The present analysis, carried out in the context of a randomized phase III trial, confirms superior outcomes for breast cancer patients for whom chemotherapy induces pathological complete response (pCR) after adjusting for other important prognostic factors. In contrast, when tumours do not achieve pCR, patients have a higher risk of relapse. This effect is observed in all intrinsic subtypes and justifies the current interest in post-neoadjuvant trials .
Keywords: breast cancer, neoadjuvant chemotherapy, TP53, landmark analysis, pathological complete response
Abstract
Background
Pathological complete response (pCR) following chemotherapy is strongly associated with both breast cancer subtype and long-term survival. Within a phase III neoadjuvant chemotherapy trial, we sought to determine whether the prognostic implications of pCR, TP53 status and treatment arm (taxane versus non-taxane) differed between intrinsic subtypes.
Patients and methods
Patients were randomized to receive either six cycles of anthracycline-based chemotherapy or three cycles of docetaxel then three cycles of eprirubicin/docetaxel (T-ET). pCR was defined as no evidence of residual invasive cancer (or very few scattered tumour cells) in primary tumour and lymph nodes. We used a simplified intrinsic subtypes classification, as suggested by the 2011 St Gallen consensus. Interactions between pCR, TP53 status, treatment arm and intrinsic subtype on event-free survival (EFS), distant metastasis-free survival (DMFS) and overall survival (OS) were studied using a landmark and a two-step approach multivariate analyses.
Results
Sufficient data for pCR analyses were available in 1212 (65%) of 1856 patients randomized. pCR occurred in 222 of 1212 (18%) patients: 37 of 496 (7.5%) luminal A, 22 of 147 (15%) luminal B/HER2 negative, 51 of 230 (22%) luminal B/HER2 positive, 43 of 118 (36%) HER2 positive/non-luminal, 69 of 221(31%) triple negative (TN). The prognostic effect of pCR on EFS did not differ between subtypes and was an independent predictor for better EFS [hazard ratio (HR) = 0.40, P < 0.001 in favour of pCR], DMFS (HR = 0.32, P < 0.001) and OS (HR = 0.32, P < 0.001). Chemotherapy arm was an independent predictor only for EFS (HR = 0.73, P = 0.004 in favour of T-ET). The interaction between TP53, intrinsic subtypes and survival outcomes only approached statistical significance for EFS (P = 0.1).
Conclusions
pCR is an independent predictor of favourable clinical outcomes in all molecular subtypes in a two-step multivariate analysis.
ClinicalTrials.gov
EORTC 10994/BIG 1-00 Trial registration number NCT00017095.
introduction
A proportion of breast cancer patients continue to be diagnosed with large tumours, and despite treatment with neoadjuvant chemotherapy, have a high rate of relapse as shown in several recently published trials including the EORTC 10994/BIG 1-00 trial [1]. There is growing recognition of the importance of pathological complete response (pCR) following chemotherapy, particularly as a surrogate for survival outcomes, and the US food and drug administration (FDA) is investigating whether pCR rates could be used to guide drug registration [2].
However, any analysis needs to consider breast cancer heterogeneity using for example the intrinsic subtype classification [3, 4]. The association between pCR and excellent prognosis has only been demonstrated for some subtypes such as triple negative (TN) [5–7] and human epidermal growth factor 2 receptor (HER2)-positive [7, 8], although with conflicting results [6].
Within the context of the EORTC 10994/BIG 1-00 randomized neoadjuvant study, we sought evidence as to whether the prognostic implications of pCR differed between intrinsic subtypes, after adjusting for other important prognostic factors. Additionally, we evaluated the impact of treatment arm (taxane versus non-taxane), and TP53 status on survival outcomes among the different molecular subtypes. To address these questions, we carried out a landmark [9, 10] and a two-step approach multivariate analyses.
methods
study design, eligibility and treatment
This was a planned secondary analysis of the EORTC 10994/BIG 1-00 trial which randomized patients between six cycles of 5-fluorouracil, epirubicin and cyclophosphamide and a taxane-based regimen, docetaxel × three cycles followed by epirubicin + docetaxel for three cycles, all given before primary surgery [1]. Eligible patients were women aged <71 years with histologically proven invasive carcinoma of the breast suitable for neoadjuvant chemotherapy, with any large operable or locally advanced/inflammatory breast cancer. Patients with HER2-positive tumours were allowed to enter adjuvant clinical trials assessing trastuzumab or to receive this treatment in the adjuvant setting once it became standard practice, and none received neoadjuvant trastuzumab.
The trial was approved by national and/or local ethics committees in all participating centres. Before registration, all patients gave signed informed consent for the clinical trial and for research on tumour samples.
For the sub-study that is the subject of this report, a subgroup of the initial population of 1856 was selected based on the following criteria: (i) patients with sufficient information available on HER2, ER, PR (progesterone receptor) and tumour grade to permit a simplified intrinsic subtype classification as defined below; (ii) patients who received at least one cycle of neoadjuvant chemotherapy and who did not receive radiotherapy before surgery; (iii) patients without inflammatory cancers (T4d). The analysis including pathological response was further confined to patients with post-treatment pathology data and whose tumours did not progress on neoadjuvant chemotherapy.
objectives
The primary objective of this analysis was to assess the effect of pCR on event-free survival (EFS) by breast cancer intrinsic subtype (the simplified intrinsic subtypes classification used in this study is detailed in the pathology section below), in a two-step multivariate analysis adjusting for important factors prognostic for EFS, as listed in the statistical section.
Secondary objectives included investigating (i) the effect of TP53 function on pCR and survival outcomes within each subtype and (ii) the effect of treatment on survival outcomes across subtypes.
pathology assessment
No central pathology review was carried out for this analysis. Grade, ER, PR and HER2 status were assessed by local pathologists from a biopsy taken at diagnosis and prospectively collected in the case report form. ER and PR, assessed by immunohistochemistry (IHC), were reported as positive or negative according to each centre's local definition. HER2 positivity was defined as either HER2 gene amplification by fluorescent in situ hybridization and/or scored 3+ by IHC. Because information on Ki-67 was not available from data collected within the main TP53 study, we replaced Ki-67 by grade in order to classify tumours in a simplified intrinsic subtype’s classification, as suggested by the St Gallen 2011 consensus [11]. We used the following classification:
Luminal A-like: ER and/or PR positive, HER2 negative and grade 1 or 2.
Luminal B-like (HER2 negative): ER and/or PR positive, HER2 negative and grade 3.
Luminal B-like (HER2 positive): ER and/or PR positive, HER2 positive (IHC3+ or amplified) and any grade (or grade unknown).
HER2 positive (non-luminal): ER and PR negative, HER2 positive (IHC3+ or amplified) and any grade (or grade unknown).
TN: ER and PR negative, HER2 negative and any grade (or grade unknown).
Pathological response was assessed by local pathologists after completion of the neoadjuvant chemotherapy. pCR was defined as no evidence of residual ‘invasive cancer’ or very few scattered tumour cells left in the primary breast tumour—with or without residual ductal carcinoma in situ (DCIS)—and in the lymph nodes. TP53 status was assessed using a functional test in yeast as previously described [1].
statistical analysis
A statistical analysis plan was prospectively defined. Eligible patients were analysed on an intent-to-treat basis. Associations between baseline characteristics and pCR were evaluated in a multivariate logistic regression model. Survival times were defined as time from randomization to an event.
In order to investigate the prognostic value of pCR on outcomes, a landmark analysis approach was used [9, 10], with the landmark chosen as the date of surgery. Of note, by definition, patients who progressed on neoadjuvant treatment were excluded from this analysis. To address our research question, the following two-step approach was adopted after adjusting for important prognostic factors (supplementary Figure S1, available at Annals of Oncology online): as a first-step, the potential effects of the three interactions (pCR and intrinsic subtypes; TP53 status and intrinsic subtypes; treatment arm and intrinsic subtypes) were simultaneously investigated in a multivariate Cox regression model, adjusting for age at diagnosis, menopausal status, cT stage, cN stage and tumour histology (invasive ductal, invasive lobular or other invasive carcinoma). Interactions with a P value of ≤0.1 were considered significant. When a significant interaction was observed, the HRs [99% confidence intervals (CI)] within the different subtypes were reported. If not, as a second-step, the multivariate model was refitted without interaction(s) and an overall (i.e. over all intrinsic subtypes) HR (99% CI) was reported. All non-interactions tests were compared with a significance level α = 0.01.
results
baseline characteristics by intrinsic subtype
Of the 1856 patients originally randomized, only 1289 were assessable for the relationship between subtype and outcome, and 1212 for the landmark analysis of pCR, subtype and outcome. The reasons for ineligibility are shown in the Consort diagram (supplementary Figure S2, available at Annals of Oncology online). The characteristics of patients included in this sub-study and those who were excluded were similar (supplementary Table S1, available at Annals of Oncology online).
The median follow-up was 56 months from randomization and 51 months from date of surgery. The subtypes repartition is given on supplementary Table S2, available at Annals of Oncology online. Among patients with HER-positive tumours, 120 of 365 (33%) received adjuvant trastuzumab and none received neoadjuvant trastuzumab.
pathological responses by intrinsic subtypes and TP53 status
A pCR was observed in 222 of 1212 (18%) assessable patients (Table 1). pCR rates differed significantly (P < 0.001) across intrinsic subtypes, with the lowest rate for luminal A-like (7.5%) and the highest rate for HER2+/non-luminal (36%). A multivariate logistic regression analysis was carried out to assess whether these differences persist when adjusting for other known predictive factors for pCR. First, the effect of two interactions (TP53 and intrinsic subtypes; treatment assigned and intrinsic subtypes) on pCR rate was examined, but neither were significant. Next, the effect of TP53 and treatment assigned were tested, and both were non-significant. Only intrinsic subtype (P < 0.001) and cT stage (P < 0.001) were found to be independent predictors for pCR (supplementary Table S3, available at Annals of Oncology online).
Table 1.
pCR rates by intrinsic subtype
| N | Non-assessable patients, N | Patients with pCR (assessable patients), N | Patients with no pCR (assessable patients), N | pCR rate (assessable patients) (%) | Odds ratios (99% CI)a | |
|---|---|---|---|---|---|---|
| Luminal A-like | 515 | 19 | 37 | 459 | (7.5) | 1.00 |
| Luminal B-like (HER2 negative) | 154 | 7 | 22 | 125 | (15.0) | 2.18 (1.04–4.58) |
| Luminal B-like (HER2 positive) | 237 | 7 | 51 | 179 | (22.2) | 3.54 (1.94–6.45) |
| HER2 positive (non-luminal) | 128 | 10 | 43 | 75 | (36.4) | 7.11 (3.67–13.8) |
| Triple negative | 255 | 34 | 69 | 152 | (31.2) | 5.63 (3.16–10.0) |
| Total | 1289 | 77 | 222 | 990 | (18.3) | P < 0.001b |
aConsidering only assessable patients.
bP value for overall test of a difference between subtypes considering only assessable patients using a logistic regression model.
pCR, pathological complete response; CI, confidence interval; HER2, human epidermal growth factor receptor 2.
survival outcome measures (EFS, DMFS and OS) by intrinsic subtype
Supplementary Figure S3, available at Annals of Oncology online shows a significant difference in the survival end points according to subtype [P < 0.001 for EFS, distant metastasis-free survival (DMFS) and overall survival (OS)]. Patients in the luminal A-like group had the best outcome across all three survival measures [12] despite experiencing the lowest pCR rates (Table 1). Events contributing to EFS are detailed in supplementary Table S5, available at Annals of Oncology online.
effect of pCR, TP53 and treatment on EFS across intrinsic subtypes (landmark analysis)
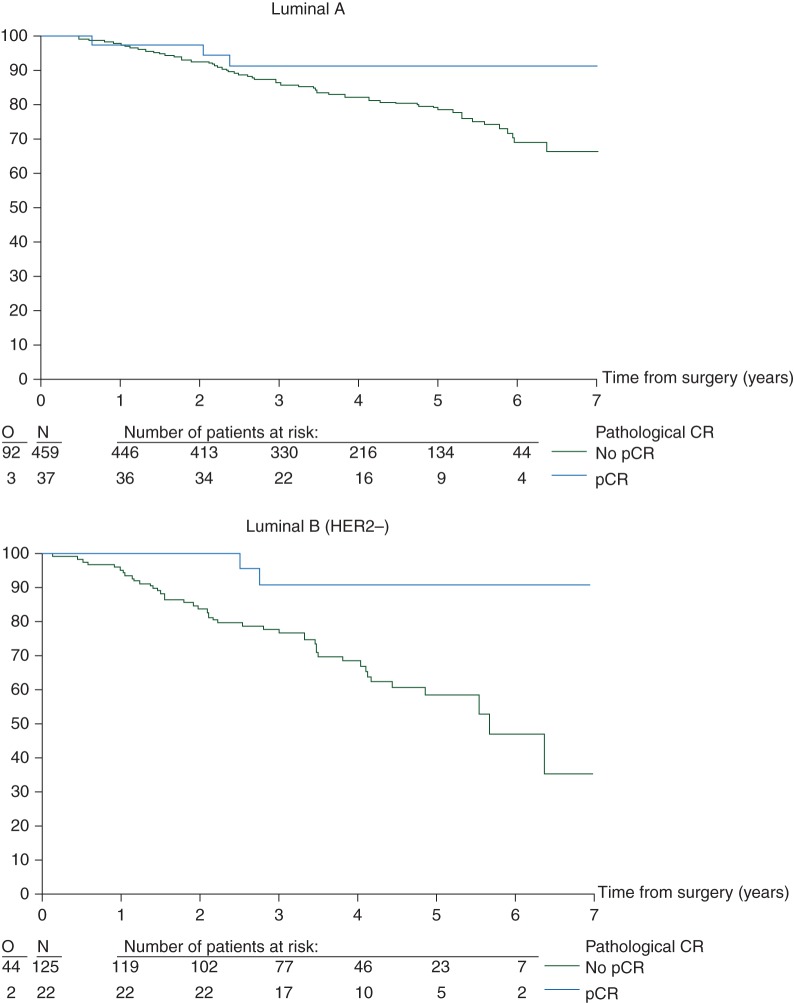
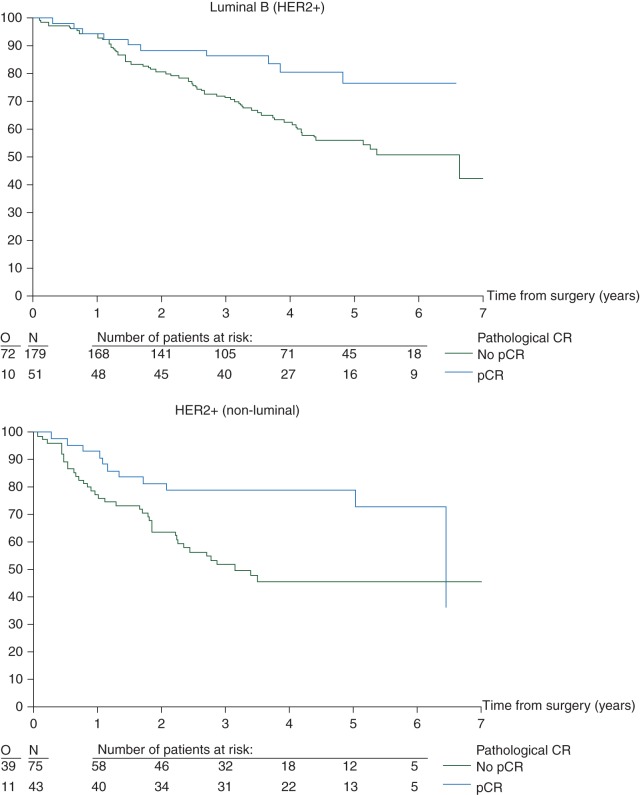
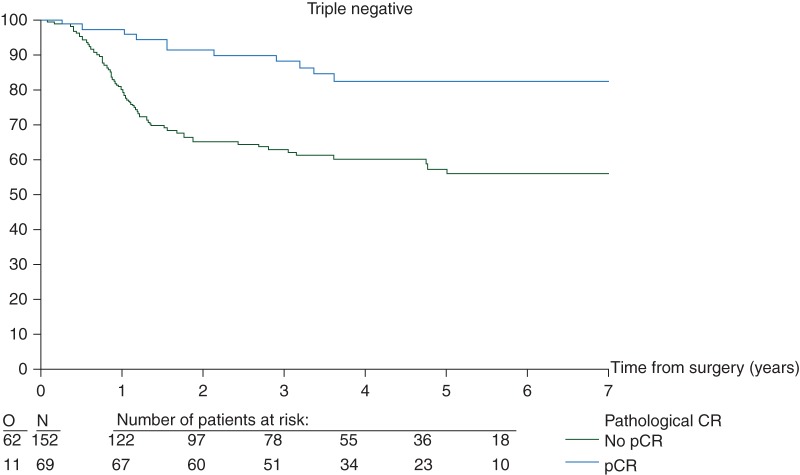
EFS curves (Figure 1) and univariate analysis (Figures 2 and 3)
Figure 1.
Effect of pCR on EFS by intrinsic subtype (N = 1212 eligible and assessable patients). pCR, pathological complete response; EFS, event-free survival.
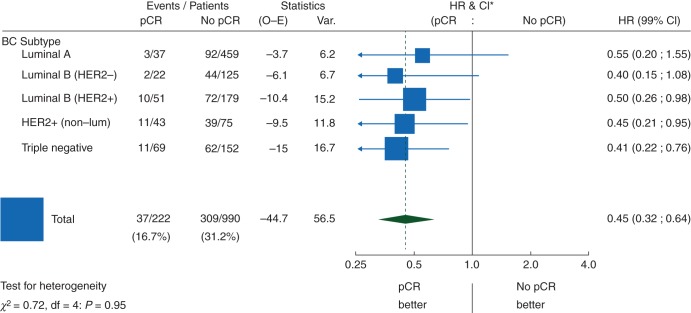
Figure 2.
Effect of pCR on EFS by intrinsic subtype: univariate Cox regression models (N = 1212 eligible and assessable patients). pCR, pathological complete response; EFS, event-free survival; HR, hazard ratio; CI, confidence interval; HER2, human epidermal growth factor 2; df, degrees of freedom.
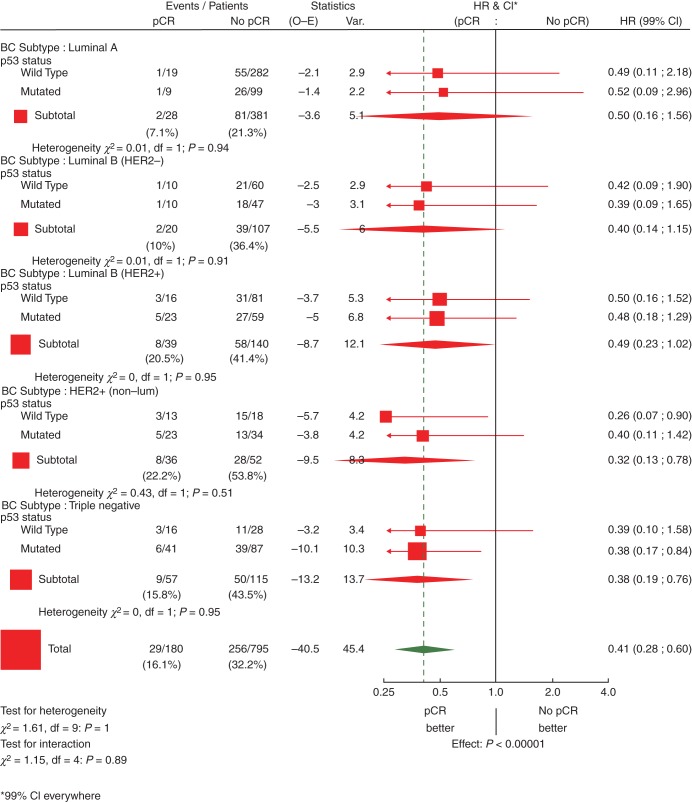
Figure 3.
Effect of pCR on event-free survival by intrinsic subtype and TP53 status: univariate Cox regression models (N = 1212 eligible and assessable patients)a. pCR, pathological complete response; HR, hazard ratio; CI, confidence interval; HER2, human epidermal growth factor 2; df, degrees of freedom. aTwo hundred and thirty-seven patients with p53 status missing were not considered in this graph.
There was no evidence of an interaction between subtype and the prognostic influence of pCR (P = 0.95) (Figure 2). Overall, regardless of subtype, pCR was prognostic for EFS (Figure 2). Furthermore, in the univariate Cox regression models, pCR predicts for a better EFS in the luminal B/HER2-positive, HER2-positive/non-luminal and TN breast cancer patients. Although a similar trend is seen for the other two subtypes, the effect is neither statistically significant on its own nor significantly different from the other subtypes (Figure 2).
Figure 3, limited to those patients with assessable TP53 status, shows that, within the subtypes, there is no evidence of an interaction between TP53 status and pCR (P ranging between 0.51 for HER2 positive/non-luminal and 0.95 for Luminal B/HER2 positive and TN). Although in all subgroups, pCR seems to be associated with better EFS, this association is significant only in HER2 positive/non-luminal with TP53 wild type and TN with p53 mutated.
two-step multivariate analysis
First, we tested the effect of the three interactions (pCR and intrinsic subtypes, TP53 and intrinsic subtypes and treatment assigned and intrinsic subtypes) on EFS. Only the interaction between TP53 and intrinsic subtypes was of borderline statistical significance (P = 0.1), with the two other P values being 0.835 and 0.926, respectively, indicating that pCR and treatment do not interact differently with EFS according to subtype. The multivariate model was thus refitted without the non-significant interactions. Table 2 shows the results of this model, with subtype-specific HRs for TP53 and overall HRs for pCR and treatment on EFS. Both pCR and treatment contribute significantly to the overall prediction of EFS [HR = 0.40, P < 0.001 in favour of pCR, and HR = 0.73, P = 0.004 in favour of three cycles of docetaxel then three cycles of eprirubicin/docetaxel (T-ET)].
Table 2.
Relationship between pCR, other important prognostic factors and EFS: a two-step multivariate Cox regression modela—HRs with 99% CI (landmark method—N = 1212)
| Factor | HR (99% CI) |
P valueb | ||
|---|---|---|---|---|
| Age (years) | ||||
| ≤40 | 1.00 | 0.024 | ||
| 41–50 | 0.67 (0.46–0.98) | |||
| 51–70 | 0.66 (0.35–1.25) | |||
| Menopausal status | ||||
| Premenopausal | 1.00 | 0.603 | ||
| Postmenopausal | 1.12 (0.63–1.99) | |||
| cT stage | ||||
| T1–T2 | 1.00 | <0.001 | ||
| T3 | 1.82 (1.33–2.49) | |||
| T4 | 2.85 (1.89–4.31) | |||
| cN stage | ||||
| N0 | 1.00 | <0.001 | ||
| N1 | 1.54 (1.13–2.10) | |||
| N2–N3 | 2.17 (1.18–4.00) | |||
| Histological type | ||||
| Lobular | 1.00 | 0.773 | ||
| Ductal | 0.93 (0.56–1.53) | |||
| Other | 0.79 (0.34–1.84) | |||
| Pathological response | ||||
| No pCR pCR |
1.00 0.40 (0.25–0.64) |
<0.001 | ||
| Treatment arm | ||||
| FEC | 1.00 | 0.004 | ||
| T-ET | 0.73 (0.54–0.97) | |||
| Intrinsic subtype | ||||
| – | – | <0.001 | ||
| TP53 | ||||
| – | – | 0.004 | ||
| TP53 by intrinsic subtype | ||||
| Wild type | Mutated | Not done or failurec | 0.100 | |
| Luminal A-like | 1.00 | 1.39 (0.75–2.55) | 0.77 (0.34–1.75) | |
| Luminal B-like (HER2 negative) | 1.00 | 1.18 (0.52–2.68) | 0.74 (0.20–2.65) | |
| Luminal B-like (HER2 positive) | 1.00 | 1.13 (0.60–2.16) | 0.91 (0.41–2.00) | |
| HER2 positive (non-luminal) | 1.00 | 0.36 (0.15–0.85) | 0.60 (0.24–1.52) | |
| Triple negative | 1.00 | 1.17 (0.53–2.59) | 0.71 (0.26–1.94) | |
Note that while subtype and TP53 were included as main effects in the model, we have not reported HR effects as these are difficult to interpret in the presence of a significant interaction between these terms.
bSignificance level α = 0.1 for interaction tests; α = 0.01 for main effects.
cTP53 not tested. The main reason was samples with <20% tumour cells.
pCR, Pathological complete response; EFS, event-free survival; HR, hazard ratios; CI, confidence interval; FEC, 5-fluorouracil, epirubicin and cyclophosphamide; T-ET, docetaxel followed by epirubicin and docetaxel.
discussion
This analysis within a randomized phase III study confirms superior outcomes for those patients whose tumours achieve pCR in multivariate analysis, with impressive HRs in favour of patients achieving pCR (0.40, 0.32 and 0.32 for EFS, DMFS and OS, respectively) (Table 2, supplementary Tables S6 and S7, available at Annals of Oncology online). Two important methodological approaches were used to avoid two potential biases. First, we assessed the predictive value of pCR in a multivariate model after adjusting for other well-known EFS prognostic factors [7, 8, 13]. Other studies have shown that tumours with a better prognosis (e.g. smaller tumours) have a higher pCR rate. The aim of our two-step approach multivariate analysis was to correct for any such bias introduced by the different subtypes. Second, we used a landmark approach when correlating pCR with clinical end points, to avoid a bias known as guarantee-time bias (GTB) [9, 10, 14]. Our concern in assessing survival outcomes following pCR was to avoid a bias in favour of the group of patients achieving pCR: this potential bias is known as GTB and occurs when an analysis compares the survival of two groups that are defined by an event occurring after the start of the follow-up period. In oncology, GTB is very well recognized in the metastatic setting where the classic classifying event is objective response [9, 10], but a recently published paper has highlighted the importance of GTB in other settings including the neoadjuvant one [14]. In our study, we used a landmark analysis which is a widely recommended approach to avoid GTB [9, 10, 14]. The findings we report within the context of a single study have been confirmed in the recent FDA meta-analysis of 13 864 patients treated in prospective neoadjuvant chemotherapy trials [7].
There is an increased recognition of the need to consider breast cancer as a collection of biologically different subtypes. The results of the two-step multivariate analysis suggest a prognostic impact of pCR on EFS across all subtypes even when adjusting for subtypes. Our study has limitations. No central pathology review of post-chemotherapy histology reports was carried out. Such an analysis was conducted recently in the context of a large multicentric trial and reported a 5% rate of misclassification involving pCR versus minimal residual disease [15]. Notwithstanding these reassuring results, this analysis highlights the need for consensus guidelines in the context of prospective trials. No central review for grade, ER, PR and HER2 was carried out. In addition, we could not use expression arrays to distinguish between luminal A and B tumours, and used the simplified classification instead, as suggested by the St Gallen 2011 consensus [11]. Reassuringly, the pCR rates observed in the different intrinsic subtypes in our series, including luminal A-like and B-like, were very similar to those reported in a large German series or in the recent meta-analysis where the same pCR definition was used [6, 7]. As with the German series and FDA meta-analysis, we did not find firm evidence that pCR was univariately predictive of better outcomes in luminal A-like tumours, though equally there was no evidence that pCR had different prognostic effects between subtypes as shown by the two-step multivariate analysis [6, 7]. We have collected centrally the luminal A-like tumours and we are in the process of analysing them at a molecular level, but we suggest that the data are consistent with pCR being predictive irrespective of subtype.
Patients with pCR had excellent prognosis, particularly for TN or luminal B/HER2-negative subgroups, and a correspondingly poor prognosis for these subgroups when pCR was not achieved. Given that all luminal B-like cases subsequently received 5 years standard endocrine treatment, it is clear from our data that a poor response to chemotherapy in this group was still clinically relevant despite subsequent endocrine therapy. Similar differences were observed in those patients with HER2-positive tumours who went on to get post-surgical adjuvant trastuzumab (supplementary Figure S5, available at Annals of Oncology online). In other words, in this study, achieving pCR remains a predictor of better survival despite the use of post-surgical targeted therapies.
We chose to define pCR as complete disappearance of invasive carcinoma in the primary tumour (or very few scattered tumour cells) and in the nodes, while allowing for persistence of DCIS. The recent FDA meta-analysis did not demonstrate a prognostic difference whether DCIS remains present or absent in the absence of residual invasive cancer [7].
Given the known relationship between TP53 mutations and ER status, the effect of TP53 function on pCR and survival outcomes was assessed within each subtype [1]. In the univariate Cox regression model, pCR predicts for a better EFS in all subtypes irrespective of TP53 status (Figure 3). Furthermore, in the multivariate Cox regression model, in all subtypes except for HER2-positive/non-luminal, patients with TP53-mutated tumours had worse outcomes. The opposite result for HER2-positive/non-luminal disease is probably a chance finding: this interaction effect approaches significance only for EFS (P = 0.1) (Table 2), this subtype has the smallest number of patients and, among those patients not receiving adjuvant trastuzumab, disproportionately more patients with wild-type TP53 relapsed 16 of 22 (72%), compared with those with mutant TP53 10 of 32 (31%).
In conclusion, this analysis of over 1200 cases within the EORTC 10994/BIG 1-00 randomized phase III trial suggests that patients who achieve a pCR following six cycles of contemporary chemotherapy have significantly better outcomes, irrespective of their intrinsic subtype and TP53 status. In contrast, when chemotherapy does not induce a pCR, which is the case for the majority of patients, outcomes are poorer. This observation across all subgroups justifies the current interest in the development of post-neoadjuvant trials, even in subgroups where effective targeted therapies exist.
funding
Research support for the study: the US National Cancer Institute (grants 2U10 CA11488-31 through 5U10 CA011488-40, Bethesda, MA); the French Ligue Nationale Contre le Cancer and Cancer Research UK (donations through the EORTC Charitable Trust); the EU (fp6 Active p53 grant); Pharmacia Corporation (Educational Grant to EORTC Headquarters) and Sanofi-Aventis (Educational Grants to EORTC Headquarters and Clinical Trial Office at Karolinska University); the Fondation Widmer. Sanofi-Aventis provided Docetaxel (Taxotere®) for this study. Financial support for the study: Fondation pour la lutte contre le cancer et pour des recherches medico-biologiques and SIRIC BRIO (Site de Recherche Intégrée sur le Cancer—Bordeaux Recherche Intégrée Oncologie) [Grant INCa-DGOS-Inserm 6046].
disclosure
JB and the Karolinska Institutet received research funding from Agmen, Bayer, Merck, Roche and Sanofi-Aventis for clinical and molecular biological studies. Other authors have declared no conflicts of interest. This publication content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Cancer Institute or the European Commission.
Supplementary Material
acknowledgements
We thank the patients, doctors and nurses involved in the EORTC 10994/BIG 1-00 study for their generous participation. We also thank the data managers from the EORTC, the Anglo-Celtic Cooperative Oncology Group (ACCOG) at the Information and Statistics Division of the Scottish NHS, the Swedish Breast Cancer Group (SweBCG) and the Swiss Group for Clinical Cancer Research (SAKK); Pippa McKelvie-Sebileau, of Institut Bergonié, for assistance in preparation and submission of the manuscript.
references
- 1.Bonnefoi H, Piccart M, Bogaerts J, et al. TP53 status for prediction of sensitivity to taxane versus non-taxane neoadjuvant chemotherapy in breast cancer (EORTC 10994/BIG 1-00): a randomised phase 3 trial. Lancet Oncol. 2011;12:527–539. doi: 10.1016/S1470-2045(11)70094-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prowell TM, Pazdur R. Pathological complete response and accelerated drug approval in early breast cancer. N Engl J Med. 2012;366:2438–2441. doi: 10.1056/NEJMp1205737. [DOI] [PubMed] [Google Scholar]
- 3.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 4.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26:1275–1281. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 6.von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796–1804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 7.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014 doi: 10.1016/S0140-6736(13)62422-8. February 14 [epub ahead of print], http://dx.doi.org/10.1016/S0140-6736(13)62422-8 . [DOI] [PubMed] [Google Scholar]
- 8.Untch M, Fasching PA, Konecny GE, et al. Pathologic complete response after neoadjuvant chemotherapy plus trastuzumab predicts favorable survival in human epidermal growth factor receptor 2-overexpressing breast cancer: results from the TECHNO trial of the AGO and GBG study groups. J Clin Oncol. 2011;29:3351–3357. doi: 10.1200/JCO.2010.31.4930. [DOI] [PubMed] [Google Scholar]
- 9.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1:710–719. doi: 10.1200/JCO.1983.1.11.710. [DOI] [PubMed] [Google Scholar]
- 10.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response and other comparisons of time-to-event by outcome variables. J Clin Oncol. 2008;26:3913–3915. doi: 10.1200/JCO.2008.16.1000. [DOI] [PubMed] [Google Scholar]
- 11.Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kennecke H, Yerushalmi R, Woods R, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28:3271–3277. doi: 10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- 13.Esserman LJ, Berry DA, DeMichele A, et al. Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: results from the I-SPY 1 TRIAL—CALGB 150007/150012, ACRIN 6657. J Clin Oncol. 2012;30:3242–3249. doi: 10.1200/JCO.2011.39.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giobbie-Hurder A, Gelber RD, Regan MM. Challenges of guarantee-time bias. J Clin Oncol. 2013;31:2963–2969. doi: 10.1200/JCO.2013.49.5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Provenzano E, Vallier AL, Champ R, et al. A central review of histopathology reports after breast cancer neoadjuvant chemotherapy in the neo-tango trial. Br J Cancer. 2013;108:866–872. doi: 10.1038/bjc.2012.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.