Abstract
Background:
In 2008, a national human papillomavirus (HPV) immunisation programme began in Scotland for 12–13 year old females with a three-year catch-up campaign for those under the age of 18. Since 2008, three-dose uptake of bivalent vaccine in the routine cohort aged 12–13 has exceeded 90% annually, while in the catch-up cohort overall uptake is 66%.
Methods:
To monitor the impact of HPV immunisation, a programme of national surveillance was established (pre and post introduction) which included yearly sampling and HPV genotyping of women attending for cervical screening at age 20. By linking individual vaccination, screening and HPV testing records, we aim to determine the impact of the immunisation programme on circulating type-specific HPV infection particularly for four outcomes: (i) the vaccine types HPV 16 or 18 (ii) types considered to be associated with cross-protection: HPV 31, 33 or 45; (iii) all other high-risk types and (iv) any HPV.
Results:
From a total of 4679 samples tested, we demonstrate that three doses (n=1100) of bivalent vaccine are associated with a significant reduction in prevalence of HPV 16 and 18 from 29.8% (95% confidence interval 28.3, 31.3%) to 13.6% (95% confidence interval 11.7, 15.8%). The data also suggest cross-protection against HPV 31, 33 and 45. HPV 51 and 56 emerged as the most prevalent (10.5% and 9.6%, respectively) non-vaccine high-risk types in those vaccinated, but at lower rates than HPV 16 (25.9%) in those unvaccinated.
Conclusions:
This data demonstrate the positive impact of bivalent vaccination on the prevalence of HPV 16, 18, 31, 33 and 45 in the target population and is encouraging for countries which have achieved high-vaccine uptake.
Keywords: human papillomavirus, vaccine, impact, prevalence
It has been estimated that 15–20% of all human cancers are preceded by viral infection (Parkin et al, 2006). Persistent infection with high-risk human papillomavirus is considered requisite for the development of certain anogenital cancers, including cervix, and a subset of oropharyngeal cancers (Smith et al, 2007; Kreimer et al, 2011a; Junor et al, 2012). Human papillomavirus (HPV) types 16 and/or 18 are implicated in the majority of cervical cancers globally and local data would suggest these types are responsible for approximately 80% of cervical cancers in Scotland (Cuschieri et al, 2010).
HPV immunisation programmes are now implemented in many countries, although the methods of delivery, uptake, gender policy and monitoring systems vary (Markowitz et al, 2012), as does the choice of vaccines which differ in valency, long-term immunogenicity and cross-protective ability (Romanowski, 2011). In Scotland, a school-based vaccination programme for 12–13-year-old girls, with an initial 3-year catch-up of 13–17 year olds, commenced in September 2008 using the bivalent prophylactic HPV vaccine, Cervarix . Uptake of vaccine in the routine cohort (12–13 year olds) has been high, with levels sustained around 90% (2010/11 uptake at August 2012—dose 1: 93% dose 2: 92% and dose 3: 90%) (Sinka et al, 2014). The 3-year catch-up campaign attained a high uptake for all three doses among those vaccinated in school, exceeding 80% (year 2 catch-up 2009/10—dose 1: 91% dose 2: 90% and dose 3: 85%), but a lower uptake of around 30% was achieved in school leavers (year 2 school leavers 2009/10—dose 1: 49% dose 2: 41% and dose 3: 29%). In order to determine the impact of HPV immunisation, a longitudinal national HPV surveillance programme was set up in Scotland, a key element of which involved yearly sampling and HPV genotyping of women attending for their cervical screen at age 20.
Although HPV immunisation has been shown to reduce the prevalence of HPV types 16 and 18 in the vaccine trials (Paavonen et al, 2009; Wheeler et al, 2012), few studies have reported on the impact of vaccination on type-specific HPV infection in national programmes and to our knowledge, this is the first which assesses the impact of the bivalent vaccination in relation to HPV genoprevalence. The PATRICIA trial of Cervarix, a double-blind randomised study of women aged 15–25 years, which considered the efficacy of the vaccine against CIN2+ lesions and 6- and 12-month persistent infection with non-vaccine types, reported end-of-trial data (Wheeler et al, 2012) of efficacy against HPV 31, 33 and 45 and a sub-group analysis of the Costa Rica Vaccine randomised control trial suggested high efficacy for prevention of infection with fewer than three doses (Kreimer et al, 2011b). If such observations are corroborated at a population level, they will convey additional public health and cost effectiveness benefits (Kim et al, 2012).
Given the timing of the catch-up programme and the fact that first screening invitation in Scotland is before aged 21, we are in a position to assess post-vaccination HPV genoprevalence in women aged 20–21 years according to their individual vaccination status through the linkage of national databases for immunisation and screening.
We aimed to compare HPV DNA type-specific prevalence in unvaccinated and vaccinated 20–21 year olds to provide information on the early impact of bivalent HPV vaccination at the population level. By performing a comprehensive genotyping assay for surveillance, as opposed to focusing on HPV 16/18 only, we aimed to gain insights into cross-protection, type replacement and unmasking by examining the occurrence of multiple high-risk infections in young vaccinated women (Tota et al, 2013).
Materials and methods
Sampling
We conducted a cross-sectional study that sampled ∼1000 women aged 20–21 years attending their cervical screening appointment over the period 2009–2012 to establish HPV prevalence in vaccinated and unvaccinated women in Scotland.
Scotland has a population of ∼5.2 million (National Records of Scotland, 2012) and an organised cytology-based cervical screening programme. Screening is currently recommended every 3 years for women aged 20–60 years who are first invited to attend shortly after their 20th birthday. All women eligible for cervical screening are recorded in the national Scottish Cervical screening Call and Recall System (SCCRS), a population-based information technology system, which supports the programme and contains pathology, recall and management information.
From 2009 to 2012, anonymised residual liquid-based cytology (LBC) samples from young women aged 20–21 years were collected from all 11 NHS cytopathology laboratories in Scotland, which served the national programme. The inclusion of samples from 2009–2010 before vaccinated girls were eligible for screening allowed similar numbers of vaccinated and unvaccinated girls to be compared as outlined in Kavanagh et al (2013). To achieve the desired sample size of 1000 specimens per year, each laboratory collected residual samples following cervical screening over a 2-month period from women aged 20–21 in that year; the exact number from each laboratory was dictated by the size of the population served by the laboratory, to ensure a geographically representative sample. The collection periods from each laboratory were staggered throughout the year, to balance workload in the HPV Reference laboratory at the Royal Infirmary of Edinburgh.
Data and linkage
At the source cytology laboratories, all LBC samples were labelled with an anonymous study identification number and sent to the HPV Reference laboratory for HPV genotyping. Separately, the study ID and the patient Community Health Index reference (CHI, a unique national patient identifier) were sent to the Information Services Division (ISD) of the National Health Service in Scotland, who used CHI to link to SCCRS and the national Scottish Immunisation call Recall System (SIRS) and Child Health Schools Programme-System (CHSP-S) data. Geographical data-zone (a small-area statistical geography see (Scottish Government, 2005)) derived from the postcode of residence, was attributed to each record and the Scottish Index of Multiple Deprivation (SIMD) (a ranking of data-zones in terms of multiple deprivation see (Scottish Government, 2012)) assigned. All personal identifiable information was removed before passing to Health Protection Scotland for analysis. Health Protection Scotland used the study ID in these records to link to genotyping results from the HPV Reference Laboratory.
HPV testing
Residual LBC samples were vortexed and a 1-ml aliquot was used for extraction. Automated extraction for LBC samples was performed used the MDX media Kit (Qiagen, Venlo, Netherlands). HPV amplification and genotyping was performed using the Multimetrix HPV Assay (Diamex, Heidelberg, Germany) (Schmitt et al, 2006). This assay is based on luminex technology and is capable of detecting 18 high-risk or putatively high-risk types according to current IARC classification (Bouvard et al, 2009), specifically the 12 types in Group1 with ‘carcinogenic' status (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59), the single type in Group 2A with ‘probably carcinogenic' status (HPV 68) and six types from Group 2B with ‘possibly carcinogenic' status (HPV 26, 53, 66, 70, 73, 82). In addition, five low-risk types are detected (6, 11, 42, 43, 44).
Statistical analysis
The sample size of 2000 LBC specimens before vaccination initiation (∼1000 in each of 2009 and 2010) and 1000 per year thereafter provided at least a 99% power to detect a 15% reduction in HPV prevalence at first cervical screen from 40 to 34%, a 25% reduction in high-risk human papillomavirus prevalence at first cervical screen from 25 to 19%, and a 40% reduction in HPV 16/18 prevalence at first cervical screen from 12 to 7%.
HPV type-specific prevalence and associated 95% confidence intervals (CI) were calculated. Differences in HPV type-specific prevalence of those receiving three doses of vaccination and no vaccination were assessed via a z-test of two proportions using the Bonferroni correction (significance level, α=0.05/22) to account for the multiple statistical testing conducted for the 22 non-vaccine HPV types. As the analysis was powered to detect changes in HPV types 16 and 18, their significance is assessed at α=0.05. The associations between the number of doses of vaccination (0, 1, 2 or 3 doses) and HPV outcome measure were assessed using logistic regression adjusted for birth cohort year and deprivation score (assessed via the SIMD quintiles of the area of residence: one is most deprived and five is least deprived) and evidence of a linear change in positivity over the range of these variables was assessed via a linear trend test. The interactions of birth cohort year and number of doses and of SIMD and number of doses were considered, but none found to be significant. HPV outcomes considered were; HPV types 16 or 18; HPV types 31, 33 or 45; all high-risk HPV (IARC Group 1 and Group 2A) excluding types 16, 18, 31, 33, 45 and finally; positivity for any HPV.
This analysis allowed us to examine whether there is a statistically significant reduction of HPV 16/18, potential cross-reactive types or other high-risk HPV types associated with number of doses of vaccine received. In addition, we evaluated the common pairings of HPV types detected and how these differ between the vaccinated and non-vaccinated women by birth cohort. In those with more than two high-risk types present, all possible pairings were counted. We examined the robustness of our conclusions on the effectiveness of the vaccine in reducing HPV 16/18 prevalence by conducting two sensitivity analyses: (i) limiting the analysis to those born in 1991 and 1992 (this removes the temporal bias that the unvaccinated are mainly those born in 1988–1990, but induces an out-of-school bias where the unvaccinated group is likely to be mainly those who had left school) and (ii) excluding the unvaccinated in 1991 and 1992 (this limits the out-of-school bias, but has unvaccinated and vaccinated individuals compared over different time frames).
Results
Overall, 4729 LBC samples were collected (∼1000 per year, distributed consistently (∼20%) over the deprivation quintiles) and tested for HPV with 4679 valid HPV test results obtained (Table 1). Overall, 57.7% (95% CI 56.2, 59.1%) of samples were positive for any HPV type and 47.4% (95% CI 46.0, 48.8%) were positive for a high-risk HPV type. Due to eligibility, vaccination status varied strongly by collection year with 38% of samples collected in 2011 being from women who were vaccinated with three doses of vaccination compared with 67% in 2012 (Table 1). In years 2009 and 2010, samples are from those born in 1988, 1989 and 1990; the majority of whom are unvaccinated due to ineligibility for the catch-up campaign. Overall, 23.4% of the 4729 LBC samples that were tested originated from fully vaccinated individuals and 3.4% from partially vaccinated individuals.
Table 1. Yearly distribution of the number of samples collected (n=4729) by number of vaccine doses received, the Scottish Index of Multiple Deprivation (SIMD) and the number of samples with viable HPV results.
|
Collection year |
||||||||
|---|---|---|---|---|---|---|---|---|
|
2009 |
2010 |
2011 |
2012 |
|||||
| Total n samples |
1672 |
1074 |
1005 |
978 |
||||
| n | % | n | % | n | % | n | % | |
|
Number of doses | ||||||||
| 0 | 1653 | 98.9% | 1012 | 94.2% | 557 | 55.4% | 241 | 24.6% |
| 1 | 4 | 0.2% | 7 | 0.7% | 18 | 1.8% | 26 | 2.7% |
| 2 | 1 | 0.1% | 7 | 0.7% | 48 | 4.8% | 50 | 5.1% |
| 3 | 14 | 0.8% | 48 | 4.5% | 382 | 38.0% | 661 | 67.6% |
|
SIMD | ||||||||
| 1: Most deprived | 386 | 23.1% | 260 | 24.2% | 235 | 23.4% | 214 | 21.9% |
| 2 | 388 | 23.2% | 208 | 19.4% | 190 | 18.9% | 197 | 20.1% |
| 3 | 335 | 20.0% | 219 | 20.4% | 185 | 18.4% | 166 | 17.0% |
| 4 | 271 | 16.2% | 193 | 18.0% | 201 | 20.0% | 187 | 19.1% |
| 5: Least deprived | 292 | 17.5% | 194 | 18.1% | 194 | 19.3% | 214 | 21.9% |
| Total samples with valid HPV results | 1651 | 98.7% | 1053 | 98.0% | 1001 | 99.6% | 974 | 99.5% |
Abbreviations: HPV=human papillomavirus; SIMD=Scottish Index of Multiple Deprivation.
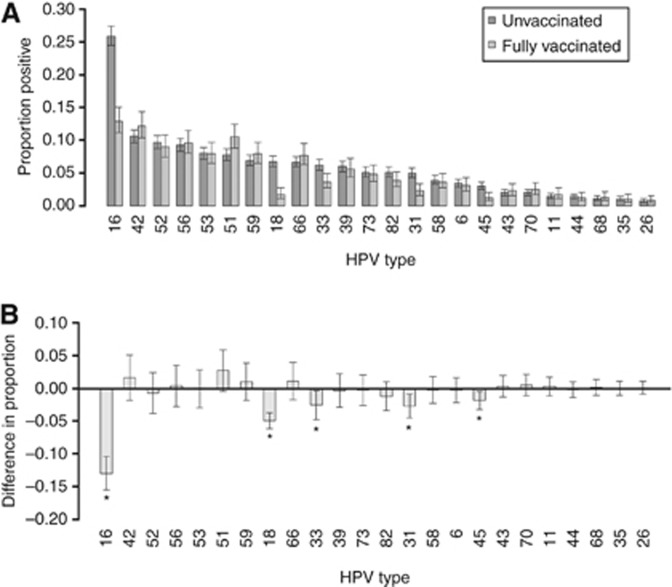
The prevalence and 95% CI of each of the HPV types detected, stratified by vaccine status, is shown in Figure 1. There was an increase in the detection of high-risk HPV types 51 and 59 in those who had received three doses of vaccination compared with the unvaccinated group, but these increases were not statistically significant. We found no decrease in HPV6/11 positivity across the study years (2009: 4.2%, 2010: 5.2%, 2011: 5%, 2012: 5.5%) nor with number of vaccine received (0 dose: 4.7%, 1 dose: 12.7%, 2 doses: 9.4%, 3 doses: 4.6%).
Figure 1.
(A) Proportion and 95% CI of anonymised 4679 LBC samples in 2009–2012, which tested positive for each HPV type in unvaccinated and fully vaccinated (three doses) women attending for their cervical screen. (B) Difference in the proportion positive and associated 95% CI for the difference by HPV type. With the exception of types 16 and 18, the 95% CIs of the difference were corrected for multiple testing using the Bonferroni correction. *statistically significant change.
HPV positivity for vaccine HPV genotypes (HPV 16 or 18)
Vaccine was associated with a statistically significant reduction in the prevalence of both HPV 16 and 18 (both P<0.0001) (Figure 1). Overall, positivity for HPV 16 or 18 was 13.6% (95% CI 11.7, 15.8%) in those receiving three doses of vaccine compared with 29.8% (95% CI 28.3, 31.3%) in those who were unvaccinated (Table 2). This is mirrored by the change over time with genoprevalence decreasing from 28.8% (95% CI 26.7, 31.1%) in the 2009 samples to 16.7% (95% CI 14.5, 19.2%) in the 2012 samples. Once adjusted for vaccination status, there was no overall significant linear trend of birth cohort year or SIMD on positivity for HPV 16 or 18 (P=0.07 and 0.1, respectively) (Table 3). There was a strong effect of vaccination for those women vaccinated, with three doses showing a significant reduction in HPV type 16 and 18 infection in the fully adjusted model (Table 3). This effect is stronger when the unadjusted effect of vaccination is considered: three doses (odds ratio (OR)=0.37, 95% CI 0.31, 0.45) and for two doses (OR=0.61, 95% CI 0.38, 0.99). Although one dose also showed a reduction in HPV type 16 and 18, (OR=0.88, 95% CI 0.48, 1.6) this was not statistically significant (P=0.68) in the unadjusted model.
Table 2. Prevalence of HPV 16 or 18, cross-protective types, high-risk HPV excluding HPV 16, 18, 31, 33 or 45 and any HPV stratified by the number of doses received and the year of collection (N=4679).
|
HPV 16 or 18 |
Cross-protective typesa |
High-risk HPV excluding vaccine and cross-protective typesb |
Any HPV |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Number positive | Prevalence | 95% CI | Number positive | Prevalence | 95% CI | Number positive | Prevalence | 95% CI | Number positive | Prevalence | 95% CI | |
|
Number of doses | |||||||||||||
| 0 | 3418 | 1018 | 29.8% | (28.3, 31.3)% | 448 | 13.1% | (12.0, 14.3)% | 1108 | 32.4% | (30.9, 34.0)% | 2041 | 59.7% | (58.1, 61.3)% |
| 1 | 55 | 15 | 27.3% | (17.3, 40.2)% | 10 | 18.2% | (10.2, 30.3)% | 21 | 38.2% | (26.5, 51.4)% | 37 | 67.3% | (54.1, 78.2)% |
| 2 | 106 | 22 | 20.8% | (14.1, 29.4)% | 8 | 7.5% | (3.9, 14.2)% | 36 | 34.0% | (25.6, 43.4)% | 64 | 60.4% | (50.9, 69.2)% |
| 3 | 1100 | 150 | 13.6% | (11.7, 15.8)% | 75 | 6.8% | (5.5, 8.5)% | 348 | 31.6% | (29.0, 34.4)% | 587 | 53.4% | (50.4, 56.3)% |
|
Collection year | |||||||||||||
| 2009 | 1651 | 476 | 28.8% | (26.7, 31.1)% | 215 | 13.0% | (11.5, 14.7)% | 480 | 29.1% | (26.9, 31.3)% | 959 | 58.1% | (55.7, 60.4)% |
| 2010 | 1053 | 333 | 31.6% | (28.9, 34.5)% | 143 | 13.6% | (11.6, 15.8)% | 364 | 34.6% | (31.8, 37.5)% | 618 | 58.7% | (55.7, 61.6)% |
| 2011 | 1001 | 233 | 23.3% | (20.8, 26.0)% | 104 | 10.4% | (8.6, 12.4)% | 330 | 33.0% | (30.1, 35.9)% | 587 | 58.6% | (55.6, 61.7)% |
| 2012 | 974 | 163 | 16.7% | (14.5, 19.2)% | 79 | 8.1% | (6.6, 10.0)% | 339 | 34.8% | (31.9, 37.9)% | 565 | 58.0% | (54.9, 61.1)% |
Abbreviations: CI=confidence interval; HPV=human papillomavirus.
Cross-protective types: HPV 31, 33 or 45.
High-risk HPV excluding vaccine and cross-protective types: HPV 35, 39, 51, 52, 56, 58, 59 or 68.
Table 3. Fully adjusted odds of positivity for various groupings of HPV type by cohort year, number of doses received and SIMD.
|
HPV 16 or 18 |
Cross-protective typesa |
High-risk HPV excluding vaccine and cross-protective typesb |
Any HPV |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | Linear trend P-value | OR | 95% CI | Linear trend P-value | OR | 95% CI | Linear trend P-value | OR | 95% CI | Linear trend P-value | |
| Birth year | 0.07 | 0.45 | 0.002 | 0.02 | ||||||||
| 1988 | 1 | — | 1 | — | 1 | — | 1 | — | ||||
| 1989 | 0.96 | (0.79, 1.17) | 0.82 | (0.63, 1.06) | 1.18 | (0.97, 1.43) | 1.08 | (0.90, 1.29) | ||||
| 1990 | 1.06 | (0.87, 1.29) | 0.99 | (0.76, 1.28) | 1.40 | (1.15, 1.70) | 1.11 | (0.93, 1.33) | ||||
| 1991 | 0.95 | (0.73, 1.23) | 0.89 | (0.62, 1.26) | 1.44 | (1.12, 1.84) | 1.44 | (1.14, 1.82) | ||||
| 1992 | 0.72 | (0.52, 0.98) | 0.79 | (0.52, 1.21) | 1.53 | (1.16, 2.01) | 1.29 | (0.99, 1.67) | ||||
| Number of doses | <0.0001 | <0.0001 | 0.08 | 0.0009 | ||||||||
| 0 | 1 | — | 1 | — | 1 | — | 1 | — | ||||
| 1 | 0.95 | (0.51, 1.76) | 1.44 | (0.70, 2.96) | 1.09 | (0.62, 1.91) | 1.18 | (0.66, 2.11) | ||||
| 2 | 0.68 | (0.42, 1.12) | 0.55 | (0.26, 1.17) | 0.90 | (0.59, 1.39) | 0.88 | (0.58, 1.33) | ||||
| 3 | 0.43 | (0.34, 0.55) | 0.53 | (0.38, 0.74) | 0.81 | (0.66, 0.99) | 0.66 | (0.54, 0.80) | ||||
| SIMD quintile | 0.1 | 0.005 | 0.48 | 0.009 | ||||||||
| 1: Most deprived | 1 | — | 1 | — | 1 | — | 1 | — | ||||
| 2 | 0.89 | (0.73, 1.08) | 1.02 | (0.79, 1.33) | 1.01 | (0.84, 1.21) | 0.94 | (0.79, 1.12) | ||||
| 3 | 0.89 | (0.73, 1.10) | 0.92 | (0.70, 1.20) | 1.04 | (0.86, 1.26) | 0.89 | (0.74, 1.06) | ||||
| 4 | 0.94 | (0.76, 1.15) | 0.66 | (0.49, 0.89) | 1.01 | (0.84, 1.23) | 0.93 | (0.77, 1.11) | ||||
| 5: Least deprived | 0.80 | (0.65, 0.98) | 0.79 | (0.59, 1.05) | 0.92 | (0.76, 1.12) | 0.77 | (0.64, 0.92) | ||||
Abbreviations: CI=confidence interval; HPV=human papillomavirus; OR=odds ratio; SIMD=Scottish Index of Multiple Deprivation. Bold values highlight the statistically significant associations.
Cross protective types: HPV 31, 33 or 45.
High-risk HPV excluding vaccine and cross-protective types: HPV 35, 39, 51, 52, 56, 58, 59 or 68.
HPV positivity for potentially cross-protective HPV types (HPV 31, 33 or 45)
Along with the vaccine-specific types, the vaccine also afforded cross-protection against other high-risk types, with statistically significant reductions in the prevalence between fully vaccinated and unvaccinated groups for types HPV 31, 33 or 45 (P=0.0002; P=0.002; P=0.001, respectively) (Figure 1). Overall positivity for HPV 31, 33 or 45 was 6.8% (95% CI 5.5, 8.5%) in those receiving three doses of vaccine compared with 13.1% (95% CI 12.0, 14.3%) in those who were unvaccinated (Table 2). There is a similar change over time with prevalence decreasing from 13.0% (95% CI 11.5, 14.7%) in the 2009 samples to 8.1% (95% CI 6.6, 10.0%) in the 2012 samples. Those who had received three doses had a significantly reduced odds of HPV types 31, 33 or 45 infection (adjusted OR=0.53, 95% CI 0.38, 0.74) and those with two doses had a reduced odds of infection, although this was not statistically significant (adjusted OR=0.55, 95% CI 0.26, 1.17). As with HPV types 16 and 18, there was no significant effect of cohort year on positivity for HPV types 31, 33 or 45 when adjusted for vaccination status (P=0.45) (Table 3). A significant linear effect of SIMD (P=0.005) remained for cross-protective type positivity whereby women residing in the least deprived areas were less likely to be infected than those in the most deprived (SIMD4 adjusted OR=0.66, 95% CI (0.49, 0.89), SIMD5 adjusted OR=0.79, 95% CI (0.59, 1.05)).
HPV positivity for non-vaccine high-risk types (any HR-HPV excluding 16, 18, 31, 33, 45)
Prevalence for any HR-HPV excluding 16, 18, 31, 33 and 45 was 31.6% (95% CI 29.0, 34.4%) in those receiving three doses of vaccine compared with 32.4% (95% CI 30.9, 34.0%) in those who were unvaccinated (Table 2). There is, however, an increase over time with prevalence rising from 29.1% (95% CI 26.9, 31.3%) in the 2009 samples to 34.8% (95% CI 31.9, 37.9%) in the 2012 samples confounding the prevalence rates. There was a significant linear trend in positivity with birth cohort (P=0.002) over and above the effect of vaccination. Those born in 1991 were 1.44 times (95% CI 1.12, 1.84) more likely to be infected with a non-vaccine high-risk type than those born in 1988 and similarly those born in 1992 were 1.53 times more likely (95% CI 1.16, 2.01). When adjusting for this, those receiving three doses of vaccine had a reduced odds of infection with high-risk types (OR=0.81, 95% CI 0.66, 0.99) (Table 3). SIMD was not found to have a significant relationship with high-risk HPV type positivity excluding types 16, 18, 31, 33 and 45 (P=0.48).
Overall HPV positivity (any HPV type)
Overall HPV positivity was generally unchanged over time—58.1% in 2009 and 58.0% in 2012 (Table 2) despite those who received three doses of vaccination being less likely to be positive for any HPV (adjusted OR=0.66, 95% CI 0.54, 0.80) (Table 3). This is due to a general increasing trend (P=0.02) with birth cohort (positivity by year: 1988; 56.6%, 1989; 58.5%, 1990; 57.9%, 1991; 59.5%, 1992; 55%). Compared with the reference year 1988, the first cohort vaccinated as part of the catch-up campaign to attend screening (birth year 1991) shows an increased adjusted odds of overall HPV positivity (OR=1.44, 95% CI 1.14, 1.82). Those in the least deprived group were significantly less likely to be positive (OR=0.77, 95% CI 0.64, 0.92).
Sensitivity analyses
For the HPV 16/18 outcome, restriction of the analysis to those in the 1991 and 1992 cohorts, thus removing the temporal imbalance between the unvaccinated and vaccinated groups, showed a stronger effect of vaccination than the baseline analysis (adjusted OR=0.34, 95% CI 0.25, 0.46) (Table 4). Limiting the out-of-school effect in the unvaccinated (using all year cohorts but excluding the unvaccinated in 1991 and 1992 birth cohorts) shows a weaker but still significant effect of vaccination (OR=0.65, 95% CI 0.44, 0.95).
Table 4. Results of two sensitivity analyses for the HPV 16 or 18 outcome.
|
Sensitivity analysis 1: limit to 1991 and 1992 cohorts |
Sensitivity analysis 2: exclude unvaccinated in 1991 and 1992 cohorts |
|||||||
|---|---|---|---|---|---|---|---|---|
| N | % positive | OR | 95% CI | N | % positive | OR | 95% CI | |
|
Birth year | ||||||||
| 1988 | — | — | — | — | 845 | 29.7% | 1 | |
| 1989 | — | — | — | — | 1195 | 28.7% | 0.96 | (0.79, 1.17) |
| 1990 | — | — | — | — | 1025 | 34.0% | 1.01 | (0.83, 1.24) |
| 1991 | 839 | 20.1% | 1 | — | 562 | 15.3% | 0.60 | (0.38, 0.95) |
| 1992 | 646 | 14.4% | 0.79 | (0.59, 1.05) | 523 | 11.1% | 0.43 | (0.26, 0.69) |
|
Number of doses | ||||||||
| 0 | 400 | 29.5% | 1 | — | 3063 | 29.4% | 1 | — |
| 1 | 41 | 21.9% | 0.65 | (0.29, 1.41) | 55 | 27.3% | 1.36 | (0.70, 2.67) |
| 2 | 85 | 21.2% | 0.64 | (0.37, 1.14) | 106 | 20.8% | 1.00 | (0.57, 1.77) |
| 3 | 959 | 12.2% | 0.34 | (0.26, 0.46) | 1105 | 13.6% | 0.64 | (0.44, 0.95) |
|
SIMD quintile | ||||||||
| 1: Most deprived | 354 | 18.9% | 1 | — | 995 | 26.7% | 1 | — |
| 2 | 310 | 20.0% | 1.07 | (0.72, 1.59) | 893 | 24.9% | 0.90 | (0.73, 1.11) |
| 3 | 246 | 17.9% | 0.96 | (0.62, 1.47) | 829 | 25.3% | 0.91 | (0.73, 1.12) |
| 4 | 271 | 16.9% | 1.01 | (0.66, 1.55) | 799 | 25.8% | 0.98 | (0.80, 1.22) |
| 5: Least deprived | 304 | 14.1% | 0.76 | (0.49, 1.17) | 813 | 22.5% | 0.82 | (0.66, 1.03) |
Abbreviations: CI=confidence interval; OR=odds ratio; SIMD=Scottish Index of Multiple Deprivation. Bold values highlight the statistically significant associations.
Most common HPV pairings
Of those infected with a high-risk HPV type, 50.0% (1123/2244) were infected with more than one high-risk HPV type. Of these individuals, 27% had two types of high-risk HPV present, 30% three, 22% four, 11% five and 10% had more than five types. Infection with non-vaccine high-risk types was common with 96.7% (1086/1123) having these types (not 16/18) present. Excluding the vaccine and cross-reactive types, 86.1% (968/1123) had another high-risk type present. Table 5 shows that in unvaccinated women in all birth cohorts, HPV 16 was the most common HPV type and was frequently detected concurrently with types 52, 59 and 56. In the fully vaccinated women in the 1991 and 1992 birth cohorts, HPV 16 no longer dominates and pairings of HPV 52 with HPV 56 and HPV 51 with HPV 56 are common (13 and 12% of those with multiple high-risk HPV types, respectively compared with 7.1 and 8.1% in unvaccinated individuals in the same birth cohort). The pairing of HPV 39 with HPV 56 was found in 10.4% of those vaccinated in the 1991/92 birth cohort with multiple high-risk types, compared with 4.7% in those unvaccinated in the 1988/89/90 birth cohort.
Table 5. Most commonly occurring pairings of HPV types in those infected with multiple types, stratified by vaccine status and year.
|
1988/89/90 cohorts
Unvaccinated
N=771 |
1988/89/90 cohorts
Fully vaccinated
N=27 |
1991/92 cohorts
Unvaccinated
N=99 |
1991/92 cohorts
Fully vaccinated
N=192 |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rank | HPV pairing | % of samples with multiple HR types | 95% CI | HPV pairing | % of samples with multiple HR types | 95% CI | HPV pairing | % of samples with multiple HR types | 95% CI | HPV pairing | % of samples with multiple HR types | 95% CI | ||||
| 1 | 16 | 52 | 19.2 | (16.6, 22.1) | 16 | 56 | 22.2 | (10.6, 40.8) | 16 | 52 | 13.1 | (7.8, 21.2) | 52 | 56 | 13 | (9, 18.5) |
| 2 | 16 | 56 | 18.3 | (15.7, 21.2) | 16 | 52 | 18.5 | (8.2, 36.7) | 16 | 59 | 10.1 | (5.6, 17.6) | 51 | 56 | 12 | (8.1, 17.3) |
| 3 | 16 | 59 | 13.1 | (10.9, 15.7) | 39 | 59 | 14.8 | (5.9, 32.5) | 16 | 45 | 10.1 | (5.6, 17.6) | 39 | 59 | 10.4 | (6.8, 15.5) |
| 4 | 16 | 51 | 11.5 | (9.5, 14.0) | 16 | 59 | 14.8 | (5.9, 32.5) | 16 | 31 | 9.1 | (4.9, 16.4) | 51 | 59 | 9.4 | (6.0, 14.3) |
| 5 | 16 | 18 | 11.4 | (9.4, 13.9) | 16 | 51 | 14.8 | (5.9, 32.5) | 51 | 56 | 8.1 | (4.2, 15.1) | 16 | 51 | 9.4 | (6.0, 14.3) |
| 6 | 16 | 31 | 10.6 | (8.7, 13.0) | 16 | 18 | 14.8 | (5.9, 32.5) | 16 | 33 | 8.1 | (4.2, 15.1) | 16 | 59 | 8.9 | (5.6, 13.7) |
| 7 | 16 | 33 | 10.0 | (8.1, 12.3) | 51 | 52 | 11.1 | (3.9, 28.1) | 52 | 56 | 7.1 | (3.5, 13.9) | 51 | 52 | 8.3 | (5.2, 13.1) |
| 8 | 16 | 39 | 9.1 | (7.2, 11.3) | 16 | 45 | 11.1 | (3.9, 28.1) | 16 | 58 | 7.1 | (3.5, 13.9) | 16 | 52 | 8.3 | (5.2, 13.1) |
| 9 | 52 | 56 | 7.3 | (5.6, 9.3) | 51 | 59 | 7.4 | (2.1, 23.4) | 16 | 56 | 7.1 | (3.5, 13.9) | 56 | 58 | 7.8 | (4.8, 12.5) |
| 10 | 16 | 45 | 6.7 | (5.2, 8.7) | 39 | 51 | 7.4 | (2.1, 23.4) | 16 | 39 | 7.1 | (3.5, 13.9) | 39 | 56 | 7.8 | (4.8, 12.5) |
Abbreviations: CI=confidence interval; HPV=human papillomavirus; HR=human risk.
Those with incomplete doses are excluded from the analysis (three individuals from the 1988/89/90 birth cohorts and 29 individuals from 1991/92 birth cohorts).
Discussion
This study has revealed the first definitive evidence of a large reduction in HPV 16 and 18 in the target population after introduction of a national bivalent HPV immunisation programme and has also shown significant cross-protective effects for HPV 31, 33 and 45. In addition, we present evidence that two doses leads to a reduction in HPV 16 and 18. However, the limited number of individuals receiving two doses gives wide variability in the estimate of this effect. Unlike Szarewski et al (2013), we found no change in HPV 6 and 11 positivity over either the time frame of the study or by vaccination status, although our results are not directly comparable as those tested were not necessarily sexually naïve at vaccination.
In Scotland, the 3-year catch-up campaign targeting girls aged 13–17 years attained a high uptake for all three doses among those vaccinated in school, exceeding 80%. However, there was lower uptake of around 30% in school leavers (Sinka et al, 2014). Nevertheless, the overall uptake of 66% in the catch-up cohort compares very favourably with other countries, such as the United States where 32% of girls aged 13–17 received the full three-dose regimen (CDC, 2011). In Australia, where vaccine uptake ranges from 50–70%, surveillance data have shown a considerable decrease in vaccine-targeted genotypes (Tabrizi et al, 2012) and ecological studies have shown a demonstrable reduction in cervical high-grade abnormalities associated with the quadrivalent HPV vaccine (Brotherton et al, 2011). With high levels of uptake, cervical screening commencement at age 20 and the ability to link individual screening and vaccination records, we are able, in Scotland, to demonstrate definitively the early impact of bivalent HPV vaccine on HPV infections in the target population.
The reduction in HPV 16/18 infection associated with two doses is lower than the efficacy found in the Costa Rica randomised control trial (Kreimer et al, 2011b). This is not unexpected as our study was not powered to detect a two-dose effect. Those receiving two doses did so only by default, leading to a limited dataset for analysis. In addition, the HPV status of each girl at the date of vaccination is unknown, whereas HPV naïve girls can be identified in the randomised control trial. Therefore, the effect we found may be smaller than the true effect. The analysis of those in the routinely vaccinated cohort, who were likely to be sexually naïve at vaccination, should present further evidence of the two-dose effect when they enter the screening programme.
There are theoretical concerns about high-risk HPV-type replacement in those who are vaccinated (Tota et al, 2013) and in our study, HPV 51, 52 and 56 emerged as the most prevalent high-risk HPV types in the vaccinated population, although at lower rates than those types which they replaced. Furthermore, it is feasible that the types we describe as having ‘emerged' have actually always been present at low-viral load and have become increasingly unmasked as a result of reduced competition for assay resources.
In addition, commensurate with the reduction in HPV 16, 18, 31, 33 and 45, we also observed a decrease in cervical intra-epithelial neoplasia (CIN) 2 and 3 in vaccinated cohorts through analysis of colposcopy data (Pollock et al, 2013). Thus, although the proportion found to be positive for any HPV type remains high even in the immunised population, we have seen no evidence of clinically significant high-risk type-replacement, at least in the short term. This finding clearly needs to be explored further with longer follow-up and associated clinical studies (Cuschieri et al, 2010; de Sanjose et al, 2010). Furthermore, the HPV immunisation surveillance strategy in Scotland also involves the longitudinal HPV typing of CIN2+, which will be essential for the quantification and monitoring of any clinically significant type replacement.
The level of HPV positivity (including HR types) in vaccinated women in this study reinforces the continuing need for cervical screening, although it is important to highlight that a relatively high proportion may have had sexual experience before vaccination—given the age range which constituted the catch-up population. One would expect the effect of vaccination to be even more profound in the routine cohort of 12/13 year olds in whom vaccine uptake is higher and this group will first present for their cervical smear is 2015. Indeed, it is a limitation of our study that we only sample those who present at routine screening. Although overall screening uptake in Scotland is relatively high (71.2% in 2012–13), it is considerably lower at 53.5% in those aged 20–24 (National Services Scotland ISD, 2013). We have previously reported HPV prevalence in a pre-vaccination group who had defaulted from routine screening, using a postal testing kits (urine or swab) survey (Sinka et al, 2011). Although defaulters were more likely to come from deprived groups, they were found to have similar patterns in HPV type prevalence compared with attenders. However, a caveat of this analysis is that different biospecimens were tested (urine and swabs vs LBC samples) and results could not be directly compared. Furthermore, the poor response rate to the survey (13.2%) precluded repetition for post-vaccination.
A further limitation of our analysis is that comparison of the unvaccinated with the vaccinated groups is partially a temporal comparison of the earlier cohorts with the later cohorts, as virtually all of the earlier cohorts are unvaccinated and most of the latter are fully vaccinated. If HPV positivity was decreasing over this time frame then this would lead to an apparent reduction in HPV prevalence among the vaccinated women, even if the vaccine had no effect. However, temporal analysis showed overall HPV positivity stayed constant over the 4 years sampled. We also explored this issue specifically using a sensitivity analysis, which indicated a stronger effect of vaccination when the analysis was restricted to the later cohorts. This implies that it is very unlikely that there has been a coincidental reduction in HPV prevalence over the time frame. It should be noted that by solely using the 1991 and 1992 cohorts, the unvaccinated group are mainly those who have left school which may contribute to an over-estimation of the effect. Furthermore, we found a significant reduction in the odds of non-vaccine high-risk type HPV in the vaccinated group, whereas a non-effect might have been expected. This indicates that those vaccinated are less likely to have been exposed to HPV before vaccination than girls from the unvaccinated group. A previous study of HPV prevalence in 12–19 year olds in Scotland (O'Leary et al, 2011) (where a self-sample was used), showed that 9% of 16–18 year olds, who were at an urban school, were HPV positive, compared with 38% of those who had left school and were in further education. Although we cannot assess when women in that cohort left school and fully adjust for it, the potential over-estimation in vaccine effect has been partially controlled for by examining unvaccinated women overall five birth cohorts to give a more representative balance of those in and out of school. The potential scale of the confounding effect is considered by sensitivity analysis, which demonstrated that excluding the unvaccinated females in 1991 and 1992 led to a reduced but still statistically significant reduction for HPV 16/18. This suggests that reduction in prevalence of HPV vaccine types is unlikely to be solely attributable to the confounding influence of age at leaving school.
Although vaccine was offered to all girls from age 13–17 during the catch-up programme, there was a statistically significant effect of deprivation on HPV positivity which existed at the baseline examination of HPV prevalence, where the most deprived girls were more likely to have HPV infection (Kavanagh et al, 2013). This differential is still present in this post-vaccination assessment. While the mean uptake of vaccine in the catch-up cohort was 66% for all three doses, it should be noted that there was a disparity in uptake between those who stayed on at school (80%) and those who left school but attended their GP surgery (30%), with those who stayed on at school more likely residing in the least deprived areas (Sinka et al, 2014). Even within the school-delivered programme, those residing in the least deprived areas were more likely to be vaccinated with three doses than those in the most deprived areas. These data reinforce the need for young women to attend cervical screening on a regular basis, as it is women from more deprived areas who are disproportionately affected by cervical malignancy (Baker and Middleton, 2003). It is somewhat reassuring that the analysis of vaccine uptake in the routine cohorts aged 12/13 does not show the same pattern, with uptake of vaccine being both high and equitable across deprivation classes (Sinka et al, 2014).
In order to estimate the impact of HPV vaccine, it is important to ascertain the effect of the vaccination programme on the whole population, with particular focus on the age group where such changes will be initially observed. We have shown here that vaccination with the bivalent HPV vaccine is associated with a significant reduction in vaccine-specific HPV types in the catch-up cohort. Furthermore, the vaccine appears to confer protection against infection with other high-risk HPV types 31, 33 and 45. It is also encouraging that there appears to be a lesser protective effect of receiving two doses, albeit the majority of these were delivered in months 0 and 1. These encouraging findings add to the evidence presented in clinical studies although these employed a two-dose regimen several months apart (Roteli-Martins et al, 2012). Our data provide a tantalising insight into the early impact of HPV vaccination at the population level and are very encouraging for countries with national programmes where high-vaccine uptake has been achieved.
Acknowledgments
Monitoring and evaluation of the HPV immunisation programme in Scotland is funded by the Scottish Government. The associated research is partially funded by the Chief Scientist Office (CZH/4/528). We also acknowledge Katy Sinka's earlier work at Health Protection Scotland in setting up the HPV surveillance programme.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V WHO International Agency for Research on Cancer Monograph Working Group (2009) A review of human carcinogens—Part B: biological agents. Lancet Oncol 10: 321–322. [DOI] [PubMed] [Google Scholar]
- Brotherton JML, Fridman M, May CL, Chappell G, Saville AM, Gertig DM (2011) Early effect of the HPV vaccination programme on cervical abnormalities in Victoria, Australia: an ecological study. Lancet 377: 2085–2092. [DOI] [PubMed] [Google Scholar]
- Baker D, Middleton E (2003) Cervical screening and health inequality in England in the 1990s. J Epidemiol Community Health 57: 417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (2011) National and state vaccination coverage among adolescents aged 13 through 17–United States, 2010. MMWR 60: 1117–1123. [PubMed] [Google Scholar]
- Cuschieri K, Brewster DH, Williams ARW, Brewster DH, Williams AR, Millan D, Murray G, Nicoll S, Imrie J, Hardie A, Graham C, Cubie HA (2010) Distribution of HPV types associated with cervical cancers in Scotland and implications for the impact of HPV vaccines. Br J Cancer 102: 930–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin HR, Vallejos CS, de Ruiz PA, Lima MA, Guimera N, Clavero O, Alejo M, Llombart-Bosch A, Cheng-Yang C, Tatti SA, Kasamatsu E, Iljazovic E, Odida M, Prado R, Seoud M, Grce M, Usubutun A, Jain A, Suarez GA, Lombardi LE, Banjo A, Menéndez C, Domingo EJ, Velasco J, Nessa A, Chichareon SC, Qiao YL, Lerma E, Garland SM, Sasagawa T, Ferrera A, Hammouda D, Mariani L, Pelayo A, Steiner I, Oliva E, Meijer CJ, Al-Jassar WF, Cruz E, Wright TC, Puras A, Llave CL, Tzardi M, Agorastos T, Garcia-Barriola V, Clavel C, Ordi J, Andújar M, Castellsagué X, Sánchez GI, Nowakowski AM, Bornstein J, Muñoz N, Bosch FX Retrospective International Survey and HPV Time Trends Study Group (2010) Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 11: 1048–1056. [DOI] [PubMed] [Google Scholar]
- Junor E, Kerr G, Oniscu A, Campbell S, Kouzeli I, Gourley C, Cuschieri K (2012) Benefit of chemotherapy as part of treatment for HPV DNA-positive but p16-negative squamous cell carcinoma of the oropharynx. Br J Cancer 106: 358–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh K, Sinka K, Cuschieri K, Love J, Potts A, Pollock KG, Cubie H, Donaghy M, Robertson C (2013) Estimation of HPV prevalence in young women in Scotland; monitoring of future vaccine impact. BMC Infect Dis 13: 519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Lowy D, Smith-McCune KK, Melief CJM (2012) The Value of HPV Vaccination. Nat Med 18: 28–29.22227663 [Google Scholar]
- Kreimer AR, Gonzalez P, Katki HA, Porras C, Schiffman M, Rodriguez AC, Solomon D, Jiménez S, Schiller JT, Lowy DR, van Doorn LJ, Struijk L, Quint W, Chen S, Wacholder S, Hildesheim A, Herrero R CVT Vaccine Group (2011. a) Efficacy of a bivalent HPV 16/18 vaccine against anal HPV 16/18 infection among young women: a nested analysis within the Costa Rica Vaccine Trial. Lancet Oncol 12: 862–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreimer AR, Rodriguez AC, Hildesheim A, Herrero R, Porras C, Schiffman M, González P, Solomon D, Jiménez S, Schiller JT, Lowy DR, Quint W, Sherman ME, Schussler J, Wacholder S CVT Vaccine Group. (2011. b) Proof-of-principle evaluation of the efficacy of fewer than three doses of 831 a bivalent HPV16/18 vaccine. J Natl Cancer Inst 103: 1444–1451, 832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz LE, Tsu V, Deeks SL, Cubie H, Wang SA, Vicari AS, Brotherton JM (2012) Human papillomavirus vaccine introduction–the first five years. Vaccine 30(Suppl 5): F139–F148. [DOI] [PubMed] [Google Scholar]
- National Records of Scotland (2012) 2011 Census: First Results on Population Estimates for Scotland - Release 1A. http://www.scotlandscensus.gov.uk/documents/censusresults/release1a/rel1asb.pdf accessed 04/07/13.
- National Services Scotland ISD (2013) Scottish Cervical Screening Programme Statistics 2012-13 https://isdscotland.scot.nhs.uk/Health-Topics/Cancer/Publications/2013-08-27/2013-08-27-Cervical-Screening-report.pdf?65131777525 [accessed 05/02/14].
- O'Leary MC, Sinka K, Robertson C, Cuschieri K, Lyman R, Lacey M, Potts A, Cubie HA, Donaghy M (2011) HPV type-specific prevalence using a urine assay in unvaccinated male and female 11- to 18-year olds in Scotland. Brit J Cancer 104: 1221–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter D, Kitchener H, Castellsague X, Teixeira JC, Skinner SR, Hedrick J, Jaisamrarn U, Limson G, Garland S, Szarewski A, Romanowski B, Aoki FY, Schwarz TF, Poppe WA, Bosch FX, Jenkins D, Hardt K, Zahaf T, Descamps D, Struyf F, Lehtinen M, Dubin G (2009) Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 374: 301–314. [DOI] [PubMed] [Google Scholar]
- Parkin DM (2006) The global health burden of infection-associated cancers in the year 2002. Int J Cancer 118: 3030–3044. [DOI] [PubMed] [Google Scholar]
- Pollock K, Potts A, Love J, Cuschieri K, Cubie H, Kavanagh K, Robertson C, Cruickshank M, Donaghy M (2013) Early effect of the HPV bivalent vaccine on high-risk HPV prevalence and high-grade cervical abnormalities in Scotland. Proc EuroGIN, http://www.eurogin.com/2013/images/pdf/EUROGIN-2013-Abstracts-Part-2.pdf [accessed 05/01/14].
- Romanowski B (2011) Long-term protection against cervical infection with human papillomavirus. Review of currently available vaccines. Hum Vaccin 7(2): 161–169. [DOI] [PubMed] [Google Scholar]
- Roteli-Martins CM, Naud P, De Borba P, Teixeira JC, De Carvalho NS, Zahaf T, Sanchez N, Geeraerts B, Descamps D (2012) Sustained immunogenicity and efficacy of the HPV-16/18 AS04-adjuvanted vaccine: up to 8.4 years of follow-up. Hum Vaccin Immunother 8(3): 390–397. [DOI] [PubMed] [Google Scholar]
- Schmitt M, Bravo IG, Snijders PJ, Gissmann L, Pawlita M, Waterboer T (2006) Bead based multiplex genotyping of human papillomaviruses. J Clin Microbiol 44: 504–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scottish Government (2005) Scottish Neighbourhood Statistics Guide. http://www.scotland.gov.uk/Publications/2005/02/20697/52626. Accessed online 28 October 2013.
- Scottish Government (2012) Scottish Index of Multiple Deprivation. http://www.scotland.gov.uk/Topics/Statistics/SIMD. Accessed online 28 October 2013.
- Sinka K, Kavanagh K, Gordon R, Love J, Potts A, Donaghy M, Robertson C (2014) Achieving high and equitable coverage of adolescent HPV vaccine in Scotland. J Epidemiol Community Health 68: 57–63. [DOI] [PubMed] [Google Scholar]
- Sinka K, Lacey M, Robertson C, Kavanagh K, Cuschieri K, Nicholson D, Donaghy M (2011) Acceptability and response to a postal survey using self-taken samples for HPV vaccine impact monitoring. Sex Transm Infect 87(7): 548–552. [DOI] [PubMed] [Google Scholar]
- Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, Clifford GM (2007) Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer 21: 621–632. [DOI] [PubMed] [Google Scholar]
- Szarewski A, Skinner SR, Garland SM, Romanowski B, Schwarz TF, Apter D, Chow SN, Paavonen J, Del Rosario-Raymundo MR, Teixeira JC, De Carvalho NS, Castro-Sanchez M, Castellsagué X, Poppe WA, De Sutter P, Huh W, Chatterjee A, Tjalma WA, Ackerman RT, Martens M, Papp KA, Bajo-Arenas J, Harper DM, Torné A, David MP, Struyf F, Lehtinen M, Dubin G (2013) Efficacy of the HPV-16/18 AS04-adjuvanted vaccine against low-risk hpv types (PATRICIA randomized trial): an unexpected observation. J Infect Dis 208(9): 1391–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tota JE, Ramanakumar AV, Jiang M, Dillner J, Walter SD, Kaufman JS, Coutlée F, Villa LL, Franco EL. Epidemiologic approaches to evaluating the potential for human papillomavirus type replacement postvaccination. Am J Epidemiol (2013) ; 178(4): 625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabrizi SN, Brotherton JM, Kaldor JM, Skinner SR, Cummins E, Liu B, Bateson D, McNamee K, Garefalakis M, Garland SM (2012) Fall in human papillomavirus prevalence following a national vaccination program. J Infect Dis 206: 1645–1651. [DOI] [PubMed] [Google Scholar]
- Wheeler CM, Castellsague X, Garland SM, Szarewski A, Paavonen J, Naud P, Salmerón J, Chow SN, Apter D, Kitchener H, Teixeira JC, Skinner SR, Jaisamrarn U, Limson G, Romanowski B, Aoki FY, Schwarz TF, Poppe WA, Bosch FX, Harper DM, Huh W, Hardt K, Zahaf T, Descamps D, Struyf F, Dubin G, Lehtinen M HPV PATRICIA Study Group (2012) Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol 13: 100–110. [DOI] [PubMed] [Google Scholar]