Abstract
Diabetic retinopathy is a leading cause of blindness worldwide. Despite laser and surgical treatments, anti-angiogenic and other therapies, and strict metabolic control, many patients progress to visual impairment and blindness. New insights are needed into the pathophysiology of diabetic retinopathy in order to develop new methods to improve the detection and treatment of disease and the prevention of blindness. Hyperglycemia and diabetes result in increased flux through the hexosamine biosynthetic pathway, which, in turn, results in increased post-translational modification of Ser/Thr residues of proteins by O-linked β-N-acetylglucosamine (O-GlcNAc). O-GlcNAcylation is involved in regulation of many nuclear and cytoplasmic proteins in a manner similar to protein phosphorylation. Altered O-GlcNAc signaling has been implicated in the pathogenesis of diabetes and may play an important role in the pathogenesis of diabetic retinopathy. The goal of this review is to summarize the biology of the hexosamine biosynthesis pathway and O-GlcNAc signaling, to present the current evidence for the role of OGlcNAc signaling in diabetes and diabetic retinopathy, and to discuss future directions for research on O-GlcNAc in the pathogenesis of diabetic retinopathy.
Keywords: diabetes, diabetic retinopathy, glucose toxicity, hexosamine biosynthesis pathway, O-GlcNAcylation
1 Introduction
Diabetic retinopathy is the leading cause of visual impairment and blindness. Worldwide, there are an estimated 93 million people with diabetic retinopathy, of whom 17 million have proliferative diabetic retinopathy and 21 million have diabetic macular edema [1]. The number of people with diabetes is growing worldwide due to the rising prevalence of obesity [2]. Diabetic retinopathy has a wide spectrum of lesions including microaneurysms, dot-blot hemorrhages, occlusion and leakage of retinal blood vessels, small microinfarcts of the neuroretina (cotton-wool spots), and macular edema that characterize nonproliferative diabetic retinopathy. The proliferative phase of diabetic retinopathy is characterized by new blood vessel formation in the retina and vitreous, vitreous hemorrhage, and tractional retinal detachment. Diabetic macular edema, a major cause of visual loss in diabetes, can occur during both the nonproliferative and proliferative phases of diabetic retinopathy.
Although laser photocoagulation, anti-angiogenic drugs, and vitreo-retinal surgery are available for the treatment of diabetic retinopathy, many people with diabetic retinopathy progress to visual loss and blindness. Current treatments to prevent or treat diabetic retinopathy include the peroxisome proliferator-activated receptor α (PPAR-α) agonist, fenofibrate [3,4], inhibitors of vascular endothelial growth factor (VEGF) such as bevacizumab [5,6] and ranibizumab [7] that are injected directly into the eye to treat macular edema, and reninangiotensin system blockers lisinopril [8] and candesartan [9,10]. Intensive glucose control can delay the onset and progression of diabetic retinopathy [11]. Even among diabetic patients with intensive metabolic control, over 20% develop proliferative diabetic retinopathy after thirty years [11]. For the last five decades, the mainstay of treatment for proliferative diabetic retinopathy remains pan-retinal photocoagulation, a destructive ablation of the peripheral retina with laser, in order to suppress pro-angiogenic factors and reduce retinal neovascularization. The procedure is expensive, uncomfortable for the patient, and has permanent side effects of reduced peripheral vision and decreased night vision. As noted by Gariano and Gardner, “it is the ocular equivalent of lower-extremity amputation and, so far, there is no clinically proven non-surgical alternative.” [12].
The hexosamine biosynthesis pathway and altered O-GlcNAc signaling are implicated in the pathogenesis of diabetic cardiomyopathy and nephropathy. Recent evidence now suggests a similar role for O-GlcNAc in diabetic retinopathy. The goal of this review is to summarize the biology of the hexosamine biosynthesis pathway and O-GlcNAc signaling, to present the current evidence for the role of O-GlcNAc signaling in diabetes and diabetic retinopathy, and to discuss future directions for research on O-GlcNAc in the pathogenesis of diabetic retinopathy.
O-GlcNAc modification of proteins
O-GlcNAcylation is an important protein post-translational modification that involves the addition of a single O-linked β-N-acetylglucosamine (O-GlcNAc) moiety to the hydroxyl groups of serine and/or threonine residues of proteins (Figure 1). O-GlcNAcylation is distinct from classical glycosylation because the sugar moiety is not further modified or elongated, and OGlcNAcylation occurs primarily in the nucleus and cytoplasm, rather than in extracellular or luminal domains of membrane or secreted glycoproteins [13]. O-GlcNAcylation is a dynamic and reversible post-translational modification that involves interplay with phosphorylation. Since the discovery of O-GlcNAc in 1984 by Torres and Hart [14], over one thousand O-GlcNAcylated proteins have been identified [15-18]. O-GlcNAcylation is analogous to phosphorylation in that it is inducible and reversible, but unlike protein phosphorylation – which involves hundreds of distinct phosphatases and kinases – the dynamic turnover of O-GlcNAc is catalyzed by only two distinct polypeptides: uridine diphospho-N-acetylglucosamine:polypeptide β-N-acetyl-glucosaminyltransfase (O-GlcNAc transferase; OGT), and β-N-acetylglucosaminidase (O-GlcNAcase; OGA). O-GlcNAcylation is characterized by the rapid attachment and removal of O-GlcNAc to a polypeptide, multiple times during the life of a polypeptide, and at different rates on different sites [19]. O-GlcNAc signaling is fundamental to many biological processes including the cell cycle, immune activation, apoptosis, stress response pathways, inflammation, protein trafficking, transcriptional regulation, and nutrient sensing [20].
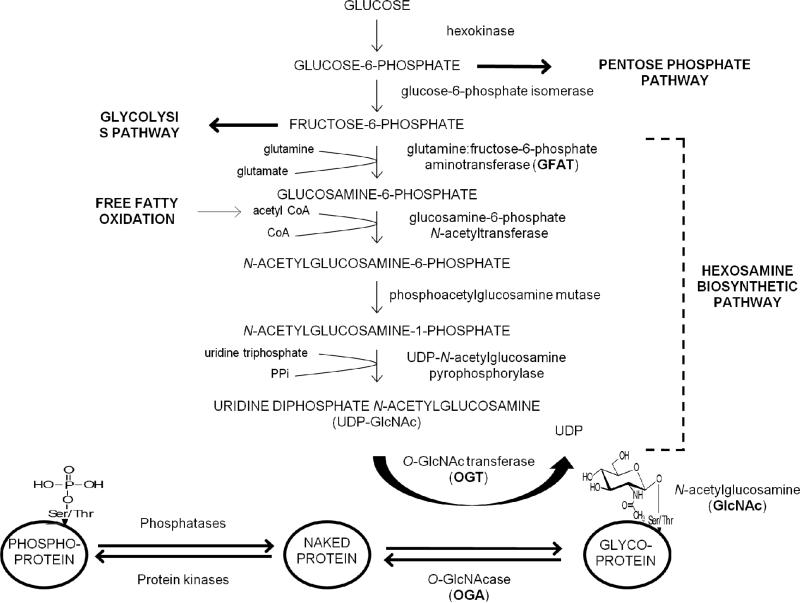
Figure 1.
Relationship of the hexosamine pathway with O-GlcNAcylation of proteins. The rate-limiting enzyme for entry into the pathway is GFAT. The major end product of the pathway is UDP-GlcNAC, which catalyzes the addition of O-GlcNAc to serine and threonine residues of proteins.
The OGT gene has been mapped to Xq13. OGT is a 1066-amino-acid protein with two domains, an N-terminal tetratricopeptide repeat (TPR) domain and a multidomain catalytic region [21]. OGT is located predominantly in the nucleus and cytoplasm [22]. Three splice forms of OGT are known: full length OGT with 13.5 TPRs, found in the nucleus and cytoplasm (ncOGT), mitochondrial OGT (mOGT) with 9 TPRs, and soluble OGT (sOGT) with 3 TPRs [23]. OGT catalyzes the addition of N-acetylglucosamine from UDP-GlcNAc to serine or threonine residues to form a β-glycosidic linkage.
The gene for OGA (MGEA5; Meningioma Express Antigen 5) has been mapped to 10q24. O-GlcNAcase is a 916-amino-acid protein with two domains, an N-terminal hexosaminidase domain and a C-terminal histone acetyltransferase (HAT) domain linked by a region containing a caspase C cleavage site [24]. OGA is widely expressed in all tissues. There are two splice variants of OGA, a full-length protein (OGA-L) that is predominantly located in the cytoplasm, and a variant that lacks the C-terminal domain (OGA-S) that is predominantly located in the nucleus [25]. OGA-S accumulates on the surface of nascent lipid droplets and may play a role in an O-GlcNAc-dependent feedback loop that regulates lipid droplet surface remodeling [26]. OGA appears to act on different protein substrates through a peptide binding groove on the protein surface [27,28] and by interacting with a large number of different targeting proteins. How OGT and OGA can act upon different binding sites in hundreds of different proteins to regulate O-GlcNAc is currently unresolved and an area of intense investigation.
3 Relationship of O-GlcNAc signaling with glucose metabolism
The hexosamine biosynthesis pathway is a nutrient sensing pathway that plays an important role in the development of glucose-induced insulin resistance [13,29]. Under normal conditions, the hexosamine biosynthesis pathway is a minor metabolic pathway that accounts for 2-5% of glucose metabolism (Figure 1) [30]. Glucose that enters the cell is converted by hexokinase into glucose-6-phosphate, which can then enter the pentose phosphate pathway or enter the glycolytic pathway via fructose-6-phosphate. The largest proportion of fructose-6-phosphate enters the glycolytic pathway and then the tricarboxylic acid cycle. The rate limiting enzyme for the entry of fructose-6-phosphate into the hexosamine biosynthesis pathway is glutamine:fructose-6-phosphate aminotransferase (GFAT). The major end product of the pathway is uridine diphosphate N-acetylglucosamine (UDP-GlcNAc), which catalyzes the addition of O-GlcNAc to serine and threonine residues of proteins as noted earlier (Figure 1). All of the glycolytic enzymes are modified by O-GlcNAc, and recently, O-GlcNAcylation was shown to be a key regulator of flux through the pathway [31].
Insulin resistance is a state in which tissues – especially adipose tissue and skeletal muscle – become desensitized to the normal action of insulin in stimulating glucose uptake by the cell. Under normal conditions in insulin-responsive tissues such as adipocytes and skeletal muscle, glucose uptake occurs through glucose transporter-4 (GLUT4) in response to insulin. The vertebrate retina, with its extremely high metabolic rate, also expresses GLUT4 [32]. In the absence of insulin, GLUT4 is located predominantly in the cytoplasm and is inactive. Glucose levels in the retina are also influenced by glucose transporter-1 (GLUT1) which is responsive to blood glucose concentrations [33].
Under conditions of sustained hyperglycemia that occur in diabetes, GFAT is upregulated, flux increases through the hexosamine biosynthesis pathway, and there is an increase in O-GlcNAc-modified proteins [30]. For example, adults with type 2 diabetes had higher GFAT activity in skeletal muscle biopsies compared with controls [34]. Even in the early stages of type 2 diabetes, so-called “pre-diabetes”, O-GlcNAcylation and OGA are increased in patients’ blood cells [35,36]. Increased flux through the hexosamine biosynthetic pathway has been linked with insulin resistance. Glucose-induced insulin resistance can be blocked by inhibiting GFAT [29] or increased by direct glucosamine infusion [37] and greater free fatty acid availability [38]. By inhibiting OGA, a global increase in O-GlcNAcylated proteins can cause insulin resistance in the absence of hyperglycemia [39]. Recent work shows that abnormal increases in O-GlcNAc disrupt phosphorylation-mediated insulin signaling of insulin receptor substrate protein 1 [40]. Another mechanism by which the hexosamine biosynthetic pathway contributes to insulin resistance may be through modulation of cholesterol synthesis. OGlcNAcylation of specificity protein 1 (Sp1), a transcription factor involved in cholesterol synthesis, increases the binding of Sp1 to 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase (HMGR). Sp1 binding of the HMGR promoter leads to elevated cholesterol synthesis and altered cytoskeletal structure that impairs insulin-mediated glucose transport [41].
4 Pathogenesis of diabetic retinopathy
The retina is a thin, delicate, transparent layer of neural tissue that lines the inner surface of the eye between the vitreous humor and the retinal pigment epithelium. The main function of the retina is to detect photons and convert photochemical energy into neural signals and transmit them to the visual cortex of the brain. Light traverses the layers of the retina from the inner limiting membrane through the inner nuclear layer, inner plexiform layer, outer nuclear layer, and outer plexiform layer to the photoreceptors. The retina is largely transparent because the nerve fibers between cells are not myelinated and the density of blood vessels is low. The inner retina, supplied by a sparse vasculature, is relatively hypoxic with a pO2 of ~25 mm Hg and relies heavily on glycolysis, whereas the outer retina, supplied by a more generous network of choroidal vessels, has a pO2 of ~80 mm Hg and relies heavily on oxidative phosphorylation to generate cellular ATP [42].
The retinal neurovascular unit has been considered the key to understanding the retinal dysfunction that occurs in diabetic retinopathy [42]. This unit consists of the close interrelationship of neurons, glia, and specialized vasculature (Figure 2). The neurovascular unit includes endothelial cells and pericytes, astrocytes, Müller cells, and retinal neurons that comprise the blood-retina barrier and regulate nutrient flow. The photoreceptor cells, upon stimulation with light, transmit signals to the brain via bipolar, amacrine, horizontal, and ganglion cells. Under normal conditions, nutrients provide energy balance and maintain retinal microenvironment of ionic balance for neural signaling, synaptic transmission, and other processes that allow vision [42]. There are two blood-retinal barriers involved in the retinal neurovascular unit, one formed by the intra-retinal vasculature in the neuroretina, and a second outer one formed by the barrier between the retinal pigment epithelium and blood vessels of the choriocapillaris.
Figure 2.
The neurovascular unit of the retina, consisting of the close relationship of neurons, glia, and specialized vasculature.
In diabetic retinopathy, one of the earliest events is the breakdown of the blood-retina barrier with leakage of retinal vessels in the inner retina. Several pathways have been implicated, including VEGF, tumor necrosis factor (TNF)-α, platelet-derived growth factor (PDGF) B, the kallikrein kinin system, advanced glycation end products, oxidative stress/inflammation, and the polyol pathway. Many of these pathways are inter-related, and some of the pathways may potentially involve altered O-GlcNAc signaling. Current knowledge regarding altered O-GlcNAc signaling in the eye and the hexosamine biosynthetic pathway is presented in a separate section that follows the presentation of these other pathways.
VEGF plays an important role in the induction of vascular permeability and angiogenesis. In the retina, VEGF is secreted by many cell types, including pericytes, endothelial cells, Müller cells, astrocytes, glial cells, and retinal pigment epithelial cells. VEGF is produced in response to hypoxia through upregulation by hypoxia-inducible factor (HIF)-1 [43]. VEGF affects tight junctions by altering occludin, an integral membrane protein that is a component of tight junctions [44]. Using a mass spectrometry and bioinformatics approach, Antonetti and colleagues identified five putative VEGF-induced phosphorylation sites on occludin, including Ser490, as validated by protein interaction studies [45]. VEGF activates protein kinase C β, which phosphorylates occludin [46]. In studies of hepatitis C viral entry and replication, the VEGF pathway is activated, and PKC phosphorylates occludin at Ser490 [47]. The Ser490 site of occludin has also been identified as a site for O-GlcNAcylation [48]. Whether there is interplay of phosphorylation and O-GlcNAcylation of occludin in the early vascular permeability of diabetic retinopathy is not known.
The effects of VEGF on vascular permeability are also partially mediated by TNF-α [49]. TNF-α plays a role in diabetes-related leukostasis, inflammation, and apoptosis [49-51]. In the TNF-α signaling pathway, TNF binds with TNF receptors 1 and 2, with activation of nuclear factor (NF)-κB and a resulting increase in genes involved in vascular inflammation. TNF-α-mediated signaling involves phosphorylation of the p65 subunit of NF-κB at Ser536, and recently it has been shown that the Ser536 is reciprocally modified by O-GlcNAc [52]. Thus, Ser536 on the p65 subunit of NF-κB could be another possible site for interplay between phosphorylation and O-GlcNAcylation in TNF-α-mediated signaling in diabetic retinopathy.
An early feature of diabetic retinopathy is loss of pericytes, which play an important role in maintaining the blood-retina barrier. Pericyte loss can result in endothelial cell proliferation and microaneurysm formation. PDGF-B and its receptor PDGF-β are essential for the recruitment of pericytes in the retinal vasculature. Hyperglycemia activates protein kinase C (PKC) delta to increase a protein tyrosine phosphatase, Src homology-2 domain-containing phosphatase (SHP-1). SHP-1 signaling leads to dephosphorylation of PDGF-β and subsequent pericyte apoptosis [53]. All PKC isozymes have been shown to be dynamically modified by O-linked beta-N-acetylglycosamine [54,55].
Recent work by Feener and colleagues has implicated carbonic anhydrase and the kallikrein kinin system in the increased vascular permeability that occurs in diabetic retinopathy [56,57]. Carbonic anhydrase is component of the vitreous humor that catalyzes the conversion of CO2 + H2O to H+ and HCO3−, thus increasing the pH of the vitreous. Carbonic anhydrase is O-GlcNAcylated in red blood cells of diabetic patients, but its modification in retina has not yet been studied [35]. Increased pH in the vitreous humor activates kallikrein, a serine protease, and the kallikrein kinin pathway that involves both tissue and plasma kallikrein acting upon bradykinin (1-9), a peptide hormone that, in turn, activates two G-protein-coupled bradykinin receptors. Proteomic analysis of the vitreous humor in patients with advanced diabetic retinopathy revealed relatively high levels of plasma kallikrein, factor XII, and high molecular weight kininogen [56]. Intravitreal injection of extracellular carbonic anhydrase in rats increased retinal vascular leakage and caused retinal edema [56]. Complement 1 inhibitor (which inhibits prekallikrein activation and the classical complement pathway), neutralizing antibodies to prekallikrein, and bradykinin receptor antagonism reduced the retinal edema induced by carbonic anhydrase.
Advanced glycation end products (AGEs) arise from the non-enzymatic chemical reaction of reducing sugars with protein amine groups or with reactive dicarbonyls such as methylglyoxal [58]. Hyperglycemia contributes to the elevated formation of AGEs in the retina during diabetes [58]. In animal models of diabetes, treatment with glyoxalase 1, which detoxifies methylglyoxal, or the AGE-inhibitor aminoguanidine, reduced AGE deposition in the retina [59,60]. AGEs interact with the receptor for AGEs (RAGE) to upregulate inflammation and the generation of reactive oxygen species [61]. AGEs have been implicated in pericyte death through RAGE signaling [62] and induction of oxidative stress [63]. AGEs have been linked with increased O-GlcNAc signaling. Treatment of fetal human cardiac myocytes with methylglyoxal and AGEs resulted in increased O-GlcNAc-modified proteins in the nucleus [64]. In mouse kidney cells, high glucose levels increased methylglyoxal modification of the corepressor mSin3A, recruitment of OGT, O-GlcNAcylation of the transcription factor Sp3, with resulting increase in angiopoietin 2 expression [65].
Increased oxidative stress and inflammation are characteristic of diabetic retinopathy [66]. Inflammatory cell infiltration, edema, and increased expression of cytokines and chemokines accompany the increased leukostasis, pericyte loss, vascular occlusion, and increased vascular permeability in the early stages of diabetic retinopathy. In addition to TNF-α, other growth factors, cytokines, and chemokines, such as insulin-like growth factor (IGF)-1, interleukin-1β, and monocyte chemoattractant protein-1, are expressed in the retina and accompany vascular damage [2,66]. The polyol pathway, by which excess intracellular glucose is metabolized to sorbitol by aldose reductase, requires NADPH as a cofactor. The conversion of fructose to sorbitol by sorbitol dehydrogenase requires NAD+ as a cofactor, which is reduced to NADH. An increase in the NADH/NAD+ ratio can lead to the production of reactive oxygen species, while the accumulation of sorbitol and fructose impairs the function of astrocytes and Müller cells [66].
5 Altered O-GlcNAc signaling in the eye
O-GlcNAcylated proteins have been described in the lens, cornea, retinal pigment epithelium, and neuroretina of the eye. αA-crystallins and αB-crystallins, members of the small heat-shock protein family, comprise a major part of the proteins in the vertebrate lens. OGlcNAcylated αA-crystallin and αB-crystallin have been described in the lens [67], and a major site of O-GlcNAcylation of αB-crystallin was identified at Thr 170 [68]. αB-crystallins also occur in heart, nervous system, skeletal muscle, and liver. In the heart, αB-crystallin is considered to play a role in cardioprotection through stress-induced translocation and binding to cytoskeletal elements, and O-GlcNAcylation appears to be an important regulator of this function [69]. In a transgenic mouse model that overexpresses GK-NCAT, a dominant-negative form of O-GlcNAcase, increased O-GlcNAcylation of lens proteins was associated with disrupted lens fiber cell differentiation and the formation of cataracts [70].
Diabetic keratopathy, a condition that is estimated to affect about half of diabetic patients, is characterized by decreased corneal sensitivity, increased risk of corneal epithelial defects, abnormal corneal wound healing, and elevated risk of corneal ulceration [71]. Corneal nerves, which are affected by diabetic neuropathy, have a trophic effect upon the corneal epithelium through the release of neurotrophins and other nerve-derived factors [72]. In a study of Wistar (control) and Goko-Kakizaki (diabetic) rats, diabetic corneas had much greater immunohistochemical staining for both O-GlcNAc-modified proteins and O-GlcNAc transferase in the epithelial, stromal, and endothelial layers of the cornea compared with control corneas [73]. Immunoreactivity for O-GlcNAc transferase was limited mainly to the nuclei of epithelial, stromal, and endothelial cells of control corneas, whereas diabetic corneas showed immunoreactivity in both the nucleus and cytoplasm. There were no differences in immunoreactivity for O-GlcNAc transferase in the stroma and endothelium of control and diabetic corneas [73]. In control cornea epithelial cells that were cultured with PUGNAc (1,5-hydroxyimolactone), an inhibitor of O-GlcNAcase, there was an increase in O-GlcNAcylated proteins with an accompanying disruption of hemidesmosome development, enlargement of intercellular spaces between epithelial cells, and localized detachment of epithelial cells from the basement membrane [73]. These findings suggest that increased O-GlcNAc signaling reduced cell-to-cell adhesion. However, the specific proteins that are O-GlcNAcylated in the cornea have not been identified. Akimoto and colleagues hypothesized that certain keratins may have been modified, since keratin-8, -13, and -18 have already been shown to be O-GlcNAcylated [73].
The clinical spectrum of proliferative diabetic retinopathy includes the formation of fibrotic epiretinal membranes on the surface of the retina or within the vitreous. Traction of the membranes can subsequently lead to retinal detachment and loss of vision. Transforming growth factor (TGF)-β has been implicated in the pathogenesis of proliferative vitreoretinal disease. Elevated TGF-β concentrations have been described in the vitreous of patients with advanced diabetic retinopathy [74]. Tubulins are small proteins that make up microtubules, and six β-tubulin isotypes occur in mammalian cells. The normal retinal pigment epithelium does not contain class III β-tubulin (βIII), which is usually expressed in neurons, but βIII has been described in abnormal retinal pigment epithelial cells found in epiretinal membranes [75]. In cultured retinal pigment epithelial cells, TGF-β stimulation led to the production of OGlcNAcylated βIII [76]. O-GlcNAcylation of tubulin has been shown to inhibit tubulin polymerization [77]. Several key microtubule regulatory proteins are modified by O-GlcNAc [78-80].
Two murine models have been used to study the role of O-GlcNAc-modified proteins in the retina: the Akita mouse model (Ins2Akita/+) and oxygen-induced ischemic retinopathy model [81]. The Akita mouse model is useful for investigating early retinal changes in diabetic retinopathy [82-84] while the oxygen-induced ischemic retinopathy model facilitates the study of the later proliferative stage of diabetic retinopathy [85]. Increased O-GlcNAcylation of retinal proteins was noted by Western blots after six weeks of age in diabetic Akita mice and in oxygen-induced ischemic mice during the phase of active neovascularization [81]. Immunohistochemical studies showed that high concentrations of O-GlcNAcylated proteins co-localized with the retinal vascular plexus in both mouse models. Retinal endothelial cells, pericytes, and astrocytes were cultured under various glucose concentrations. The level of O-GlcNAcylation of proteins increased with higher glucose concentrations in retinal endothelial cells and pericytes but not in retinal astrocytes [81]. Migration of retinal pericytes is important in establishing healthy capillaries in the retina. Further studies of cultured retinal pericytes showed that high glucose levels mediated an increase in O-GlcNAcylation and impaired migration of retinal pericytes [81]. The regulation of GFAT in the diabetic retina has not been well studied. However, total levels of O-GlcNAc modified proteins in retinal pericytes cultured with high glucose concentrations was decreased by addition of the GFAT inhibitor, DON [81].
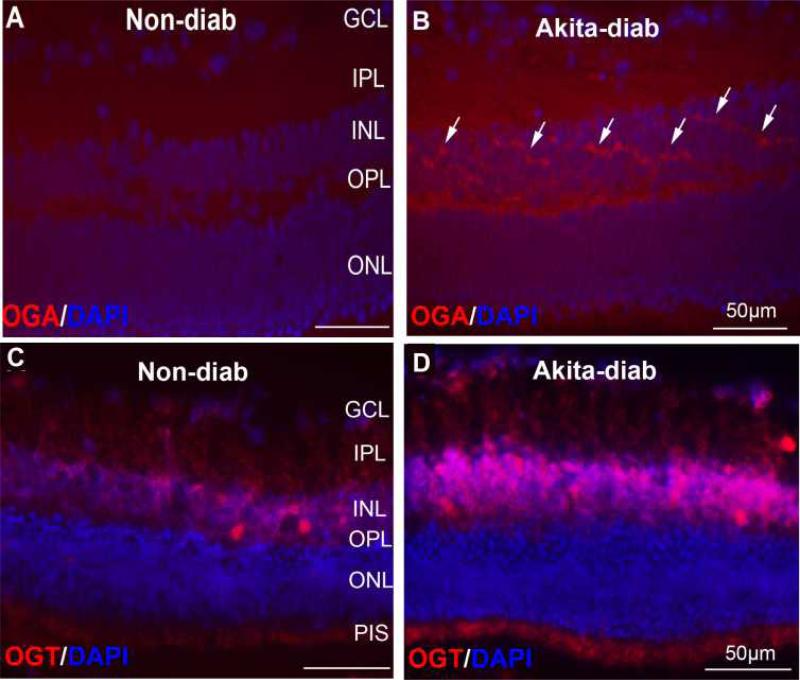
Our immunohistochemical studies in the diabetic Akita mouse model show that staining for OGA and OGT is increased in the retina (Figure 3). Three 5- to 6-month old Akita mice (with diabetes of 4-5 mos duration) and three age-matched, non-diabetic control mice were used for the examination of OGT and OGA protein expression and localization in the retina cells. Anti-OGT (AL25) and anti-OGA (345) (provided by Gerald W. Hart), were used for immunofluorescence staining. The OGT-positive cells localize predominantly in the inner nuclear layer (INL) with some in the inner plexiform layer (IPL), ganglion cell layer (GCL) and photoreceptor inner segment (PIS). The immunofluorescence intensity of OGT was dramatically increased in all the OGT-positive cells of diabetic retinas compared to non-diabetic controls. The OGA-positive cells were only detected in the INL of diabetic retina but not in the non-diabetic control. Compared to OGT, the staining intensity of OGA was much weaker. These staining patterns suggest that diabetes induce the increased protein expression of OGT and OGA in the retina.
Figure 3.
OGA and OGT in diabetic Akita mouse retina. Retinal cryo-sections were prepared from the 5~6-month-old Akita diabetic mice or non-diabetic control mice. Immunofluorescence images of OGA in non-diabetic (A) and control (B) mice. Arrows indicate OGA-positive cells. Immunofluorescence images of OGT in non-diabetic (C) and control (D) mice. GCL: Ganglion cell layer; IPL: inner plexiform layer; INL: inner nuclear layer; OPL: outer plexiform layer; ONL: outer nuclear layer. Counterstaining with diamidino-2-phenylindole (DAPI) was used to highlight the three nuclear layers in the retina: ONL, INL, and GCL.
The changes in O-GlcNAc signaling that have been reported in the eye above are summarized in Table 1. Although investigations into specific O-GlcNAcylated proteins in the human eye have been limited, the potential number of functional groups and proteins in the eye that are modified by O-GlcNAc is large. For example, over sixty proteins in the human retina that have been identified using mass spectrometry have also been identified in non-retinal tissues as O-GlcNAc-modified proteins (Table 2) [86-88]. It is interesting to note that many of the proteins are involved in glucose metabolism.
Table 1.
O-GlcNAc Signaling Reported in the Eye
| Tissue | O-GlcNAcylated proteins | OGA staining | OGT staining | References |
|---|---|---|---|---|
| Lens | α-A-crystallins α-B-crystallins |
?? | ?? | 67, 68 |
| Cornea | increased in epithelial cells cultured with PUGNAc; keratins-8, -13, -18 | ?? | nuclei in epithelium, stroma, endothelium | 73 |
| Neuroretina | increased total proteins in diabetic mice, especially retinal vascular plexus | inner nuclear layer | inner nuclear layer; inner plexiform layer; ganglion cell layer; photoreceptor inner segments | 81, present study |
| Retinal pigment epithelium | class III beta tubulin in cells cultured with TGF-β | ?? | ?? | 76 |
Table 2.
Examples of Proteins Found in the Human Retina That Are Reported to be O-GlcNAcylated in Non-Retinal Tissues1
| UniProt ID | Description |
|---|---|
| P14618 | pyruvate kinase isozymes M1/M2 |
| P40926 | malate dehydrogenase, mitochondrial |
| P40925 | malate dehydrogenase, cytoplasmic |
| Q00610 | clathrin heavy chain 1 |
| P49411 | elongation factor Tu, mitochondrial |
| P38671 | phosphoglucomutase-1 |
| P04075 | fructose-bisphosphate aldolase A |
| P23528 | cofilin-1 |
| P11137 | microtubule-associated protein 2 |
| P12814 | alpha-actinin-1 |
| Q14204 | cytoplasmic dynein 1 heavy chain 1 |
| P09972 | fructose-bisphosphate aldolase C |
| P63261 | actin, cytoplasmic 2 |
| P06744 | glucose-6-phosphate isomerase |
| P08237 | 6-phosphofructokinase, muscle type |
| Q92945 | far upstream element-binding protein 2 |
| P06748 | nucleophosmin |
| Q15366 | poly(rC)-binding protein 2 |
| P07355 | annexin A2 |
| P52209 | 6-phosphogluconate dehydrogenase, decarboxylating |
| P35579 | myosin-9 |
| P27797 | calreticulin |
| Q15233 | non-POU domain-containing octamer-binding protein |
| P13010 | x-ray repair cross-complementing protein 5 |
| P50990 | T-complex protein 1 subunit theta |
| Q01082 | spectrin beta chain, non-erythrocytic 1 |
| Q06830 | peroxiredoxin-1 |
| P51991 | heterogeneous nuclear ribonucleoprotein A3 |
| P68104 | elongation factor 1-alpha 1 |
| P50991 | T-complex protein 1 subunit delta |
| P32119 | peroxiredoxin-2 |
| P07737 | profilin-1 |
| P06733 | alpha-enolase |
| Q96AE4 | far upstream element-binding protein 1 |
| P35613 | basigin |
| P40227 | T-complex protein 1 subunit zeta |
| P49368 | T-complex protein 1 subunit gamma |
| P26639 | threonine-tRNA ligase, cytoplasmic |
| P61604 | 10 kDa heat shock protein, mitochondrial |
| P22314 | ubiquitin-like modifier-activating enzyme 1 |
| O75376 | nuclear receptor corepressor 1 |
| P23526 | adenosylhomocysteinase |
| P38646 | stress-70 protein, mitochondrial |
| Q13838 | splicesome RNA helicase DDX39B |
| P63244 | guanine nucleotide-binding protein subunit beta-2-like 1 |
| Q9P2J5 | leucine-tRNA ligase, cytoplasmic |
| P68371 | tubulin beta-4B chain |
| Q8WWM7 | ataxin-2-like protein |
| Q04637 | eukaryotic translation initiation factor 4 gamma 1 |
| P27816 | microtubule-associated protein 4 |
| Q9UPN6 | protein SCAF8 |
| Q6XRS2 | helicase SRCAP |
| Q14980 | nuclear mitotic apparatus protein 1 |
| P12270 | nucleoprotein TPR |
| Q9P2N6 | KAT8 regulatory NSL complex subunit 3 |
| Q14157 | ubiquitin-associated protein 2-like |
| Q09666 | neuroblast differentation-associated protein AHNAK |
| Q96HC4 | PDZ and LIM domain protein 5 |
| P49792 | E3 SUMO-protein ligase RanBP2 |
| Q9Y520 | protein PRRC2C |
| P02545 | prelamin-A/C |
| Q8WWI1 | LIM domain only protein 7 |
| P18583 | protein SON |
This list was compiled by comparison of the UniProt IDs of known proteins in the human retina [86] with documented O-GlcNAcylated proteins reported by the Hu Lab, Georgetown University website (http://cbsb.lombardi.georgetown.edu/hulab/OGAP.html, accessed July 4, 2013) and [88].
6 Altered O-GlcNAc signaling in the kidney and heart in diabetes
Diabetic retinopathy and diabetic nephropathy are two common microvascular complications of diabetes. Similar to the eye, diabetic nephropathy is characterized by increased production of TGF-β [89,90]. Increased O-GlcNAc-modified proteins have been demonstrated in the nucleus and cytosol of renal glomeruli and tubules of kidney biopsy specimens from diabetic nephropathy patients [91]. Increased O-GlcNAcylation enhanced profibrotic signaling in rat mesangial cells, suggesting a role for the accumulation of extracellular matrix that occurs in diabetic nephropathy [92].
Altered O-GlcNAc signaling has also been implicated in heart failure and in diabetic cardiomyopathy [93,94]. Increased global O-GlcNAcylation has been shown in left ventricular tissue from patients with aortic stenosis and in hearts of hypertensive rats with hypertrophic and failing hearts [93]. In cardiomyocytes isolated from type 2 diabetic (db/db) and control mice, increased O-GlcNAc levels were found in diabetic compared with control cardiomyocytes [95]. Impaired agonist-induced hypertrophic and cell signaling responses were seen in diabetic compared to control cardiomyocytes [95]. These findings suggest that increased flux through the hexosamine biosynthesis pathway and altered O-GlcNAc signaling occur in the heart in type 2 diabetes.
7 Analytical Considerations
Although O-GlcNAcylation was discovered nearly thirty years ago [14,96], and despite the fact that it is abundant and wide-spread throughout the nucleus and cytoplasm and is involved in most essential cellular processes, our knowledge about the specific roles of this ubiquitous post-translational modification is still limited [97]. O-GlcNAc remained undiscovered for decades, and progress has been slow for several reasons: (1) O-GlcNAcylation does not generally affect the migration of proteins upon gel electrophoresis, even when proteins are separated on high-resolution 2D-gels [98]; (2) Early studies of protein glycosylation used radiolabeled sugars and proteolysis or chemical methods to release glycans. Many earlier researchers likely concluded that the released O-GlcNAc was unincorporated radioactive sugar. (3); If precautions are not taken, O-GlcNAc is rapidly removed by both lysosomal hexosaminidases and by O-GlcNAcase when cells are damaged or homogenized; (4) Detection and analysis of O-GlcNAc by standard collision-induced dissociation (CID) mass spectrometry simply does not work effectively. O-GlcNAc is an mass spectrometry-labile modification and thus is severely under-detected to due ion suppression when unmodified peptides are also present, and site mapping by CID is limited as the glycosidic bond is much more labile in the gas-phase than the peptide bond [99]. Even with matrix-assisted laser desorption mass spectrometry (MALDI), O-GlcNAcylated peptides are typically not detected in mixtures of unmodified peptides due to lower surface activity; (5) Unlike other PTMs, such as phosphorylation and ubiquitination, proteomic tools such as site-specific O-GlcNAc-dependent antibodies, pan-specific antibodies and kits for detection and study of O-GlcNAcylation have only recently become available [98]; (6) Even so, most tools for altering O-GlcNAcylation do so on a global scale. The only significant tool currently available to study protein or site-specific functions of O-GlcNAc is site-directed mutagenesis of the O-GlcNAc site to alanine or other non-hydroxyl containing amino acid. No O-GlcNAc mimic exists, like glutamic acid is used to mimic phosphorylation.
Nonetheless, in recent years, substantial progress has been made in approaches to elucidate the functions of O-GlcNAcylation. Several pan-specific [100-102] and a handful of site-specific antibodies [36,103-105] to O-GlcNAc have been generated and are readily available. The development of electron capture dissociation (ECD) and electron transfer dissociation (ETD) mass spectrometry have improved our ability to site-map O-GlcNAc sites of individual purified proteins (or very simple mixtures) [106]. However, these powerful methods still do not solve the ion-suppression and lability issues described above. Since the biological functions of PTMs are not only protein-specific but also site-specific on a protein, site-mapping and quantification of stoichiometry at a site are critical toward elucidating the functions of the modification.
Fortunately, two approaches have proven highly useful in enriching O-GlcNAcylated peptides away from other peptides to allow for site mapping by ETD-MS/MS. Enrichment of OGlcNAc peptides using long columns of a GlcNAc-binding lectin, wheat germ agglutinin (WGA), has allowed site mapping of over seventeen hundred O-GlcNAc sites in proteins from brain [107,108]. Enzymatically tagging O-GlcNAc peptides using a mutant galactosyltransferase [99,109] and UDP-GalNAz as the donor has also proven a powerful method to attach biotin or other molecules to O-GlcNAc peptides via ‘Click chemistry’ for enrichment via affinity chromatography [110]. When a mass spectrometer capable of ETD or ECD is not available, beta-elimination Michael Addition using dithiothreitol (BEMAD) is also a useful enrich OGlcNAcylated peptides using thio-affinity chromatography and standard CID MS/MS to map the location of the added dithiothreitol moiety [111]. However, the major drawback to BEMAD is the potential for false positives due to other Ser(Thr) modifications. Since BEMAD site mapping is indirect, this approach requires pre-treatment with phosphatases and many controls and confirmation of the mapped sites by other methods. Highly specific O-GlcNAcase inhibitors have proven to be very useful in evaluating OGlcNAc’s functions [112]. Amongst the most specific and useful are thiamet-G (TMG) [113] and GlcNAcstatin [114]. PUGNAc, while somewhat less selective than TMG or GlcNAcstatin, has also proven useful in many studies [115,116]. The drawback – not only to these OGlcNAcase inhibitors but also to genetic approaches, such as RNAi or gene knockouts – is that O-GlcNAcylation is elevated on thousands of proteins, making it difficult to sort out site-specific functions.
Some studies have used OGT inhibitors to globally lower O-GlcNAcylation. Unfortunately, the more specific inhibitors, such as those developed by Suzanne Walker's group [117], generally don't work well in most living cells or animals. Ac5-thio-GlcNAc has proven to be a highly useful inhibitor of OGT in living cells [118], but its off-target affects have yet to be systematically investigated. A uridine analog, alloxan has also been used to inhibit OGT in cells [119], but it is known to be fairly non-specific, affecting many enzymes that use uridine nucleotides [120]. As with any inhibitors, careful controls and alternative approaches are required to validate the significance of any findings. Again, the problem with all of these OGT inhibitors, is that they affect O-GlcNAcylation globally, making the determination of protein or site specific functions of the PTM very difficult.
The use of inhibitors or genetic approaches to reduce the activity of GFAT, the rate limiting enzyme of the hexosamine biosynthetic pathway (HBP) is a powerful way to determine if the conversion of glucose to glucosamine is required for a biological process [121,122]. Studies have used deoxynorleucine (DON) or azaserine to inhibit GFAT and prevent glucose conversion to glucosamine [123,124]. In both inhibitor studies and in knockout studies of GFAT, it is an essential control to show that the addition of exogenous glucosamine by-passes the block in the pathway. GFAT inhibition would most rapidly affect O-GlcNAc, as O-GlcNAcylation is sensitive across the range of intracellular UDP-GlcNAc concentrations. For luminal glycosylation, such as classical N-glycans and O-glycans, UDP-GlcNAc antiporters are saturated in the low micromolar range and N-glycan lipid-linked precursors are in excess, thus it would take longer and more severe GFAT inhibition to affect other glycoproteins and glycolipids. Since O-GlcNAcylation is the major intracellular endpoint of the HBP, biological affects in nucleocytoplasmic functions caused by GFAT inhibition are generally the result of defective OGlcNAcylation.
8 Future Directions
Current data would suggest that a limited global reduction of OGT activity in diabetic tissues would like ameliorate glucose toxicity, however if OGT is inhibited by more than 50-60%, it would likely be toxic. Lowering flux through the HBP by preventing the biosynthesis of UDP-GlcNAc would also appear to ameliorate glucose toxicity, but would also likely produce unexpected side effects. While OGT is highly sensitive to UDP-GlcNAc concentrations in cells, it is targeted to its specific substrates by myriad cell type specific binding proteins [125-131]. The most specific therapeutic agents will likely be small molecules that block the protein:protein interactions between OGT and its targeting partners. For example, we have proposed that blocking PGC-1a's interaction with OGT in hyperglycemic individuals would prevent OGT from O-GlcNAcylating the FOXO transcription factors in the liver that drive inappropriate gluconeogenesis in the liver of diabetic individuals [127]. Blocking the OGT:PGC-1a interaction should lower blood glucose in diabetic individuals.
Before the study of O-GlcNAcylation in disease – including diabetic retinopathy – can make rapid progress, more facile tools need to become available. Most laboratories do not have access to high-end mass spectrometry, for example. Clearly, a great tool that would advance the field would be a large number site-specific antibodies, as are now available for protein phosphorylation [129]. Tools to artificially target OGT or OGA to specific substrates or even sites, would allow the rapid advance of our understanding of the roles of this modification. Even the ability to prepare site-specific O-GlcNAcylated proteins, either using methods similar to that employed to introduce artificial amino acids [130] or by chemical ligation methods [131], when combined with protein transfection, would prove to be more specific and powerful tool than the global approaches now used in the field. Some methods to study O-GlcNAc signaling in real-time in living cells have been developed [132,133]. However, much more work in this area will be needed to understand the dynamics of O-GlcNAcylation and its relationships to other PTMs.
Thus, while it is clear that O-GlcNAcylation is fundamentally important to glucose toxicity in diabetes, and indeed increased O-GlcNAcylation might be the major molecular cause of myriad dysfunctions seen in hyperglycemia, we need much more effort by more people to develop better tools and to apply them to specific biological processes in order to realize the promise of the unexpected avenue for future development of drugs to treat diabetes and diabetic retinopathy.
Acknowledgements
This paper was supported by the National Institutes of Health grants R01 AG27012, R01 HL111271, P50 HL084946, and NHLBI Contract TAS::75 0872::TAS.
List of abbreviations
- AGEs
advanced glycation end products
- BEMAD
beta-elimination Michael Addition using dithiothreitol
- DAPI
diamidino-2-phenylindole
- DON
deoxynorleucine
- ECD
electron capture dissociation
- ETD
electron transfer dissociation
- GCL
ganglion cell layer
- GFAT
glutamine:fructose-6-phosphate aminotransferase
- GLUT1
glucose transporter-1
- GLUT4
glucose transporter-4
- HAT
histone acetyltransferase
- HBP
hexosamine biosynthetic pathway
- HIF-1
hypoxia-inducible factor-1
- HMGR
3-hydroxy-3-methyl-glutaryl-coenzyme A reductase
- IGF-1
insulin-like growth factor-1
- INL
inner nuclear layer
- IPL
inner plexiform layer
- mOGT
mitochondrial OGT
- ncOGT
nucleus and cytoplasm
- NF-κB
nuclear factor-κB
- O-GlcNAc
O-linked β-N-acetylglucosamine
- OGA
β-N-acetylglucosaminidase; O-GlcNAcase
- OGT
uridine diphospho-N-acetylglucosamine:polypeptide β-N-acetyl-glucosaminyltransfase; O-GlcNAc transferase
- ONL
outer nuclear layer
- PDGF
platelet-derived growth factor B
- PIS
photoreceptor inner segment
- PKC
protein kinase C
- PPAR-α
peroxisome proliferator-activated receptor α
- RAGE
receptor for advanced glycation end products
- SHP-1
Src homology-2 domain-containing phosphatase 1
- sOGT
soluble OGT
- TGF-β
Transforming growth factor-β
- TMG
thiamet G
- TNF-α
tumor necrosis factor-α
- TPR
N-terminal tetratricopeptide repeat
- UDP-GlcNAc
uridine diphosphate N-acetylglucosamine
- VEGF
vascular endothelial growth factor
- WGA
wheat germ agglutinin
Footnotes
The authors declare no conflicts of interest.
References
- 1.Yau JW, Roger SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund J, Haffner S, Hamman RF, Ikram MK, Kayama T, Klein BE, Klein R, Krishnaiah S, Mayurasakorn K, O'Hare JP, Orchard TJ, Porta M, Rema M, Roy MS, Sharma T, Mitchell P, Wong TY. Meta-Analysis for Eye Disease (META-EYE) Study Group, Global prevalence and major risk factors for diabetic retinopathy. Diabetes Care. 2012;35:556–564. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N. Engl. J. Med. 2012;366:1227–1239. doi: 10.1056/NEJMra1005073. [DOI] [PubMed] [Google Scholar]
- 3.Keech AC, Mitchell P, Summanen PA, O'Day J, Davis TM, Moffitt MS, Taskinen MR, Simes RJ, Tse D, Williamson E, Merrifield A, Laatikainen LT, d'Emden MC, O'Connell RL, Colman PG. FIELD Study Investigators, Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet. 2007;370:1687–1697. doi: 10.1016/S0140-6736(07)61607-9. [DOI] [PubMed] [Google Scholar]
- 4.ACCORD Study Group ACCORD, Eye Study Group. Chew EY, Ambrosius WT, Davis MD, Danis RP, Gangaputra S, Greven CM, Hubbard L, Esser BA, Lovato JF, Perdue LH, Goff DC, Jr., Cushman WC, Ginsberg HN, Elam MB, Genuth S, Gerstein HC, Schubart U, Fine LJ. Effects of medical therapies on retinopathy progression in type 2 diabetes. N. Engl. J. Med. 2010;363:233–244. doi: 10.1056/NEJMoa1001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michaelides M, Kaines A, Hamilton RD, Fraser-Bell S, Rajendram R, Quhill F, Boos CJ, Xing W, Egan C, Peto T, Bunce C, Leslie RD, Hykin PG. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12-month data: report 2. Ophthalmology. 2010;117:1078–1086.e2. doi: 10.1016/j.ophtha.2010.03.045. [DOI] [PubMed] [Google Scholar]
- 6.Rajendram R, Fraser-Bell S, Kaines A, Michaelides M, Hamilton RD, Esposti SD, Peto T, Egan C, Bunce C, Leslie RD, Hykin PG. A 2-year prospective randomized controlled trial of intravitreal bevacizumab or laser therapy (BOLT) in the management of diabetic macular edema: 24-month data: report 3. Arch. Ophthalmol. 2012;130:972–979. doi: 10.1001/archophthalmol.2012.393. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen QE, Shah SM, Khwaja AA, Channa R, Hatef E, Do DV, Boyer D, Heier JS, Abraham P, Thach AB, Lit ES, Foster BS, Kruger E, Dugel P, Chang T, Das A, Ciulla TA, Pollack JS, Lim JI, Elliot D, Campochiaro PA. READ-2 Study Group, Two-year outcomes of the ranibizumab for edema of the macula in diabetes (READ-2) study. Ophthalmology. 2010;117:2146–2151. doi: 10.1016/j.ophtha.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 8.Chaturvedi N, Sjolie AK, Stephenson JM, Abrahamian H, Keipes M, Castellarin A, Rogulja-Pepeonik Z, Fuller JH. Effect of lisinopril on progression of retinopathy in normotensive people with type 1 diabetes. The EUCLID Study Group. EURODIAB Controlled Trial of Lisinopril in Insulin-Dependent Diabetes Mellitus. Lancet. 1998;351:28–31. doi: 10.1016/s0140-6736(97)06209-0. [DOI] [PubMed] [Google Scholar]
- 9.Sjolie AK, Klein R, Porta M, Orchard T, Fuller J, Parving HH, Bilous R, Chaturvedi N, DIRECT Programme Study Group Effect of candesartan on progression and regression of retinopathy in type 2 diabetes (DIRECT-Protect 2): a randomised placebo-controlled trial. Lancet. 2008;372:1385–1393. doi: 10.1016/S0140-6736(08)61411-7. [DOI] [PubMed] [Google Scholar]
- 10.Chaturvedi N, Porta M, Klein R, Orchard T, Fuller J, Parving HH, Bilous R, Sjolie AK, DIRECT Programme Study Group Effect of candesartan on prevention (DIRECT-Prevent 1) and progression (DIRECT-Protect 1) of retinopathy in type 1 diabetes: randomised, placebo-controlled trials. Lancet. 2008;372:1394–1402. doi: 10.1016/S0140-6736(08)61412-9. [DOI] [PubMed] [Google Scholar]
- 11.Nathan DM, Zinman B, Cleary PA, Backlund JY, Genuth S, Miller R, Orchard TJ, DCCT/EDIC Research Group Modern-day clinical course of type 1 diabetes mellitus after 30 years’ duration: the Diabetes Controls and Complications Trial/Epidemiology of Diabetes Interventions and Complications and Pittsburgh Epidemiology of Diabetes Complications Experience (1983-2005). Arch. Intern. Med. 2009;169:1307–1316. doi: 10.1001/archinternmed.2009.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gariano RF, Gardner TW. Retinal angiogenesis in development and disease. Nature. 2005;438:960–966. doi: 10.1038/nature04482. [DOI] [PubMed] [Google Scholar]
- 13.Copeland RJ, Bullen JW, Hart GW. Cross-talk between GlcNAcylation and phosphorylation: roles in insulin resistance and glucose toxicity. Am. J. Physiol. Endocrinol. Metab. 2008;295:E17–E28. doi: 10.1152/ajpendo.90281.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torres CR, Hart GW. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J. Biol. Chem. 1984;259:3308–3317. [PubMed] [Google Scholar]
- 15.Vosseller K, Trinidad JC, Chalkley RJ, Specht CG, Thalhammer A, Lynn AJ, Snedecor JO, Guan S, Medzihradszky KF, Maltby DA, Schoepfer R, Burlingame AL. O-linked N-acetylglucosamine proteomics of postsynaptic density preparations using lectin weak affinity chromatography and mass spectrometry. Mol. Cell. Proteomics. 2006;5:923–934. doi: 10.1074/mcp.T500040-MCP200. [DOI] [PubMed] [Google Scholar]
- 16.Alfaro JF, Gong CX, Monroe ME, Aldrich JT, Clauss TR, Purvine SO, Wang Z, Camp DG, Shabanowitz J, Stanley P, Hart GW, Hunt DF, Yang F, Smith RD. Tandem mass spectrometry identifies many mouse brain O-GlcNAcylated proteins including EGF domain-specific O-GlcNAc transferase targets. Proc. Natl. Acad. Sci. 2012;109:7280–7285. doi: 10.1073/pnas.1200425109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagel AK, Schilling M, Comte-Walters S, Berkaw MN, Ball LE. Identification of O-linked N-acetyleglucosamine (O-GlcNAc)-modified osteoblast proteins by electron transfer dissociation mass spectrometry reveals proteins critical for bone formation. Mol. Cell. Proteomics. 2013;12:945–955. doi: 10.1074/mcp.M112.026633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hahne H, Sobotzki N, Nyberg T, Helm D, Borodkin VS, van Aalten DM, Agnew B, Kuster B. Proteome wide purification and identification of O-GlcNAc-modified proteins using click chemistry and mass spectrometry. J. Proteome Res. 2013;12:927–936. doi: 10.1021/pr300967y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hart GW, Akimoto Y. The O-GlcNAc modification. In: Varki A, Cummings RD, Esko JD, et al., editors. Essentials of Glycobiology. 2nd edition Cold Spring Harbor Laboratory Press; Cold Spring Harbor (NY): 2009. [Google Scholar]
- 20.Zachara NE, Hart GW. Cell signaling, the essential role of O-GlcNAc! Biochim. Biophys. Acta. 2006;1761:599–617. doi: 10.1016/j.bbalip.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Lazarus MB, Nam Y, Jiang J, Sliz P, Walker S. Structure of human O-GlcNAc transferase and its complex with a peptide structure. Nature. 2011;469:564–567. doi: 10.1038/nature09638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kreppel LK, Blomberg MA, Hart GW. Dynamic glycosylation of nuclear and cytosolic proteins. Cloning and characterization of a unique O-GlcNAc transferase with multiple tetratricopeptide repeats. J. Biol. Chem. 1997;272:9308–9315. doi: 10.1074/jbc.272.14.9308. [DOI] [PubMed] [Google Scholar]
- 23.Vocadlo DJ. O-GlcNAc processing enzymes: catalytic mechanisms, substrate specificity, and enzyme regulation. Curr. Opin. Chem. Biol. 2012;16:488–497. doi: 10.1016/j.cbpa.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 24.Butkinaree C, Cheung WD, Park S, Park K, Barber M, Hart GW. Characterization of beta-N-acetylglucosaminidase cleavage by caspase-3 during apoptosis. J. Biol. Chem. 2008;283:23557–23566. doi: 10.1074/jbc.M804116200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Comtesse N, Maldener E, Meese E. Identification of a nuclear variant of MGEA5, a cytoplasmic hyaluronidase and a beta-N-acetylglucosaminidase. Biochem. Biophys. Res. Comm. 2001;283:634–640. doi: 10.1006/bbrc.2001.4815. [DOI] [PubMed] [Google Scholar]
- 26.Keembiyehetty CN, Krzeslak A, Love DC, Hanover JA. A lipid-droplet-targeted O-GlcNAcase isoform is a key regulator of the proteasome. J. Cell Sci. 2011;124:2851–2860. doi: 10.1242/jcs.083287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schimpl M, Schüttelkopf AW, Borodkin VS, van Aalten DM. Human OGA binds substrates in a conserved peptide recognition groove. Biochem. J. 2010;432:1–7. doi: 10.1042/BJ20101338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schimpl M, Borodkin VS, Gray LJ, van Aalten DM. Synergy of peptide and sugar in O-GlcNAcase substrate recognition. Chem. Biol. 2012;19:173–178. doi: 10.1016/j.chembiol.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marshall S, Bacote V, Traxinger RR. Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. J. Biol. Chem. 1991;266:4706–4712. [PubMed] [Google Scholar]
- 30.Buse MG. Hexosamines, insulin resistance and the complications of diabetes: current status. Am. J. Physiol. Endocrinol. Metab. 2006;290:E1–E8. doi: 10.1152/ajpendo.00329.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yi W, Clark PM, Mason DE, Keenan MC, Hill C, goddard WA, 3rd, Peters EC, Driggers EM, Hsieh-Wilson LC. Phosphofructokinase 1 glycosylation regulates cell growth and metabolism. Science. 2012;337:975–980. doi: 10.1126/science.1222278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sánchez-Chávez G, Peña-Rangel MT, Riesgo-Escovar JR, Martínez-Martínez A, Salceda R. Insulin stimulated-glucose transporter Glut 4 is expressed in the retina. PLoS One. 2012;7:e52959. doi: 10.1371/journal.pone.0052959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu L, Seidel CP, Iwase T, Stevens RK, Gong YY, Want X, Hackett SF, Campochiaro PA. Suppression of GLUT1; a new strategy to prevent diabetic complications. J. Cell Physiol. 2013;228:251–257. doi: 10.1002/jcp.24133. [DOI] [PubMed] [Google Scholar]
- 34.Yki-Järvinen H, Daniels MC, Virkamäki A, Mäkimattila S, DeFronzo RA, McClain D. Increased glutamine:fructose-6-phosphate amidotransferase activity in skeletal muscle of patients with NIDDM. Diabetes. 1996;45:302–307. doi: 10.2337/diab.45.3.302. [DOI] [PubMed] [Google Scholar]
- 35.Wang Z, Park K, Comer F, Hsieh-Wilson LC, Saudek CD, Hart GW. Site-specific GlcNAcylation of human erythrocyte proteins: potential biomarker(s) for diabetes. Diabetes. 2009;58:309–317. doi: 10.2337/db08-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park K, Saudek CD, Hart GW. Increased expression of beta-N-acetylglucosaminidase in erythrocytes from individuals with pre-diabetes and diabetes. Diabetes. 2010;59:1845–1850. doi: 10.2337/db09-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rossetti L, Hawkins M, Chen W, Gindi J, Barzilai N. In vivo glucosamine infusion induces insulin resistance in normoglycemic but not in hyperglycemic conscious rats. J. Clin. Invest. 1995;96:132–140. doi: 10.1172/JCI118013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hawkins M, Barzilai N, Liu R, Hu M, Chen W, Rossetti L. Role of the glucosamine pathway in fat-induced insulin resistance. J. Clin. Invest. 1997;99:2173–2182. doi: 10.1172/JCI119390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vosseller K, Wells L, Lane MD, Hart GW. Elevated nucleocytoplastmic glycosylation by O-GlcNAc results in insulin resistance associated with defects in Akt activation in 3T3-L1 adipocytes. Proc. Natl. Acad. Sci. U. S. A. 2002;99:5313–5318. doi: 10.1073/pnas.072072399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whelan SA, Dias WB, Thiruneelakantapillai L, Lane MD, Hart GW. Regulation of insulin receptor substrate 1 (IRS-1)/AKT kinase-mediated insulin signaling by O-linked β-N-acetylglucosamine in 3T3-L1 adipocytes. J. Biol. Chem. 2010;285:5204–5211. doi: 10.1074/jbc.M109.077818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Penque BA, Hoggat AM, Herring BP, Elmendorf JS. Hexosamine biosynthesis impairs insulin action via a cholesterolgenic response. Mol. Endocrinol. 2013:536–547. doi: 10.1210/me.2012-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Antonetti DA, Barber AJ, Bronson SK, Freeman WM, Gardner TW, Jefferson LS, Kester M, Kimball SR, Krady JK, LaNoeu KF, Norbury CC, Quinn PG, Sandirasegarane L, Simpson IA. JDRF Diabetic Retinopathy Center Group, Diabetic retinopathy: seeing beyond glucose-induce microvascular disease. Diabetes. 2006;55:2401–2411. doi: 10.2337/db05-1635. [DOI] [PubMed] [Google Scholar]
- 43.Levy AP, Levy NS, Goldberg MA. Post-transcriptional regulation of vascular endothelial growth factor by hypoxia. J. Biol. Chem. 1996;272:2746–2753. doi: 10.1074/jbc.271.5.2746. [DOI] [PubMed] [Google Scholar]
- 44.Murakami T, Felinski EA, Antonetti DA. Occludin phosphorylation and ubiquination regulate tight junction trafficking and vascular endothelial growth factor-induced permeability. J. Biol. Chem. 2009;284:21036–21046. doi: 10.1074/jbc.M109.016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sundstrom JM, Tash BR, Murakami T, Flanagan JM, Bewley MC, Stanley BA, Gonsar KB, Antonetti DA. Identification and analysis of occludin phosphosites: a combined mass spectrometry and bioinformatics approach. J. Proteome Res. 2009;8:808–817. doi: 10.1021/pr7007913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harhaj NS, Felinski EA, Wolpert EB, Sundstrom JM, Gardner TW, Antonetti DA. VEGF activation of protein kinase C stimulates occludin phosphorylation and contributes to endothelial permeability. Invest. Ophthalmol. Vis. Sci. 2006;47:5106–5115. doi: 10.1167/iovs.06-0322. [DOI] [PubMed] [Google Scholar]
- 47.Mee CJ, Farquhar MJ, Harris HJ, Hu K, Ramma W, Ahmed A, Maurel P, Bicknell R, Balfe P, McKeating JA. Hepatitis C infection reduces hepatocellular polarity in a vascular endothelial growth factor-dependent manner. Gastroenterology. 2010;138:1134–1142. doi: 10.1053/j.gastro.2009.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Butt AM, Feng D, Nasrullah I, Tahir S, Idrees M, Tong Y, Lu J. Computational identification of interplay between phosphorylation and O-β-glycosylation of human occludin as a potential mechanism to impair hepatitis C viral entry. Infect. Genet. Evol. 2012;12:1235–1245. doi: 10.1016/j.meegid.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 49.Huang H, Gandhi JK, Zhong X, Wei Y, Gong J, Duh EJ, Vinores SA. TNFα is required for late BRB breakdown in diabetic retinopathy, and its inhibition prevents leukostasis and protects vessels and neurons from apoptosis. Invest. Ophthalmol. Vis. Sci. 2011;52:1336–1344. doi: 10.1167/iovs.10-5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Joussen AM, Doehmen S, Le ML, Koizumi K, Radetzky S, Krohne TU, Poulaki V, Semkova I, Kociok N. TNF-alpha mediated apoptosis plays an important role in the development of early diabetic retinopathy and long-term histopathological alterations. Mol. Vis. 2009;15:1418–1428. [PMC free article] [PubMed] [Google Scholar]
- 51.Costa GN, Vindeirinho J, Cavadas C, Ambrósio AF, Santos PF. Contribution of TNF receptor 1 to retinal neural cell death induced by elevated glucose. Mol. Cell. Neurosci. 2012;50:113–123. doi: 10.1016/j.mcn.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 52.Xing D, Gong K, Feng W, Nozell SE, Chen YF, Chatham JC, Oparil S. OGlcNAc modification of NFκB p65 inhibits TNF-α-induced inflammatory mediator expression in rat aortic smooth muscle cells. PLoS One. 2011;6:e24021. doi: 10.1371/journal.pone.0024021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Geraldes P, Hiraoka-Yamamoto J, Matsumoto M, Clermont A, Leitges M, Marette A, Aiello LP, Kern TS, King GL. Activation of PKC-delta and SHP-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nat. Med. 2009;15:1298–1306. doi: 10.1038/nm.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matthews JA, Acevedo-Duncan M, Potter RL. Selective decrease of membrane-associated PKC-alpha and PCK-epsilon in response to elevated intracellular O-GlcNAc levels in transformed human glial cells. Biochim. Biophys. Acta. 2005;1743:305–315. doi: 10.1016/j.bbamcr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 55.Robles-Flores M, Meléndez L, García W, Mendoza-Hernández G, Lam TT, Castañeda-Patlán C, González-Aguilar H. Posttranslational modifications on protein kinase C enzymes. Effects of epinephrine and phorbol esters. Biochim. Biophys. Acta. 2008;1783:695–712. doi: 10.1016/j.bbamcr.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 56.Gao BB, Clermont A, Rook S, Fonda SJ, Srinivasan VJ, Wojtkowski M, Fujimoto JG, Avery RL, Arrigg PG, Bursell SE, Aiello LP, Feener EP. Extracellular carbonic anhydrase mediates hemorrhagic retinal and cerebral vascular permeability through prekallikrein activation. Nat. Med. 2007;13:181–188. doi: 10.1038/nm1534. [DOI] [PubMed] [Google Scholar]
- 57.Feener EP. Plasma kallikrein and diabetic macular edema. Curr. Diab. Rep. 2010;10:270–275. doi: 10.1007/s11892-010-0127-1. [DOI] [PubMed] [Google Scholar]
- 58.Milne R, Brownstein S. Advanced glycation end products and diabetic retinopathy. Amino Acids. 2013;44:1397–1407. doi: 10.1007/s00726-011-1071-3. [DOI] [PubMed] [Google Scholar]
- 59.Berner AK, Brouwers O, Pringle R, Klaassen I, Colhoun L, McVicar C, Brockbanks S, Curry JW, Miyata T, Brownlee M, Schlingemann RO, Schalkwijk C, Stitt AW. Protection against methylglyoxal-derived AGEs by regulation of glyoxalase 1 prevents retinal neuroglial and vasodegenerative pathology. Diabetologia. 2012;55:845–854. doi: 10.1007/s00125-011-2393-0. [DOI] [PubMed] [Google Scholar]
- 60.Luo D, Fan Y, Xu W. The effects of aminoguanidine on retinopathy in STZ-induced diabetic rats. Bioorg. Med. Chem. Lett. 2012;22:4386–4390. doi: 10.1016/j.bmcl.2012.04.130. [DOI] [PubMed] [Google Scholar]
- 61.Semba RD, Nicklett EJ, Ferrucci L. Does accumulation of advanced glycation end products contribute to the aging phenotype? J. Gerontol. A. Biol. Sci. Med. Sci. 2010;65:963–975. doi: 10.1093/gerona/glq074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamagishi S, Hsu CC, Taniguchi M, Harada S, Yamamoto Y, Ohsawa K, Kobayashi K, Yamamoto H. Receptor-mediated toxicity to pericytes of advanced glycosylation end products: a possible mechanism of pericyte loss in diabetic microangiopathy. Biochem. Biophys. Res. Commun. 2004;213:681–687. doi: 10.1006/bbrc.1995.2185. [DOI] [PubMed] [Google Scholar]
- 63.Liu B, Bhat M, Padival AK, Smith DG, Nagaraj RH. Effect of dicarbonyl modification of fibronectin on retinal capillary pericytes. Invest. Ophthalmol. Vis. Sci. 2004;45:1983–1995. doi: 10.1167/iovs.03-0995. [DOI] [PubMed] [Google Scholar]
- 64.Li SY, Sigmon VK, Babcock SA, Ren J. Advanced glycation endproduct induces ROS accumulation, apoptosis, MAP kinase activation and nuclear O-GlcNAcylation in human cardiac myocytes. Life Sci. 2007;80:1051–1056. doi: 10.1016/j.lfs.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 65.Yao D, Taguchi T, Matsumura T, Pestell R, Edelstein D, Giardino I, Suske G, Rabbani N, Thornalley PJ, Sarthy VP, Hammes HP, Brownlee M. High glucose increases angiopoietin-2 transcription in microvascular endothelial cells through methylglyoxal modification of mSin3A. J. Biol. Chem. 2007;282:31038–31045. doi: 10.1074/jbc.M704703200. [DOI] [PubMed] [Google Scholar]
- 66.Frey T, Antonetti DA. Alterations to the blood-retinal barrier in diabetes: cytokines and reactive oxygen species. Antioxid. Redox Signal. 2011;15:1271–1284. doi: 10.1089/ars.2011.3906. [DOI] [PubMed] [Google Scholar]
- 67.Roquemore EP, Dell A, Morris HR, Panico M, Reason AJ, Savoy LA, Wistow GJ, Zigler JS, Jr., Earles BJ, Hart GW. Vertebrate lens α-crystallins are modified by O-linked N-acetylglucosamine. J. Biol. Chem. 1992;267:555–563. [PubMed] [Google Scholar]
- 68.Roquemore EP, Chevrier MR, Cotter RJ, Hart GW. Dynamic O-GlcNAcylation of the small heat shock protein αB-cystallin. Biochemistry. 1996;35:3578–3586. doi: 10.1021/bi951918j. [DOI] [PubMed] [Google Scholar]
- 69.Krishnamoorthy V, Donofrio AJ, Martin JL. O-GlcNAcylation of αB-crystallin regulates its stress-induced translocation and cytoprotection. Mol. Cell. Biol. 2013;279:59–68. doi: 10.1007/s11010-013-1627-5. [DOI] [PubMed] [Google Scholar]
- 70.Wang K, Ho SR, Mao W, Huang P, Zhang F, Schwiebert EM, Kudlow JE, Paterson AJ. Increased O-GlcNAc causes disrupted lens fiber cell differentiation and cataracts. Biochem. Biophys. Res Comm. 2009;387:70–76. doi: 10.1016/j.bbrc.2009.06.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Abdelkader H, Patel DV, McGhee CNJ, Alany RG. New therapeutic approaches in the treatment of diabetic keratopathy: a review. Clin. Exp. Ophthalmol. 2011;39:259–270. doi: 10.1111/j.1442-9071.2010.02435.x. [DOI] [PubMed] [Google Scholar]
- 72.Müller LJ, Marfurt CF, Kruse F, Tervo TMT. Corneal nerves: structure, contents and function. Exp. Eye Res. 2003;76:521–542. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 73.Akimoto Y, Kawakami H, Yamamoto K, Munetomo E, Hida T, Hirano H. Elevated expression of O-GlcNAc-modified proteins and O-GlcNAc transferase in corneas of diabetic Goto-Kakizaki rats. Invest. Ophthalmol. Vis. Sci. 2003;44:3802–3809. doi: 10.1167/iovs.03-0227. [DOI] [PubMed] [Google Scholar]
- 74.Patel JI, Tombran-Tink J, Hykin PG, Gregor ZJ, Cree IA. Vitreous and aqueous concentrations of proangiogenic, antiangiogenic factors and other cytokines in diabetic retinopathy patients with macular edema: implications for structural differences in macular profiles. Exp. Eye Res. 2006;82:798–806. doi: 10.1016/j.exer.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 75.Vinores SA, Derevjanik NL, Mahlow J, Hackett SF, Haller JA, deJuan E, Frankfurter A, Campochiaro PA. Class III beta-tubulin in human retinal pigment epithelial cells in culture and in epiretinal membranes. Exp. Eye Res. 1995;60:385–400. doi: 10.1016/s0014-4835(05)80095-8. [DOI] [PubMed] [Google Scholar]
- 76.Chung EJ, Chun JN, Jung SA, Cho JW, Lee JH. TGF-β-stimulated aberrant expression of class III β-tubulin via the ERK signaling pathway in cultured retinal pigment epithelial cells. Biochem. Biophys. Res. Comm. 2011;415:367–372. doi: 10.1016/j.bbrc.2011.10.074. [DOI] [PubMed] [Google Scholar]
- 77.Ji S, Kang JG, Park SY, Lee JH, Oh YJ, Cho JW. O-GlcNAcylation of tubulin inhibits its polymerization. Amino Acids. 2011;40:809–818. doi: 10.1007/s00726-010-0698-9. [DOI] [PubMed] [Google Scholar]
- 78.Ding M, Vandré DD. High molecular weight microtubule-associated proteins contain O-linked-n-acetylglucosamine. J. Biol. Chem. 1996;271:12555–12561. doi: 10.1074/jbc.271.21.12555. [DOI] [PubMed] [Google Scholar]
- 79.Arnold CS, Johnson GV, Cole RN, Dong DL, Lee M, Hart GW. The microtubule-associated protein tau is extensively modified with O-linked N-acetylglucosamine. J. Biol. Chem. 1996;271:28741–28744. doi: 10.1074/jbc.271.46.28741. [DOI] [PubMed] [Google Scholar]
- 80.Yuzwa SA, Macauley MS, Heinonen JE, Shan X, Dennis RJ, He Y, Whitworth GE, Stubbs KA, McEachern EJ, Davies GJ, Vocadlo DJ. A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo. Nat. Chem. Biol. 2008;4:483–490. doi: 10.1038/nchembio.96. [DOI] [PubMed] [Google Scholar]
- 81.Gurel Z, Sieg KM, Shallow KD, Sorenson CM, Sheibani N. Retinal O-linked N-acetylglucosamine protein modifications: implications for postnatal retinal vascularization and the pathogenesis of diabetic retinopathy. Mol. Vis. 2013;19:1047–1059. [PMC free article] [PubMed] [Google Scholar]
- 82.Barber AJ, Antonetti DA, Kern TS, Reiter CEN, Soans RS, Krady JK, Levison SW, Gardner TW, Bronson SK. The Ins2Akita mouse as a model of early retinal complications in diabetes. Invest. Ophthalmol. Vis. Sci. 2005;46:2210–2218. doi: 10.1167/iovs.04-1340. [DOI] [PubMed] [Google Scholar]
- 83.Gastinger MJ, Kunselman AR, Conboy EE, Bronson SK, Barber AJ. Dendrite remodeling and other abnormalities in retinal ganglion cells of Ins2Akita diabetic mice. Invest. Ophthalmol. Vis. Sci. 2008;49:2635–2642. doi: 10.1167/iovs.07-0683. [DOI] [PubMed] [Google Scholar]
- 84.Huang H, Gandhi JK, Zhong X, Wei Y, Gong J, Duh EJ, Vinores SA. TNFα is required for late BRB breakdown in diabetic retinopathy, and its inhibition prevents leukostasis and protects vessels and neurons from apoptosis. Invest. Ophthalmol. Vis. Sci. 2011;52:1336–1344. doi: 10.1167/iovs.10-5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stahl A, Connor KM, Sapieha P, Chen J, Dennison RJ, Krah NM, Seaward MR, Willett KL, Aderman CM, Guerin KI, Hua J, Löfqvist C, Hellström A, Smith LEH. The mouse retina as an angiogenesis model. Invest. Ophthalmol. Vis. Sci. 2010;51:2813–2826. doi: 10.1167/iovs.10-5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Semba RD, Enghild JJ, Venkatramana V, Dyrlund TF, Van Eyk JE. The Human Eye Proteome Project: perspectives on an emerging proteome. Proteomics. 2013;13:2500–2511. doi: 10.1002/pmic.201300075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lab Hu. Georgetown University; [July 4, 2013]. ( http://cbsb.lombardi.georgetown.edu/hulab/OGAP.html) [Google Scholar]
- 88.Wang Z, Udeshi ND, Slawson C, Compton PD, Sakabe K, Cheung WD, Shabanowitz J, Hunt DF, Hart GW. Extensive crosstalk between O-GlcNAcylation and phosphorylation regulates cytokinesis. Sci. Signal. 2010;3:ra2. doi: 10.1126/scisignal.2000526. doi: 10.1126/scisignal.200052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schleicher ED, Weigert C. Role of the hexosamine biosynthetic pathway in diabetic nephropathy. Kidney Int. Suppl. 2000;77:S13–S18. doi: 10.1046/j.1523-1755.2000.07703.x. [DOI] [PubMed] [Google Scholar]
- 90.Weigert C, Friess U, Brodbeck K, Häring HU, Schleicher ED. Glutamine:fructose-6-phosphate aminotransferase enzyme activity is necessary for the induction of TGF-beta1 and fibronectin expression in mesangial cells. Diabetologia. 2003;46:852–855. doi: 10.1007/s00125-003-1122-8. [DOI] [PubMed] [Google Scholar]
- 91.Degrell P, Cesh J, Mohá M, Molnár GA, Pajor L, Chatham JC, Fülöp N, Wittmann I. Evidence of O-linked N-acetylglucosamine in diabetic nephropathy. Life Sci. 2009;84:389–393. doi: 10.1016/j.lfs.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 92.Goldberg H, Whiteside C, Fantus IG. O-linked β-N-acetylglucosamine supports p38 MAPK activation by high glucose in glomerular mesangial cells. Am. J. Physiol. Endocrinol. Metab. 2011;301:E713–E726. doi: 10.1152/ajpendo.00108.2011. [DOI] [PubMed] [Google Scholar]
- 93.Lunde IG, Aronsen JM, Kvaloy H, Qvigstad E, Sjaastad I, Tonnessen T, Christensen G, Gronning-Wang LM, Carlson CR. Cardiac O-GlcNAc signaling is increased in hypertrophy and heart failure. Physiol. Genomics. 2012;44:162–172. doi: 10.1152/physiolgenomics.00016.2011. [DOI] [PubMed] [Google Scholar]
- 94.McLarty JL, Marsh SA, Chatham JC. Post-translational protein modification by O-linked N-acetyl-glucosamine: its role in mediating the adverse effects of diabetes on the heart. Life Sci. 2013;92:621–627. doi: 10.1016/j.lfs.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Marsh SA, Dell'Italia LJ, Chatham JC. Activation of the hexosamine biosynthesis pathway and protein O-GlcNAcylation modulate hypertrophic and cell signaling pathways in cardiomyoctes from diabetic mice. Amino Acids. 2011;40:819–828. doi: 10.1007/s00726-010-0699-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Holt GD, Hart GW. The subcellular distribution of terminal N-acetylglucosamine moieties. Localization of a novel protein-saccharide linkage, O-linked GlcNAc. J. Biol. Chem. 1986;261:8049–8057. [PubMed] [Google Scholar]
- 97.Hart GW, Slawson C, Ramirez-Correa G, Lagerlof O. Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease. Annu. Rev. Biochem. 2011;80:825–858. doi: 10.1146/annurev-biochem-060608-102511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zachara NE, Vosseller K, Hart GW. Detection and analysis of proteins modified by O-linked N-acetylglucosamine. Curr. Protoc. Mol. Biol. 2011:17.6. doi: 10.1002/0471142727.mb1706s95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang Z, Hart GW. Glycomic approaches to study GlcNAcylation: protein identification, site-mapping, and site-specific O-GlcNAc quantitation. Clin. Proteomics. 2008;4:5–13. [Google Scholar]
- 100.Comer FI, Vosseller K, Wells L, Accavitti MA, Hart GW. Characterization of mouse monoclonal antibody specific for O-linked N-acetylglucosamine. Anal. Biochem. 2001;293:169–177. doi: 10.1006/abio.2001.5132. [DOI] [PubMed] [Google Scholar]
- 101.Holt GD, Snow CM, Senior A, Haltiwanger RS, Gerace L, Hart GW. Nuclear pore complex glycoproteins contain cytoplasmically disposed O-linked N-acetylglucosamine. J. Cell Biol. 1987;104:1157–1164. doi: 10.1083/jcb.104.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Teo CF, Ingale S, Wolfert MA, Elsayed GA, Not LG, Chatham JC, Wells L, Boons GJ. Glycopeptide-specific monoclonal antibodies suggest new roles for O-GlcNAc. Nat. Chem. Biol. 2010;6:338–343. doi: 10.1038/nchembio.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kamemura K, Hayes BK, Comer FI, Hart GW. Dynamic interplay between O-glycosylation and O-phophorylation of nucleocytoplasmic proteins. Alternative glycosylation/phosphorylation of Thr58, a known mutational hot spot of c-Myc in lymphomas, is regulated by mitogens. J. Biol. Chem. 2002;277:19229–19235. doi: 10.1074/jbc.M201729200. [DOI] [PubMed] [Google Scholar]
- 104.Pathak S, Borodkin VS, Albarbarawi O, Campbell DG, Ibrahim A, van Aalten DM. O-GlcNAcylation of TAB1 modulates TAK1-mediated cytokine release. EMBO J. 2012;31:1393–1404. doi: 10.1038/emboj.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yuzwa SA, Yadav AK, Skorobogatko Y, Clark T, Vosseller K, Vocadlo DJ. Mapping O-GlcNAc modification sites on tau and generation of a site-specific O-GlcNAc tau antibody. Amino Acids. 2011;40:857–868. doi: 10.1007/s00726-010-0705-1. [DOI] [PubMed] [Google Scholar]
- 106.Wang Z, Udeshi N, O'Malley M, Shabanowitz J, Hunt DF, Hart GW. Highly specific enrichment and site-mapping of O-GlcNAcylation using chemoenzymatic tagging, solid-phase photocleavage, and electron transfer dissociation mass spectrometry. Mol. Cell. Proteomics. 2010;9:153–160. doi: 10.1074/mcp.M900268-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vosseller K, Trinidad JC, Chalkley RJ, Specht CG, Thalhammer A, Lynn AJ, Snedecor JO, Guan S, Medzihradszky KF, Malby DA, Schoepfer R, Burlingame AL. O-linked N-acetylglucosamine proteomics of postsynaptic density preparations using lectin weak affinity chromatography and mass spectrometry. Mol. Cell. Proteomics. 2006;5:923–834. doi: 10.1074/mcp.T500040-MCP200. [DOI] [PubMed] [Google Scholar]
- 108.Trinidad JC, Barkan DT, Gulledge BF, Thalhammer A, Sali A, Schoepfer R, Burlingame AL. Global identification and characterization of both O-GlcNAcylation and phosphorylation at the murine synapse. Mol. Cell. Proteomics. 2012;11:215–229. doi: 10.1074/mcp.O112.018366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Khidekel N, Arndt S, Lamarre-Vincent N, Lippert A, Poulin-Kerstien KG, Ramakrishnan B, Qasba PK, Hsieh-Wilson LC. A chemoenzymatic approach toward the rapid and sensitive detection of O-GlcNAc posttranslational modifications. J. Am. Chem. Soc. 2003;125:16162–16163. doi: 10.1021/ja038545r. [DOI] [PubMed] [Google Scholar]
- 110.Hahne H, Sobotzki N, Nyberg T, Helm D, Borodkin VS, van Aalten DM, Agnew B, Kuster B. Proteome wide purification and identification of O-GlcNAc-modified proteins using click chemistry and mass spectrometry. J. Proteome Res. 2013;12:927–936. doi: 10.1021/pr300967y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wells L, Vosseller K, Cole RN, Cronshaw JM, Matunis MJ, Hart GW. Mapping sites of O-GlcNAc modification using affinity tags for serine and threonine post-translational modifications. Mol. Cell. Proteomics. 2002;1:791–804. doi: 10.1074/mcp.m200048-mcp200. [DOI] [PubMed] [Google Scholar]
- 112.Macauley MS, Vocadlo DJ. Increasing O-GlcNAc levels: an overview of small-molecule inhibitors of O-GlcNAcase. Biochim. Biophys. Acta. 2010;1800:107–121. doi: 10.1016/j.bbagen.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 113.Yuzwa SA, Macauley MS, Heinonen JE, Shan X, Dennis RJ, He Y, Whitworth GE, Stubbs KA, McEachern EJ, Davies GJ, Vocadlo DJ. A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo. Nat. Chem. Biol. 2008;4:483–490. doi: 10.1038/nchembio.96. [DOI] [PubMed] [Google Scholar]
- 114.Dorfmueller HC, Borodkin VS, Schimpl M, Shepherd SM, Shpiro NA, van Aalten DM. GlcNAcstatin: a picomolar, selective O-GlcNAcase inhibitor that modulates intracellular O-GlcNAcylation levels. J. Am. Chem. Soc. 2006;128:16484–16485. doi: 10.1021/ja066743n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Arias EB, Kim J, Cartee GD. Prolonged incubation in PUGNAc results in increased protein O-linked glycosylation and insulin resistance in rat skeletal muscle. Diabetes. 2004;53:921–930. doi: 10.2337/diabetes.53.4.921. [DOI] [PubMed] [Google Scholar]
- 116.Park SY, Ryu J, Lee W. O-GlcNAc modification on IRS-1 and Akt2 by PUGNAc inhibits their phosphorylation and induces insulin resistance in rat primary adipocytes. Exp. Mol. Med. 2005;37:220–229. doi: 10.1038/emm.2005.30. [DOI] [PubMed] [Google Scholar]
- 117.Gross BJ, Kraybill BC, Walker S. Discovery of O-GlcNAc transferase inhibitors. J. Am. Chem. Soc. 2005;127:14588–14589. doi: 10.1021/ja0555217. [DOI] [PubMed] [Google Scholar]
- 118.Gloster TM, Zandberg WF, Heinonen JE, Shen DL, Deng L, Vocadlo DJ. Hijacking a biosynthetic pathway yields a glycosyltransferase inhibitor within cells. Nat. Chem. Biol. 2011;7:174–181. doi: 10.1038/nchembio.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dorfmueller HC, Borodkin VS, Blair PE, Pathak S, Navratilova I, van Aalten DM. Substrate and product analogues as human O-GlcNAc transferase inhibitors. Amino Acids. 2011;40:781–792. doi: 10.1007/s00726-010-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lenzen S, Panten U. Alloxan: history and mechanism of action. Diabetologia. 1988;31:337–342. doi: 10.1007/BF02341500. [DOI] [PubMed] [Google Scholar]
- 121.Housley MP, Rodgers JT, Udeshi ND, Kelly TJ, Shabanowitz J, Hunt DF, Puigserver P, Hart GW. O-GlcNAc regulates FoxO activation in response to glucose. J. Biol Chem. 2008;283:16283–16292. doi: 10.1074/jbc.M802240200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jensen RV, Zachara NE, Nielsen PH, Kimose HH, Kristiansen SB, Botker HD. Impact of O-GlcNAc on cardioprotection by remote ischaemic preconditioning in non-diabetic and diabetic patients. Cardiovasc. Res. 2013;97:369–378. doi: 10.1093/cvr/cvs337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Majumdar G, Harmon A, Candelaria R, Martinez-Hernandez A, Raghow R, Solomon SS. O-glycosylation of Sp1 and transcriptional regulation of the calmodulin gene by insulin and glucagon. Am. J. Physiol. Endocrinol. Metab. 2003;285:E584–591. doi: 10.1152/ajpendo.00140.2003. [DOI] [PubMed] [Google Scholar]
- 124.Liu J, Marchase RB, Chatham JC. Glutamine-induced protection of isolated rat heart from ischemia/reperfusion injury is mediated via the hexosamine biosynthesis pathway and increased protein O-GlcNAc levels. J. Mol. Cell. Cardiol. 2007;27:177–185. doi: 10.1016/j.yjmcc.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hart GW, Slawson C, Ramirez-Correa G, Lagerlof O. Crosstalk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription and chronic disease. Ann. Rev. Biochem. 2011;80:825–858. doi: 10.1146/annurev-biochem-060608-102511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cheung WD, Sakabe K, Housley MP, Dias WB, Hart GW. O-GlcNAc transferase substrate specificity is regulated by MYPT1 and other interacting proteins. J. Biol. Chem. 2008;283:33935–33941. doi: 10.1074/jbc.M806199200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Housley MP, Rodgers JT, Puigserver P, Hart GW. A Pgc-1∝:O-GlcNAc transferase regulates FoxO transcription factor activity in response to glucose. J. Biol. Chem. 2009;284:5148–5157. doi: 10.1074/jbc.M808890200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cheung WD, Hart GW. AMP-activated protein kinase and p38 activate O-GlcNAcylation of neuronal proteins during glucose deprivation. J. Biol. Chem. 2008;283:13009–13020. doi: 10.1074/jbc.M801222200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Al-Dhaheri MH, Rowan BG. Application of phosphorylation site-specific antibodies to measure nuclear receptor signaling: characterization of novel phosphoantibodies for estrogen receptor alpha. Nucl. Rec. Signal. 2006;4:e007. doi: 10.1621/nrs.04007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Noren CJ, Anthony-Cahill SJ, Griffith MC, Schultz PG. A general method for site-specific incorporation of unnatural amino acids into proteins. Science. 1989;244:182–188. doi: 10.1126/science.2649980. [DOI] [PubMed] [Google Scholar]
- 131.Muir TW, Sondhi D, Cole PA. Expressed protein ligation: a general method for protein engineering. Proc. Natl. Acad. Sci. U.S.A. 1998;95:6705–6710. doi: 10.1073/pnas.95.12.6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Carrillo LD, Krishnamoorthy L, Mahal LK. A cellular FRET-based sensor for beta-O-GlcNAc, a dynamic carbohydrate modification involved in signaling. J. Am. Chem. Soc. 2006;128:14768–14769. doi: 10.1021/ja065835+. [DOI] [PubMed] [Google Scholar]
- 133.Carrillo LD, Froemming JA, Mahal LK. Targeted in vivo O-GlcNAc sensors reveal discrete compartment-specific dynamics during signal transduction. J. Biol. Chem. 2011;286:6650–6658. doi: 10.1074/jbc.M110.191627. [DOI] [PMC free article] [PubMed] [Google Scholar]