Abstract
Virus-like particles (VLPs) offer a platform to test the hypothesis that, since antibody binding to native envelope glycoprotein (Env) trimers results in HIV-1 neutralization, that native Env trimers presented in membranes may be useful for inducing neutralizing antibodies (nAbs) in a vaccine setting. So far, VLPs have not fulfilled this potential. Here, using a “shotgun” approach, we evaluated a wide cross-section of variables in a series of VLP immunizations. We identified 3 tentative leads. First, that VLP doses may not have been sufficient for optimal nAb induction. Second, that dampening the antigenicity of non-functional Env (for example uncleaved gp160) using either protease digests or IgG masking may be useful. Third, that guinea pig sera preferentially target non-conserved epitopes and exhibit relatively high background activity, suggesting that rabbits may be preferable as small animal vaccine models. Recent immunogenicity studies in rabbits appear to bear out all 3 of these leads.
Keywords: HIV, vaccine, antibody, neutralization, VLPs, gp120, gp41, Env
INTRODUCTION
Designing a vaccine for human immunodeficiency virus type 1 (HIV-1) remains a major challenge of modern biomedical research (Schiffner et al., 2013). Broadly neutralizing antibodies (bnAbs) are expected to be a key component of the protective immunity imparted by an effective HIV-1 vaccine (Mascola and Montefiori, 2010). However, current vaccine candidates usually fail to elicit effective tier 2 neutralization even against the vaccine strain - and when they do so, it is usually elicited inconsistently in only a few immunized animals and largely fails to cross-neutralize other tier 2 strains (Klasse et al., 2012; Mascola and Montefiori, 2010; McCoy and Weiss, 2013; Schiffner et al., 2013; van Gils and Sanders, 2013).
HIV-1 neutralization occurs when antibodies bind to native, functional Env spikes arrayed on viral membranes, thereby interfering in their engagement with cellular receptors (Mascola and Montefiori, 2010). Functional Env spikes consist of trimers of gp120/gp41 heterodimers in which the surface gp120 subunit mediates receptor binding and the membrane-anchoring gp41 subunit mediates fusion. These two subunits derive from a gp160 precursor that is glycosylated co-translationally (Earl et al., 1990), and later processed by a furin-like enzyme to form native gp120/gp41 trimers that are incorporated into nascent particles.
Mature Env spikes are compact and heavily glycosylated, thus allowing them to evade antibody binding and neutralization (Kwong and Mascola, 2012; Moore et al., 2006; van Gils and Sanders, 2013). Env can exist in various other forms, including the uncleaved (UNC) gp160 precursor, soluble monomeric gp120 and gp41 stumps, all of which are relatively accessible to antibody binding (Tong et al., 2012; Tong et al., 2013). It is perhaps not altogether surprising then that vaccines based on these and other relatively "accessible" forms of Env elicit antibodies that are largely unable to penetrate the compact native trimer's natural defenses (Klasse et al., 2012; Mascola and Montefiori, 2010; McCoy and Weiss, 2013; Schiffner et al., 2013). Conversely, the native Env trimer itself, presented in its natural context on lipid membranes might have the requisite stringency as an immunogen to consistently induce antibodies capable of penetrating its own highly sophisticated defenses - if optimal immunization conditions can be identified (Emini and Koff, 2004).
A natural lipid membrane context may be important for a fully native Env trimer conformation. Considering that all currently licensed vaccines against infectious diseases and several others under development are particle-based, many of which incorporate lipid membranes, this general approach has a strong track record (Garrone et al., 2011; Kanekiyo et al., 2013; Kulkarni et al., 2012; Kushnir et al., 2012; Roldao et al., 2010). Particle vaccines presenting native HIV-1 Env spikes have so far been explored in the form of live inactivated viruses, VLPs, liposomes and virosomes (Bomsel et al., 2011; Buonaguro et al., 2005; Chen et al., 2005; Crooks et al., 2007; Dennison et al., 2011; Doan et al., 2005; Evans et al., 2005; Grovit-Ferbas et al., 2000; Grundner et al., 2002; Hammonds et al., 2003; Hammonds et al., 2005; Hammonds et al., 2007; Hicar et al., 2010; Kamdem Toukam et al., 2012; Lifson et al., 2004; McBurney et al., 2007; McKenna et al., 2004; McKenna et al., 2003; Montero et al., 2012; Pastori et al., 2012; Poon et al., 2005; Visciano et al., 2011; Vzorov et al., 1999; Yang et al., 2012; Zhou et al., 2011). However, none have yet demonstrated a great potential to elicit tier 2 nAbs. One explanation for this lack of progress may be that the native Env trimer's compact nature renders it an inherently poor immunogen (see Fig. 4 in (Tong et al., 2013)). It therefore remains possible that higher native Env trimer doses and/or the use of powerful dose-sparing adjuvants can address problem (Li et al., 2006; VanCott et al., 1997).
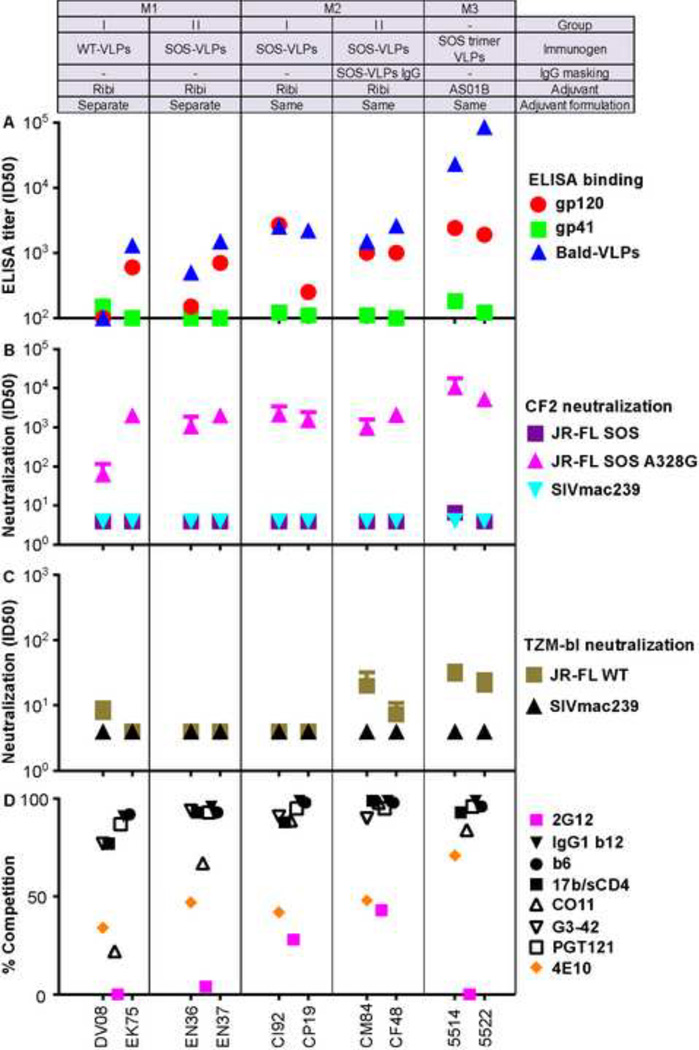
Fig. 4. Profile of M1 and M2 sera.
Macaque sera were analyzed in an identical manner as the guinea pig sera described in Fig. 3.
Another problem with particle-based vaccines is that they carry non-functional Env on their surfaces, principally uncleaved (UNC) gp160 and gp41 stumps that are relatively accessible to binding by non-neutralizing antibodies and may therefore interfere with the development of neutralizing responses to the native Env trimer (Agrawal et al., 2011; Crooks et al., 2007; Hicar et al., 2010; Joyner et al., 2011; Leaman et al., 2010; Moore et al., 2006; Nyambi et al., 1998; Tong et al., 2012; Tong et al., 2013). One solution may be to extract and purify soluble native Env trimers from membranes. However, this is hampered by the instability of the isolated product and its tendency to aggregate. Another solution may be to mask non-functional Env using IgG. This idea has some precedents (Denisova et al., 1996; Keller and Arora, 1999; Liao et al., 2004; Visciano et al., 2008). Furthermore, IgG complexing may facilitate VLP adsorption to follicular dendritic cells (Abdel-Motal et al., 2010; Forthal et al., 2007; van Montfort et al., 2007) and antigen processing (Hioe et al., 2009). A more recent option is to treat VLPs with proteases to selectively digest and thereby remove non-functional Env, leaving native Env trimers intact (Crooks et al., 2011; Tong et al., 2012).
A growing number of broadly neutralizing monoclonal antibodies recovered from infected donors to date are considered to be blueprints for vaccine discovery. Their sophisticated features, however, raise questions about whether model species used for vaccine testing have sufficient germline immunoglobulin repertoire complexity to develop similar nAbs (Pinheiro et al., 2011). This uncertainty calls for the continued testing of nAb vaccine candidates in multiple animal models.
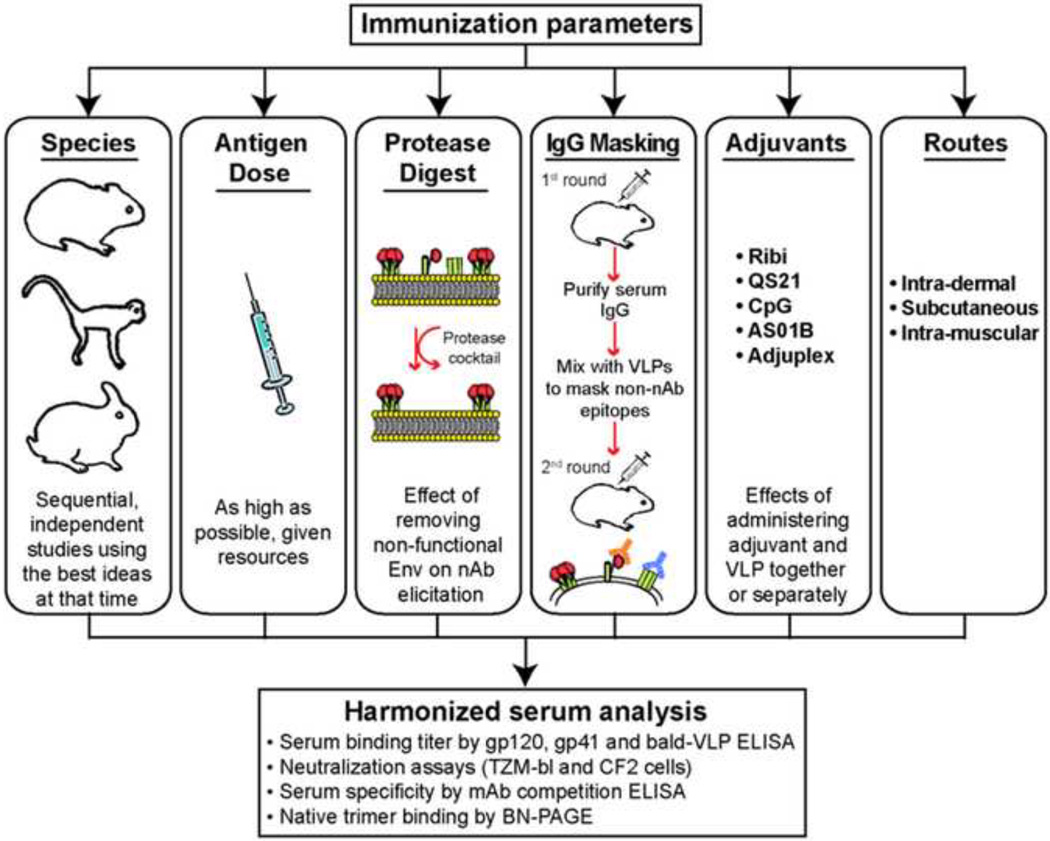
The goal in this study was to identify leads important for eliciting tier 2 nAbs by VLP immunogens. Investigating all of the abovementioned variables would require very large animal numbers and high costs. Here, using limited resources, and building on our previous VLP immunogenicity study in guinea pigs (Crooks et al., 2007), as outlined in Fig. 1, we examined a wide cross-section of variables in a series of immunization studies in guinea pigs, macaques and rabbits to try to partition factors that might associate with nAb development. Our findings suggest that i) higher VLP doses ii) dampening the antigenicity of non-functional Env from VLP surfaces and iii) the preferential use of rabbits over guinea pigs as a small animal may be important. All three of these leads appear to be validated in our more recent VLP immunogenicity studies in rabbits.
Fig. 1. Overview of immunization parameters.
Various immunization parameters, as indicated, were assessed in a shotgun approach to try to determine those that might partition with nAb development. A consistent algorithm to analyze sera was used to enhance our ability to compare strategies between different immunization groups and species.
MATERIALS AND METHODS
Monoclonal antibodies
Monoclonal antibodies (mAbs) were obtained from their producers, the NIH AIDS reagent repository, or commercial suppliers. They included the following (originators in parentheses): 17b and E51 (J. Robinson), directed to CD4-inducible (CD4i) epitopes of gp120 (Labrijn et al., 2003; Xiang et al., 2002); 2G12 (H. Katinger), directed to a glycan-dependent epitope of gp120 (Sanders et al., 2002; Scanlan et al., 2002); F2A3, CO11, 39F (J. Robinson), directed to the gp120 V3 loop (Crooks et al., 2005); PGT121 (D. Burton), directed to an N332-dependent gp120 epitope (Walker et al., 2011); G3–42 (M. Fung), directed to a C4-V3 epitope (Moore et al., 1994; Moore et al., 1993; Sun et al., 1989); b6, b12 (D. Burton), and 15e (J. Robinson), directed to the CD4 binding site (CD4bs) (Burton et al., 1994); 2F5 and 4E10 (H. Katinger), directed to the gp41 membrane-proximal ectodomain region (MPER) (Huang et al., 2012; Zwick et al., 2004); 7B2 and 2.2B, directed to the gp41 cluster I and II epitopes, respectively (Moore et al., 2006).
Recombinant Env glycoproteins, soluble CD4 and p24
Recombinant monomeric JR-FL gp120 and soluble CD4 (sCD4), consisting of all the 4 outer domains were gifts from Progenics Pharmaceuticals (Tarrytown, NY). Recombinant HXB2 gp41 (Catalog#VTI310; residues 546–682) was obtained from Meridian Life Science (Saco, Maine). Recombinant p24 was purchased from ProSpec-Tany TechnoGene Ltd. (Israel).
Antisera and HIV+ donor plasmas and HIVIG
The HIV-1-infected donor plasmas 1702, N160, BB68, 1686 and an uninfected control (donor 210) were described previously (Binley et al., 2008; Crooks et al., 2005). HIVIG was obtained from J. Mascola (NIH). Since these specimens were not generated specifically for this study, the Torrey Pines Institutional Review Board (IRB-12-001-JB) waived informed consent.
Plasmids and mutagenesis
The JR-FL clone was used as our parent Env (Binley et al., 2003; Crooks et al., 2007; Crooks et al., 2005; Crooks et al., 2011; Moore et al., 2006; Tong et al., 2012; Tong et al., 2013), as it expresses well and exhibits efficient gp120/gp41 processing. The pCAGGS plasmid was used to express various mutants (Binley et al., 2003; Moore et al., 2006), all with a gp41 tail truncation (gp160ΔCT) that leads to higher expression without significantly affecting neutralization sensitivity compared to full length Env (Crooks et al., 2005). Mutants were generated by Quikchange (Agilent Technologies) and numbered according to the HXB2 reference strain (Binley et al., 2008). "SOS" mutations introduce an intermolecular disulfide between gp120 and gp41 (Binley et al., 2003; Binley et al., 2000; Crooks et al., 2005). The A328G mutation increases neutralization sensitivity to nAbs as well as non-nAbs (Tomaras et al., 2011). The double mutant K510S/R511S ablates gp120-gp41 processing, resulting in uncleaved gp160 (UNC) (Crooks et al., 2011). Env-deficient sub-genomic plasmids pNL4-3.Luc.R-E- and pSG3ΔEnv were described previously (Binley et al., 2002; Li et al., 2005). A JR-FL gp120 D368R mutant was made in the PPI4 plasmid (Binley et al., 2000).
VLP Production
WT-VLPs, SOS-VLPs, or UNC-VLPs were produced by co-transfecting 293T cells with pNL4-3.Luc.R-E- and pCAGGS-based Env-expressing plasmids, as described previously (Tong et al., 2012). "Bald-VLPs" formerly termed "naked VLPs" (Crooks et al., 2007) bearing no Env were produced by transfecting pNL4-3.Luc.R-E- alone. VLPs were inactivated using 1mM aldrithiol (AT-2) (Rossio et al., 1998) and in some cases, were digested with a protease cocktail including proteinase K, subtilisin, chymotrypsin and trypsin, as described previously (Tong et al., 2012).
Animal immunizations
i) Species
Dunkin Hartley guinea pig and New Zealand white rabbit immunizations were performed at the Pocono Rabbit Farm (Canadensis, PA). Rhesus macaque immunizations were performed at the Tulane University Primate Center or Bioqual Inc (Rockville, MD). These sites are approved by the Association for Assessment and Accreditation of Laboratory Animal Care. All animals were fed, housed and handled in strict accordance with the recommendations of the NIH Guide for the Care and Use of Laboratory Animals and the Animal Welfare Act. Macaques were housed indoors in stainless steel cages, had a 12h/12h daily light cycle, were fed twice daily, provided water ad libitum, and were given various toys and feeding enrichment.
Immunization protocols were approved by all Institutional Animal Care and Use Committees (protocols TPI 10-03, TPI 13-01 and Tulane 3309), for which Animal Welfare Assurance numbers are: A4193-01 (Torrey Pines Institute), A4499-01 (Tulane University Primate Center), A-3086-01 (Bioqual) and A3886-01 (Pocono Rabbit Farm and Laboratory). For all immunization and bleed protocols, pain and distress was slight and momentary and did not affect animal health. Discomfort and injury to animals was limited to that which is unavoidable in the conduct of scientifically valuable research. Analgesics, anesthetics, and tranquilizing drugs were used as necessary by veterinary staff. For macaques, ketamine (10mg/kg) was administered intramuscularly as an anesthesia to immobilize animals for all procedures. Macaque health was monitored by complete physical examinations under ketamine anesthesia before each immunization and bleed.
Rabbits and guinea pigs were all female. Macaques were all male. Immunization routes were intramuscular (IM), intradermal (ID) and subcutaneous (SC), as indicated in Fig. 2. Each animal was immunized 3 times, at ~6 week intervals. An exception was the macaque M3 group who received VLPs at months 0, 1 and 6. Final sera were collected two weeks after the 3rd immunization. At the end of each study, rabbits and guinea pigs were euthanized according to NIH guidelines by administering ketamine and xyalzine intramuscularly at 35mg/kg and 5mg/kg, respectively, followed by exsanguination via cardiac puncture. Macaques were returned to the colony.
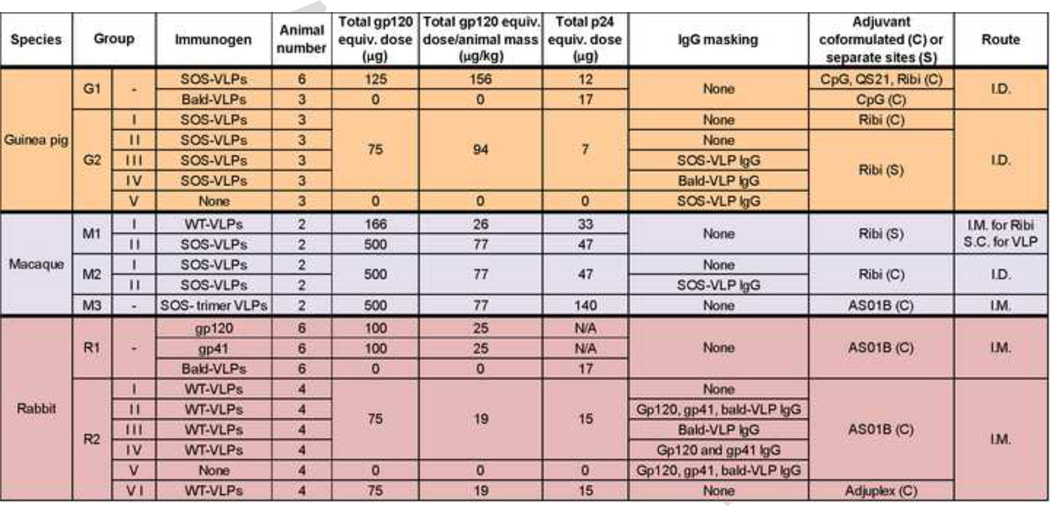
Fig. 2. Summary of immunization studies.
Seven immunization groups G1, G2, M1, M2, M3, R1 and R2 were coded according to species (i.e. G = guinea pigs, M = macaque and R = rabbit). Immunization studies were performed consecutively from top to bottom, starting from group G1, with the exception that the M3 study was performed last. In each study, groups consisting of 2–6 animals were inoculated with the immunogens indicated. Env-VLP doses are expressed as an approximate gp120 equivalent dose determined by ELISA (see Fig. S1). Gp120 equivalent dose/kg of average animal mass was calculated by assuming average masses as follows: guinea pigs (0.8kg), rabbits (4kg) and macaques (6.5kg). Total VLP p24 equivalent doses were estimated by SDS-PAGE-Western blot (Fig. S2). Adjuvants were either formulated with VLPs or administered separately. In some groups (G2, M2 and R2), VLPs were complexed with masking IgG purified from the sera of animals of their respective preceding groups (i.e. G1, M1 and R1). Control subgroups received only masking IgG (i.e. G2-V and R2-V). M3 animals received SOS-VLPs that were digested with proteases to remove non-functional Env.
ii) Immunogens
Immunogens included WT-VLPs, SOS-VLPs, bald-VLPs, recombinant JR-FL gp120 and HXB2 gp41. Some animals were immunized with VLPs mixed with polyclonal IgG or with polyclonal IgG alone (e.g. guinea pig groups G2-III and G2-V, respectively, in Fig. 2). This polyclonal IgG was isolated by protein A-agarose chromatography (GE Healthcare) from the immune sera of an initial round of immunizations. It was then adjusted to 10mg/ml to approximately match the IgG concentration of the parent sera. For animals receiving VLP-IgG complexes, VLP pellets were resuspended in purified serum IgG and incubated for 1h at room temperature (RT). Macaque group M3 animals received "SOS trimer VLPs" that had been protease digested to clear non-functional Env (Fig. 2). Total immunogen volumes were 0.3ml for guinea pigs and 0.5ml for rabbits and macaques. When more than one type of masking IgG was used (e.g. group R2-II), total immunogen volumes comprised of equal volumes of each 10mg/ml IgG stock.
iii) Adjuvants
Adjuvants included the CpG-based adjuvant ImmunEASY (Qiagen), Quillaja saponaria-derived QS21, the Ribi adjuvant system (Ras3C) (Sigma), Adjuplex (Advanced BioAdjuvants) and AS01B (GlaxoSmithKline). Ras3C is a 2.0% v/v squalene oil-in-water emulsion containing 4'monophosphoryl lipid A (MPL) derived from the lipopolysaccharide of Salmonella minnesota R595, cell wall skeleton from Mycobacterium phlei and synthetic trehalose dicorynomycolate. Adjuplex consists of detergent-free lecithin and carbomer homopolymer. AS01B consists of liposomes containing deacylated MPL and QS21. In most cases, adjuvants and immunogens were co-formulated. However, in some cases were administered separately (Fig. 2).
iv) Dose
Gp120 and gp41 were administered in 100µg doses. Doses of the Env component of VLPs were estimated in terms of “gp120 equivalents” by ELISA (see below). Immulon II plates were coated overnight at 4°C with graded dilutions of monomeric gp120 or Env-VLPs. Following a PBS wash and blocking, a panel of mAbs were titrated against each antigen. Alkaline phosphatase anti-Fc conjugates (Accurate, Westbury, NY) and solubilized SigmaFAST p-nitrophenyl phosphate tablets (Sigma) were then used to detect binding. Plates were read at 405nm.
VLP Gag p24 doses were determined in reference to a recombinant p24 standard by reducing SDS-PAGE-Western blots that were probed first by HIVIG diluted to 1µg/ml in PBS containing 2% nonfat milk, then by an alkaline phosphatase-labeled anti-human Fc conjugate (Jackson) and finally developed using SigmaFast BCIP/NBT (5-Bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium) substrate (Sigma). Band densities were quantified using NIH Image software.
Serum analysis
ELISA
a) Gp120, gp41 and bald-VLPs
Immulon II plates were coated overnight at 4°C with recombinant gp120 or gp41 at 5µg/ml. Bald-VLPs were coated at 20x their concentration in transfection supernatants. In some experiments, bald-VLPs were protease-digested (Tong et al., 2012). Species-matched anti-Fc conjugates were used to detect serum binding. The serum dilution resulting in an OD of 0.5 (~5 times background) was recorded as its titer. Each assay was performed at least three times.
b) Binding specificity
In competitive VLP ELISAs (Tong et al., 2013), pooled serum groups at a 1:20 dilution were used to inhibit the binding of graded concentrations of biotinylated mAbs to VLPs coated at 20× on ELISA wells. A prebleed was used as a control competitor. MAbs were biotinylated using NHS-X-biotin reagent (Calbiochem). Soluble CD4 was used at 2µg/ml. Biotinylated mAb binding was detected using streptavidin-alkaline phosphatase (Vector, Burlingame, CA). Competition was expressed as a percentage relative to biotinylated mAb binding in the presence of the prebleed control.
Neutralization assays
Neutralization assays were performed at least 3 times in duplicate to ensure consistency.
a) CF2.CD4.CCR5 assays
Virus was incubated with graded dilutions of antibody for 1 h at 37°C and then added to CD4.CD4.CCR5 cells (CF2 for short), incubated for 2h at 37°C, after which the medium was changed. For SOS viruses, infection was activated by adding 5mM DTT for 5 minutes, followed by a PBS wash and the addition of fresh medium. This is termed the “wash out” format. After 3 days, luciferase activity was measured in cell lysates, as described previously (Crooks et al., 2005).
b) TZM-bl assays
Two versions of a neutralization assay using human cervical HeLa-derived TZM-bl cells (CD4 and CCR5 positive) were used: 1) a standard or “leave in” format (Li et al., 2005), in which trypsinized TZM-bl cells were added to virus-antibody mixtures and left for 3 days without a change of medium and 2) a "wash out" format in which virus-antibody mixtures were added to wells pre-seeded with TZM-bl cells and the medium was changed after a 2h incubation at 37°C. Three days later, luciferase activity was measured in lysates.
c) Gp120 interference
Monomeric JR-FL gp120 D368R was made by transfecting 293T cells with a PPI4-based plasmid and were purified over Galanthus nivalis-agarose then added to neutralization assays at 10µg/ml.
Blue Native PAGE (BN-PAGE) shift assays
BN-PAGE “shift” assays were used to measure the ability of antibodies to bind VLP Env (Crooks et al., 2008; Crooks et al., 2007; Crooks et al., 2005; Moore et al., 2006). Briefly, VLPs were incubated with antibody for 1h at 37°C, then washed with PBS, solubilized in 0.12% Triton X-100, loaded onto a 4–12% Bis-Tris NuPAGE gel (Life Technologies, Inc.) and separated at 4°C for 3 hours at 100V. Ferritin (GE Healthcare) was used as a size standard. Gels were blotted onto polyvinylidene difluoride membranes, destained, immersed in blocking buffer (4% nonfat milk in PBS) and probed by mAbs 2G12, b12, 39F, 2F5 and 4E10 at 1µg/ml each. When WT-VLPs were used, this mAb cocktail was supplemented with mAbs 7B2 and 2.2B. Blots were then probed by an anti-human Fc alkaline phosphatase conjugate (Jackson) and developed using SigmaFast BCIP/NBT substrate (Sigma). Binding was then assessed by the loss of the unliganded trimer density as it forms complexes with nAbs, using NIH Image software.
Flow cytometry
293T cells were incubated with sera for 1h at RT. FITC-labeled species-specific anti-Fc conjugates (Jackson) were used for detection.
RESULTS
Our previous VLP immunogenicity study in guinea pigs (group G1 in Fig. 2 (Crooks et al., 2007)) resulted in weak and sporadic tier 2 neutralization of the JR-FL index virus. Here, we sought to identify areas for improvement. Since our VLPs express native Env trimers on their surfaces (Crooks et al., 2007; Crooks et al., 2005; Moore et al., 2006; Tong et al., 2012), we suggest that they should be capable of consistently eliciting nAbs. A multitude of factors could, however, limit the native trimer's immunogenicity, including an effective dose, adjuvant, stability, immunogenic interference and the competency of model species' immunoglobulin germline repertoires to recognize this difficult antigen.
A thorough investigation of the above parameters would require large numbers of animals. Since our resources were limited, we used a shotgun approach to investigate a wide cross-section of variables to try to partition those associated with nAb development (Fig. 1). Thus, we immunized a further group of guinea pigs (G2), three groups of macaques (M1, M2 and M3) and two groups of rabbits (R1 and R2). Immunization studies were performed consecutively over a number of years in the sequence shown in Fig. 2, with the exception that the M3 macaques were immunized most recently. As information accumulated, we built implicit improvements and tested new parameters in each successive study. Thus, IgG masking strategies differed between studies, as did adjuvants and other conditions. Although our resources restricted us to only n=3 animals per group for guinea pigs and n=2 for macaques, we were able to use n=4 animals in rabbits and a thorough, harmonized serum analysis enabled us to mine data for useful leads (Fig. 1).
Guinea pig VLP immunizations
Overview
A major goal of our G2 guinea pig immunization study was to use IgG masking to prevent putative immunogenic interference by non-functional Env on VLP surfaces (Fig. 1). Two protein A-purified IgGs were isolated from the pooled sera of guinea pigs that were immunized with SOS-VLPs (animals S9–S12 and P13–P15) or bald-VLPs (animals P16–P18) in our previous G1 study ((Fig. 2; (Crooks et al., 2007)). VLP doses were estimated as described in Fig. S1, S2. As shown in Fig. 2, G2 groups were stratified to receive VLPs either alone or mixed with either of the two masking IgGs above. One group (group V) received only SOS-VLP IgG (no VLPs) as a control to monitor its decay.
At the time of the G2 study, we were concerned that the detergent in Ribi adjuvant might destabilize VLPs and/or Env trimers. To avoid this possibility, most G2 animals received VLPs and Ribi at separate sites (Fig. 2, groups G2 II–V), in the hope that Ribi's immune stimulatory effect alone would be sufficient (i.e. with no depot effect).
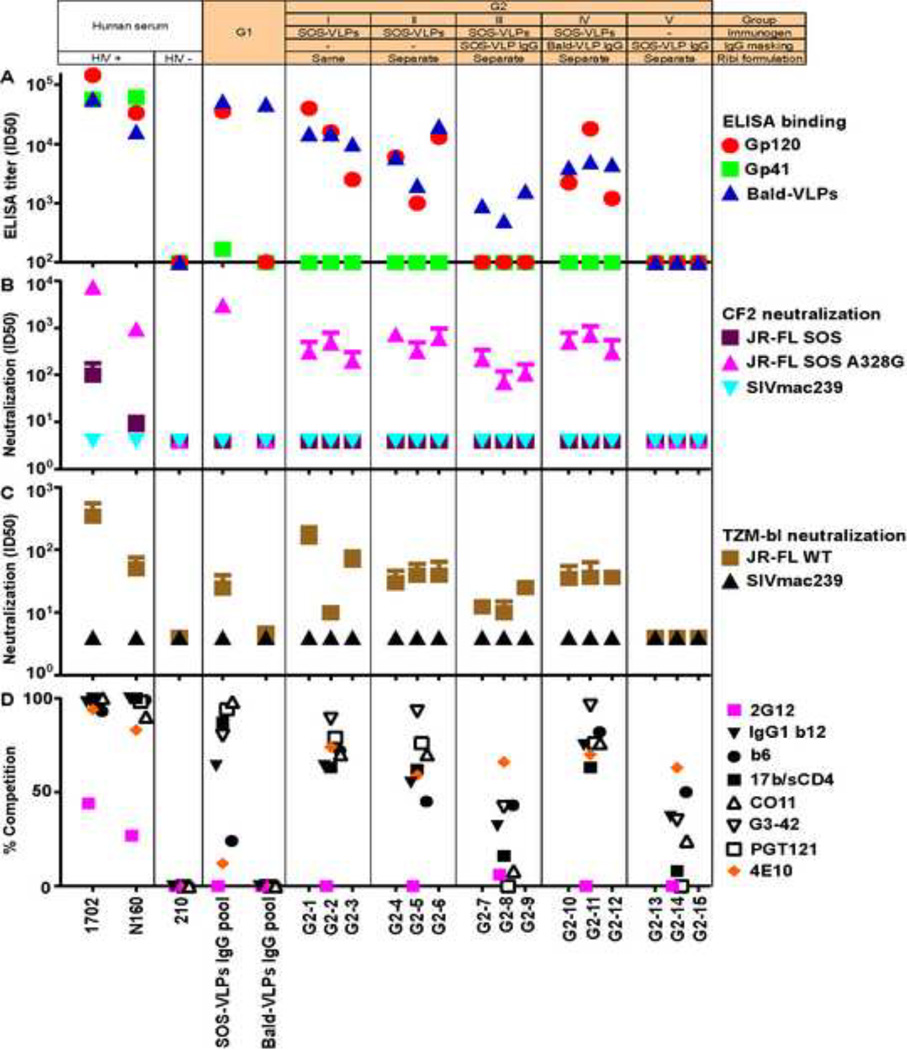
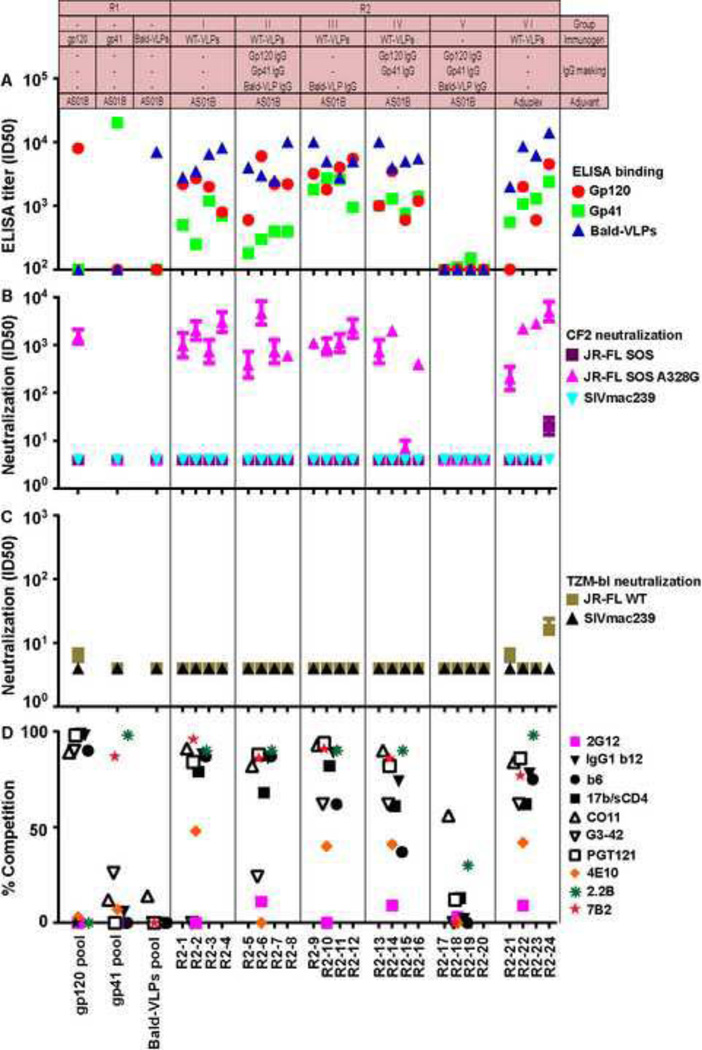
Fig. 3 shows a composite analysis of the two G1 masking IgGs, G2 sera, two human HIV-1-infected donor (HIV+) sera (1702 and N160) and one HIV-1-seronegative serum (210). Fig. 3A shows ELISA binding to various antigens, Fig. 3B and C show their neutralizing activities and Fig. 3D shows competition with various biotinylated mAbs in ELISA.
Fig. 3. Profile of purified G1 IgGs and G2 sera.
A) ELISA titers using recombinant JR-FL gp120, gp41 and bald-VLPs. Purified IgGs from pooled mixtures of SOS-VLP (animals S9–S12) or bald-VLP (animals P13–P15) immune sera from our earlier G1 study are shown (Crooks et al., 2007). Data points are the arithmetic means of at least two repeats. B) Neutralization in the CF2 assay (Crooks et al., 2007), against the SOS parent virus, a "globally sensitive" SOS A328G mutant and SIVmac239 control virus, using a "wash out" format. C) Neutralization in the TZM-bl assay against JR-FL WT and SIVmac239 viruses, using a "leave in" format. All neutralization assays were performed at least three times in duplicate. Arithmetic mean titers and standard deviations are shown. D) Ability of pooled IgGs (0.5µg/ml) and pooled group sera (1:20 dilution) to inhibit the binding of various biotinylated mAbs to UNC SOS-VLPs in ELISA. Data are expressed as percentages, via the calculation: {(IC50 of biotin-mAb binding in the presence of control prebleed serum)/(IC50 of biotin-mAb binding in the presence of competitor serum)} × 100. Each assay was repeated twice and showed similar competition trends.
Guinea pig serum binding to JR-FL gp120, gp41 and bald-VLPs
HIV-1 donor sera 1702 and N160 bound strongly to gp120, gp41 and bald-VLPs (Fig. 3A). However, the uninfected donor serum 210 recognized none of these antigens. The G1 SOS-VLP IgG pool bound strongly to gp120 and bald-VLPs, but not gp41, presumably because major gp41 immunodominant epitopes are not exposed on SOS-VLPs (Tong et al., 2013). The G1 bald-VLP IgG only reacted with bald-VLPs, as expected (Fig. 3A). Most of the G2 sera bound somewhat more weakly to gp120 and bald-VLPs than the G1 SOS-VLP IgG pool, perhaps due to differences in dose and/or adjuvants (Fig. 2). The marginal differences in binding titers between groups G2-I and II (Fig. 3A), suggest that adjuvant co-formulation was not crucial in this study. The lack of detectable binding in the G2-V animals suggests that SOS-VLP IgG was diluted and/or decayed to near background levels and therefore would not be expected to contribute to the titers of responses generated in groups G2-III and IV. In fact, SOS-VLP IgG masking completely blocked the development of new gp120 responses and partially blocked bald-VLP responses of group G2-III. Surprisingly, bald-VLP IgG masking had less impact on titers of group G2-IV. HIV-1-infected donor sera and VLP sera reacted strongly with 293T producer cells (Fig. S3A), apparently due in large part to lipid reactivity (Fig. S3B).
Guinea pig serum neutralization
In Fig. 3B and C, we used various assays to detect nAbs. An appraisal of JR-FL WT and SOS virus neutralization assays using human TZM-bl and canine CF2 cells is shown in Figs S4, S5. Briefly, JR-FL SOS and WT viruses exhibit similar nAb sensitivities (Fig. S5; (Crooks et al., 2005)). However, the TZM-bl assay is ~5 fold more sensitive, largely because of a "leave in" protocol rather than the "wash out" protocol used in the CF2 assay (Fig. S4). The A328G mutation renders the JR-FL isolate into a phenotype we term "globally neutralization sensitive" (Fig. S5).
The various assays used in Fig. 3B and C provide a range of sensitivities. The high sensitivity of the WT/TZM-bl format (Fig. 3C) is reflected by the relatively high titers of HIV+ sera 1702 and N160 (compare Figs 3B and C) and mAbs (Fig. S4A). WT/TZM-bl activity was detected using the G1 SOS-VLP IgG pool and by all the G2 VLP sera, except the G2-V control group (Fig. 3C). However, none of the VLP sera neutralized the JR-FL SOS virus in a more stringent washout format using CF2 target cells (Fig. 3B). Purified G2 serum IgG exhibited nAb activity equivalent to the parent sera, suggesting that the nAb activities in Fig. 3B and C are IgG-mediated (data not shown). None of the sera neutralized the SIVmac239 virus, thus ruling out non-specific activity (Fig. 3B, C). In our previous report, bald-VLP sera exhibited some non-specific nAb activity (Crooks et al., 2007). However, in the current study, IgG purification seems to have eliminated this problem (Fig. 3B, C). As expected, all the G2 sera neutralized the globally sensitive JR-FL mutant A328G mutant (Fig. 3B). The G1 SOS-VLP IgG pool exhibited a higher titer, perhaps due to the use of a higher dose and/or different adjuvants (Fig. 2).
A comparison of groups G2-I and G2-II suggests that adjuvant co-formulation is not necessary (Fig. 3B, C), reflecting the similarly comparable binding responses (Fig. 3A). SOS-VLP IgG masking led to lower nAb titers in G2-III sera, perhaps because the weak nAbs present in the G1 SOS-VLP masking IgG (Fig. 3C) partially blocked de novo nAb development, just as they blocked gp120 binding titers (Fig. 3A). In contrast, bald-VLP masking had little effect (Fig. 3B, C). Representative neutralization assay titrations of serum G2–12 (Fig. S6A) reveal dose-dependent neutralization in both the WT/TZM-bl and A328G/CF2 assays. Weak, non-specific background activity against SIV control viruses (Fig. S6A) was a common characteristic of sera from this model species.
Guinea pig serum binding to native JR-FL Env trimer
Previously, we showed that native trimer binding in BN-PAGE shifts tracks with neutralization (Binley et al., 2010; Binley et al., 2008; Crooks et al., 2008; Crooks et al., 2007; Crooks et al., 2005; Crooks et al., 2011; Kang et al., 2009; Moore et al., 2006; Tong et al., 2012; Vaine et al., 2008). In Fig. S7A, some G2 sera, particularly the potent G2-1 serum, bound to the native Env trimer. Pooled G2 group sera also bound convincingly to globally sensitive JR-FL A328G Env trimers (Fig. 3B), as expected considering the potent neutralization of this virus (Fig. S7B).
Guinea pig serum mapping
Recently, we investigated mAb-mAb binding relationships on VLP Env by ELISA (Tong et al., 2013), providing reference information to interpret the competitive activities of our VLP sera in the same assay. In Fig. 3D, each point represents serum competition of a given mAb that may indicate either epitope overlap or a conformational binding relationship. For simplicity, however, we infer competitions to indicate overlaps.
Some symbols in Fig. 3D are coded in black or white to indicate epitope clusters. The black symbols identify those that overlap the CD4 and/or coreceptor binding sites (receptor cluster). The white symbols identify those incorporating elements of the V3 loop and N332 "super site" (V3 cluster) (Kong et al., 2013). Although 2G12 binding is N332-dependent, its specificity is unique, so it was excluded from the latter cluster. 4E10 was used to represent MPER mAbs.
Both HIV-1 donor sera (1702 and N160) competed quite effectively with both the receptor and V3 clusters (Fig. 3D). Our previous study suggests that competition of mAbs CO11, G3–42 and PGT121 but not 2G12 is best explained by V3 specificities (see Fig. 8B in ref. (Tong et al., 2013)). 4E10-overlapping responses were also strong. However, neither the 210 serum nor the G1 bald-VLP IgG pool competed with any of the mAbs, confirming the specificity of this assay.
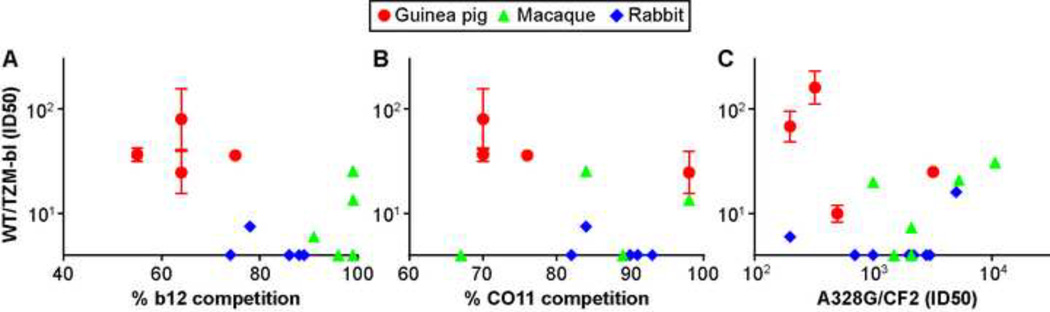
Fig. 8. Relationship of serum specificity and neutralization titers.
Serum pool competition of A) b12 and B) CO11 mAbs was correlated with WT/TZMbl ID50s. C) Mean serum group A328G/CF2 ID50s were correlated with WT/TZM-bl ID50s. Animals receiving IgG masking only (i.e. G2-V and R2-V) were excluded from this analysis.
The G1 SOS-VLP pooled IgG competed effectively with the V3 cluster. However, competition with the receptor cluster mAbs was somewhat weaker and little or no competition with either of mAbs 2G12 or 4E10 was observed (Fig. 3D; (Crooks et al., 2007; Derby et al., 2006). The G2-I, II and IV pooled sera showed somewhat weaker competition than the G1 SOS-VLP pool (Fig. 3D). G2-III and V serum competition was weak but still measureable (Fig. 3D), in keeping with their weak binding (Fig. 3A). It may be inferred, therefore, that the SOS-VLP masking IgG did not completely decay in these animals. Unexpectedly, all G2 sera competed quite well with mAb 4E10, but the G1 SOS-VLPs IgG pool did not.
Macaque VLP immunizations
Overview
VLP immunogenicity was evaluated in 10 rhesus macaques, consisting of 2 groups of 4 animals, M1 and M2 and a third group, M3, consisting of 2 animals (Fig. 2). Although cost and availability precluded our use of greater animal numbers, we hoped to still identify factors that partitioned with nAb development.
Macaque serum binding to JR-FL gp120, gp41 and bald-VLPs
At the time of the M1 study, we were still concerned about possible adverse effects of adjuvant detergent, so we administered VLPs and Ribi at separate sites. We initially compared the immunogenicities of WT and SOS Envs. Three of the 4 M1 sera recognized monomeric gp120 and bald-VLPs in ELISA, albeit at modest titers (Fig. 4A). Gp41 titers were undetectable, except for serum DV08, that lacked detectable gp120 binding, but recognized gp41, albeit weakly (Fig. 3A). This serum also did not bind to bald-VLPs and may therefore be considered as a non-responder.
In the M2 study, SOS-VLPs were co-formulated with Ribi. For group M2-II, VLPs were also mixed with protein A-purified IgG from SOS-VLP-immune M1 sera EN36 and EN37 (Fig. 2). Mean gp120 and bald-VLP titers in the M2 study were higher than in the M1 study. M2 sera also exhibited somewhat better binding to 293T cells (Fig. S3A). However, IgG masking had little effect on serum reactivity with gp120, bald-VLPs or 293T cells, perhaps because of the weak antibody titers of the M1 sera that were used to make the masking IgG (Fig. 4A, Fig. S3A).
In the M3 study, two macaques were immunized with protease digested SOS-VLPs formulated in AS01B. The protease treatment selectively clears non-functional Env, leaving the native Env trimer intact (Crooks et al., 2011; Tong et al., 2012). The VLP dose in M3 animals (Fig. 2) was normalized to adjust for the modest overall loss in mAb binding after protease digests (Tong et al., 2012). Gp120 and bald-VLP titers were higher in the M3 sera than any other group (Fig. 4A).
Macaque serum neutralization
In the WT/TZM-bl format, 5 of the 10 macaque sera exhibited detectable neutralization (Fig. 4C). Serum 5514 was the most potent and was also the only one to detectably neutralize in the more stringent SOS/CF2 neutralization assay (Fig. 4B). As expected, all 10 macaque sera neutralized in the A328G/CF2 format (Fig. 4B), the 5514 serum yet again being the most potent. None of the sera neutralized the control SIVmac239 virus. A sample neutralization titration of serum CM84 is shown in Fig. S6B. Notably, all 4 sera from macaques that received VLPs that were either masked by SOS-VLP IgG (M2–II) or protease digested (M3) exhibited detectable nAbs in the WT/TZM-bl format, suggesting that these strategies that were intended to refocus responses on the native trimer may in fact be effective. The lack of nAbs in the EN36 and EN37 sera used to make the masking IgG may have been an important factor in this partial success. The CM84 and CF48 sera both bound to the native trimer binding in BN-PAGE (Fig. S8 and supplemental text), as did both M3 sera (data not shown), consistent with these nAb activities.
Macaque serum specificity mapping
All 10 macaque sera strongly competed with both the receptor and V3 clusters, in many cases to saturation (Fig. 4D). Competition against V3 mAb CO11 was, however, relatively weak in the M1 sera. Unlike the guinea pig sera, the M2 sera, particularly subgroup II, but not the M3 sera, competed with mAb 2G12. 4E10-competing activity was present in all sera, particularly in the M3 animals, despite the undetectable gp41 binding by ELISA (Fig. 4A).
Rabbit VLP immunizations
Rabbit study R1 serum analysis
At the time we initiated rabbit immunogenicity studies, we had not yet developed protease digests as a means to eliminate non-functional Env (Crooks et al., 2011) and IgG masking was still our principle strategy for dealing with antigenic interference. To try to optimize IgG masking, we considered the results of the IgG masking attempts described above, which suggested that it may be effective (M2 study), as long as the masking IgG is not neutralizing (as in the G2 study). In an attempt to comprehensively conceal non-neutralizing Env epitopes, while minimizing nAb elicitation, we immunized R1 rabbits with recombinant gp120 monomer and gp41 in two groups of six animals. A further six animals were immunized with bald-VLPs. All 3 immunogens were co-formulated with AS01B (Fig. 2). Each R1 serum pool exhibited high binding titers to their respective immunogens (Fig. 5A). The neutralizing activity of the gp41 and bald-VLP serum pools was undetectable (Fig. 5B, C). However, the R1 gp120 serum pool neutralized weakly in the WT/TZM-bl and strongly in the A328G/CF2 format (Fig. 5B, C).
Fig. 5. Profile of R1 and R2 sera.
Rabbit sera were analyzed as in Figs 3 and 4, except that UNC WT-VLPs were used for competition mapping instead of UNC SOS-VLPs in part D.
The R1 gp120 serum pool overlapped with the receptor and V3 clusters, but not with 2G12 or 4E10 (Fig. 5D). The gp41 serum pool overlapped the 7B2 and 2.2B epitopes (gp41 clusters I and II, respectively), but not 4E10. As expected, the bald-VLP serum pool did not appreciably compete with any of the mAbs. In BN-PAGE shifts, the R1 gp120 serum pool recognized the gp160 monomer and the gp41 serum pool bound the gp41 stumps. However, none of the serum pools appeared to bind the native Env trimer (Fig. S9A).
Rabbit study R2 immunizations
R1 pooled IgGs were purified over protein A and were then mixed with WT-VLP immunogens used for R2 rabbits (Fig. 2). A control group (R2-V) received only masking IgG (no VLPs). AS01B was used in groups I–V. Group VI received WT-VLPs co-formulated with Adjuplex. An interim check of masking efficiency after the second R2 immunization by BN-PAGE suggested that it was at least partly effective (Fig. S9B).
Rabbit (R2) serum binding to gp120, gp41 and bald-VLPs
R2-I group sera exhibited lower mean gp120 and gp41 binding titers than the R1 gp120 and gp41 serum pools (Fig. 5A). This was not unexpected, given the lower antigenicity of VLPs Env compared to soluble gp120 (Tong et al., 2013). In contrast, the bald-VLP titers in group R2-I sera were similar to that of R1 bald-VLP serum pool. Similar gp120 and bald-VLP mean ID50s in R2 groups I and II were observed, although gp41 titers were slightly lower in group II, consistent with some gp41 masking (Fig. 5A and S9B). Group R2-III sera exhibited modestly higher gp120 and gp41 titers that may have been augmented by bald-VLP IgG complexing. Bald-VLP ID50s were similar, however, suggesting that masking was incomplete. R2-IV serum gp120, gp41 and bald-VLP mean ID50s were similar to those of R2-I, despite IgG masking. As expected, sera from R2-V animals that received only masking IgG did not bind any of the antigens effectively. The R2-VI animals that received WT-VLPs in Adjuplex exhibited highly variable titers in individual animals. In two cases, R2–22 and 24, gp120 titers were similar to those of the R2-I control group, but in the other cases (R2–21 and 23), the titers were substantially lower. Despite the apparent group-specific differences observed above, Mann-Whitney tests did not suggest any statistical significance.
Rabbit (R2) serum neutralization
Most of the R2 sera exhibited no neutralization in either the SOS/CF2 or WT/TZM-bl assays (Fig. 5B, C). Only two sera in subgroup VI (animals 21 and 24) exhibited detectable nAbs. In particular, serum R2–24 neutralized in both CF2 and TZM-bl assays (Fig. S6C). It may not be a coincidence that this serum also strongly bound all 3 antigens by ELISA (Fig. 5A). In contrast, serum R2–21 exhibited undetectable gp120 binding (Fig. 5A), yet neutralized in the TZM/bl assay (Fig. 5C).
Most R2 sera exhibited A328G/CF2 titers of ~1:1,000, with no obvious differences between groups (Fig. 5B). However, serum R2–15 did not neutralize this virus, suggesting that it must target epitopes that are unusually absent on these globally sensitive trimers. None of the sera neutralized the SIVmac239 virus. We did not detect any trimer binding by the R2 sera in BN-PAGE shifts, except for serum R2–24 (not shown). Further BN-PAGE shifts revealed that IgG masking did not block the development of new responses to UNC gp160 (Fig. S9C, D).
Rabbit (R2) specificity mapping
Mapping of R2 serum group pools by ELISA (Fig. 5D) revealed receptor and V3 cluster overlap by all VLP sera. Anti-gp120 masking led to somewhat weaker gp120 mAb competitions (Fig. 5D; group IV sera and to some extent the group II). 7B2 and 2.2B competition was consistently high (Fig. 5D), regardless of gp41 IgG masking or gp41 binding differences (Fig. 5A). All sera except those of groups II and V competed with 4E10. Little or no 2G12 competition was detected. The somewhat weaker overall competition by group VI serum pool than the group I serum pool may be due to the different adjuvants used and/or to the weak responses in some group VI sera (i.e animals 21 and 23).
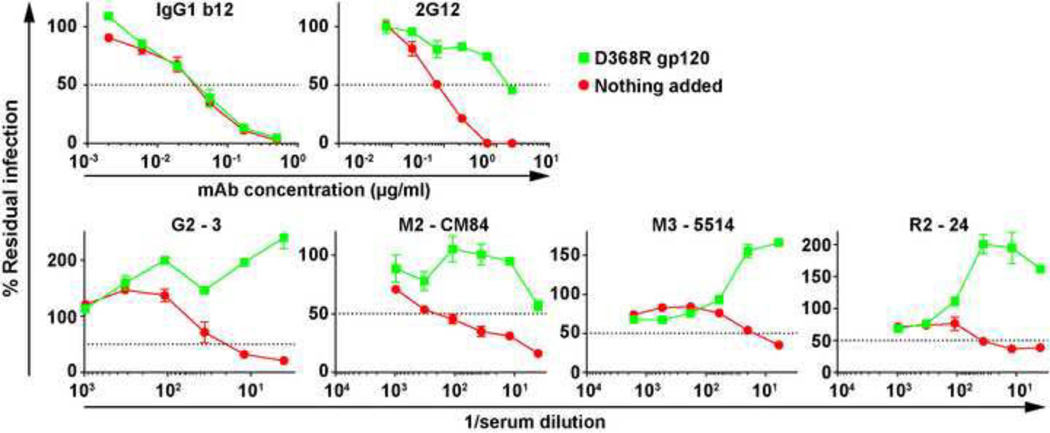
Neutralization interference by monomeric JR-FL gp120 D368R
We next investigated the susceptibility of nAbs in our VLP sera to soluble gp120 D368R monomer interference. This mutant gp120 does not bind cellular CD4 and therefore does not interfere with infection. Fig. 6 shows that mAb 2G12 neutralization was blocked by this gp120, but mAb b12 was unaffected because it does not bind to the D368R mutant. The neutralizing activities of sera from all 3 species were blocked by gp120 monomer. Thus, nAbs in these sera recognize epitopes that are present on the gp120 monomer and are impartial to the D368R mutation.
Fig. 6. Neutralization interference by monomeric JR-FL gp120 D368R.
Neutralization assays were performed using the control mAbs b12 and 2G12 and the sera indicated, in the presence or absence of added JR-FL gp120 D368R at an approximate concentration of 10µg/ml. The WT/TZM-bl assay was used in each case except for serum 5514, in which the SOS/CF2 assay was used.
Inter-species VLP serum meta-analysis
To try to determine any useful patterns from the above studies, we used a meta-analysis to mine our data. It is important to first acknowledge the caveat that this was a cross-sectional study in which we used different VLP doses, different adjuvants, with or without co-formulation, and various forms of IgG masking. Nevertheless, by comparing two variables at a time and ignoring all others, we hoped to uncover the most important limitations of our vaccine regimen.
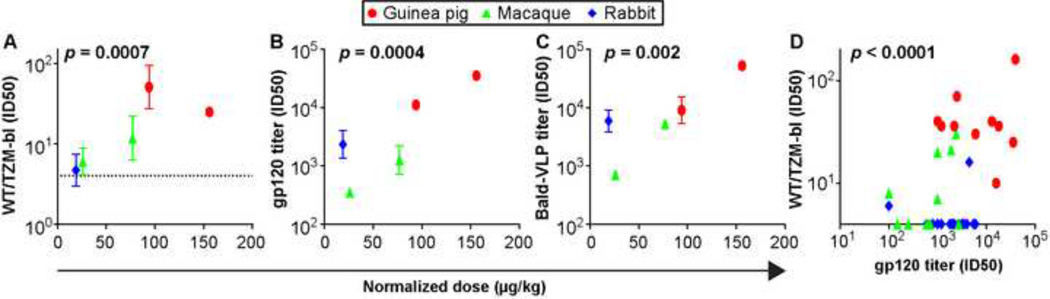
We first compared normalized VLP dose (Fig. 2) with mean nAb titers of VLP immune sera from all 3 species (Fig. 7A). The trend in Fig. 7A suggests higher normalized VLP doses correlates with higher nAb titers. Higher VLP doses also correlated with higher gp120 and bald-VLP binding titers (Fig. 7B and C). Furthermore, higher gp120 binding titers correlated with higher WT/TZM-bl titers (Fig. 7D). Overall, these data suggest that neither the binding nor the neutralizing responses had reached an upper threshold, due to our use of insufficient VLP doses. These correlations appear to be driven by the increased frequency and titer of nAbs in the macaques and guinea pigs that received higher normalized doses than the rabbits.
Fig. 7. Relationship of normalized VLP dose with binding and neutralizing titers.
The normalized VLP dose per animal mass was compared in a scatterplot against A) WT neutralization titer in TZM-bl assay; B) gp120 binding titer in ELISA and C) bald VLP binding titer in ELISA. D) A scatterplot to show the correlative effect of gp120 binding titer in all immunized animals and their respective WT neutralization titer by TZM-bl assay. Animals receiving IgG masking only (i.e. G2-V and R2-V) and the SOS-VLP IgG masked group G2-III were excluded from this analysis.
We next compared mean WT/TZM-bl ID50s and serum competition of mAbs IgG1 b12 (Fig. 8A) and CO11 (Fig. 8B). The b12 and CO11 mAbs represent the receptor and V3 super site epitope clusters (see Fig. 7B of (Tong et al., 2013)). Despite their high gp120 binding and WT/TZM-bl neutralization titers (Fig. 7D), guinea pig sera competed weakly with both b12 (Fig. 8A) and CO11 compared to the sera from other model species (Fig. 8B). Guinea pig sera also exhibited relatively low A328G/CF2 titers (Fig. 8C), suggesting poor reactivity with known epitopes (most known mAbs to conserved epitopes neutralize the A328G mutant effectively). Overall, this suggests that guinea pig sera preferentially bind non-conserved epitopes that do not overlap the receptor or V3 supersite clusters. Thus, guinea pigs may not be ideal models for future attempts to induce nAbs against conserved epitopes.
Finally, we examined the effect of the adjuvant used in immunizations on serum nAb titers (Fig. S10). The only significant difference was between the Ribi and AS01B groups. However, this may well be due to the relatively high normlaized dose used in guinea pigs. Overall, we suggest that the adjuvant was not a limiting factor in the current study and that the use of higher VLP doses in macaques or rabbit models may in future be able to elicit strong nAbs to conserved epitopes.
DISCUSSION
We hypothesize that VLPs expressing native HIV-1 Env trimers may be able to induce nAbs. However, one or more undefined factors may have so far limited their efficacy as nAb-inducing vaccines. These factors may include, but are not limited to, immune interference by non-functional Env, uncertainties regarding the effective dose, a need for an optimal adjuvant to compensate for the inherently poor immunogenicity of the native Env trimer, uncertainties regarding VLP stability in adjuvant formulations, and inherent differences in model species to mount nAb responses or to recognize conserved Env epitopes. A fundamental and underappreciated problem in vaccine studies to date is that autologous, vaccine-matched tier 2 nAbs tend to develop inconsistently only in a subset of vaccinated animals. In many cases, the underlying cause appears to be that the immunogen insufficiently resembles the native Env trimer and therefore largely elicits "off target" responses. While this problem may be intractable for many immunogens, it is eliminated for VLP immunogens because any antibodies generated to the native trimer on their surfaces should also neutralize. The present study tracks our attempts over several years to identify any factor(s) that might limit the immunogenicity of native trimers.
To accelerate the discovery process, we attempted to cover all of the most likely factors (Fig. 1). However, a principle drawback was that we were prevented from investigating all the parameters comprehensively and with statistical strength both by our limited access to large animal numbers and by our VLP production capacity. We therefore evaluated a large cross-section of scenarios in the hope of mining data to identify leads to prioritize in future, statistically powered VLP immunogenicity studies. Our approach in each animal group (Fig. 2) was based on our best ideas and information available at the time of their initiation. Although each study was self-inclusive and was not designed expressly for the inter-study comparisons we undertook here, we used a consistent algorithm to investigate all sera in the hope of making some general observations. Despite the obvious drawbacks of our small group numbers, we felt that this was a worthwhile compromise compared to the alternative of evaluating few variables in larger numbers of animals. A case in point is the general failure of our R2 rabbit VLP immunizations, despite our use of n=4 animals per group, probably because our production capacity led us to use an insufficient VLP dose.
Consistent with our previous study (Crooks et al., 2007), VLP immune sera, at best, only modestly neutralized the tier 2 JR-FL autologous virus and did not neutralize other tier 2 isolates (not shown). Nevertheless, we were able to identify some useful leads, as follows. First, a post-hoc analysis unexpectedly suggested that higher VLP doses correlate with higher nAb titers (Fig. 7). This suggests that we may not have reached a threshold beyond which higher doses would not induce more robust nAb titers. VLP dose estimations are complicated by the fact that Env is only a minor component. Furthermore, given the strong binding responses we observed in our previous study (Crooks et al., 2007), we did not anticipate dose as a possible limiting factor. Therefore the present studies were not designed formally to test dose response.
The observations in Fig. 7 add to our growing realization that immunogenicity might be governed by antigenic accessibility. Since membrane-expressed Env is far less accessible to antibody binding than soluble forms of Env like the gp120 monomer (Ota et al., 2012; Tong et al., 2013), logically, it may also be less immunogenic. Therefore, the common perception that particulate antigens might have immunogenic advantages over their soluble counterparts must be balanced by the reality that the forms of Env presented on membranes are far more compact and therefore probably less immunogenic. Thus, in Fig. 3, while the VLP doses used may have been sufficient to elicit binding responses, presumably against the moderately accessible non-functional Env antigens on VLP membranes, higher doses might be necessary to trigger responses to the more compact native trimer. We are now evaluating various methods to improve VLP yield and native trimer incorporation. This should facilitate an exploration of the ability of higher VLP doses to induce nAbs. A limitation may be that even with higher doses, IgG germline repertoires may limit the frequency in which nAbs can be elicited. It may therefore be useful to remove key glycans from the trimer to increase their accessibility to germline precursors of broad nAbs (Hoot et al., 2013; McGuire et al., 2013).
A second lead was that nAb development may be favored by eliminating responses to non-functional Env. This was apparent in two pairs of macaques that received either IgG masked VLPs or protease digested "trimer VLPs", all of which developed modest nAbs (Fig. 4). IgG masking was not, however, effective in either guinea pigs or rabbits. In both cases, the presence of nAbs in the masking IgG could explain this failure. In the rabbits, this problem may have been compounded by incomplete IgG masking and the relatively low VLP dose. Our IgG masking strategy was launched as the only practical approach to address the perceived problem of non-functional Env interference at the time these studies were initiated. However, in practice, it proved to be rather labor intensive and difficult to control. In some cases, masking may have been insufficient, especially after immunization, whereupon the masking IgG becomes substantially diluted. Nevertheless, SOS-VLP IgG masking completely prevented the development of binding responses in guinea pigs (Fig. 3A), despite not resulting in nAb development in this instance. IgG masking efficiency could possibly be improved by tailoring it to specifically mask only non-functional Env, perhaps using VLPs that express only these forms of Env (e.g. UNC-VLPs) in the first round of immunizations. Masking of non-Env VLP components by bald-VLP IgG had negligible impact on nAb responses, suggesting that these responses are independently regulated.
Protease digested "trimer VLPs" provided a far more convenient solution for non-functional Env interference. This idea emerged only recently and was therefore only tested in 2 macaques here (group M3). The fact that both M3 macaques developed modest titers was promising. However, we can not rule out the possible benefits of the expanded immunization schedule and different adjuvant used distinctly in the M3 group.
A third lead was that guinea pigs may not have great long term potential as small animal vaccine models. Although detectable nAbs were prevalent in guinea pig sera (Fig. 3C), this may be largely a reflection of the relatively high normalized VLP dose they received. A major concern was that guinea pig sera exhibited poor competition with conserved CD4bs and V3 epitopes (Fig. 3D and Fig. 8A, B), which was mirrored by their similarly weak A328G/CF2 ID50s (Fig. 8C) and suggests a likely preoccupation with type-specific epitopes. A further problem was a greater tendency for non-specific neutralization by guinea pig sera, as exemplified by the effect of the G2–12 serum on SIVmac239 in the TZM-bl assay (Fig. S6A). Rabbits may be preferable as small animal models (Pan et al., 2013; Pinheiro et al., 2011) because they lack this serum background problem and also react better with conserved targets that may ultimately be important for the development of nAbs of any breadth. However, it is important to point out that the VH family diversity in both rabbits and guinea pigs is quite limited compared to primates and that their antibody diversity is generated principally by gene conversion, contrasting with the largely combinatorial origins of IgG diversity in primates (somatic hypermutation is also used in all cases). Thus, the germline prototypes of broad nAbs identified in HIV+ humans are unlikely to be relevant in these small animal models, that will inevitably need to develop species-specific solutions for generating antibodies that react with the native trimer.
The effects of other immunization factors were less clear (Fig. 1), perhaps because of the overriding effects of dose and non-functional Env interference. Once the current limitations are addressed, these other factors may be worth further study. For example, contemporary adjuvants such as AS01B and Adjuplex may be more effective than Ribi. Our concerns at the time of our earlier immunogenicity studies here regarding the potential destabilizing effects of Ribi and AS01B were more recently eliminated experimentally (see Fig. 1 of (Crooks et al., 2011)). Adjuplex was, however, difficult to evaluate, because its viscous nature rendered it difficult to separate from VLPs to evaluate what, if any, effects it might have had on their integrity. However, the nAbs observed in 2 of 4 rabbits (Fig. 5C) in which Adjuplex was used suggest that it probably has no adverse effects.
A thorough and conservative approach was important to confirm the modest nAbs in our VLP sera. Thus, neutralization i) should be confirmed in repeat assays, ii) should ideally be detectable in multiple neutralization assay formats, iii) should not neutralize control viruses (e.g. those bearing SIV Env), iv) should vary in potency in a dose-dependent manner with serum dilution, v) should be retained in IgG purified from sera, vi) should ideally be reflected by binding to the native Env trimer in BN-PAGE shift assays (Crooks et al., 2005) and vii) might also be adsorbed by monomeric gp120. Of these approaches, the latter convincingly demonstrated that serum neutralization was specifically adsorbed (Fig. 8). This method also revealed that the neutralization in our VLP sera did not depend either on quaternary Env trimer structure or the D368 residue of the CD4 binding loop. The nAb titers were, however, of insufficient titer for detailed mapping efforts.
Several other assays centered on VLPs were developed to comprehensively monitor the serum responses, to wit: bald-VLP ELISAs, A328G neutralization and mapping studies. These assays have the distinct advantages of matching the immunogens and may be a useful part a comprehensive serum analysis strategy to mine our data.
When neutralization was absent, infection enhancement was sometimes observed, (e.g. Fig. S6B and Fig. 6). SIV enhancement may arise from the reactivity of sera with target cells (Fig. S3A) rather than with virus, which could affect cell growth and/or viability and therefore the infection readout in neutralization assays.
Our observations that serum reactivity to bald VLPs is resistant to protease digests (Fig. S3B) and that protease-digested VLPs also induce high titer bald-VLP responses (Fig. 4A, M3 sera) suggest serum reactivity to membrane lipids. Previously, in PBMC neutralization assays, we observed robust non-specific neutralization by VLP sera (see Fig. 6 in (Crooks et al., 2007)), perhaps because lipid-reactive serum antibodies trigger β-chemokine expression in macrophages that block infection (Brown et al., 2007; Jobe et al., 2012; Moody et al., 2010).
HIV+ donor sera appear to exhibit lipid reactivity similar to the VLP sera (Fig. 3 and Fig. S3B and Fig. 9D of ref. (Crooks et al., 2007)). This challenges the common perception that membrane lipid is immunosilent and raises the question of why these responses develop and whether they are safe. One explanation is provided by the observation that enveloped viruses expose certain phospholipids on their outer membrane leaflets, such as anionic phosphatidyl serine that are normally sequestered on the inner leaflet of host cell membranes (Jobe et al., 2012; Matyas et al., 2010; Soares et al., 2008)(Lorizate et al., 2013). These lipids are flipped "inside out" in a scrambling effect induced by cancer, inflammation, or infection (Sims and Wiedmer, 2001) and commonly induce antibodies as part of the array of early immune responses. The lack of any adverse effects of VLP immunizations and the similar lack of problems with other licensed vaccines that incorporate lipid membranes, such as those for influenza and hepatitis B, suggest a good safety profile. Lipid antibodies are, in fact, benign and ubiquitous in nature (Alving, 2006, 2008; Jobe et al., 2012; Matyas et al., 2010). The expression of these unusual lipids on permanent (i.e. cancerous) cell lines could explain their staining by our VLP sera (Fig. S3A).
The 4E10 epitope overlap by many VLP sera was unexpected (Fig. 3–5), especially given the low gp41 binding titers in guinea pigs and macaques (Fig. 3, 4). The particularly strong 4E10 competition by M3 sera could be partly result of the 10-fold increase in MPER epitope exposure following protease digestion of VLPs (Tong et al., 2012). On the other hand, the gp120 interference of M3 sera suggests that MPER nAbs do not contribution to neutralization (Fig. 6). The possibility that VLP-elicited anti-lipid antibodies non-specifically compete with 4E10 appears to be ruled out because bald-VLP sera do not compete (Fig. 3, 5).
In summary, our shotgun approach to discover factors important for nAb induction by VLP immunogens suggested that higher VLP doses and removing interference by non-functional Env may be worth testing, preferably in rabbit models. Indeed, in studies conducted since the work described here, we have observed potent autologous tier 2 nAbs (WT/TZM-bl titers of ~1:1,000) in two of 8 rabbits immunized with a high dose (>150µg/kg) of protease-digested trimer VLPs. However, little or no nAbs were observed in 4 guinea pigs immunized with a similar high dose of trimer VLPs. Furthermore, no nAbs were observed in 4 rabbits immunized with a high dose of undigested VLPs. These findings appear to validate the leads we identified here. The continued use of our algorithm to characterize VLP sera should allow us to track factors important for further improving nAb responses in the future.
Supplementary Material
Research highlights for VIRO-14-67 manuscript.
Rabbit are animal model superior to guinea pigs in eliciting neutralizing responses against native trimers.
High VLP doses required to elicit neutralizing responses against native trimers.
Protease-treated VLPs to remove non-functional Env improves neutralizing responses.
ACKNOWLEDGEMENTS
This work was supported by grants RO1AI93278, RO1AI58763 and R21AI84714 (JMB; NIH/NIAID/DAIDS) and Center for HIV/AIDS Vaccine Immunology and Immunogen Discovery (CHAVI-ID) grant AI100663-01. We thank Ping Zhu, Carl Alving, and Gary Matyas for advice, and IAVI and the NIH AIDS Reagent Repository for providing mAbs, Clarisse Lorin (Glaxo Pharmaceuticals) for providing AS01B and Emily Carrow (Advanced BioAdjuvants) for providing Adjuplex, Progenics Pharmaceuticals, Inc. for providing gp120 and sCD4 and Scott Conklin at the Pocono Rabbit Farm for assistance with animal immunizations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abdel-Motal UM, Wang S, Awad A, Lu S, Wigglesworth K, Galili U. Increased immunogenicity of HIV-1 p24 and gp120 following immunization with gp120/p24 fusion protein vaccine expressing alpha-gal epitopes. Vaccine. 2010;28:1758–1765. doi: 10.1016/j.vaccine.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal N, Leaman DP, Rowcliffe E, Kinkead H, Nohria R, Akagi J, Bauer K, Du SX, Whalen RG, Burton DR, Zwick MB. Functional stability of unliganded envelope glycoprotein spikes among isolates of human immunodeficiency virus type 1 (HIV-1) PLoS One. 2011;6:e21339. doi: 10.1371/journal.pone.0021339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alving CR. Antibodies to lipids and liposomes: immunology and safety. Journal of liposome research. 2006;16:157–166. doi: 10.1080/08982100600848553. [DOI] [PubMed] [Google Scholar]
- Alving CR. 4E10 and 2F5 monoclonal antibodies: binding specificities to phospholipids, tolerance, and clinical safety issues. AIDS. 2008;22:649–651. doi: 10.1097/QAD.0b013e3282f51922. [DOI] [PubMed] [Google Scholar]
- Binley JM, Ban YE, Crooks ET, Eggink D, Osawa K, Schief WR, Sanders RW. Role of complex carbohydrates in human immunodeficiency virus type 1 infection and resistance to antibody neutralization. Journal of virology. 2010;84:5637–5655. doi: 10.1128/JVI.00105-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binley JM, Cayanan CS, Wiley C, Schulke N, Olson WC, Burton DR. Redox-triggered infection by disulfide-shackled human immunodeficiency virus type 1 pseudovirions. J Virol. 2003;77:5678–5684. doi: 10.1128/JVI.77.10.5678-5684.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binley JM, Lybarger EA, Crooks ET, Seaman MS, Gray E, Davis KL, Decker JM, Wycuff D, Harris L, Hawkins N, Wood B, Nathe C, Richman D, Tomaras GD, Bibollet-Ruche F, Robinson JE, Morris L, Shaw GM, Montefiori DC, Mascola JR. Profiling the specificity of neutralizing antibodies in a large panel of plasmas from patients chronically infected with human immunodeficiency virus type 1 subtypes B and C. Journal of virology. 2008;82:11651–11668. doi: 10.1128/JVI.01762-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binley JM, Sanders RW, Clas B, Schuelke N, Master A, Guo Y, Kajumo F, Anselma DJ, Maddon PJ, Olson WC, Moore JP. A recombinant human immunodeficiency virus type 1 envelope glycoprotein complex stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits is an antigenic mimic of the trimeric virion- associated structure. J Virol. 2000;74:627–643. doi: 10.1128/jvi.74.2.627-643.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binley JM, Sanders RW, Master A, Cayanan CS, Wiley CL, Schiffner L, Travis B, Kuhmann S, Burton DR, Hu SL, Olson WC, Moore JP. Enhancing the proteolytic maturation of human immunodeficiency virus type 1 envelope glycoproteins. J Virol. 2002;76:2606–2616. doi: 10.1128/JVI.76.6.2606-2616.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blish CA, Nguyen MA, Overbaugh J. Enhancing exposure of HIV-1 neutralization epitopes through mutations in gp41. PLoS medicine. 2008;5:e9. doi: 10.1371/journal.pmed.0050009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomsel M, Tudor D, Drillet AS, Alfsen A, Ganor Y, Roger MG, Mouz N, Amacker M, Chalifour A, Diomede L, Devillier G, Cong Z, Wei Q, Gao H, Qin C, Yang GB, Zurbriggen R, Lopalco L, Fleury S. Immunization with HIV-1 gp41 subunit virosomes induces mucosal antibodies protecting nonhuman primates against vaginal SHIV challenges. Immunity. 2011;34:269–280. doi: 10.1016/j.immuni.2011.01.015. [DOI] [PubMed] [Google Scholar]
- Brown BK, Karasavvas N, Beck Z, Matyas GR, Birx DL, Polonis VR, Alving CR. Monoclonal antibodies to phosphatidylinositol phosphate neutralize human immunodeficiency virus type 1: role of phosphate-binding subsites. J Virol. 2007;81:2087–2091. doi: 10.1128/JVI.02011-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonaguro L, Visciano ML, Tornesello ML, Tagliamonte M, Biryahwaho B, Buonaguro FM. Induction of systemic and mucosal cross-clade neutralizing antibodies in BALB/c mice immunized with human immunodeficiency virus type 1 clade A virus-like particles administered by different routes of inoculation. J Virol. 2005;79:7059–7067. doi: 10.1128/JVI.79.11.7059-7067.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton DR, Pyati J, Koduri R, Sharp SJ, Thornton GB, Parren PW, Sawyer LS, Hendry RM, Dunlop N, Nara PL, et al. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science. 1994;266:1024–1027. doi: 10.1126/science.7973652. [DOI] [PubMed] [Google Scholar]
- Chen X, Rock MT, Hammonds J, Tartaglia J, Shintani A, Currier J, Slike B, Crowe JE, Jr, Marovich M, Spearman P. Pseudovirion particle production by live poxvirus human immunodeficiency virus vaccine vector enhances humoral and cellular immune responses. J Virol. 2005;79:5537–5547. doi: 10.1128/JVI.79.9.5537-5547.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks ET, Jiang P, Franti M, Wong S, Zwick MB, Hoxie JA, Robinson JE, Moore PL, Binley JM. Relationship of HIV-1 and SIV envelope glycoprotein trimer occupation and neutralization. Virology. 2008;377:364–378. doi: 10.1016/j.virol.2008.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks ET, Moore PL, Franti M, Cayanan CS, Zhu P, Jiang P, de Vries RP, Wiley C, Zharkikh I, Schulke N, Roux KH, Montefiori DC, Burton DR, Binley JM. A comparative immunogenicity study of HIV-1 virus-like particles bearing various forms of envelope proteins, particles bearing no envelope and soluble monomeric gp120. Virology. 2007;366:245–262. doi: 10.1016/j.virol.2007.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks ET, Moore PL, Richman D, Robinson J, Crooks JA, Franti M, Schulke N, Binley JM. Characterizing anti-HIV monoclonal antibodies and immune sera by defining the mechanism of neutralization. Hum Antibodies. 2005;14:101–113. [PMC free article] [PubMed] [Google Scholar]
- Crooks ET, Tong T, Osawa K, Binley JM. Enzyme digests eliminate nonfunctional Env from HIV-1 particle surfaces, leaving native Env trimers intact and viral infectivity unaffected. Journal of virology. 2011;85:5825–5839. doi: 10.1128/JVI.00154-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denisova G, Stern B, Raviv D, Zwickel J, Smorodinsky NI, Gershoni JM. Humoral immune response to immunocomplexed HIV envelope glycoprotein 120. AIDS Res Hum Retroviruses. 1996;12:901–909. doi: 10.1089/aid.1996.12.901. [DOI] [PubMed] [Google Scholar]
- Dennison SM, Sutherland LL, Jaeger FH, Anasti KM, Parks R, Stewart S, Bowman C, Xia SM, Zhang R, Shen X, Scearce RM, Ofek G, Yang Y, Kwong PD, Santra S, Liao HX, Tomaras G, Letvin NL, Chen B, Alam SM, Haynes BF. Induction of antibodies in rhesus macaques that recognize a fusion-intermediate conformation of HIV-1 gp41. PLoS One. 2011;6:e27824. doi: 10.1371/journal.pone.0027824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derby NR, Kraft Z, Kan E, Barnett SW, Srivastava IK, Binley JM, L S. Comparative analysis of antibody responses elicited in macaques immunized with HIV-1 SF162-derived gp140 envelope immunogens with those elicited during homologous SHIVSF162 and heterologous HIV-1 infection. J.Virol. 2006;80:8745–8762. doi: 10.1128/JVI.00956-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doan LX, Li M, Chen C, Yao Q. Virus-like particles as HIV-1 vaccines. Reviews in medical virology. 2005;15:75–88. doi: 10.1002/rmv.449. [DOI] [PubMed] [Google Scholar]
- Earl PL, Doms RW, Moss B. Oligomeric structure of the human immunodeficiency virus type 1 envelope glycoprotein. Proc Natl Acad Sci U S A. 1990;87:648–652. doi: 10.1073/pnas.87.2.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emini EA, Koff WC. AIDS/HIV. Developing an AIDS vaccine: need, uncertainty, hope. Science. 2004;304:1913–1914. doi: 10.1126/science.1100368. [DOI] [PubMed] [Google Scholar]
- Evans DT, Bricker JE, Sanford HB, Lang S, Carville A, Richardson BA, Piatak M, Jr, Lifson JD, Mansfield KG, Desrosiers RC. Immunization of macaques with single-cycle simian immunodeficiency virus (SIV) stimulates diverse virus-specific immune responses and reduces viral loads after challenge with SIVmac239. J Virol. 2005;79:7707–7720. doi: 10.1128/JVI.79.12.7707-7720.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forthal DN, Gilbert PB, Landucci G, Phan T. Recombinant gp120 vaccine-induced antibodies inhibit clinical strains of HIV-1 in the presence of Fc receptor-bearing effector cells and correlate inversely with HIV infection rate. Journal of immunology. 2007;178:6596–6603. doi: 10.4049/jimmunol.178.10.6596. [DOI] [PubMed] [Google Scholar]
- Garrone P, Fluckiger AC, Mangeot PE, Gauthier E, Dupeyrot-Lacas P, Mancip J, Cangialosi A, Du Chene I, LeGrand R, Mangeot I, Lavillette D, Bellier B, Cosset FL, Tangy F, Klatzmann D, Dalba C. A prime-boost strategy using virus-like particles pseudotyped for HCV proteins triggers broadly neutralizing antibodies in macaques. Sci Transl Med. 2011;3:94ra71. doi: 10.1126/scitranslmed.3002330. [DOI] [PubMed] [Google Scholar]
- Grovit-Ferbas K, Hsu JF, Ferbas J, Gudeman V, Chen IS. Enhanced binding of antibodies to neutralization epitopes following thermal and chemical inactivation of human immunodeficiency virus type 1. J Virol. 2000;74:5802–5809. doi: 10.1128/jvi.74.13.5802-5809.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundner C, Mirzabekov T, Sodroski J, Wyatt R. Solid-phase proteoliposomes containing human immunodeficiency virus envelope glycoproteins. J Virol. 2002;76:3511–3521. doi: 10.1128/JVI.76.7.3511-3521.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammonds J, Chen X, Ding L, Fouts T, De Vico A, zur Megede J, Barnett S, Spearman P. Gp120 stability on HIV-1 virions and Gag-Env pseudovirions is enhanced by an uncleaved Gag core. Virology. 2003;314:636–649. doi: 10.1016/s0042-6822(03)00467-7. [DOI] [PubMed] [Google Scholar]
- Hammonds J, Chen X, Fouts T, DeVico A, Montefiori D, Spearman P. Induction of neutralizing antibodies against human immunodeficiency virus type 1 primary isolates by Gag-Env pseudovirion immunization. J Virol. 2005;79:14804–14814. doi: 10.1128/JVI.79.23.14804-14814.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammonds J, Chen X, Zhang X, Lee F, Spearman P. Advances in methods for the production, purification, and characterization of HIV-1 Gag-Env pseudovirion vaccines. Vaccine. 2007;25:8036–8048. doi: 10.1016/j.vaccine.2007.09.016. [DOI] [PubMed] [Google Scholar]
- Haynes BF, Fleming J, St Clair EW, Katinger H, Stiegler G, Kunert R, Robinson J, Scearce RM, Plonk K, Staats HF, Ortel TL, Liao HX, Alam SM. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science. 2005;308:1906–1908. doi: 10.1126/science.1111781. [DOI] [PubMed] [Google Scholar]
- Hicar MD, Chen X, Briney B, Hammonds J, Wang JJ, Kalams S, Spearman PW, Crowe JE., Jr Pseudovirion particles bearing native HIV envelope trimers facilitate a novel method for generating human neutralizing monoclonal antibodies against HIV. Journal of acquired immune deficiency syndromes. 2010;54:223–235. doi: 10.1097/QAI.0b013e3181dc98a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hioe CE, Visciano ML, Kumar R, Liu J, Mack EA, Simon RE, Levy DN, Tuen M. The use of immune complex vaccines to enhance antibody responses against neutralizing epitopes on HIV-1 envelope gp120. Vaccine. 2009;28:352–360. doi: 10.1016/j.vaccine.2009.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoot S, McGuire AT, Cohen KW, Strong RK, Hangartner L, Klein F, Diskin R, Scheid JF, Sather DN, Burton DR, Stamatatos L. Recombinant HIV envelope proteins fail to engage germline versions of anti-CD4bs bNAbs. PLoS Pathog. 2013;9:e1003106. doi: 10.1371/journal.ppat.1003106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Ofek G, Laub L, Louder MK, Doria-Rose NA, Longo NS, Imamichi H, Bailer RT, Chakrabarti B, Sharma SK, Alam SM, Wang T, Yang Y, Zhang B, Migueles SA, Wyatt R, Haynes BF, Kwong PD, Mascola JR, Connors M. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature. 2012;491:406–412. doi: 10.1038/nature11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobe O, Peachman KK, Matyas GR, Asher LV, Alving CR, Rao M. An anti-phosphoinositide-specific monoclonal antibody that neutralizes HIV-1 infection of human monocyte-derived macrophages. Virology. 2012;430:110–119. doi: 10.1016/j.virol.2012.04.017. [DOI] [PubMed] [Google Scholar]
- Joyner AS, Willis JR, Crowe JE, Jr, Aiken C. Maturation-induced cloaking of neutralization epitopes on HIV-1 particles. PLoS Pathog. 2011;7:e1002234. doi: 10.1371/journal.ppat.1002234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamdem Toukam D, Tenbusch M, Stang A, Temchura V, Storcksdieck Genannt Bonsmann M, Grewe B, Koch S, Meyerhans A, Nchinda G, Kaptue L, Uberla K. Targeting antibody responses to the membrane proximal external region of the envelope glycoprotein of human immunodeficiency virus. PLoS One. 2012;7:e38068. doi: 10.1371/journal.pone.0038068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanekiyo M, Wei CJ, Yassine HM, McTamney PM, Boyington JC, Whittle JR, Rao SS, Kong WP, Wang L, Nabel GJ. Self-assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies. Nature. 2013 doi: 10.1038/nature12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang YK, Andjelic S, Binley JM, Crooks ET, Franti M, Iyer SP, Donovan GP, Dey AK, Zhu P, Roux KH, Durso RJ, Parsons TF, Maddon PJ, Moore JP, Olson WC. Structural and immunogenicity studies of a cleaved, stabilized envelope trimer derived from subtype A HIV-1. Vaccine. 2009;27:5120–5132. doi: 10.1016/j.vaccine.2009.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller MA, Arora Y. Inhibition of the anti-V3 loop response to a recombinant gp120SF2 vaccine by preexisting monoclonal antibody. AIDS Res Hum Retroviruses. 1999;15:855–860. doi: 10.1089/088922299310764. [DOI] [PubMed] [Google Scholar]
- Klasse PJ, Sanders RW, Cerutti A, Moore JP. How can HIV-type-1-Env immunogenicity be improved to facilitate antibody-based vaccine development? AIDS Res Hum Retroviruses. 2012;28:1–15. doi: 10.1089/aid.2011.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L, Lee JH, Doores KJ, Murin CD, Julien JP, McBride R, Liu Y, Marozsan A, Cupo A, Klasse PJ, Hoffenberg S, Caulfield M, King CR, Hua Y, Le KM, Khayat R, Deller MC, Clayton T, Tien H, Feizi T, Sanders RW, Paulson JC, Moore JP, Stanfield RL, Burton DR, Ward AB, Wilson IA. Supersite of immune vulnerability on the glycosylated face of HIV-1 envelope glycoprotein gp120. Nat Struct Mol Biol. 2013;20:796–803. doi: 10.1038/nsmb.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni PS, Manjunath K, Agarkhedkar S Group of, S.I.I.I.I.V.S. Safety and immunogenicity of an adjuvanted whole virion, inactivated A (H1N1) 2009 influenza vaccine in young and elderly adults, and children. Vaccine. 2012;31:20–22. doi: 10.1016/j.vaccine.2012.10.081. [DOI] [PubMed] [Google Scholar]
- Kushnir N, Streatfield SJ, Yusibov V. Virus-like particles as a highly efficient vaccine platform: Diversity of targets and production systems and advances in clinical development. Vaccine. 2012;31:58–83. doi: 10.1016/j.vaccine.2012.10.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong PD, Mascola JR. Human Antibodies that Neutralize HIV-1: Identification, Structures, and B Cell Ontogenies. Immunity. 2012;37:412–425. doi: 10.1016/j.immuni.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrijn AF, Poignard P, Raja A, Zwick MB, Delgado K, Franti M, Binley J, Vivona V, Grundner C, Huang CC, Venturi M, Petropoulos CJ, Wrin T, Dimitrov DS, Robinson J, Kwong PD, Wyatt RT, Sodroski J, Burton DR. Access of Antibody Molecules to the Conserved Coreceptor Binding Site on Glycoprotein gp120 Is Sterically Restricted on Primary Human Immunodeficiency Virus Type 1. J Virol. 2003;77:10557–10565. doi: 10.1128/JVI.77.19.10557-10565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaman DP, Kinkead H, Zwick MB. In-solution virus capture assay helps deconstruct heterogeneous antibody recognition of human immunodeficiency virus type 1. J Virol. 2010;84:3382–3395. doi: 10.1128/JVI.02363-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Gao F, Mascola J, Stamatatos L, Polonis VR, Koutsoukos M, Goepfert P, Gilbert P, Greene KM, Bilska M, Kothe DL, Salazar-Gonzalez JF, Wei X, Decker JM, Hahn B, Montefiori D. Human Immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J.Virol. 2005;79:10108–10125. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Svehla K, Mathy NL, Voss G, Mascola JR, Wyatt R. Characterization of antibody responses elicited by human immunodeficiency virus type 1 primary isolate trimeric and monomeric envelope glycoproteins in selected adjuvants. J Virol. 2006;80:1414–1426. doi: 10.1128/JVI.80.3.1414-1426.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao HX, Alam SM, Mascola JR, Robinson J, Ma B, Montefiori DC, Rhein M, Sutherland LL, Scearce R, Haynes BF. Immunogenicity of constrained monoclonal antibody A32-human immunodeficiency virus (HIV) Env gp120 complexes compared to that of recombinant HIV type 1 gp120 envelope glycoproteins. J Virol. 2004;78:5270–5278. doi: 10.1128/JVI.78.10.5270-5278.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifson JD, Rossio JL, Piatak M, Jr, Bess J, Jr, Chertova E, Schneider DK, Coalter VJ, Poore B, Kiser RF, Imming RJ, Scarzello AJ, Henderson LE, Alvord WG, Hirsch VM, Benveniste RE, Arthur LO. Evaluation of the safety, immunogenicity, and protective efficacy of whole inactivated simian immunodeficiency virus (SIV) vaccines with conformationally and functionally intact envelope glycoproteins. AIDS Res Hum Retroviruses. 2004;20:772–787. doi: 10.1089/0889222041524661. [DOI] [PubMed] [Google Scholar]
- Lorizate M, Sachsenheimer T, Glass B, Habermann A, Gerl MJ, Krausslich HG, Brugger B. Comparative lipidomics analysis of HIV-1 particles and their producer cell membrane in different cell lines. Cellular microbiology. 2013;15:292–304. doi: 10.1111/cmi.12101. [DOI] [PubMed] [Google Scholar]
- Mascola JR, Montefiori DC. The role of antibodies in HIV vaccines. Annu Rev Immunol. 2010;28:413–444. doi: 10.1146/annurev-immunol-030409-101256. [DOI] [PubMed] [Google Scholar]
- Matyas GR, Wieczorek L, Bansal D, Chenine AL, Sanders-Buell E, Tovanabutra S, Kim JH, Polonis V, Alving CR. Inhibition of HIV-1 infection of peripheral blood mononuclear cells by a monoclonal antibody that binds to phosphoinositides and induces secretion of beta-chemokines. Biochem Biophys Res Commun. 2010;402:808–812. doi: 10.1016/j.bbrc.2010.10.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBurney SP, Young KR, Ross TM. Membrane embedded HIV-1 envelope on the surface of a virus-like particle elicits broader immune responses than soluble envelopes. Virology. 2007;358:334–346. doi: 10.1016/j.virol.2006.08.032. [DOI] [PubMed] [Google Scholar]
- McCoy LE, Weiss RA. Neutralizing antibodies to HIV-1 induced by immunization. J Exp Med. 2013;210:209–223. doi: 10.1084/jem.20121827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire AT, Hoot S, Dreyer AM, Lippy A, Stuart A, Cohen KW, Jardine J, Menis S, Scheid JF, West AP, Schief WR, Stamatatos L. Engineering HIV envelope protein to activate germline B cell receptors of broadly neutralizing anti-CD4 binding site antibodies. J Exp Med. 2013;210:655–663. doi: 10.1084/jem.20122824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna PM, Aye PP, Dietzschold B, Montefiori DC, Martin LN, Marx PA, Pomerantz RJ, Lackner A, Schnell MJ. Immunogenicity study of glycoprotein-deficient rabies virus expressing simian/human immunodeficiency virus SHIV89.6P envelope in a rhesus macaque. Journal of virology. 2004;78:13455–13459. doi: 10.1128/JVI.78.24.13455-13459.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna PM, Pomerantz RJ, Dietzschold B, McGettigan JP, Schnell MJ. Covalently linked human immunodeficiency virus type 1 gp120/gp41 is stably anchored in rhabdovirus particles and exposes critical neutralizing epitopes. J Virol. 2003;77:12782–12794. doi: 10.1128/JVI.77.23.12782-12794.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero M, Gulzar N, Klaric KA, Donald JE, Lepik C, Wu S, Tsai S, Julien JP, Hessell AJ, Wang S, Lu S, Burton DR, Pai EF, Degrado WF, Scott JK. Neutralizing epitopes in the membrane-proximal external region of HIV-1 gp41 are influenced by the transmembrane domain and the plasma membrane. J Virol. 2012;86:2930–2941. doi: 10.1128/JVI.06349-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody MA, Liao HX, Alam SM, Scearce RM, Plonk MK, Kozink DM, Drinker MS, Zhang R, Xia SM, Sutherland LL, Tomaras GD, Giles IP, Kappes JC, Ochsenbauer-Jambor C, Edmonds TG, Soares M, Barbero G, Forthal DN, Landucci G, Chang C, King SW, Kavlie A, Denny TN, Hwang KK, Chen PP, Thorpe PE, Montefiori DC, Haynes BF. Anti-phospholipid human monoclonal antibodies inhibit CCR5-tropic HIV-1 and induce beta-chemokines. The Journal of experimental medicine. 2010;207:763–776. doi: 10.1084/jem.20091281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JP, Sattentau QJ, Wyatt R, Sodroski J. Probing the structure of the human immunodeficiency virus surface glycoprotein gp120 with a panel of monoclonal antibodies. Journal of virology. 1994;68:469–484. doi: 10.1128/jvi.68.1.469-484.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JP, Thali M, Jameson BA, Vignaux F, Lewis GK, Poon SW, Charles M, Fung MS, Sun B, Durda PJ, et al. Immunochemical analysis of the gp120 surface glycoprotein of human immunodeficiency virus type 1: probing the structure of the C4 and V4 domains and the interaction of the C4 domain with the V3 loop. Journal of virology. 1993;67:4785–4796. doi: 10.1128/jvi.67.8.4785-4796.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore PL, Crooks ET, Porter L, Zhu P, Cayanan CS, Grise H, Corcoran P, Zwick MB, Franti M, Morris L, Roux KH, Burton DR, Binley JM. Nature of nonfunctional envelope proteins on the surface of human immunodeficiency virus type 1. Journal of virology. 2006;80:2515–2528. doi: 10.1128/JVI.80.5.2515-2528.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyambi PN, Gorny MK, Bastiani L, van der Groen G, Williams C, Zolla-Pazner S. Mapping of epitopes exposed on intact human immunodeficiency virus type 1 (HIV-1) virions: a new strategy for studying the immunologic relatedness of HIV-1. J Virol. 1998;72:9384–9391. doi: 10.1128/jvi.72.11.9384-9391.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rourke SM, Schweighardt B, Scott WG, Wrin T, Fonseca DP, Sinangil F, Berman PW. Novel ring structure in the gp41 trimer of human immunodeficiency virus type 1 that modulates sensitivity and resistance to broadly neutralizing antibodies. J Virol. 2009;83:7728–7738. doi: 10.1128/JVI.00688-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota T, Doyle-Cooper C, Cooper AB, Huber M, Falkowska E, Doores KJ, Hangartner L, Le K, Sok D, Jardine J, Lifson J, Wu X, Mascola JR, Poignard P, Binley JM, Chakrabarti BK, Schief WR, Wyatt RT, Burton DR, Nemazee D. Anti-HIV B Cell lines as candidate vaccine biosensors. J Immunol. 2012;189:4816–4824. doi: 10.4049/jimmunol.1202165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan R, Sampson JM, Chen Y, Vaine M, Wang S, Lu S, Kong XP. Rabbit anti-HIV-1 monoclonal antibodies raised by immunization can mimic the antigen-binding modes of antibodies derived from HIV-1-infected humans. J Virol. 2013;87:10221–10231. doi: 10.1128/JVI.00843-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastori C, Tudor D, Diomede L, Drillet AS, Jegerlehner A, Rohn TA, Bomsel M, Lopalco L. Virus like particle based strategy to elicit HIV-protective antibodies to the alpha-helic regions of gp41. Virology. 2012;431:1–11. doi: 10.1016/j.virol.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Pinheiro A, Lanning D, Alves PC, Mage RG, Knight KL, van der Loo W, Esteves PJ. Molecular bases of genetic diversity and evolution of the immunoglobulin heavy chain variable region (IGHV) gene locus in leporids. Immunogenetics. 2011;63:397–408. doi: 10.1007/s00251-011-0533-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon B, Safrit JT, McClure H, Kitchen C, Hsu JF, Gudeman V, Petropoulos C, Wrin T, Chen IS, Grovit-Ferbas K. Induction of humoral immune responses following vaccination with envelope-containing, formaldehyde-treated, thermally inactivated human immunodeficiency virus type 1. J Virol. 2005;79:4927–4935. doi: 10.1128/JVI.79.8.4927-4935.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roldao A, Mellado MC, Castilho LR, Carrondo MJ, Alves PM. Virus-like particles in vaccine development. Expert Rev Vaccines. 2010;9:1149–1176. doi: 10.1586/erv.10.115. [DOI] [PubMed] [Google Scholar]
- Rossio JL, Esser MT, Suryanarayana K, Schneider DK, Bess JW, Jr, Vasquez GM, Wiltrout TA, Chertova E, Grimes MK, Sattentau Q, Arthur LO, Henderson LE, Lifson JD. Inactivation of human immunodeficiency virus type 1 infectivity with preservation of conformational and functional integrity of virion surface proteins. J Virol. 1998;72:7992–8001. doi: 10.1128/jvi.72.10.7992-8001.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders RW, Venturi M, Schiffner L, Kalyanaraman R, Katinger H, Lloyd KO, Kwong PD, Moore JP. The mannose-dependent epitope for neutralizing antibody 2G12 on human immunodeficiency virus type 1 glycoprotein gp120. J Virol. 2002;76:7293–7305. doi: 10.1128/JVI.76.14.7293-7305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlan CN, Pantophlet R, Wormald MR, Ollmann Saphire E, Stanfield R, Wilson IA, Katinger H, Dwek RA, Rudd PM, Burton DR. The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2G12 recognizes a cluster of a1-->2 mannose residues on the outer face of gp120. J Virol. 2002;76:7306–7321. doi: 10.1128/JVI.76.14.7306-7321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffner T, Sattentau QJ, Dorrell L. Development of prophylactic vaccines against HIV-1. Retrovirology. 2013;10:72. doi: 10.1186/1742-4690-10-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, Dennison SM, Liu P, Gao F, Jaeger F, Montefiori DC, Verkoczy L, Haynes BF, Alam SM, Tomaras GD. Prolonged exposure of the HIV-1 gp41 membrane proximal region with L669S substitution. Proc Natl Acad Sci U S A. 2010;107:5972–5977. doi: 10.1073/pnas.0912381107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims PJ, Wiedmer T. Unraveling the mysteries of phospholipid scrambling. Thrombosis and haemostasis. 2001;86:266–275. [PubMed] [Google Scholar]
- Soares MM, King SW, Thorpe PE. Targeting inside-out phosphatidylserine as a therapeutic strategy for viral diseases. Nat Med. 2008;14:1357–1362. doi: 10.1038/nm.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun NC, Ho DD, Sun CR, Liou RS, Gordon W, Fung MS, Li XL, Ting RC, Lee TH, Chang NT, et al. Generation and characterization of monoclonal antibodies to the putative CD4-binding domain of human immunodeficiency virus type 1 gp120. Journal of virology. 1989;63:3579–3585. doi: 10.1128/jvi.63.9.3579-3585.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaras GD, Binley JM, Gray ES, Crooks ET, Osawa K, Moore PL, Tumba N, Tong T, Shen X, Yates NL, Decker J, Wibmer CK, Gao F, Alam SM, Easterbrook P, Abdool Karim S, Kamanga G, Crump JA, Cohen M, Shaw GM, Mascola JR, Haynes BF, Montefiori DC, Morris L. Polyclonal B cell responses to conserved neutralization epitopes in a subset of HIV-1-infected individuals. J Virol. 2011;85:11502–11519. doi: 10.1128/JVI.05363-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong T, Crooks ET, Osawa K, Binley JM. HIV-1 virus-like particles bearing pure env trimers expose neutralizing epitopes but occlude nonneutralizing epitopes. Journal of virology. 2012;86:3574–3587. doi: 10.1128/JVI.06938-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong T, Osawa K, Robinson JE, Crooks ET, Binley JM. Topological Analysis of HIV-1 Glycoproteins Expressed in situ on Virus Surfaces Reveals Tighter Packing but Greater Conformational Flexibility Compared to Soluble Gp120. J Virol. 2013;87:9233–9249. doi: 10.1128/JVI.01145-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaine M, Wang S, Crooks ET, Jiang P, Montefiori DC, Binley J, Lu S. Improved induction of antibodies against key neutralizing epitopes by human immunodeficiency virus type 1 gp120 DNA prime-protein boost vaccination compared to gp120 protein-only vaccination. Journal of virology. 2008;82:7369–7378. doi: 10.1128/JVI.00562-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gils MJ, Sanders RW. Broadly neutralizing antibodies against HIV-1: templates for a vaccine. Virology. 2013;435:46–56. doi: 10.1016/j.virol.2012.10.004. [DOI] [PubMed] [Google Scholar]
- van Montfort T, Nabatov AA, Geijtenbeek TB, Pollakis G, Paxton WA. Efficient capture of antibody neutralized HIV-1 by cells expressing DC-SIGN and transfer to CD4+ T lymphocytes. J Immunol. 2007;178:3177–3185. doi: 10.4049/jimmunol.178.5.3177. [DOI] [PubMed] [Google Scholar]
- VanCott TC, Mascola JR, Kaminski RW, Kalyanaraman V, Hallberg PL, Burnett PR, Ulrich JT, Rechtman DJ, Birx DL. Antibodies with specificity to native gp120 and neutralization activity against primary human immunodeficiency virus type 1 isolates elicited by immunization with oligomeric gp160. J Virol. 1997;71:4319–4330. doi: 10.1128/jvi.71.6.4319-4330.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visciano ML, Diomede L, Tagliamonte M, Tornesello ML, Asti V, Bomsel M, Buonaguro FM, Lopalco L, Buonaguro L. Generation of HIV-1 Virus-Like Particles expressing different HIV-1 glycoproteins. Vaccine. 2011;29:4903–4912. doi: 10.1016/j.vaccine.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Visciano ML, Tuen M, Gorny MK, Hioe CE. In vivo alteration of humoral responses to HIV-1 envelope glycoprotein gp120 by antibodies to the CD4-binding site of gp120. Virology. 2008;372:409–420. doi: 10.1016/j.virol.2007.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vzorov AN, Lea-Fox D, Compans RW. Immunogenicity of full length and truncated SIV envelope proteins. Viral Immunol. 1999;12:205–215. doi: 10.1089/vim.1999.12.205. [DOI] [PubMed] [Google Scholar]
- Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien JP, Wang SK, Ramos A, Chan-Hui PY, Moyle M, Mitcham JL, Hammond PW, Olsen OA, Phung P, Fling S, Wong CH, Phogat S, Wrin T, Simek MD, Koff WC, Wilson IA, Burton DR, Poignard P. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang S-H, Doka N, Choudhary RK, Sodroski J, Robinson JE. Characterization of CD4-Induced Epitopes on the HIV-1 gp120 Envelope Glycoprotein Recognized by Neutralizing Human Monoclonal Antibodies. AIDS Research and Human Retroviruses. 2002;18:1207–1217. doi: 10.1089/08892220260387959. [DOI] [PubMed] [Google Scholar]
- Yang L, Song Y, Li X, Huang X, Liu J, Ding H, Zhu P, Zhou P. HIV-1 virus-like particles produced by stably transfected Drosophila S2 cells: a desirable vaccine component. Journal of virology. 2012;86:7662–7676. doi: 10.1128/JVI.07164-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou ZR, Wang ML, Deng F, Li TX, Hu ZH, Wang HL. Production of CCHF virus-like particle by a baculovirus-insect cell expression system. Virol Sin. 2011;26:338–346. doi: 10.1007/s12250-011-3209-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwick MB, Saphire EO, Burton DR. gp41: HIV's shy protein. Nat Med. 2004;10:133–134. doi: 10.1038/nm0204-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.