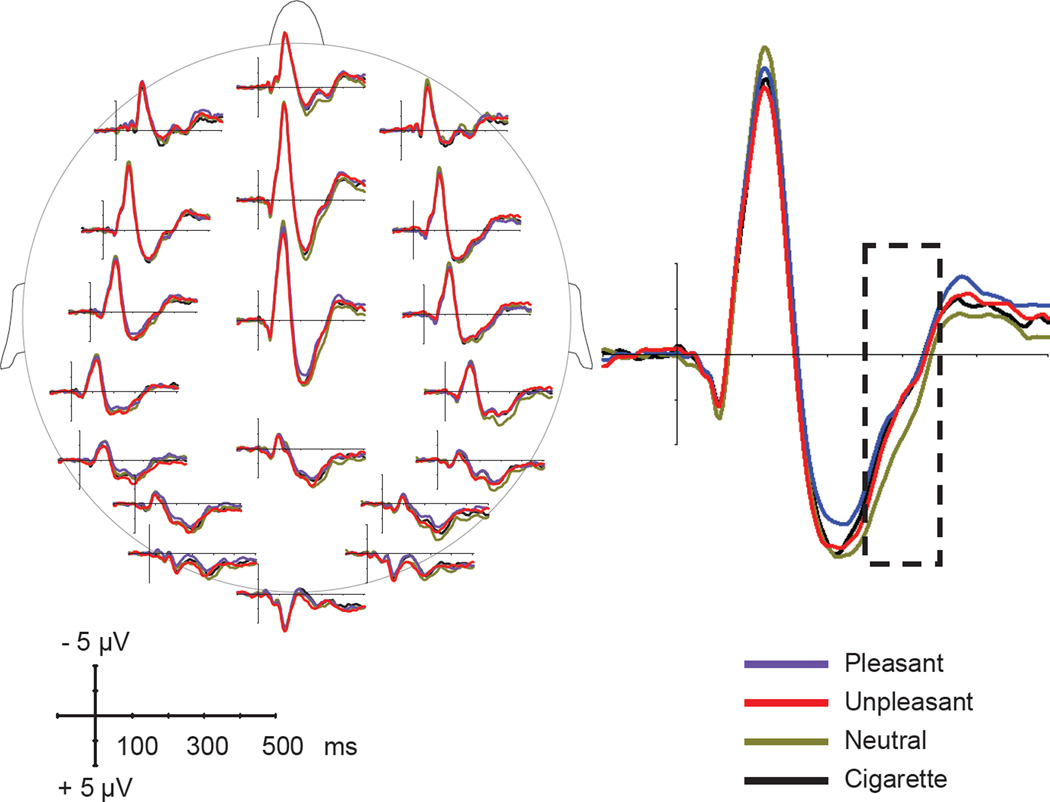
The ERPs to the startle probes delivered during presentation of pleasant, neutral, unpleasant, and cigarette-related pictures are shown in Figure 1.
Figure 1.
Left: Event-related potentials (at selected locations) in response to startle probes presented during viewing of cigarette-related, neutral, pleasant, and unpleasant pictures. Right: ERPs to startle probes at electrode Cz. The dotted line indicates the time window used for statistical analyses.
Consistent with previous studies (Cuthbert et al., 1998; Keil et al., 2007; Schupp et al., 1997), startle probes elicited late positivity in the P3 range and the amplitude of this component was modulated by the content of the picture being viewed by the smoker when the startle probe was delivered. The statistical analyses performed on the global field power showed that startle probes delivered during the neutral condition resulted in higher levels of overall EEG activity than the emotional and cigarette related conditions and this difference was maximal in a time window between 250 and 350 ms after probe onset. No other component in the ERPs to the startle probe showed differential modulation as a function of the visual content.
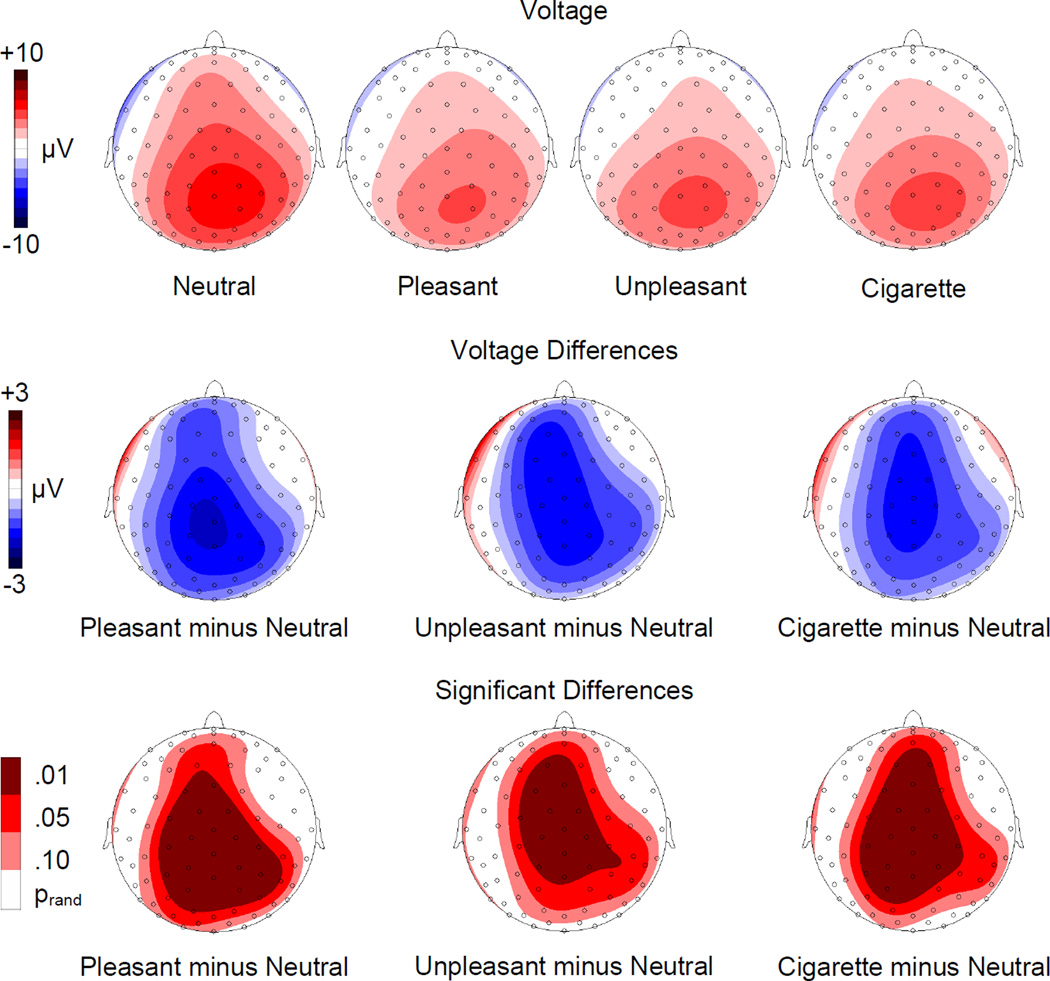
When the average voltage at each scalp electrode during the 250–350 ms time window was analyzed all emotional conditions showed a significant reduction of the probe P3 amplitude over central, parietal and frontal areas (see Figure 2). The analysis of the scalp topographies led to comparable results in all three emotional conditions.
Figure 2.
Top row: Topographic distribution of the voltages for startle probe P3 (250–350 ms) during presentation of neutral, pleasant, unpleasant, and cigarette-related pictures. Middle row: Topographic distribution of the voltage differences (motivationally significant minus neutral). Bottom row: Topographic distribution of the statistically significant differences.
The results observed for the pleasant and unpleasant conditions confirm previous studies and can be attributed to a larger amount of attentional resources being captured by the emotional stimuli. In keeping with our hypothesis, a significant reduction of the probe P3 component relative to the neutral condition was also observed when cigarette-related cues were presented. The effect observed in the cigarette-related condition is comparable to the modulation of the P3 produced by viewing other emotionally evocative stimuli (pleasant and unpleasant pictures): None of the contrasts comparing the three motivationally significant conditions (i.e. cigarette-related, pleasant, and unpleasant) was significant. Our results show that for smokers, cigarette cues capture attentional resources similarly to other inherently motivationally relevant stimuli.
Surface Laplacian
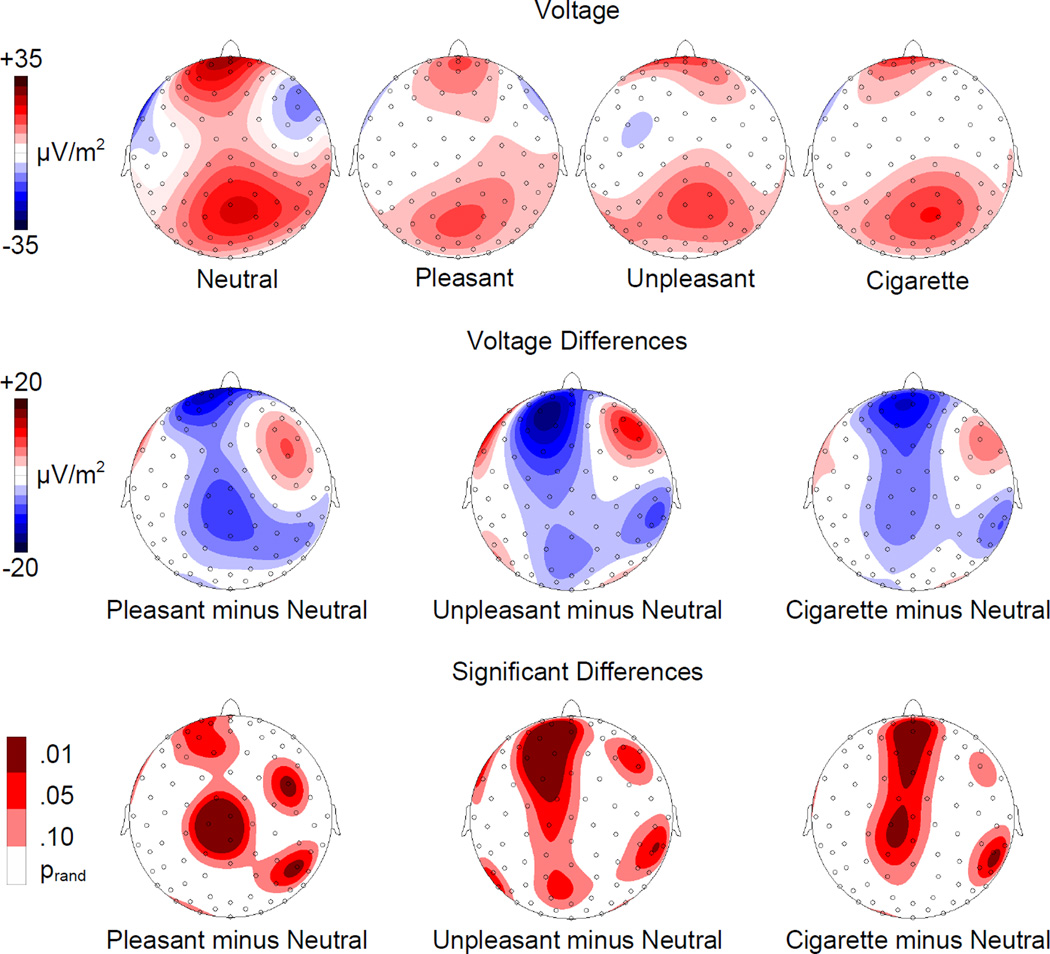
The surface Laplacian estimates showed two main sources of activation localized under parietal and frontal sensors, across all conditions (see Figure 3). The statistical analyses revealed a significantly reduced activation of the frontal electrocortical sources when cigarette-related, pleasant, or unpleasant vs. neutral images were present at the moment of the startle probe delivery. In addition to these frontal sources, significant differences between motivationally significant (i.e., cigarette-related, pleasant, and unpleasant) and neutral images were associated with central and right parietal sources of activation (see Figure 3). When the three motivationally significant conditions were compared, none of the contrasts was significant.
Figure 3.
Top row: Topographic distribution of the Surface Laplacian Estimates for startle probe P3 (250–350 ms) during presentation of neutral, pleasant, unpleasant, and cigarette-related pictures. Middle row: Topographic distribution of the voltage differences (motivationally significant minus neutral). Bottom row: Topographic distribution of the statistically significant differences.
Discussion
This study used the amplitude of the P3 ERP component evoked by an acoustic startle probe to investigate neural mechanisms associated with the distribution of attentional resources when smokers are exposed to motivationally relevant stimuli. The results show that cigarette-related images, similar to both pleasant and unpleasant images, capture attentional resources and reduce the smoker’s ability to process other competing stimuli. The amplitude of the P3 component elicited by the startle probe in the presence of pleasant, unpleasant, or cigarette-related pictures was significantly reduced compared to that observed for neutral pictures. The surface Laplacian estimates suggest that sources of electrocortical activity under frontocentral and parietal sensors mediated this effect.
Cigarette-related cues are hypothesized to capture smokers’ attentional resources by exploiting the same brain mechanisms that allow motivationally relevant stimuli to receive prioritized processing (Baker et al., 2004; Everitt et al., 2001; Hyman, 2005; Robinson & Berridge, 2001; Robinson & Berridge, 2003). Hence, an important feature of our experimental design involves the inclusion of intrinsically pleasant and unpleasant stimuli in addition to the cigarette-related and neutral ones. Previous studies conducted with healthy individuals showed that, relative to neutral stimuli, emotional stimuli automatically engage neural processing systems associated with approach and avoidance motivation, and capture a larger amount of attentional resources (Cuthbert et al., 1998; Keil et al., 2007; Schupp et al., 1997). Thus, fewer resources may be available to process concurrently presented competing stimuli, in this case the startle probe, resulting in a reduction of the elicited probe P3 amplitude. Our results are consistent with this observation and extend the findings to another category of motivationally relevant stimuli, cigarette cues, for a group of current smokers. Although the lack of a non-smokers control group does not allow us to exclude that even non-addicted individuals might have found cigarette-related cues motivationally significant, we think that this possibility is unlikely given that drug-related and neutral cues lead to similar brain activation in non-addicted individuals in previous studies (Due, Huettel, Hall, & Rubin, 2002; Wolfling, Flor, & Grusser, 2008). Future studies comparing ERPs to emotional, cigarette-related, and neutral cues in smokers and non-smokers will provide further data about this issue.
The findings from the surface Laplacian analysis that neural sources of activation associated with the emotionally relevant stimuli, including cigarette cues, were localized in frontocentral and parietal cortical areas confirm the idea that probe P3 modulation is due to differences in attentional resources allocation (Keil et al., 2007; Lang et al., 1997). Functional brain imaging studies have identified frontal and parietal areas as the main nodes of the visuospatial attention network and have showed that these areas are activated in a variety of visuospatial tasks and, via feedback projections, modulate the activity of the visual system (Kastner & Ungerleider, 2000; Knudsen, 2007).
Overall, our results suggest that, among smokers, cigarette cues receive prioritized attention, thereby exploiting the same neural mechanisms associated with the processing of intrinsically motivationally relevant stimuli (pleasant and unpleasant images) (Baker et al., 2004; Everitt et al., 2001; Hyman, 2005; Robinson & Berridge, 2001; Robinson & Berridge, 2003). In fact we found no differences between cigarette-related, pleasant, and unpleasant conditions, in either P3 amplitude or neural sources associated with its modulation.
Previous fMRI studies comparing patterns of brain activation following the presentation of smoking-related vs. neutral cues reported increased activation in anterior cingulate/orbitofrontal cortex, superior frontal gyrus, and intraparietal sulcus (David et al., 2005; Due et al., 2002). The statistical analyses performed on the surface Laplacian estimates calculated on our data show a pattern entirely consistent with localization of the activity within these areas. Hence, similar to what has been noted for intrinsically motivating stimuli, areas involved in the control of visuospatial attention are less available to process concurrent stimuli when cigarette-related cues become the focus of attention. This finding indicates that smokers may have greater difficulty attending to concurrent stimuli when cigarette cues are present than when they are not. Critically, the reduction of attentional resources in the presence of a cigarette related cue would make it more difficult for a smoker trying to quit to suppress an automatic response (i.e. lighting a cigarette) and engage in non-smoking alternative behaviors (Tiffany, 1990).
Brain imaging studies have also shown that the presence of cigarette-related cues increase activation in subcortical areas, in particular the mesolimbic dopamine reward circuits (David et al., 2005; David et al., 2007; Due et al., 2002). Given the limited sensitivity of the surface Laplacian estimate to detect subcortical sources of activation, we cannot make any direct inference about activity within these structures. However, following theories developed in basic affective neuroscience (Bradley, Codispoti, Cuthbert, & Lang, 2001; Keil et al., 2002; Lang et al., 1997; Pessoa, 2008), it seems reasonable to suggest that these subcortical circuits engage cortical structures to mobilize resources towards cigarette-related stimuli and grant them prioritized processing. Future studies investigating connectivity between these areas will shed light on this issue.
Another issue deserving further investigation is the effect that nicotine and nicotine deprivation might have on different attentional components. Our results showed that intrinsically motivating and cigarette-related cues reduce attentional resources equally, but when Gilbert and co-workers (Gilbert et al., 2007) used emotional pictures as distracters in a detection task, they observed that nicotine deprivation increased bias towards negative pictures. As Gilbert and colleagues pointed out, their relatively small sample (N=16) and the higher arousal level that characterized their unpleasant compared to their pleasant pictures might limit the possibility to generalize their results to different samples. On the other hand, given that for our non deprived participants attending the visual stimuli was the primary task, it is possible that differences between motivationally significant cues were masked by a ceiling effect. (The presence of a ceiling effect might also explain the lack of a significant correlation between probe P3 amplitude and FTND scores in our results). Additional studies about these issues will certainly contribute to refining our understanding of the interactions among nicotine, emotion, and attention, and to improving the effectiveness of smoking cessation interventions.
The data presented here support the idea that cigarette-related stimuli receive prioritized attentional processing and may “hijack” the same brain mechanisms that evolved to process stimuli that inherently trigger approach and avoidance motivational predisposition. The involvement of frontal structures, observed in the current study, is consistent with the idea that part of the power cigarette-related cues have in triggering a relapse stems from the altered functionality of frontal areas involved in decision making and inhibitory control (Robinson & Berridge, 2003). The high salience attributed to cigarette-related stimuli, the reduced availability of attentional resources to process alternative information, and the reduced ability to inhibit over-reinforced and over-learned (automatic) drug seeking behaviors are all factors conspiring against the explicit goal of abstinence to which a smoker might aspire. Future studies examining the effects of medications and behavioral strategies on these cognitive processes may help to identify potentially clinically relevant interventions aimed at better preparing the smoker for encountering cigarettes and other motivationally relevant stimuli in their environment as they navigate a smoking cessation attempt.
Acknowledgments
This work was supported in part by grant # 1R01DA017073-01 to Paul Cinciripini. The authors wish to thank Krystle Bartley, Paul Longoria, Kevin Mulpur, Cissette Muster, and Susana Torres for their help in data collection.
Footnotes
The numbers of IAPS pictures presented were as follows. Pleasant: 1722, 2040, 2058, 2303, 2340, 2346, 2550, 4599, 4601, 4608, 4614, 4640, 4687, 5260, 5450, 5460, 5626, 5830, 7230, 7502, 7508, 8090, 8170, 8300, 8501, 8503, 8510. Neutral: 2305, 2393, 2397, 2518, 2579, 5395, 5500, 5532, 5534, 5740, 7035, 7055, 7056, 7080, 7190, 7205, 7207, 7217, 7233, 7235, 7248, 7285, 7500, 7550, 7820, 7830, 7950. Unpleasant: 2352.2, 2683, 2688, 2710, 3017, 3061, 3140, 3160, 3300, 3550, 6243, 6360, 6571, 6838, 8485, 9000, 9181, 9301, 9331, 9405, 9424, 9430, 9433, 9520, 9560, 9902, 9910.
References
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychological Review. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Research Reviews. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. Emotion and motivation I: defensive and appetitive reactions in picture processing. Emotion. 2001;1:276–298. [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Lang PJ. A multi-process account of startle modulation during affective perception. Psychophysiology. 2006;43:486–497. doi: 10.1111/j.1469-8986.2006.00412.x. [DOI] [PubMed] [Google Scholar]
- Carter BL, Robinson JD, Lam CY, Wetter DW, Day SX, Tsan JY, Cinciripini PM. A psychometric evaluation of cigarette stimuli used in a cue reactivity study. Nicotine & Tobacco Research. 2006;8:361–369. doi: 10.1080/14622200600670215. [DOI] [PubMed] [Google Scholar]
- Cinciripini PM, Robinson JD, Carter BL, Lam CY, Wu X, De Moor CA, Baile WS, Wetter DW. The Effects of smoking deprivation and nicotine administration on emotional reactivity. Nicotine & Tobacco Research. 2006;8:379–392. doi: 10.1080/14622200600670272. [DOI] [PubMed] [Google Scholar]
- Curtin JJ, McCarthy DE, Piper ME, Baker TB. Implicit and explicit drug motivational processes: A model of boundary conditions. In: Reinout R, Stacy A, editors. Handbook of implicit cognition and addiction. Thousand Oaks, CA: Sage Publications; 2006. pp. 233–250. [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley M, McManis M, Lang PJ. Probing affective pictures: attended startle and tone probes. Psychophysiology. 1998;35:344–347. doi: 10.1017/s0048577298970536. [DOI] [PubMed] [Google Scholar]
- David SP, Munafo MR, Johansen-Berg H, Mackillop J, Sweet LH, Cohen RA, Niaura R, Rogers RD, Matthews PM, Walton RT. Effects of Acute Nicotine Abstinence on Cue-elicited Ventral Striatum/Nucleus Accumbens Activation in Female Cigarette Smokers: A Functional Magnetic Resonance Imaging Study. Brain Imaging Behav. 2007;1:43–57. doi: 10.1007/s11682-007-9004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David SP, Munafo MR, Johansen-Berg H, Smith SM, Rogers RD, Matthews PM, Walton RT. Ventral striatum/nucleus accumbens activation to smoking-related pictorial cues in smokers and nonsmokers: a functional magnetic resonance imaging study. Biological Psychiatry. 2005;58:488–494. doi: 10.1016/j.biopsych.2005.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G. Drug addiction as dopamine-dependent associative learning disorder. Eur J Pharmacol. 1999;375:13–30. doi: 10.1016/s0014-2999(99)00372-6. [DOI] [PubMed] [Google Scholar]
- Due DL, Huettel SA, Hall WG, Rubin DC. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: evidence from functional magnetic resonance imaging. Am. J. Psychiatry. 2002;159:954–960. doi: 10.1176/appi.ajp.159.6.954. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res Brain Res Rev. 2001;36:129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, Rabinovich NE. The international smoking image series (with neutral counterparts), v. 1.2. Carbondale, IL: Department of Psychology, Southern Illinois University; 1999. [Google Scholar]
- Gilbert DG, Sugai C, Zuo Y, Rabinovich NE, McClernon FJ, Froeliger B. Brain indices of nicotine's effects on attentional bias to smoking and emotional pictures and to task-relevant targets. Nicotine & Tobacco Research. 2007;9:351–363. doi: 10.1080/14622200701188810. [DOI] [PubMed] [Google Scholar]
- Hamburger HL, vd Burgt MA. Global field power measurement versus classical method in the determination of the latency of evoked potential components. Brain Topogr. 1991;3:391–396. doi: 10.1007/BF01129642. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström test for nicotine dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hyman SE. Addiction: a disease of learning and memory. Am. J Psychiatry. 2005;162:1414–1422. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- Johnsen BH, Thayer JF, Laberg JC, Asbjornsen AE. Attentional bias in active smokers, abstinent smokers, and nonsmokers. Addictive Behaviors. 1997;22:813–817. doi: 10.1016/s0306-4603(97)00010-5. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annu Rev Neurosci. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Keil A, Bradley MM, Hauk O, Rockstroh B, Elbert T, Lang PJ. Large-scale neural correlates of affective picture processing. Psychophysiology. 2002;39:641–649. doi: 10.1017.S0048577202394162. [DOI] [PubMed] [Google Scholar]
- Keil A, Bradley MM, Junghofer M, Russmann T, Lowenthal W, Lang PJ. Cross-modal attention capture by affective stimuli: evidence from event-related potentials. Cogn Affect.Behav Neurosci. 2007;7:18–24. doi: 10.3758/cabn.7.1.18. [DOI] [PubMed] [Google Scholar]
- Knudsen EI. Fundamental components of attention. Annu Rev Neurosci. 2007;30:57–78. doi: 10.1146/annurev.neuro.30.051606.094256. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention, and the startle reflex. Psychological Review. 1990;97:377–395. [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Motivated attention: Affect, activation, and action. In: Lang PJ, Simons RF, Balaban M, editors. Attention and orienting: Sensory and motivational processes. Mahwah, NJ: Lawrence Erlbaum; 1997. pp. 97–136. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report no. A-6. Gainesville, FL: University of Florida; 2005. International Affective Picture System (IAPS): Affective ratings of pictures and instruction manual. [Google Scholar]
- Maris E. Randomization tests for ERP topographies and whole spatiotemporal data matrices. Psychophysiology. 2004;41:142–151. doi: 10.1111/j.1469-8986.2003.00139.x. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. Selective processing of smoking-related cues in smokers: Manipulation of deprivation level and comparison of three measures of processing bias. Journal of Psychopharmacology. 2002;16:385–392. doi: 10.1177/026988110201600416. [DOI] [PubMed] [Google Scholar]
- Munafo M, Mogg K, Roberts S, Bradley BP, Murphy M. Selective processing of smoking-related cues in current smokers, ex-smokers and never-smokers on the modified Stroop task. Journal of Psychopharmacology. 2003;17:310–316. doi: 10.1177/02698811030173013. [DOI] [PubMed] [Google Scholar]
- Pessoa L. On the relationship between emotion and cognition. Nat. Rev Neurosci. 2008;9:148–158. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Neurobehavioural mechanisms of reward and motivation. Curr. Opin. Neurobiol. 1996;6:228–236. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Birbaumer N, Lang PJ. Probe P3 and blinks: Two measures of affective startle modulation. Psychophysiology. 1997;34:1–6. doi: 10.1111/j.1469-8986.1997.tb02409.x. [DOI] [PubMed] [Google Scholar]
- Srinivasan R. High-resolution EEG: Theory and practice. In: Handy TC, editor. Event-related potentials A methods handbook. Cambridge, Massachusetts: The MIT Press; 2005. pp. 167–188. [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: Role of automatic and nonautomatic processes. Psychological Review. 1990;97:147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Feyerabend C. Determinants and effects of attentional bias in smokers. Psychology of Addictive Behaviors. 2000;14:111–120. doi: 10.1037//0893-164x.14.2.111. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Bradley BP, Mogg K. Attentional shifts to smoking cues in smokers. Addiction. 2003;98:1409–1417. doi: 10.1046/j.1360-0443.2003.00465.x. [DOI] [PubMed] [Google Scholar]
- Wolfling K, Flor H, Grusser SM. Psychophysiological responses to drug-associated stimuli in chronic heavy cannabis use. Eur J Neurosci. 2008;27:976–983. doi: 10.1111/j.1460-9568.2008.06051.x. [DOI] [PubMed] [Google Scholar]