This manuscript presents exploratory analyses of the incidence and time to development of CNS metastases in patients with first-line HER2-positive metastatic breast cancer in the CLEOPATRA study of pertuzumab, trastuzumab, and docetaxel. The results showed that pertuzumab, trastuzumab, and docetaxel delayed the onset of CNS disease compared with placebo, trastuzumab, and docetaxel.
Keywords: central nervous system, HER2, metastatic breast cancer, pertuzumab, trastuzumab
Abstract
Background
Results from the phase III trial CLEOPATRA in human epidermal growth factor receptor 2-positive first-line metastatic breast cancer demonstrated significant improvements in progression-free and overall survival with pertuzumab, trastuzumab, and docetaxel over placebo, trastuzumab, and docetaxel. We carried out exploratory analyses of the incidence and time to development of central nervous system (CNS) metastases in patients from CLEOPATRA.
Patients and methods
Patients received pertuzumab/placebo: 840 mg in cycle 1, then 420 mg; trastuzumab: 8 mg/kg in cycle 1, then 6 mg/kg; docetaxel: initiated at 75 mg/m2. Study drugs were administered i.v. every 3 weeks. The log-rank test was used for between-arm comparisons of time to CNS metastases as first site of disease progression and overall survival in patients with CNS metastases as first site of disease progression. The Kaplan–Meier approach was used to estimate median time to CNS metastases as first site of disease progression and median overall survival.
Results
The incidence of CNS metastases as first site of disease progression was similar between arms; placebo arm: 51 of 406 (12.6%), pertuzumab arm: 55 of 402 (13.7%). Median time to development of CNS metastases as first site of disease progression was 11.9 months in the placebo arm and 15.0 months in the pertuzumab arm; hazard ratio (HR) = 0.58, 95% confidence interval (CI) 0.39–0.85, P = 0.0049. Overall survival in patients who developed CNS metastases as first site of disease progression showed a trend in favor of pertuzumab, trastuzumab, and docetaxel; HR = 0.66, 95% CI 0.39–1.11. Median overall survival was 26.3 versus 34.4 months in the placebo and pertuzumab arms, respectively. Treatment comparison of the survival curves was not statistically significant for the log-rank test (P = 0.1139), but significant for the Wilcoxon test (P = 0.0449).
Conclusions
While the incidence of CNS metastases was similar between arms, our results suggest that pertuzumab, trastuzumab, and docetaxel delays the onset of CNS disease compared with placebo, trastuzumab, and docetaxel.
ClinicalTrials.gov
introduction
Breast cancer is the second most common neoplasm metastasizing to the brain, second only to lung cancer [1, 2], and the most common solid tumor associated with leptomeningeal carcinomatosis [3, 4]. Approximately 10%–16% of patients with metastatic breast cancer develop symptoms from brain metastases during the course of their disease [5]. However, autopsy studies have revealed that the true incidence of brain metastases from breast cancer is ∼30% [5].
Overexpression of human epidermal growth factor receptor 2 (HER2), described in around 20% of patients with breast cancer [6], is among the risk factors for developing central nervous system (CNS) metastases [5, 7]. Between 25% and 48% of patients with HER2-positive metastatic breast cancer develop CNS metastases [8–14], an incidence that is considerably higher than that reported for breast cancer overall. In a systematic review of trastuzumab in early breast cancer, which analyzed data from five randomized trials (HERA/BIG 1-01, NSABP B-31, NCCTG N9831, FinHer, PACS-04), an increased risk of brain metastases as first site of relapse was reported for patients who had received trastuzumab compared with those who had not [risk ratio 1.75, 90% confidence interval (CI) 1.29–2.38; P = 0.002] [15]. It is thought that this observation is due to limited penetration of trastuzumab through the blood–brain barrier combined with better control of extracranial disease. Disease-free and overall survival were significantly prolonged with trastuzumab-based treatment compared with chemotherapy alone [15]. In addition, in a prospective, observational study continuation of trastuzumab after diagnosis of CNS metastases has been associated with improved survival outcomes [9]. After a longer follow-up, data from the HERA/BIG 1-01 trial showed a similar incidence of CNS relapse as first disease-free survival event with and without trastuzumab treatment and did not confirm an increased risk of CNS relapse with adjuvant trastuzumab [16]. Median overall survival from time of diagnosis of CNS disease to death ranged from 17.1 to 23.5 months in patients with HER2-positive breast cancer [17–19]. In comparison, median overall survival was shorter in patients with brain metastases from HER2-negative breast cancer (5.2 [17] and 9.4 months [18], respectively).
Results from CLEOPATRA, a phase III trial in first-line HER2-positive metastatic breast cancer, have demonstrated significant and clinically meaningful improvements in progression-free [20] and overall survival [21] with the combination of pertuzumab, trastuzumab, and docetaxel compared with placebo, trastuzumab, and docetaxel. In this article, we report exploratory analyses of the development of CNS metastases in CLEOPATRA.
patients and methods
study design and treatment
CLEOPATRA was a randomized, double-blind, placebo-controlled phase III trial designed to compare efficacy and safety of placebo, trastuzumab, and docetaxel (placebo arm) with pertuzumab, trastuzumab, and docetaxel (pertuzumab arm) in patients with HER2-positive metastatic breast cancer. Primary end point was independently assessed progression-free survival. Secondary end points included overall survival, defined as the time from randomization to death from any cause, investigator-assessed progression-free survival, objective response rate, and safety. Pertuzumab/placebo was given at 840 mg in cycle 1, followed by 420 mg in subsequent cycles. Trastuzumab was administered at 8 mg/kg in cycle 1 and 6 mg/kg in subsequent cycles. Docetaxel was initiated at 75 mg/m2. All study drugs were administered intravenously on a 3-weekly schedule. Pertuzumab/placebo and trastuzumab were given until investigator-assessed disease progression or unmanageable toxicity. At least six cycles of docetaxel were recommended; before cycle 6 docetaxel was discontinued for disease progression or unmanageable toxicity, while more cycles could be given at the discretion of the investigator.
The study was conducted according to Good Clinical Practice and the Declaration of Helsinki. Protocol approval was obtained from independent ethics committees at each site. Participants provided written informed consent.
patients
Eligible patients had centrally confirmed HER2-positive locally recurrent, unresectable, or metastatic breast cancer without prior chemotherapy or biologic therapy for their advanced disease. Patients with current clinical or radiographic evidence of CNS metastases were excluded. Computed tomography (CT) or magnetic resonance imaging (MRI) scan of the brain was mandatory within 28 days of randomization in patients with clinical suspicion of brain metastases only.
assessments
Tumor assessments were based on RECIST and carried out every 9 weeks. Adverse events were monitored continuously and graded according to NCI-CTCAE v3.0. CT or MRI scans of the brain and/or spine were carried out when CNS metastases were clinically suspected only.
statistics
In patients who developed CNS metastases as first site of disease progression the Kaplan–Meier approach was used to estimate median time to CNS metastases and median overall survival. The log-rank test was used to compare time to CNS metastases as first site of disease progression and overall survival between arms. Overall survival was also compared between arms using the Wilcoxon test to assess the sensitivity of the log-rank test results. The Wilcoxon test places more weight on early events. A Cox proportional hazards model was utilized to estimate hazard ratios (HRs) and 95% CI. Univariate and multivariate Cox regression analyses of time to CNS metastases as first site of disease progression were carried out to test the treatment effect adjusted for selected covariates and to investigate the association of selected baseline characteristics, irrespective of study treatment, with the development of CNS metastases as first site of disease progression. SAS version 9.2 was used for statistical analyses. All analyses were exploratory only. The trial is registered with ClinicalTrials.gov, NCT00567190.
results
study population
Between February 2008 and July 2010, 808 patients were enrolled and randomized to treatment with placebo, trastuzumab, and docetaxel or with pertuzumab, trastuzumab, and docetaxel. The median follow-up was 30 months. Baseline characteristics of patients who experienced first disease progression either in the CNS or other sites are presented in Table 1 and were generally balanced between subgroups. However, a higher proportion of patients with disease progression outside the CNS carried mutant phosphatidylinositol-3-kinase (PI3K) compared with patients who developed CNS metastases as first site of disease progression. A protocol violation occurred with the enrollment of one patient with brain lesions at baseline. The patient received one cycle of study treatment in the pertuzumab arm and was withdrawn to receive radiotherapy to the brain. This patient was not included in our analyses.
Table 1.
Baseline characteristics in patients with disease progression
| Placebo + trastuzumab + docetaxel |
Pertuzumab + trastuzumab + docetaxel |
|||
|---|---|---|---|---|
| PD in CNS (n = 51) | PD outside CNS (n = 218) | PD in CNS (n = 55) | PD outside CNS (n = 186) | |
| Age, years | ||||
| Median (range) | 55.0 (28–78) | 52.0 (27–79) | 54.0 (26–71) | 53.0 (22–80) |
| Hormone receptor status,a n (%) | ||||
| ER- and/or PR-positive | 22 (43.1) | 107 (49.1) | 26 (47.3) | 91 (48.9) |
| ER- and PR-negative | 28 (54.9) | 105 (48.2) | 28 (50.9) | 95 (51.1) |
| Data not available | 1 (2.0) | 6 (2.8) | 1 (1.8) | 0 (0.0) |
| Tumor grade,a n (%) | ||||
| Well differentiated (G1) | 3 (5.9) | 9 (4.1) | 0 (0.0) | 9 (4.8) |
| Moderately differentiated (G2) | 17 (33.3) | 64 (29.4) | 19 (34.5) | 51 (27.4) |
| Poorly differentiated (G3) | 19 (37.3) | 68 (31.2) | 22 (40.0) | 62 (33.3) |
| Anaplastic (G4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Unknown | 12 (23.5) | 77 (35.3) | 14 (25.5) | 64 (34.4) |
| Measurable tumor burden (target lesions), mm | ||||
| Median (range) | 80.5 (12–301) | 90.5 (10–455) | 98.0 (14–326) | 79.0 (10–422) |
| Number of patients | 48 | 198 | 52 | 175 |
| Number of metastatic sites | ||||
| Patients with ≤3 sites, n (%) | 35 (68.6) | 160 (73.4) | 32 (58.2) | 127 (68.3) |
| Patients with >3 sites, n (%) | 16 (31.4) | 58 (26.6) | 23 (41.8) | 59 (31.7) |
| Disease type, n (%) | ||||
| Visceral disease | 42 (82.4) | 176 (80.7) | 45 (81.8) | 148 (79.6) |
| Nonvisceral disease | 9 (17.6) | 42 (19.3) | 10 (18.2) | 38 (20.4) |
| Disease-free interval, months | ||||
| Median (range) | 33.0 (0–117) | 29.0 (0–181) | 32.0 (0–102) | 30.5 (1–276) |
| Number of patients | 23 | 107 | 25 | 78 |
| PI3K status,b n (%) | ||||
| Wildtype | 23 (45.1) | 92 (42.2) | 30 (54.5) | 76 (40.9) |
| Mutant | 7 (13.7) | 56 (25.7) | 7 (12.7) | 50 (26.9) |
| Data not available | 21 (41.2) | 70 (32.1) | 18 (32.7) | 60 (32.3) |
aAssessed locally.
bAssessed centrally.
CNS, central nervous system; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; PD, progressive disease; PI3K, phosphatidylinositol-3-kinase; PR, progesterone receptor.
incidence and time to development of CNS metastases
Evaluation of tumor response by an independent review facility stopped after the target of 381 progression-free survival events had been reached for the primary analysis (data cutoff in May 2011) [20]. Investigators continued to evaluate tumor response, and data presented here are based on investigator-assessed disease progression with a cutoff date in May 2012. Based on the whole intention-to-treat (ITT) population, a similar proportion of patients in the placebo (51 of 406; 12.6%) and pertuzumab (55 of 402; 13.7%) arms developed CNS metastases as first site of disease progression. In an additional 11 patients in the placebo arm and 8 patients in the pertuzumab arm, disease progression was detected in the CNS and other sites at the same visit. Overall, 15.3% (62 of 406) of patients in the placebo arm and 15.7% (63 of 402) of patients in the pertuzumab arm experienced disease progression in the CNS.
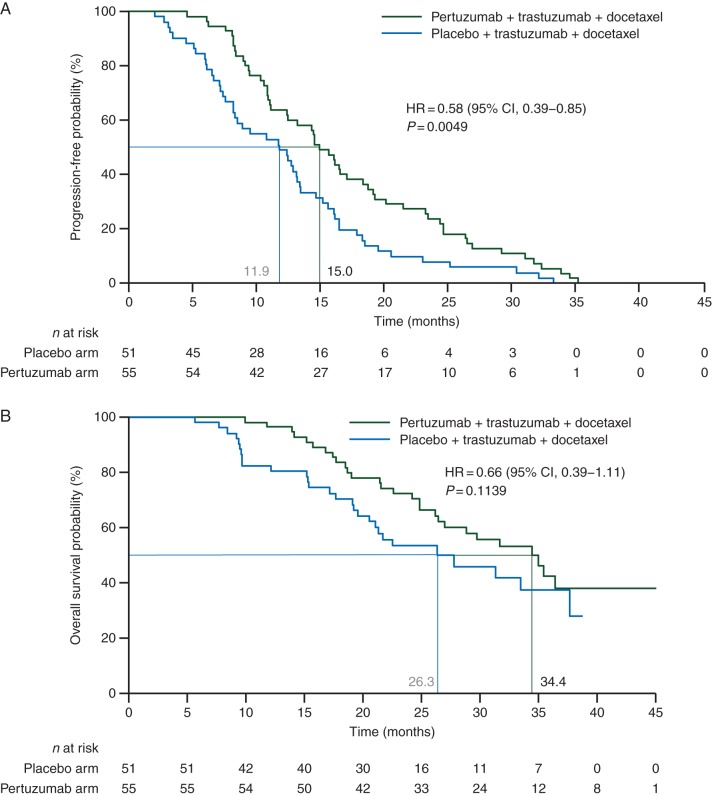
Kaplan–Meier estimates of time to development of CNS metastases as first site of disease progression are presented in Figure 1A. The median time to progression in the CNS was 11.9 months in the placebo arm and 15.0 months in the pertuzumab arm; HR= 0.58, 95% CI 0.39–0.85, P = 0.0049. Only patients with CNS metastases as first and only site of disease progression were included in time-to-event analyses to investigate the impact of pertuzumab-based treatment on the development of CNS metastases in patients with controlled systemic, extracranial disease.
Figure 1.
Kaplan–Meier estimates of time to CNS metastases as first site of disease progression (A) and overall survival in patients who developed CNS metastases as first site of disease progression (B).
overall survival
Overall survival, defined as time from randomization to death from any cause, was analyzed in patients with CNS metastases as first site of disease progression. Overall survival showed a trend in favor of pertuzumab, trastuzumab, and docetaxel (Figure 1B). Median overall survival was 26.3 months in the placebo arm and 34.4 months in the pertuzumab arm; HR = 0.66, 95% CI 0.39–1.11. The differences observed were not statistically significant for the log-rank test (P = 0.1139) but were significant for the Wilcoxon test (P = 0.0449).
Following disease progression, patients were taken off study treatment. Breast cancer treatments received by patients with CNS metastases as first site of disease progression are presented in supplementary Table S2, available at Annals of Oncology online. Treatments were generally balanced between arms, although it should be noted that the sample size is low, which limits the interpretation of results.
treatment effect and association of baseline characteristics with the development of CNS metastases
We analyzed the treatment effect of pertuzumab, trastuzumab, and docetaxel versus placebo, trastuzumab, and docetaxel on time to development of CNS metastases as first site of disease progression adjusted for selected baseline characteristics (supplementary Figure S1, available at Annals of Oncology online). The treatment effect (HR = 0.57) was not influenced by the addition of any of the covariates, with HRs ranging from 0.56 to 0.59 when each individual covariate was added to the model.
A univariate Cox regression analysis was carried out to assess the association of selected baseline characteristics [age (≥50 versus <50 years), hormone receptor status, tumor grade, measurable tumor burden, number of metastatic sites (≤3 versus >3), disease type (visceral versus nonvisceral), PI3K status (mutant versus wildtype), and disease-free interval] with development of CNS metastases as first site of disease progression irrespective of study treatment (supplementary Table S3, available at Annals of Oncology online). The hazard of developing CNS metastases was significantly reduced in patients with ≤3 metastatic sites compared with patients with >3 metastatic sites (HR = 0.42; 95% CI 0.28–0.63; P < 0.0001). The risk of developing CNS metastases increased by 4% per cm increase in the total sum of the longest diameters of target lesions (HR = 1.004; 95% CI 1.001–1.006; P = 0.0066). There was a trend for an increased risk of development of CNS metastases in patients with visceral disease at baseline (HR = 1.55; 95% CI 0.94–2.55; P = 0.0858). In a multivariate Cox regression analysis, the number of metastatic sites (≤3 versus >3) remained significantly associated with the development of CNS metastases; however, measurable tumor burden was no longer statistically significant.
discussion
We analyzed the incidence and time to development of CNS metastases as first site of disease progression in patients with first-line HER2-positive metastatic breast cancer who received placebo, trastuzumab, and docetaxel or pertuzumab, trastuzumab, and docetaxel in the CLEOPATRA study. The incidence of CNS metastases was similar between arms. However, median time to development of CNS metastases as first site of disease progression was prolonged in the pertuzumab arm compared with the placebo arm. The analysis of overall survival in patients who developed CNS metastases as first site of disease progression showed a trend in favor of pertuzumab, trastuzumab, and docetaxel.
HER2 positivity [5, 7, 22], hormone receptor negativity [23, 24], higher tumor grade [25], and younger age [3, 23] have been identified as risk factors for development of CNS metastases from breast cancer. In CLEOPATRA, a higher number of metastatic sites at baseline (>3 versus ≤3) was significantly associated with development of CNS metastases; patients with visceral disease showed a trend for an increased risk of development of CNS metastases. It should be noted that the relatively small number of CNS events as first site of disease progression (n = 106) limits the sensitivity of the analysis to detect differences in time to event by subgroups. The interpretation of our results is limited as analyses were carried out post hoc and are therefore hypothesis-generating only. In addition, there was no screening for asymptomatic CNS metastases, which may result in an underestimation of the true incidence of CNS metastases in CLEOPATRA.
There is some evidence from registry data and from a systematic review of adjuvant trastuzumab trials that the incidence of CNS metastases as first site of disease progression is increased in patients with HER2-positive breast cancer who received trastuzumab-based treatment compared with nontrastuzumab-based therapies [15, 26]. However, the systematic review does also note that when the analysis was stratified by type of trastuzumab administration (sequential or concurrent), the higher risk of brain metastases in patients receiving trastuzumab was only observed where trastuzumab therapy was sequential to chemotherapy [15]. Furthermore, improvements in systemic, extracranial disease control and overall survival associated with trastuzumab-based therapy are likely to result in an unmasking of the CNS as sanctuary for late disease recurrence that would otherwise remain clinically silent before a patient's death.
Treatment with trastuzumab delays the occurrence of CNS disease [27] and continued trastuzumab therapy following the development of CNS metastases has been associated with improved survival outcomes compared with those seen in patients who do not receive trastuzumab-based treatment [9]. Lapatinib, a small molecule dual inhibitor of HER2 and epidermal growth factor receptor (EGFR), has shown moderate antitumor activity in the CNS in patients with HER2-positive metastatic breast cancer, especially in combination with capecitabine [28, 29]. In a direct comparison with trastuzumab plus capecitabine in patients with HER2-positive metastatic breast cancer, a similar proportion of patients in both arms experienced CNS metastases as first site of disease progression [30]. However, progression-free and overall survival were longer in patients who received trastuzumab plus capecitabine compared with lapatinib plus capecitabine [30]. Recent exploratory results from TH3RESA, a phase III study comparing trastuzumab emtansine (T-DM1) with treatment of physician's choice in patients with HER2-positive metastatic breast cancer, showed that, in patients with brain metastases at baseline, the risk of disease progression or death was reduced with T-DM1 compared with treatment according to physician's choice [31].
Results from CLEOPATRA have demonstrated that the combination of pertuzumab with trastuzumab and docetaxel further improves systemic disease control by trastuzumab plus docetaxel, resulting in a significant and clinically meaningful prolongation of median progression-free [20] and overall survival [21]. Our results presented here suggest that this improvement in systemic disease control with pertuzumab-based treatment delays the onset of CNS disease. Advances in systemic disease control and overall survival may result in an increase in the number of patients who will eventually develop CNS metastases; therefore, better treatment options for CNS disease are needed to increase quality of life and prolong survival of these patients. Based on results from CLEOPATRA, further investigation into the activity of HER2-targeted antibodies in patients with CNS disease from HER2-positive breast cancer is warranted.
funding
The study was funded by F. Hoffmann-La Roche Ltd, Basel, Switzerland and Genentech, Inc., South San Francisco, CA, USA, a member of the Roche group.
disclosure
SMS acts as an uncompensated consultant/advisor for Roche/Genentech. Her institution has received research funding from Roche/Genentech, Pfizer, Puma, Sanofi-Aventis, and Bristol-Myers Squibb. JB acts as a consultant/advisor for Roche/Genentech. CQ and LFL are Genentech employees. LFL owns shares in Genentech. JC acts as a consultant/advisor for Roche, Novartis, and Celgene and has received honoraria from Roche, Novartis, Celgene, and Eisai. DM and Y-HI have no conflicts of interest to disclose.
Supplementary Material
acknowledgements
The authors acknowledge Ken Dana (Genentech, Inc., South San Francisco, CA, USA) for his contribution to statistical programming and data quality assurance. Targos Molecular Pathology (Kassel, Germany) carried out central HER2 testing. Funding for third-party writing assistance, furnished by Vilma Graupner, PhD, CMPP, was provided by F. Hoffmann-La Roche.
references
- 1.Zimm S, Wampler GL, Stablein D, et al. Intracerebral metastases in solid-tumor patients: natural history and results of treatment. Cancer. 1981;48:384–394. doi: 10.1002/1097-0142(19810715)48:2<384::aid-cncr2820480227>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 2.Nussbaum ES, Djalilian HR, Cho KH, et al. Brain metastases. Histology, multiplicity, surgery, and survival. Cancer. 1996;78:1781–1788. [PubMed] [Google Scholar]
- 3.Lee YT. Breast carcinoma: pattern of metastasis at autopsy. J Surg Oncol. 1983;23:175–180. doi: 10.1002/jso.2930230311. [DOI] [PubMed] [Google Scholar]
- 4.Kesari S, Batchelor TT. Leptomeningeal metastases. Neurol Clin. 2003;21:25–66. doi: 10.1016/s0733-8619(02)00032-4. [DOI] [PubMed] [Google Scholar]
- 5.Lin NU, Bellon JR, Winer EP. CNS metastases in breast cancer. J Clin Oncol. 2004;22:3608–3617. doi: 10.1200/JCO.2004.01.175. [DOI] [PubMed] [Google Scholar]
- 6.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007;131:18–43. doi: 10.5858/2007-131-18-ASOCCO. [DOI] [PubMed] [Google Scholar]
- 7.Miller KD, Weathers T, Haney LG, et al. Occult central nervous system involvement in patients with metastatic breast cancer: prevalence, predictive factors and impact on overall survival. Ann Oncol. 2003;14:1072–1077. doi: 10.1093/annonc/mdg300. [DOI] [PubMed] [Google Scholar]
- 8.Park YH, Park MJ, Ji SH, et al. Trastuzumab treatment improves brain metastasis outcomes through control and durable prolongation of systemic extracranial disease in HER2-overexpressing breast cancer patients. Br J Cancer. 2009;100:894–900. doi: 10.1038/sj.bjc.6604941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brufsky AM, Mayer M, Rugo HS, et al. Central nervous system metastases in patients with HER2-positive metastatic breast cancer: incidence, treatment, and survival in patients from registHER. Clin Cancer Res. 2011;17:4834–4843. doi: 10.1158/1078-0432.CCR-10-2962. [DOI] [PubMed] [Google Scholar]
- 10.Bendell JC, Domchek SM, Burstein HJ, et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer. 2003;97:2972–2977. doi: 10.1002/cncr.11436. [DOI] [PubMed] [Google Scholar]
- 11.Bartsch R, De Vries C, Pluschnig U, et al. Predicting for activity of second-line trastuzumab-based therapy in her2-positive advanced breast cancer. BMC Cancer. 2009;9:367. doi: 10.1186/1471-2407-9-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Altaha R, Crowell E, Hobbs G, et al. Increased risk of brain metastases in patients with HER-2/neu-positive breast carcinoma. Cancer. 2005;103:442–443. doi: 10.1002/cncr.20813. [DOI] [PubMed] [Google Scholar]
- 13.Stemmler HJ, Kahlert S, Siekiera W, et al. Characteristics of patients with brain metastases receiving trastuzumab for HER2 overexpressing metastatic breast cancer. Breast. 2006;15:219–225. doi: 10.1016/j.breast.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 14.Clayton AJ, Danson S, Jolly S, et al. Incidence of cerebral metastases in patients treated with trastuzumab for metastatic breast cancer. Br J Cancer. 2004;91:639–643. doi: 10.1038/sj.bjc.6601970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moja L, Tagliabue L, Balduzzi S, et al. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev. 2012;4:CD006243. doi: 10.1002/14651858.CD006243.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pestalozzi BC, Holmes E, de Azambuja E, et al. CNS relapses in patients with HER2-positive early breast cancer who have and have not received adjuvant trastuzumab: a retrospective substudy of the HERA trial (BIG 1-01) Lancet Oncol. 2013;14:244–248. doi: 10.1016/S1470-2045(13)70017-2. [DOI] [PubMed] [Google Scholar]
- 17.Eichler AF, Kuter I, Ryan P, et al. Survival in patients with brain metastases from breast cancer: the importance of HER-2 status. Cancer. 2008;112:2359–2367. doi: 10.1002/cncr.23468. [DOI] [PubMed] [Google Scholar]
- 18.Kirsch DG, Ledezma CJ, Mathews CS, et al. Survival after brain metastases from breast cancer in the trastuzumab era. J Clin Oncol. 2005;23:2114–2116. doi: 10.1200/JCO.2005.05.249. [DOI] [PubMed] [Google Scholar]
- 19.Gori S, Rimondini S, De Angelis V, et al. Central nervous system metastases in HER-2 positive metastatic breast cancer patients treated with trastuzumab: incidence, survival, and risk factors. Oncologist. 2007;12:766–773. doi: 10.1634/theoncologist.12-7-766. [DOI] [PubMed] [Google Scholar]
- 20.Baselga J, Cortes J, Kim S-B, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swain SM, Kim SB, Cortes J, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14:461–471. doi: 10.1016/S1470-2045(13)70130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chien AJ, Rugo HS. Emerging treatment options for the management of brain metastases in patients with HER2-positive metastatic breast cancer. Breast Cancer Res Treat. 2013;137:1–12. doi: 10.1007/s10549-012-2328-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans AJ, James JJ, Cornford EJ, et al. Brain metastases from breast cancer: identification of a high-risk group. Clin Oncol (R Coll Radiol) 2004;16:345–349. doi: 10.1016/j.clon.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 24.Slimane K, Andre F, Delaloge S, et al. Risk factors for brain relapse in patients with metastatic breast cancer. Ann Oncol. 2004;15:1640–1644. doi: 10.1093/annonc/mdh432. [DOI] [PubMed] [Google Scholar]
- 25.Pestalozzi BC, Zahrieh D, Price KN, et al. Identifying breast cancer patients at risk for central nervous system (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG) Ann Oncol. 2006;17:935–944. doi: 10.1093/annonc/mdl064. [DOI] [PubMed] [Google Scholar]
- 26.Musolino A, Ciccolallo L, Panebianco M, et al. Multifactorial central nervous system recurrence susceptibility in patients with HER2-positive breast cancer: epidemiological and clinical data from a population-based cancer registry study. Cancer. 2011;117:1837–1846. doi: 10.1002/cncr.25771. [DOI] [PubMed] [Google Scholar]
- 27.Dawood S, Broglio K, Esteva FJ, et al. Defining prognosis for women with breast cancer and CNS metastases by HER2 status. Ann Oncol. 2008;19:1242–1248. doi: 10.1093/annonc/mdn036. [DOI] [PubMed] [Google Scholar]
- 28.Cameron D, Casey M, Press M, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Res Treat. 2008;112:533–543. doi: 10.1007/s10549-007-9885-0. [DOI] [PubMed] [Google Scholar]
- 29.Lin NU, Dieras V, Paul D, et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res. 2009;15:1452–1459. doi: 10.1158/1078-0432.CCR-08-1080. [DOI] [PubMed] [Google Scholar]
- 30.Pivot X, Semiglazov V, Zurawski B, et al. CEREBEL (EGF111438): an open label randomized phase III study comparing the incidence of CNS metastases in patients (pts) with HER2+ metastatic breast cancer (MBC), treated with lapatinib plus capecitabine (LC) versus trastuzumab plus capecitabine (TC); LBA11. Ann Oncol. 2012;23:ixe1–ixe30. [Google Scholar]
- 31.Wildiers H, Kim SB, Gonzalez-Martin A, et al. T-DM1 for HER2-positive metastatic breast cancer (MBC): Primary results from TH3RESA, a phase 3 study of T-DM1 vs treatment of physician's choice. Presented at European Cancer Congress 2013; (Abstr LBA15). Amsterdam, The Netherlands, 27 September–1 October 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.