Abstract
Background:
We aimed to better discriminate (occult) metastasised from non-metastasised seminoma based on transcriptional changes of small RNAs in the primary tumour.
Methods:
Total RNAs including small RNAs were isolated from five testicular tumours of each, lymphogenic, occult and non-metastasised patients. Next-generation sequencing (SOLID, Life Technologies) was used to examine transcriptional changes. Small RNAs showing ⩾50 reads and a significant ⩾2-fold difference using non-metastasised tumours as the reference group were examined in univariate logistic regression analysis and combinations of two small RNAs were further examined using support vector machines.
Results:
On average, 1.3 × 107, 1.4 × 107 and 1.7 × 107 small RNA reads were detectable in non-metastasised, occult and lymphogenic metastasised seminoma, respectively, of which 30–32% remained after trimming. Between 59 and 68% represented annotated reads and between 8.6 and 11% were annotated small RNA tags. Of them, 137 small RNAs showed>50 reads and a two-fold difference to the reference. In univariate analysis, 32–38 small RNAs significantly discriminated lymphogenic/occult from non-metastasised seminoma, and among these different comparisons, it were the same small RNAs in 51–88%. Many combinations of two of these small RNAs allowed a complete discrimination of metastasised from non-metastasised seminoma irrespective of the metastasis subtype.
Conclusions:
Metastasised and non-metastasised seminoma can be completely discriminated with a combination of two small RNAs.
Keywords: germ cell tumour, testis, gene expression, small RNA, metastasised, seminoma, next-generation sequencing, risk factor
Testicular tumour, as the most common tumour in young men, is associated with a 5-year survival rate close to 100% in early stages. Pure seminoma are the most frequent histological subtype (55%) nowadays and up to 70% were present without visible metastasis at primary staging (Ruf et al, 2013a). Gold standard for primary staging is computed tomography (CT) of the chest, abdomen and pelvis to detect metastases. In truly non-metastasised clinical stage I (cSI), patients are cured by orchidectomy alone; however, despite modern staging and classification procedures, up to 30% of cSI seminoma patients bear occult metastasis in primary staging and relapse after orchidectomy alone (Krege et al, 2008; Albers et al, 2012). Until now, no reliable biological parameters exist and the clinical parameter shows a concordance of 65% only in differentiating occult metastasised stages from non-metastasised seminoma (Ruf et al, 2013b). Identification of occult metastasised patients is one of the main goals to prevent toxicity (e.g. cardiovascular and kidney disease, secondary malignancies and decreased fertility) caused by unnecessary adjuvant treatment or diagnostic procedures during follow-up (Kollmannsberger et al, 2011).
Recent publications facilitate the idea of certain demographic/clinical histological risk factors to be associated with both detectable and occult seminoma metastasis (Warde et al, 1997, 2002; Valdevenito et al, 2007; Krege et al, 2008; Albers et al, 2012; Ruf et al, 2013b). We also showed both metastasis subtypes to be indistinguishable on the transcriptional level using a whole genome screening, suggesting that they are not representing different metastasis subtypes (Ruf et al, 2014). Recently, other authors started to examine whether a certain set of microRNAs (gnostic approach) might be suitable for discriminating between seminoma-bearing patients and healthy persons (Gillis et al, 2007; Palmer et al, 2010; Dieckmann et al, 2012).
This to our knowledge provides first indications on the diagnostic potential of microRNAs in the field of urology and eventually in the discrimination of metastasised from non-metastasised urological tumours, although not shown so far. Following this approach, we used an agnostic approach where we searched the whole genome for any kind of small RNA species suitable to discriminate metastasised (either lymphogenic, occult or a combination of both subtypes) from non-metastasised seminoma using next-generation sequencing (NGS).
Materials and methods
Patient selection
Non-metastasised seminoma (n=5) received no adjuvant treatment and were free of relapse/progress for at least 2 years of follow-up. Occult metastasised patients (n=5) presented without visible metastasis at primary staging, received no adjuvant treatment and developed retroperitoneal tumour progress during follow-up. For patients with detectable metastasis at primary staging (n=5), we focused on clinical stage IIb and IIc to include lymphogenic metastatic spread only and to provide a high level of diagnostic accuracy (avoiding doubtful lymph nodes). Lymphogenic metastasis and non-metastasised patients were matched with occult metastasised patients considering demographic and histological parameters if possible (Table 1).
Table 1. Characteristics of patients, their biopsies and RNA isolates.
| No. | Metastasis detection at time of primary tumour's diagnosis | Descriptive statistics | Age at diagnosis (years) | Tumour size (mm) | pL | pV | pT | Infiltration rete testis | Initial clinical stage | Total RNA (μg) | RIN |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Non-metastasised | 38 | 14 | 0 | 0 | 1 | N | cSI | 16.8 | 7.9 | |
| 2 | 50 | 22 | 0 | 0 | 1 | N | cSI | 10.2 | 8.8 | ||
| 3 | 31 | 19 | 1 | 0 | 2 | N | cSI | 25.7 | 8.2 | ||
| 4 | 42 | 45 | 0 | 0 | 1 | Y | cSI | 31.2 | 8.4 | ||
| 5 | 35 | 19 | 0 | 0 | 1 | Y | cSI | 41.8 | 8.6 | ||
| Mean | 39.1 | 23.8 | 25.1 | 8.4 | |||||||
| |
|
s.d. |
7.2 |
12.2 |
|
|
|
|
|
12.3 |
0.3 |
| 1 | Lymphogenic metastasised | 32 | 40 | 0 | 0 | 1 | N | cSIIb | 59.9 | 8.8 | |
| 2 | 50 | 12 | 0 | 0 | 3 | N | cSIIc | 30.1 | 9.2 | ||
| 3 | 43 | 50 | 0 | 0 | 1 | N | cSIIb | 57.0 | 8.6 | ||
| 4 | 42 | 45 | 1 | 0 | 2 | Y | cSIIb | 30.3 | 8.9 | ||
| 5 | 61 | 42 | 0 | 0 | 1 | Y | cSIIc | 46.5 | 8.7 | ||
| Mean | 45.2 | 37.8 | 44.8 | 8.8 | |||||||
| |
|
s.d. |
10.8 |
14.9 |
|
|
|
|
|
14.2 |
0.2 |
| 1 | Occult metastasised | 33 | 55 | 1 | 0 | 2 | N | cSI | 68.8 | 9.4 | |
| 2 | 37 | 35 | 0 | 0 | 1 | Y | cSI | 67.9 | 9.1 | ||
| 3 | 37 | 30 | 0 | 0 | 1 | N | cSI | 13.8 | 9 | ||
| 4 | 31 | 18 | 0 | 0 | 1 | N | cSI | 30.9 | 8.7 | ||
| 5 | 23 | 55 | 0 | 0 | 1 | N | cSI | 41.1 | 8.4 | ||
| Mean | 32.1 | 38.6 | 44.5 | 8.9 | |||||||
| s.d. | 5.4 | 16.2 | 23.9 | 0.4 |
Abbreviations: cSI=clinical stage I; N=no; pL=lymphatic vessel invasion; pT=tumour stage; pV=blood vessel invasion; RIN=RNA integrity number; Y=yes.
Mean values of age and tumour size of the metastasised groups are not statistically different from the non-metastasised seminoma reference group (P>0.1, t-test).
Tissue samples and histological examination
Testicular tumour biopsies (n=15) were incubated into RNAlater solution (Qiagen, Hilden, Germany) during the operation and later stored at –20 °C. All tissue samples were examined by an experienced pathologist for histological and TNM classification (Table 1). The local ethical commission approved the study and all human samples were obtained with informed consent.
RNA isolation
Tissues frozen in RNAlater solution (Qiagen) were carefully thawed, homogenised (Homogenizer; Omni, Warrenton, VA, USA) and digested using proteinase K. Total RNA including small RNAs was isolated (mirVana Kit; Life Technologies, Darmstadt, Germany) and remaining DNA digested. RNA was stored at –80 °C until its use. Quality and quantity of isolated total RNA was measured spectrophotometrically (NanoDrop; PeqLab Biotechnology, Erlangen, Germany), whereas RNA integrity was assessed by the 2100 Agilent Bioanalyser (Life Science Group, Penzberg, Germany). For analysis, we used only RNA specimens with a ratio of A260/A280 ⩾2.0 (NanoDrop) and RNA integrity number (RIN) ⩾7.9 for small RNA NGS (IMGM Laboratories, Martinsried, Germany/CeGat, Tübingen, Germany).
Small RNA NGS and data analysis
We performed a genome-wide small RNA sequencing using the SOLiD5500xl NGS Technology (Life Technologies). In brief, total RNA was purified (PureLink miRNA Isolation Kit, Life Technologies), enriched small RNAs were ligated to SOLiD adaptors, reverse transcribed (SOLiD RT primer and ArrayScript RT, Life Technologies), cDNA was purified (MinElute PCR Purification Kit; Qiagen), a cutoff size of 60–80 nt was selected (Novex precast gel products; Invitrogen, Life Technologies), cDNA was in-gel amplified and samples were barcoded using SOLiD 3′Barcode Primer (Life Technologies) at the same time. Amplified cDNA was purified (PureLink PCR Micro Kit; Life Technologies) and used in emulsion PCR (SOLiD EZ Bead, Life Technologies). Thereafter, the emulsion was broken to recover enriched beads and the so-called di-base probes were used by the SOLiD system in the sequencing-by-ligation procedure. Besides the SOLiD5500xl inherent software (LifeScope, Life Technologies) used for image and signal processing, software CLC Genomics Workbench 5.1 (CLC bio, Aarhus, Denmark) was used for clustering, counting and annotation of all generated 75 bp reads. After discarding reads without adaptor sequence and being shorter than 15 bp (trimming), reads were assigned to known miRNAs (Sanger miRBase release 18; http://www.mirbase.org/) and known non-coding RNAs (ensembl database Homo_sapiens.GRCh37.67.ncrna.fa; http://www.ensembl.org). Small RNAs showing a significant and ⩾2-fold differences in gene expression among groups and at least 50 reads were further analysed on their ability to separate both groups using univariate logistic regression analysis (Baggerly et al, 2004). Combinations of small RNAs were examined using support vector machines (linear kernel).
Results
Characteristics of seminoma groups
The average age at diagnosis was 39.1 (±7.2) years for non-metastasised, higher (45.2 years, ±10.8) for lymphogenic metastasised and lower (32.1 years, ±5.4) for occult metastasised seminoma. Primary tumour size was comparable between lymphogenic and occult metastasised seminoma (37.8 and 38.6 mm, respectively), but smaller (23.8 mm) in non-metastasised seminoma (Table 1).
RNA isolation
On average, we isolated almost two times as much total RNA from 10 to 20 mg of our lymphogenic (44.8 μg)/occult (44.5 μg) metastasised biopsy samples in comparison with the non-metastasised seminoma (22.6 μg; Table 1). The average RIN ranged between 8.0 and 8.9 for all of our samples. No DNA contamination could be detected in our samples.
Analysis of small RNA NGS results
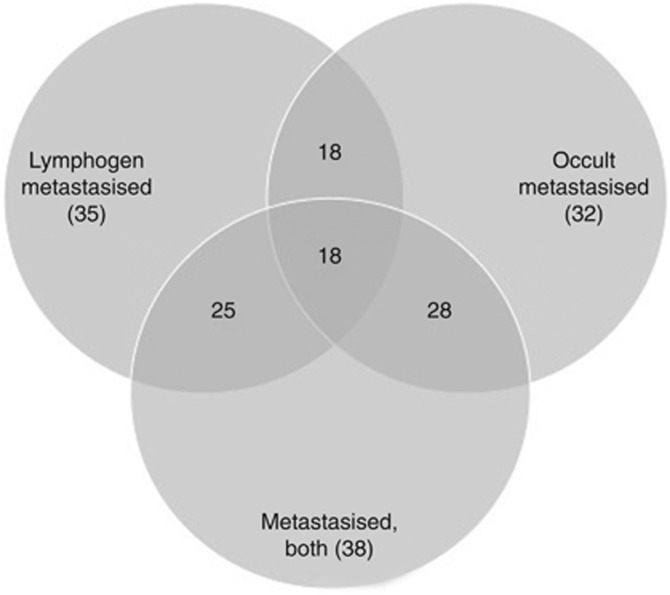
The average total number of reads for lymphogenic/occult metastasised and non-metastasised seminoma was 1.3 × 107, 1.4 × 107 and 1.7 × 107 and on average 30–32% remained for further analysis after trimming of the reads (Table 2). From these reads, 59–68% appeared annotated reads, with 8.6–11% (3.6–5.7 × 104) representing annotated small RNAs. Of them, 137 small RNAs showed >50 reads and a two-fold difference to the reference. In univariate analysis, we identified 35, 32 and 38 small RNA species, which significantly discriminated lymphogenic/occult metastasised and both metastasis subtypes combined from non-metastasised seminoma, respectively (Table 3). The overlap of small RNAs separating either lymphogenic or occult metastasis from non-metastasised seminoma was 51–56% and increased up to 88% when comparing lymphogenic and occult small RNAs candidates with the small RNAs based on the combined metastasis subtypes (Figure 1). We finally used support vector machines enabling us to separate completely lymphogenic, occult metastasis and the combined metastasis subtypes from non-metastasised seminoma using a combination of two small RNA species only (Table 4). Altogether, 125, 52 and 6 combinations allowed a complete separation of lymphogenic (n=5), occult metastasis (n=5) and the combined metastasis subtypes (n=10) from non-metastasised seminoma (n=5), respectively.
Table 2. Descriptive statistics of the number of reads before and after trimming and the percentage of annotated small RNAs for non-metastasised, lymphogenic and occult metastasised seminoma.
| |
|
Reads after trimming |
Annotated reads |
Small RNA tags |
||||
|---|---|---|---|---|---|---|---|---|
| Seminoma metastasis status | Total no. of reads | Abs. | % | Abs. | % | Abs. | Abs. annotated | % |
|
Non-metastasised, n=5 | ||||||||
| Mean | 1 266 4336.2 | 3 986 210.6 | 41 517 | 2 767 766.2 | 68.22 | 444 222.2 | 36 390.2 | — |
| S.d. | 3.9E+14 | 2.6E+14 | — | 2.0E+13 | — | 2.1E+14 | 1.3E+13 | — |
| Min | 7.1E+06 | 1.2E+06 | 41 469 | 8.3E+05 | 60.4 | 2.1E+05 | 2.1E+04 | 7.0 |
| Max |
1.6E+07 |
8.0E+06 |
63.2 |
6.0E+06 |
75.4 |
7.1E+05 |
5.0E+04 |
— |
|
Lymphogenic metastasised, n=5 | ||||||||
| Mean | 16 996 933.6 | 5.4E+06 | 30.36 | 3 379 924.4 | 62.46 | 643 430.4 | 56 876.6 | — |
| S.d. | 2.3E+14 | 3.1E+14 | — | 2.2E+14 | — | 3.4E+14 | 3.1E+14 | — |
| Min | 1.4E+07 | 1.7E+06 | 41 317 | 1.1E+06 | 53.3 | 2.6E+05 | 2.6E+04 | — |
| Max |
2.0E+07 |
9.2E+06 |
47.2 |
6.4E+06 |
69.6 |
1.1E+06 |
9.8E+04 |
— |
|
Occult metastasised, n=5 | ||||||||
| Mean | 13 943 654.4 | 4 347 341.6 | 41 638 | 2 631 565.4 | 59.16 | 4.9E+05 | 49 741.4 | — |
| S.d. | 2.0E+14 | 2.6E+14 | — | 1.8E+14 | — | 2.2E+14 | 1.9E+14 | — |
| Min | 1.1E+07 | 1.2E+06 | 41 463 | 7.1E+05 | 53.2 | 2.4E+05 | 3.2E+04 | — |
| Max | 1.7E+07 | 8.3E+06 | 49.6 | 5.5E+06 | 66.1 | 7.9E+05 | 8.0E+04 | — |
Abbreviations: Abs.=absolute; Min=minimum; max=maximum; s.d.=standard deviation.
Table 3. Summary of significantly associated small RNAs with metastasis status per group.
|
Non-
vs
metastasised (lymphogenic and occult,
n=38) |
Non-
vs
lymphogenic metastasised (n=35) |
Non-
vs
occult metastasised (n=32) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Small RNA | P-value | Fold change | 95% Confidence interval | Small RNA | P-value | Fold change | 95% Confidence interval | Small RNA | P-value | Fold change | 95% Confidence interval | |||
| Let-7b |
0.043 |
0.48 |
0.25 |
0.91 |
Mir-18a |
0.003 |
5.00 |
2.31 |
10.82 |
Mir-18a |
0.034 |
3.19 |
1.31 |
7.79 |
| Mir-17 |
0.044 |
2.34 |
1.11 |
4.96 |
Mir-25 |
0.001 |
3.57 |
2.17 |
5.89 |
Mir-25 |
0.004 |
3.81 |
1.97 |
7.38 |
| Mir-18a |
0.008 |
4.10 |
1.70 |
9.85 |
Mir-29a |
0.005 |
0.42 |
0.27 |
0.65 |
Mir-29a |
0.019 |
0.36 |
0.18 |
0.71 |
| Mir-25 |
0.0004 |
3.69 |
2.15 |
6.34 |
Mir-92a-1 |
0.017 |
2.65 |
1.41 |
5.00 |
Mir-92a-1 |
0.043 |
2.10 |
1.15 |
3.84 |
| Mir-29a |
0.001 |
0.39 |
0.25 |
0.60 |
Mir-92a-2 |
0.015 |
2.73 |
1.44 |
5.18 |
Mir-92a-2 |
0.042 |
2.14 |
1.16 |
3.95 |
| Mir-92a-1 |
0.007 |
2.38 |
1.41 |
4.02 |
Mir-93 |
0.050 |
6.24 |
1.32 |
29.48 |
Mir-99a |
0.029 |
0.18 |
0.05 |
0.64 |
| Mir-92a-2 |
0.006 |
2.43 |
1.42 |
4.16 |
Mir-99a |
0.011 |
0.18 |
0.06 |
0.50 |
Mir-29b-1 |
0.046 |
0.37 |
0.16 |
0.85 |
| Mir-99a |
0.001 |
0.18 |
0.08 |
0.40 |
Mir-197 |
0.011 |
2.38 |
1.42 |
4.00 |
Mir-29b-2 |
0.044 |
0.36 |
0.16 |
0.84 |
| Mir-29b-1 |
0.012 |
0.45 |
0.27 |
0.77 |
Mir-148a |
0.036 |
2.51 |
1.23 |
5.13 |
Mir-182 |
0.018 |
3.43 |
1.52 |
7.76 |
| Mir-29b-2 |
0.012 |
0.46 |
0.27 |
0.77 |
Mir-30c-2 |
0.033 |
2.81 |
1.28 |
6.17 |
Mir-125b-1 |
0.040 |
0.22 |
0.07 |
0.74 |
| Mir-30c-2 |
0.038 |
2.41 |
1.14 |
5.08 |
Mir-30d |
0.024 |
2.27 |
1.27 |
4.05 |
Mir-128-1 |
0.029 |
3.19 |
1.36 |
7.49 |
| Mir-182 |
0.008 |
3.35 |
1.57 |
7.14 |
Mir-7-1 |
0.028 |
3.64 |
1.41 |
9.40 |
Mir-125b-2 |
0.040 |
0.22 |
0.07 |
0.74 |
| Mir-183 |
0.037 |
5.22 |
1.29 |
21.04 |
Mir-182 |
0.015 |
3.26 |
1.54 |
6.90 |
Mir-320a |
0.035 |
2.59 |
1.24 |
5.41 |
| Mir-15b |
0.024 |
2.65 |
1.26 |
5.58 |
Mir-183 |
0.003 |
4.39 |
2.18 |
8.86 |
Mir-128-2 |
0.034 |
3.20 |
1.31 |
7.81 |
| Mir-125b-1 |
0.006 |
0.31 |
0.16 |
0.63 |
Mir-15b |
0.002 |
2.77 |
1.77 |
4.32 |
Mir-106b |
0.001 |
5.22 |
2.69 |
10.13 |
| Mir-128-1 |
0.012 |
3.86 |
1.56 |
9.55 |
Mir-128-1 |
0.009 |
4.53 |
1.91 |
10.74 |
Mir-29c |
0.019 |
0.30 |
0.13 |
0.67 |
| Mir-145 |
0.023 |
0.50 |
0.29 |
0.85 |
Mir-128-2 |
0.009 |
4.77 |
1.97 |
11.54 |
Mir-296 |
0.017 |
4.01 |
1.62 |
9.90 |
| Mir-125b-2 |
0.006 |
0.31 |
0.16 |
0.63 |
Mir-106b |
0.003 |
6.63 |
2.71 |
16.23 |
Mir-326 |
0.004 |
2.61 |
1.63 |
4.17 |
| Mir-150 |
0.028 |
0.36 |
0.16 |
0.81 |
Mir-30c-1 |
0.033 |
2.80 |
1.28 |
6.12 |
Mir-151a |
0.015 |
2.35 |
1.37 |
4.04 |
| Mir-128-2 |
0.014 |
3.98 |
1.53 |
10.36 |
Mir-296 |
0.019 |
3.78 |
1.55 |
9.20 |
Mir-331 |
0.033 |
2.58 |
1.25 |
5.30 |
| Mir-106b |
0.001 |
5.92 |
2.49 |
14.11 |
Mir-130b |
0.045 |
2.95 |
1.21 |
7.18 |
Mir-484 |
0.016 |
2.06 |
1.29 |
3.27 |
| Mir-29c |
0.005 |
0.40 |
0.24 |
0.68 |
Mir-378a |
0.015 |
2.66 |
1.43 |
4.95 |
Mir-505 |
0.006 |
2.65 |
1.59 |
4.42 |
| Mir-30c-1 |
0.037 |
2.41 |
1.15 |
5.06 |
Mir-340 |
0.0002 |
3.42 |
2.34 |
4.98 |
Mir-92b |
0.014 |
3.10 |
1.53 |
6.31 |
| Mir-296 |
0.007 |
3.89 |
1.71 |
8.87 |
Mir-326 |
0.002 |
3.63 |
2.12 |
6.21 |
Mir-652 |
0.022 |
3.23 |
1.44 |
7.24 |
| Mir-378a |
0.028 |
2.32 |
1.19 |
4.53 |
Mir-331 |
0.001 |
3.05 |
1.96 |
4.74 |
ENST00000516461 |
0.045 |
2.53 |
1.18 |
5.44 |
| Mir-340 |
0.006 |
3.12 |
1.58 |
6.18 |
Mir-345 |
0.035 |
3.26 |
1.31 |
8.12 |
ENST00000516775 |
0.045 |
2.54 |
1.18 |
5.47 |
| Mir-326 |
0.001 |
3.12 |
1.82 |
5.33 |
Mir-423 |
0.001 |
2.24 |
1.65 |
3.02 |
ENST00000517209 |
0.046 |
7.07 |
1.39 |
35.98 |
| Mir-151a |
0.023 |
2.32 |
1.22 |
4.39 |
Mir-484 |
0.015 |
2.28 |
1.35 |
3.85 |
ENST00000387347 |
0.050 |
2.77 |
1.17 |
6.57 |
| Mir-331 |
0.003 |
2.81 |
1.61 |
4.92 |
Mir-505 |
0.002 |
3.62 |
2.03 |
6.47 |
ENST00000474885 |
0.001 |
3.38 |
2.05 |
5.56 |
| Mir-484 |
0.003 |
2.17 |
1.43 |
3.29 |
Mir-92b |
0.015 |
4.76 |
1.76 |
12.89 |
ENST00000459522 |
0.001 |
3.35 |
2.04 |
5.52 |
| Mir-505 |
0.002 |
3.13 |
1.72 |
5.71 |
Mir-625 |
0.041 |
3.47 |
1.28 |
9.42 |
ENST00000488123 |
0.039 |
2.89 |
1.24 |
6.72 |
| Mir-92b |
0.016 |
3.93 |
1.48 |
10.40 |
Mir-421 |
0.025 |
2.77 |
1.34 |
5.70 |
ENST00000463796 |
0.013 |
4.19 |
1.73 |
10.17 |
| Mir-652 |
0.035 |
2.77 |
1.19 |
6.45 |
Mir-744 |
0.023 |
2.14 |
1.26 |
3.63 |
|
|
|
|
|
| ENST00000387347 |
0.013 |
2.84 |
1.39 |
5.79 |
ENST00000387347 |
0.005 |
2.91 |
1.68 |
5.06 |
|
|
|
|
|
| ENST00000474885 |
0.014 |
2.99 |
1.40 |
6.41 |
ENST00000466665 |
0.027 |
2.50 |
1.28 |
4.87 |
|
|
|
|
|
| ENST00000459522 |
0.015 |
2.97 |
1.39 |
6.37 |
|
|
|
|
|
|
|
|
|
|
| ENST00000463796 |
0.034 |
3.79 |
1.26 |
11.44 |
|
|
|
|
|
|
|
|
|
|
| ENST00000466665 | 0.046 | 2.27 | 1.09 | 4.71 | ||||||||||
Figure 1.
Venn diagram showing the number of significantly associated small RNAs (Table 1) to discriminate different metastasis subtypes (lymphogenic and occult) and the combination of both subtypes from non-metastasised seminoma.
Table 4. Summary on combinations of two small RNAs, which together allow to discriminate completely metastasis subtypes (lymphogenic and occult) and their combination from non-metastasised seminoma.
|
Non-
vs
metastasised (lymphogenic and occult,
n=6) |
Non- vs
lymphogenic metastasised (n=125) |
Non-
vs
occult metastasised (n=52) |
|||
|---|---|---|---|---|---|
| Small RNA1 | Small RNA2 | Small RNA1 | Small RNA2 | Small RNA1 | Small RNA2 |
| Mir-29a |
Enst00000387347 |
Let-7a-1 |
Mir-99a |
Let-7b |
ENST00000466665 |
| Mir-150 |
Mir-3676 |
Let-7a-2 |
Mir-99a |
Mir-15a |
ENST00000387347 |
| Mir-150 |
Enst00000516461 |
Let-7a-3 |
Mir-99a |
Mir-16-1 |
Mir-128-1 |
| Mir-150 |
Enst00000516775 |
Let-7b |
Mir-331 |
Mir-16-1 |
Mir-128-2 |
| Mir-29c |
Enst00000387347 |
Mir-15a |
Mir-331 |
Mir-16-1 |
Mir-361 |
| Mir-342 |
Mir-326 |
Mir-16-1 |
Mir-331 |
Mir-16-1 |
Mir-652 |
| |
|
Mir-18a |
Mir-99a |
Mir-19b-2 |
Mir-25 |
| |
|
Mir-22 |
Mir-99a |
Mir-24-1 |
ENST00000466665 |
| |
|
Mir-23a |
Mir-99a |
Mir-24-2 |
ENST00000466665 |
| |
|
Mir-24-1 |
Mir-326 |
Mir-25 |
Mir-126 |
| |
|
Mir-24-1 |
Mir-331 |
Mir-25 |
Mir-342 |
| |
|
Mir-24-2 |
Mir-326 |
Mir-26a-1 |
ENST00000466665 |
| |
|
Mir-24-2 |
Mir-331 |
Mir-29a |
Mir-326 |
| |
|
Mir-25 |
Mir-29a |
Mir-29a |
Mir-4454 |
| |
|
Mir-25 |
Mir-29b-1 |
Mir-29a |
ENST00000474885 |
| |
|
Mir-25 |
Mir-29b-2 |
Mir-29a |
ENST00000459522 |
| |
|
Mir-25 |
Mir-222 |
Mir-29a |
ENST00000459949 |
| |
|
Mir-25 |
Mir-29c |
Mir-29a |
ENST00000463796 |
| |
|
Mir-29a |
Mir-148a |
Mir-29a |
ENST00000466665 |
| |
|
Mir-29a |
Mir-183 |
Mir-92a-1 |
Mir-150 |
| |
|
Mir-29a |
Mir-15b |
Mir-92a-2 |
Mir-150 |
| |
|
Mir-29a |
Mir-128-1 |
Mir-99a |
Mir-320a |
| |
|
Mir-29a |
Mir-128-2 |
Mir-99a |
ENST00000466665 |
| |
|
Mir-29a |
Mir-296 |
Mir-29b-1 |
Mir-326 |
| |
|
Mir-29a |
Mir-378a |
Mir-29b-1 |
Mir-505 |
| |
|
Mir-29a |
Mir-340 |
Mir-29b-1 |
ENST00000474885 |
| |
|
Mir-29a |
Mir-331 |
Mir-29b-1 |
ENST00000459522 |
| |
|
Mir-29a |
Mir-423 |
Mir-29b-2 |
Mir-326 |
| |
|
Mir-29a |
Mir-425 |
Mir-29b-2 |
Mir-505 |
| |
|
Mir-29a |
Mir-625 |
Mir-29b-2 |
ENST00000474885 |
| |
|
Mir-29a |
Mir-421 |
Mir-29b-2 |
ENST00000459522 |
| |
|
Mir-29a |
Mir-744 |
Mir-182 |
Mir-342 |
| |
|
Mir-29a |
ENST00000387347 |
Mir-181a-1 |
Mir-106b |
| |
|
Mir-92a-2 |
Mir-29b-1 |
Let-7g |
Mir-128-2 |
| |
|
Mir-92a-2 |
Mir-29b-2 |
Let-7g |
Mir-106b |
| |
|
Mir-93 |
Mir-182 |
Let-7g |
Mir-378a |
| |
|
Mir-93 |
Mir-183 |
Let-7g |
Mir-505 |
| |
|
Mir-93 |
Mir-340 |
Let-7g |
ENST00000387347 |
| |
|
Mir-93 |
ENST00000488123 |
Mir-30b |
Mir-30e |
| |
|
Mir-93 |
ENST00000463796 |
Mir-150 |
Mir-320a |
| |
|
Mir-93 |
ENST00000466665 |
Mir-150 |
Mir-3676 |
| |
|
Mir-99a |
Mir-107 |
Mir-150 |
ENST00000516461 |
| |
|
Mir-99a |
Mir-30c-2 |
Mir-150 |
ENST00000516775 |
| |
|
Mir-99a |
Mir-15b |
Mir-106b |
Mir-660 |
| |
|
Mir-99a |
Mir-128-1 |
Mir-29c |
Mir-505 |
| |
|
Mir-99a |
Mir-128-2 |
Mir-29c |
ENST00000474885 |
| |
|
Mir-99a |
Mir-30c-1 |
Mir-29c |
ENST00000459522 |
| |
|
Mir-99a |
Mir-361 |
Mir-296 |
Mir-342 |
| |
|
Mir-99a |
Mir-331 |
Mir-26a-2 |
ENST00000466665 |
| |
|
Mir-99a |
Mir-339 |
Mir-342 |
Mir-151a |
| |
|
Mir-99a |
Mir-425 |
Mir-342 |
ENST00000474885 |
| |
|
Mir-99a |
Mir-505 |
Mir-342 |
ENST00000459522 |
| |
|
Mir-99a |
ENST00000516881 |
|
|
| |
|
Mir-29b-1 |
Mir-30c-2 |
|
|
| |
|
Mir-29b-1 |
Mir-30d |
|
|
| |
|
Mir-29b-1 |
Mir-15b |
|
|
| |
|
Mir-29b-1 |
Mir-128-1 |
|
|
| |
|
Mir-29b-1 |
Mir-128-2 |
|
|
| |
|
Mir-29b-1 |
Mir-30c-1 |
|
|
| |
|
Mir-29b-1 |
Mir-331 |
|
|
| |
|
Mir-29b-1 |
Mir-425 |
|
|
| |
|
Mir-29b-1 |
Mir-505 |
|
|
| |
|
Mir-29b-1 |
ENST00000387347 |
|
|
| |
|
Mir-29b-2 |
Mir-30c-2 |
|
|
| |
|
Mir-29b-2 |
Mir-30d |
|
|
| |
|
Mir-29b-2 |
Mir-15b |
|
|
| |
|
Mir-29b-2 |
Mir-128-1 |
|
|
| |
|
Mir-29b-2 |
Mir-128-2 |
|
|
| |
|
Mir-29b-2 |
Mir-30c-1 |
|
|
| |
|
Mir-29b-2 |
Mir-331 |
|
|
| |
|
Mir-29b-2 |
Mir-425 |
|
|
| |
|
Mir-29b-2 |
Mir-505 |
|
|
| |
|
Mir-29b-2 |
ENST00000387347 |
|
|
| |
|
Mir-16-2 |
Mir-331 |
|
|
| |
|
Mir-16-2 |
ENST00000387347 |
|
|
| |
|
Mir-197 |
Mir-150 |
|
|
| |
|
Mir-182 |
Mir-142 |
|
|
| |
|
Mir-183 |
Mir-142 |
|
|
| |
|
Mir-183 |
ENST00000365160 |
|
|
| |
|
Mir-181a-1 |
Mir-331 |
|
|
| |
|
Mir-221 |
Mir-331 |
|
|
| |
|
Mir-222 |
Mir-128-2 |
|
|
| |
|
Mir-222 |
Mir-106b |
|
|
| |
|
Mir-222 |
Mir-331 |
|
|
| |
|
Mir-222 |
ENST00000387347 |
|
|
| |
|
Let-7g |
Mir-15b |
|
|
| |
|
Let-7g |
Mir-128-1 |
|
|
| |
|
Let-7g |
Mir-331 |
|
|
| |
|
Mir-23b |
Mir-331 |
|
|
| |
|
Mir-125b-1 |
Mir-331 |
|
|
| |
|
Mir-128-1 |
Mir-29c |
|
|
| |
|
Mir-142 |
Mir-130b |
|
|
| |
|
Mir-142 |
Mir-326 |
|
|
| |
|
Mir-142 |
Mir-421 |
|
|
| |
|
Mir-142 |
Mir-1260b |
|
|
| |
|
Mir-142 |
ENST00000387347 |
|
|
| |
|
Mir-142 |
ENST00000516881 |
|
|
| |
|
Mir-145 |
Mir-331 |
|
|
| |
|
Mir-125b-2 |
Mir-331 |
|
|
| |
|
Mir-126 |
Mir-331 |
|
|
| |
|
Mir-150 |
Mir-130b |
|
|
| |
|
Mir-150 |
Mir-331 |
|
|
| |
|
Mir-150 |
Mir-345 |
|
|
| |
|
Mir-150 |
Mir-421 |
|
|
| |
|
Mir-150 |
Mir-766 |
|
|
| |
|
Mir-150 |
Mir-744 |
|
|
| |
|
Mir-150 |
Mir-1260b |
|
|
| |
|
Mir-150 |
Mir-3676 |
|
|
| |
|
Mir-150 |
ENST00000516461 |
|
|
| |
|
Mir-150 |
ENST00000516775 |
|
|
| |
|
Mir-150 |
ENST00000387347 |
|
|
| |
|
Mir-150 |
ENST00000410361 |
|
|
| |
|
Mir-150 |
ENST00000431311 |
|
|
| |
|
Mir-128-2 |
Mir-29c |
|
|
| |
|
Mir-29c |
Mir-378a |
|
|
| |
|
Mir-29c |
Mir-326 |
|
|
| |
|
Mir-29c |
Mir-331 |
|
|
| |
|
Mir-29c |
Mir-425 |
|
|
| |
|
Mir-29c |
Mir-744 |
|
|
| |
|
Mir-29c |
ENST00000387347 |
|
|
| |
|
Mir-328 |
Mir-331 |
|
|
| |
|
Mir-148b |
ENST00000365160 |
|
|
| |
|
Mir-331 |
Mir-574 |
|
|
| |
|
Mir-331 |
Mir-660 |
|
|
| Mir-425 | Mir-660 | ||||
The total number of small RNA combinations per group is shown within parentheses.
Discussion
In our study, we aimed to better discriminate metastasised (either lymphogenic or occult subtypes or both combined) from non-metastasised seminoma based on small RNA copy number changes examined in the primary tumour. A whole genome screening on small RNA species was performed using NGS. We demonstrated each metastasis subtype and its combination to be completely discriminated from non-metastasised seminoma using two small RNAs combined.
MiRNAs are previously described to be involved in different distant (e.g. migration, angiogenesis and colonisation of distant organs) and local (e.g. changes in tumour microenvironment) processes in metastatic cascade. Interestingly, the miRNAs identified in our study are involved in local tumour microenvironmental changes only. For instance, miRNAs 221 and 222 are involved in degradation of extracellular matrix via regulation of MMP-1 and associated with more aggressive lung cancer (Garofalo et al, 2009; Liu et al, 2009). Let-7 and miR-181 are involved in inflammatory processes as a part of the metastatic cascade. The Let-7 family targets oncogenes such as HMGA2 and KRAS and seems to be a key component in the so-called epigenetic switch (Johnson et al, 2005; Mayr et al, 2007; Iliopoulos et al, 2009). MicroRNA-181 inhibits tumour suppressor genes PTEN and CYLD (Iliopoulos et al, 2010). Cancer-associated fibroblasts are involved in tumour formation and progression where miR-15 and miR-16 regulate FGF2 and FGFR1 in prostate cancer (Musumeci et al, 2011), miR-18 and miR-19 in breast cancer (Yu et al, 2010) and miR-320 is reprogramming the tumour microenvironment by PTEN regulation (Bronisz et al, 2012). Owing to the link of our miRNAs with metastatic processes identified in other tumour entities, it could be speculated about their significance for identification of metastasis in other tumour entities. However, separate studies such as ours are necessary to prove that.
We did also identify miRNA changes being associated with cisplatin resistance in germ cell tumours (Port et al, 2011a). Recently, other authors demonstrated that miRNA 371-73 cluster and miRNA 302 allowed discriminating between seminoma-bearing patients and healthy persons (Gillis et al, 2007; Palmer et al, 2010; Dieckmann et al, 2012). In the next step, we examined the suitability of these miRNA to predict the metastasis status, but these miRNAs in our analysis appeared not significantly associated with the metastasis status.
The complete separation of metastasis from non-metastasised seminoma using a combination of two small RNA species points to the significant diagnostic potential of these biological markers, which is in line with previous examinations on the transcriptional level showing the superiority of molecular marker over epidemiological or clinical–histological parameter (Port et al, 2011b; Ruf et al, 2012).
Interestingly, the discrimination in our study occurred irrespective of the metastasis subtype, a finding that was expected, as we recently demonstrated lymphogenic and occult metastasised seminoma to be indistinguishable on the transcriptional level (Ruf et al, 2014).
The tumour size in this study appears different between metastasised and non-metastasised seminoma, indicating a potential for discriminating both groups. According to previous studies performed on larger groups (n=527), the association of tumour size with metastasis status appeared significant, but the discriminatory capacity of this and other clinical–histological parameter (e.g. tumour/testicular volume, tumour length) did not exceed a concordance of 65% (Ruf et al, 2013b), indicating a limited discrimination ability of tumour size in contrast to the small RNAs examined in this study. Moreover, according to our experiences RNA examinations should be performed in cryopreserved tissues or using solvents to protect RNA. We cannot recommend performing RNA analysis on paraffin-embedded tissue because of rapid and tissue-specific RNA degradation taking place during the fixation process with alcohol (Port et al, 2007).
Our study has certain weaknesses such as the low number of cases examined (total n=15). However, the molecular biological methodology applied provides a deepness that under financial considerations does not allow large-scale studies. Hence, this study provides hints towards certain small RNA species comprising a significant diagnostic potential for prediction of metastasis in seminoma, but certainly these candidate small RNA species have to be examined on a larger independent group for validation purposes using qRT–PCR as the gold standard for gene expression measurements.
Conclusion
In summary, metastasised and non-metastasised seminoma can be completely discriminated with a combination of two small RNAs irrespective of apparent lymphogenic or occult metastasis.
Acknowledgments
We thank S Senf for his skilful technical assistance. This work was supported by the German Ministry of Defense.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Albers P, Albrecht W, Algaba F, Bokemeyer C, Cohn-Cedermark G, Fizazi K, Horwich A, Laguna MP. [EAU guidelines on testicular cancer: 2011 update. European Association of Urology] Actas Urol Esp. 2012;36 (3:127–145. doi: 10.1016/j.acuro.2011.06.017. [DOI] [PubMed] [Google Scholar]
- Baggerly KA, Deng L, Morris JS, Aldaz CM. Overdispersed logistic regression for SAGE: modelling multiple groups and covariates. BMC Bioinform. 2004;5:144. doi: 10.1186/1471-2105-5-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronisz A, Godlewski J, Wallace JA, Merchant AS, Nowicki MO, Mathsyaraja H, Srinivasan R, Trimboli AJ, Martin CK, Li F, Yu L, Fernandez SA, Pecot T, Rosol TJ, Cory S, Hallett M, Park M, Piper MG, Marsh CB, Yee LD, Jimenez RE, Nuovo G, Lawler SE, Chiocca EA, Leone G, Ostrowski MC. Reprogramming of the tumour microenvironment by stromal PTEN-regulated miR-320. Nat Cell Biol. 2012;14 (2:159–167. doi: 10.1038/ncb2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieckmann KP, Spiekermann M, Balks T, Flor I, Loning T, Bullerdiek J, Belge G. MicroRNAs miR-371-3 in serum as diagnostic tools in the management of testicular germ cell tumours. Br J Cancer. 2012;107 (10:1754–1760. doi: 10.1038/bjc.2012.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofalo M, Di Leva G, Romano G, Nuovo G, Suh SS, Ngankeu A, Taccioli C, Pichiorri F, Alder H, Secchiero P, Gasparini P, Gonelli A, Costinean S, Acunzo M, Condorelli G, Croce CM. miR-221&222 regulate TRAIL resistance and enhance tumorigenicity through PTEN and TIMP3 downregulation. Cancer Cell. 2009;16 (6:498–509. doi: 10.1016/j.ccr.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gillis AJ, Stoop HJ, Hersmus R, Oosterhuis JW, Sun Y, Chen C, Guenther S, Sherlock J, Veltman I, Baeten J, van der Spek PJ, de Alarcon P, Looijenga LH. High-throughput microRNAome analysis in human germ cell tumours. J Pathol. 2007;213 (3:319–328. doi: 10.1002/path.2230. [DOI] [PubMed] [Google Scholar]
- Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139 (4:693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliopoulos D, Jaeger SA, Hirsch HA, Bulyk ML, Struhl K. STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Mol cell. 2010;39 (4:493–506. doi: 10.1016/j.molcel.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A, Labourier E, Reinert KL, Brown D, Slack FJ. RAS is regulated by the let-7 microRNA family. Cell. 2005;120 (5:635–647. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Kollmannsberger C, Tyldesley S, Moore C, Chi KN, Murray N, Daneshmand S, Black P, Duncan G, Hayes-Lattin B, Nichols C. Evolution in management of testicular seminoma: population-based outcomes with selective utilization of active therapies. Ann Oncol. 2011;22 (4:808–814. doi: 10.1093/annonc/mdq466. [DOI] [PubMed] [Google Scholar]
- Krege S, Beyer J, Souchon R, Albers P, Albrecht W, Algaba F, Bamberg M, Bodrogi I, Bokemeyer C, Cavallin-Stahl E, Classen J, Clemm C, Cohn-Cedermark G, Culine S, Daugaard G, De Mulder PH, De Santis M, de Wit M, de Wit R, Derigs HG, Dieckmann KP, Dieing A, Droz JP, Fenner M, Fizazi K, Flechon A, Fossa SD, del Muro XG, Gauler T, Geczi L, Gerl A, Germa-Lluch JR, Gillessen S, Hartmann JT, Hartmann M, Heidenreich A, Hoeltl W, Horwich A, Huddart R, Jewett M, Joffe J, Jones WG, Kisbenedek L, Klepp O, Kliesch S, Koehrmann KU, Kollmannsberger C, Kuczyk M, Laguna P, Galvis OL, Loy V, Mason MD, Mead GM, Mueller R, Nichols C, Nicolai N, Oliver T, Ondrus D, Oosterhof GO, Ares LP, Pizzocaro G, Pont J, Pottek T, Powles T, Rick O, Rosti G, Salvioni R, Scheiderbauer J, Schmelz HU, Schmidberger H, Schmoll HJ, Schrader M, Sedlmayer F, Skakkebaek NE, Sohaib A, Tjulandin S, Warde P, Weinknecht S, Weissbach L, Wittekind C, Winter E, Wood L, von der Maase H. European consensus conference on diagnosis and treatment of germ cell cancer: a report of the second meeting of the European Germ Cell Cancer Consensus group (EGCCCG): part I. Eur Urol. 2008;53 (3:478–496. doi: 10.1016/j.eururo.2007.12.024. [DOI] [PubMed] [Google Scholar]
- Liu X, Yu J, Jiang L, Wang A, Shi F, Ye H, Zhou X. MicroRNA-222 regulates cell invasion by targeting matrix metalloproteinase 1 (MMP1) and manganese superoxide dismutase 2 (SOD2) in tongue squamous cell carcinoma cell lines. Cancer Genom Proteom. 2009;6 (3:131–139. [PMC free article] [PubMed] [Google Scholar]
- Mayr C, Hemann MT, Bartel DP. Disrupting the pairing between let-7 and Hmga2 enhances oncogenic transformation. Science. 2007;315 (5818:1576–1579. doi: 10.1126/science.1137999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musumeci M, Coppola V, Addario A, Patrizii M, Maugeri-Sacca M, Memeo L, Colarossi C, Francescangeli F, Biffoni M, Collura D, Giacobbe A, D'Urso L, Falchi M, Venneri MA, Muto G, De Maria R, Bonci D. Control of tumor and microenvironment cross-talk by miR-15a and miR-16 in prostate cancer. Oncogene. 2011;30 (41:4231–4242. doi: 10.1038/onc.2011.140. [DOI] [PubMed] [Google Scholar]
- Palmer RD, Murray MJ, Saini HK, van Dongen S, Abreu-Goodger C, Muralidhar B, Pett MR, Thornton CM, Nicholson JC, Enright AJ, Coleman N, Children's C, Leukaemia G. Malignant germ cell tumors display common microRNA profiles resulting in global changes in expression of messenger RNA targets. Cancer Res. 2010;70 (7:2911–2923. doi: 10.1158/0008-5472.CAN-09-3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Port M, Glaesener S, Ruf C, Riecke A, Bokemeyer C, Meineke V, Honecker F, Abend M. Micro-RNA expression in cisplatin resistant germ cell tumor cell lines. Mol Cancer. 2011;10:52. doi: 10.1186/1476-4598-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Port M, Schmelz HU, Stassen T, Mueller K, Stockinger M, Obermair R, Abend M. Correcting false gene expression measurements from degraded RNA using RTQ–PCR. Diagn Mol Pathol. 2007;16 (1:38–49. doi: 10.1097/01.pdm.0000213472.70054.94. [DOI] [PubMed] [Google Scholar]
- Port M, Wang Y, Schmelz HU, Pottek T, Meineke V, Ruf C, Abend M. A gene signature of primary tumor identifies metastasized seminoma. Urol Oncol. 2011;29 (6:764–773. doi: 10.1016/j.urolonc.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Ruf CG, Isbarn H, Wagner W, Fisch M, Matthies C, Dieckmann KP. Changes in epidemiologic features of testicular germ cell cancer: age at diagnosis and relative frequency of seminoma are constantly and significantly increasing. Urol Oncol. 2013;32 (1:33.e1–6. doi: 10.1016/j.urolonc.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Ruf CG, Khalili-Harbi N, Sachs S, Isbarn H, Wagner W, Matthies C, Meineke V, Fisch M, Chun FK, Abend M. The search for biomarkers of metastatic seminoma. J Urol. 2013;190 (3:1046–1051. doi: 10.1016/j.juro.2013.04.022. [DOI] [PubMed] [Google Scholar]
- Ruf CG, Linbecker M, Port M, Riecke A, Schmelz HU, Wagner W, Meineke V, Abend M. Predicting metastasized seminoma using gene expression. BJU Int. 2012;110 (Part 2:E14–E20. doi: 10.1111/j.1464-410X.2011.10778.x. [DOI] [PubMed] [Google Scholar]
- Ruf CG, Port M, Schmelz H-U, Wagner W, Müller F, Senf S, Matthies C, Müller-Myhsok B, Meineke V, Abend M.2014Clinically apparent and occult metastasized seminoma: almost indistinguishable on the transcriptional level PLoS Onee-pub ahead of print 1 May 2014;doi: 10.1371/journal.pone.0095009 [DOI] [PMC free article] [PubMed]
- Valdevenito JP, Gallegos I, Fernandez C, Acevedo C, Palma R. Correlation between primary tumor pathologic features and presence of clinical metastasis at diagnosis of testicular seminoma. Urology. 2007;70 (4:777–780. doi: 10.1016/j.urology.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Warde P, Gospodarowicz MK, Banerjee D, Panzarella T, Sugar L, Catton CN, Sturgeon JF, Moore M, Jewett MA.1997Prognostic factors for relapse in stage I testicular seminoma treated with surveillance J Urol 157(51705–1709.; discussion 1709–1710. [PubMed] [Google Scholar]
- Warde P, Specht L, Horwich A, Oliver T, Panzarella T, Gospodarowicz M, von der Maase H. Prognostic factors for relapse in stage I seminoma managed by surveillance: a pooled analysis. J Clin Oncol. 2002;20 (22:4448–4452. doi: 10.1200/JCO.2002.01.038. [DOI] [PubMed] [Google Scholar]
- Yu Z, Willmarth NE, Zhou J, Katiyar S, Wang M, Liu Y, McCue PA, Quong AA, Lisanti MP, Pestell RG. MicroRNA 17/20 inhibits cellular invasion and tumor metastasis in breast cancer by heterotypic signaling. Proc Natl Acad Sci USA. 2010;107 (18:8231–8236. doi: 10.1073/pnas.1002080107. [DOI] [PMC free article] [PubMed] [Google Scholar]