Abstract
The aging-suppressor gene klotho encodes a single-pass transmembrane protein that is predominantly secreted by the choroid plexus of the brain and in the kidney. Klotho-deficient mice develop multiple aging phenotypes, including impaired cognition. Klotho concentrations have not been described in the CSF of humans. We measured klotho in the CSF of 20 older adults with Alzheimer's disease and in 20 older and 20 younger adults with normal cognition. In 10 adults, aged 38-87 years, CSF klotho measurements were made at baseline and every 6 hours up to 18-30 hours later. Mean (95% Confidence Interval [C.I.]) CSF klotho in men versus women were 899 (814, 983) and 716 (632, 801) pg/mL, respectively (P = 0.002). Mean (95% C.I.) CSF klotho in older adults with and without Alzheimer's disease were 664 (603, 725) and 776 (705, 828) pg/mL, respectively (P = 0.02), adjusting for sex. Mean (95% C.I.) klotho in older versus younger adults were 766 (658, 874) and 992 (884, 1100) pg/mL, respectively (P = 0.005), adjusting for sex. In the longitudinal study of CSF klotho, no significant circadian fluctuations were found in CSF klotho levels. This study suggests that CSF klotho concentrations are lower in females compared with males, in Alzheimer's disease, and in older versus younger adults.
Keywords: Aging, Alzheimer's Disease, Brain, Cerebrospinal Fluid, Klotho
Introduction
The aging suppressor gene klotho encodes a single-pass transmembrane protein that is predominantly expressed in the choroid plexus of the brain, distal tubule cells of the kidney, and parathyroid glands. The klotho gene, named after the Greek goddess who spins the thread of life, was originally identified in a mutant mouse strain that could not express klotho, developed multiple disorders resembling human aging, and had a shortened life span [1]. The aging phenotypes included impaired cognition, arteriosclerosis, decreased bone mineral density, and sarcopenia [2]. Overexpression of klotho in transgenic mice resulted in suppression of aging phenotypes and a significant extension of life span compared with wild-type mice [3]. Further studies have shown that klotho is involved in regulation of calcium and phosphate homeostasis and inhibition of intracellular insulin and insulin-like growth factor-1 signaling [2].
Klotho has been implicated in the regulation of brain aging because of the impaired cognition and abnormal brain pathology noted in klotho mutant mice [4,5] and gene profile analysis of aging changes in the brain white matter of rhesus monkeys [6]. Increased lipid peroxidation and oxidative DNA damage occur in the hippocampus of klotho mutant mice prior to the appearance of cognition deficits [7]. In 2004, Imura and colleagues demonstrated that soluble klotho was present in human cerebrospinal fluid and blood [8]. The relationship of klotho in cerebrospinal fluid to neurological diseases in humans has not been studied because of the lack of a reliable assay for the measurement of secreted klotho protein. Recently, a sensitive and specific assay was developed for the measurement of soluble klotho in humans [9]. Recently, the designation α-klotho has been used in the literature to describe the original klotho gene and its product, the secreted circulating klotho hormone [10], and to distinguish it from a homolog that was named β-klotho [11,12]. Throughout this paper, the term klotho will refer to α-klotho.
Our specific aims were to characterize klotho concentrations in the CSF in men versus women, in older versus younger adults, and in adults with and without Alzheimer's disease. We also sought to determine whether circadian fluctuations occur with CSF klotho levels. To address these aims, we measured CSF klotho in men and women, older and younger adults, and older adults with Alzheimer's disease. We also measured CSF klotho over time in 10 patients undergoing evaluation for normal pressure hydrocephalus.
Materials and Methods
The study participants consisted of two cohorts. The first consisted of seventy patients with a single spinal tap. Twenty were older adults (10 men, 10 women) with Alzheimer's disease, twenty were older adults (10 men, 10 women) who were cognitively normal and had spinal taps for clinical indications that proved benign, twenty were younger adults (10 men, 10 women) who were cognitively normal and had spinal taps for clinical indications that proved benign, and 10 had idiopathic normal pressure hydrocephalus. The second cohort consisted of ten patients with an indwelling lumbar catheter as part of a detailed examination of normal pressure hydrocephalus (n = 9) or pseudotumor cerebri (n = 1). Mini-Mental State Examination (MMSE) was administered to all participants [13]. In the second cohort, the subjects underwent insertion of a catheter into the lumbar subarachnoid space on the first day of hospitalization. After monitoring of intracranial pressures for 18 hours, drainage of CSF was initiated at noon the following day. Collection of CSF for analysis began at 6 PM on the first day of drainage. Forty mL of CSF were withdrawn from the lumbar catheter every 6 hours for a period of 24 or 36 continuous hours. Differences in the duration of CSF collection were due to the investigator availability. The first 10 mL of CSF collected at each time point was discarded to eliminate CSF that may have pooled in the lumbar catheter. The following 30 mL of CSF was drip collected in 2 mL aliquots and promptly stored at –80° C until further analysis. All participants were asleep and remained asleep when the midnight sample was collected and were awake at the noon and 6 PM collection of CSF. All participants gave written, informed consent for participation in the study. The Johns Hopkins University School of Medicine Institutional Review Board approved the protocol for the study.
Soluble α-klotho was measured in CSF using a solid phase sandwich enzyme-linked immunosorbent assay (ELISA) (Immuno-Biological Laboratories, Takasaki, Japan) [9]. The minimum level of detectability of the assay is 6.15 pg/mL. The minimum level is below the plasma concentrations that were found in our study. The intra-assay and inter-assay coefficients of variation were 4.1% and 8.9%, respectively, for klotho measurements in the investigator's (R.D.S.) laboratory.
Klotho concentrations in the CSF were described using means and 95% confidence intervals. ANOVA was used to compare mean klotho concentrations between groups. Since there were significant difference in mean klotho concentrations between men and women, further comparisons between groups were adjusted in the ANOVA for sex. Linear regression was used to examine CSF klotho concentrations over time. Spearman correlation was used to examine the relationship between CSF klotho concentrations and MMSE score.
Results
The characteristics of the study participants in the first cohort are shown in Table 1. There were significant differences in age, CSF klotho concentrations, and MMSE score across the three cohorts. We compared differences in CSF klotho concentrations between men and women, between older adults with and without Alzheimer's disease, and between younger and older normal adults. Mean (95% Confidence Interval [C.I.]) CSF klotho in men versus women were 899 (8144, 983) and 716 (632, 801) pg/mL, respectively (P = 0.002) (Figure 1). Mean (95% C.I.) CSF klotho in older adults with and without Alzheimer's disease were 664 (603, 725) and 776 (705, 828) pg/mL, respectively (P = 0.02), adjusting for sex. Mean (95% C.I.) klotho in older versus younger adults were 766 (658, 874) and 992 (884, 1100) pg/mL, respectively (P = 0.005), adjusting for sex. In all participants in the first cohort combined, Spearman correlation between age and CSF klotho concentrations was -0.37 (P = 0.003) and Spearman correlation between MMSE score and CSF klotho concentrations was 0.30 (P = 0.02) (Figure 2).
Table 1. Characteristics of participants in the three study groups.
| Characteristic1 | Alzheimer's disease (n = 20) | Normal older adults (n = 20) | Normal younger adults (n = 20) | P2 | |
|---|---|---|---|---|---|
| Age, years | 76.9 (73.8, 80.1) | 76.8 (74.2, 79.4) | 30.1 (27.0, 33.2) | <0.0001 | |
| Sex | Male | 10 | 10 | 10 | 1.00 |
| Female | 10 | 10 | 10 | ||
| MMSE score | 22.1 (21.1, 23.1) | 28.6 (28.2, 29.1) | not measured | <0.0001 | |
| CSF klotho (pg/mL) | 664 (603, 725) | 776 (705, 828) | 922 (844, 1100) | 0.001 | |
Mean (95% CI) shown for continuous variables
For continuous variables, by ANOVA.
Figure 1.

CSF klotho concentrations in men versus women. Boxplots represent median (25th, 75th percentile) and bars represent maximum and minimum values.
Figure 2.
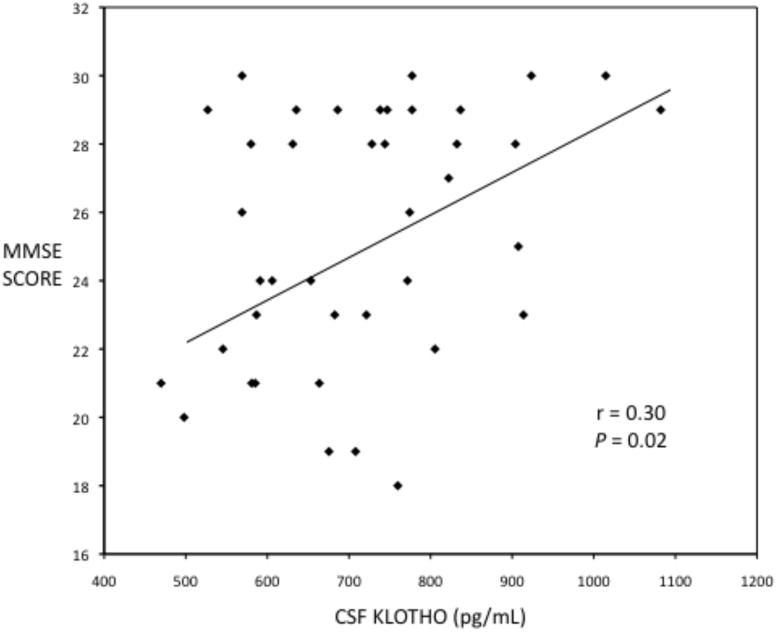
Scatterplot of MMSE score versus CSF klotho concentrations.
In the second cohort, the median age of the subjects was 75 years (range 38-87 years). Median MMSE score was 27.0. Overall in the ten subjects, mean (SD) CSF klotho concentrations were 1018 (253) pg/mL. CSF klotho concentrations in the ten subjects, measured over time, are shown in Figure 3. There were no significant circadian fluctuations in CSF klotho concentrations.
Figure 3.

CSF Klotho concentrations over time in ten adults.
Discussion
The present study showed that CSF klotho concentrations were significantly lower in older adults with Alzheimer's disease compared with older adults with normal cognition. In addition, CSF klotho concentrations were higher in men than women and higher in older versus younger normal adults. The present study showed that CSF klotho concentrations showed no apparent circadian pattern. CSF klotho measurements were highly reproducible in CSF over a period ranging from 18 to 30 hours. To our knowledge, this is the first clinical study to describe klotho concentrations in the CSF of humans. The findings of the present study corroborate and extend the initial description of a 130 KDa form of secreted klotho protein detected in both human sera and CSF by Imura and colleagues in 2004 using western blot analysis [8].
As noted previously, impaired cognition and abnormal brain pathology have been observed in klotho mutant mice [4,5]. The findings from murine models may be relevant to humans because there is 80% homology in klotho between humans and mice [14]. Genetic variants of klotho have been associated with longevity and health in humans [15,16]. The klotho V/V genotype was associated with impaired cognitive ability in older women in a combined analysis of the Lothian Birth Cohort 1921 and Aberdeen Birth Cohort 1936 in Scotland [17]. Whether polymorphisms in the klotho gene are related to plasma or CSF klotho is not known. Low plasma klotho is associated with cardiovascular disease [18] and is an independent predictor of all-cause mortality in older adults [19].
A recent study shows that klotho may play an important role in maturation and myelination in the brain [20]. Chen and colleagues have hypothesized that klotho secreted by the choroid plexus may play a role in protecting myelin integrity and prevent myelin degeneration in the aging brain [20]. Further studies are needed to characterize the potential biological roles that CSF klotho may play in Alzheimer's disease and aging.
Highlight.
the “anti-aging hormone” klotho has been implicated in aging of the brain and cognition klotho levels are lower in cerebrospinal fluid in Alzheimer's disease compared with controls
klotho levels are lower in cerebrospinal fluid of older compared with younger adults there is no apparent circadian pattern to klotho levels in cerebrospinal fluid
Acknowledgments
This work was supported by National Institutes of Health grants R01 AG027012, R01 HL111271, R01 HL094507, P50 AG05146, U01 AG033655, and the Burroughs Wellcome Trust for Translational Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima Y. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- Kuro-o M. Klotho. Eur J Physiol. 2010;459:333–343. doi: 10.1007/s00424-009-0722-7. [DOI] [PubMed] [Google Scholar]
- Kurosu H, Yamamoto M, Clark JE, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o K. Suppression of aging in mice by the hormone klotho. Science. 2005;308:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida A, Komiya T, Tashiro T, Yorifuji H, Kishimoto T, Nabeshima Y, Hisanaga S. Neurofilaments of klotho, the mutant mouse prematurely displaying symptoms resembling human aging. J Neurosci Res. 2001;64:363–370. doi: 10.1002/jnr.1087. [DOI] [PubMed] [Google Scholar]
- Shiozaki M, Yoshimura K, Shibata M, Koike M, Matsuura N, Uchiyama Y, Gotow T. Morphological and biochemical signs of age-related neurodegenerative changes in klotho mutant mice. Neurosci. 2008;152:924–941. doi: 10.1016/j.neuroscience.2008.01.032. [DOI] [PubMed] [Google Scholar]
- Duce JA, Podvin S, Hollander W, Kipling D, Rosene DL, Abraham CR. Gene profile analysis implicates klotho as an important contributor to aging changes in brain white matter of the rhesus monkey. Glia. 2008;56:106–117. doi: 10.1002/glia.20593. [DOI] [PubMed] [Google Scholar]
- Nagai T, Yamada K, Kim HC, Kim YS, Noda Y, Imura A, Nabeshima Y, Nabeshima T. Cognition impairment in the genetic model of aging klotho gene mutana mice: a role of oxidative stress. FASEB J. 2003;17:50–52. doi: 10.1096/fj.02-0448fje. [DOI] [PubMed] [Google Scholar]
- Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, Hashimoto N, Fujimori T, Nabeshima Y. Secreted klothoprotein in sera and CSF: implications for post-translational cleavage in release of klotho protein from cell membrane. FEBS Lett. 2004;565:143–147. doi: 10.1016/j.febslet.2004.03.090. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Imura A, Urakawa I, Shimada T, Murakami J, Aono Y, Hasegawa H, Yamashita T, Nakatani K, Saito Y, Okamoto N, Kurumatani N, Namba N, Kitaoka T, Ozono K, Sakai T, Hataya H, Ichikawa S, Imel EA, Econs MJ, Nabeshima YI. Establishment of a sandwich ELISA for soluble alpha-klotho measurements: age-dependent change of soluble alpha-klotho levels in healthy subjects. Biochem Biophys Res Commun. 2010;398:513–518. doi: 10.1016/j.bbrc.2010.06.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Comm. 1998;242:626–630. doi: 10.1006/bbrc.1997.8019. [DOI] [PubMed] [Google Scholar]
- Ito S, Kinoshita S, Shiraishi N, Nakagawa S, Sekine S, Fujimori T, Nabeshima YI. Molecular cloning and expression analyses of mouse betaklotho, which encodes a novel Klotho family protein. Mech Dev. 2000;98:115–119. doi: 10.1016/s0925-4773(00)00439-1. [DOI] [PubMed] [Google Scholar]
- Nabeshima Y, Imura H. α-klotho: a regulator that integrates calcium homeostasis. Am J Nephrol. 2008;28:455–464. doi: 10.1159/000112824. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Shiraki-Iida T, Aizawa H, Matsumura Y, Sekine S, Iida A, Anazawa H, Nagai R, Kuro-o M, Nabeshima Y. Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett. 1998;424:6–10. doi: 10.1016/s0014-5793(98)00127-6. [DOI] [PubMed] [Google Scholar]
- Arking DE, Krebsova A, Macek M., Sr Association of human aging with a functional variant of klotho. Proc Natl Acad Sci USA. 2002;99:856–861. doi: 10.1073/pnas.022484299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arking DE, Atzmon G, Arking A, Barzilai N, Dietz HC. Association between a functional variant of the KLOTHO gene and high-density lipoprotein cholesterol, blood pressure, stroke, and longevity. Circ Res. 2005;96:412–418. doi: 10.1161/01.RES.0000157171.04054.30. [DOI] [PubMed] [Google Scholar]
- Deary IS, Harris SE, Fox HC, Hayward C, Wright AF, Starr JM, Whalley JJ. KLOTHO genotype and cognitive ability in childhood and old age in the same individuals. Neuroscience Lett. 2005;378:22–27. doi: 10.1016/j.neulet.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Semba RD, Cappola AR, Sun K, Bandinelli S, Dalal M, Crasto C, Guralnik JM, Ferrucci L. Plasma klotho and cardiovascular disease in older community-dwelling adults. J Am Geriatr Soc. 2011;59:1596–1601. doi: 10.1111/j.1532-5415.2011.03558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba RD, Cappola AR, Sun K, Bandinelli S, Dalal M, Crasto C, Guralnik JM, Ferrucci L. Plasma klotho and mortality risk in older community-dwelling adults. J Gerontol A Biol Sci Med Sci. 2011;66:794–800. doi: 10.1093/gerona/glr058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CD, Sloane JA, Li H, Aytan N, Giannaris EL, Zeldich E, Hinman JD, Dedeoglu A, Rosene DL, Bansal R, Luebke JI, Kuro-o M, Abraham CR. The antiaging protein klotho enhances oligodendrocyte maturation and myelination in the CNS. J Neurosci. 2013;33:1927–1939. doi: 10.1523/JNEUROSCI.2080-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]


