Abstract
Bone remodeling in health and disease is carried out by osteoblasts and osteoclasts, which respectively produce bone matrix and resorb it. Endocrine and paracrine control of these cells can be direct, but they are also exerted indirectly, either by influencing progenitor cell differentiation or by stimulating paracrine signals from local accessory cells including osteocytes (which form a critical communication and regulation network within the bone matrix), macrophages and T lymphocytes. Here we review the osteotropic actions of the interleukin-6 family member cytokine oncostatin M (OSM), which is of particular interest because of its ability to stimulate bone accrual. OSM is produced within the bone microenvironment by cells of both mesenchymal and hematopoietic origin, including osteocytes, osteoblasts, macrophages and T lymphocytes, and can act via two receptor complexes: OSM receptor:gp130 and leukemia inhibitory factor receptor (LIFR):gp130. Although OSM can directly stimulate osteoblast mineralization activity and differentiation, it can also stimulate mesenchymal stem cell osteoblastic commitment at the expense of adipogenesis. In osteocytes, OSM can suppress the production of the bone formation inhibitor sclerostin, an action that is mediated by LIFR:gp130. OSM also stimulates the production of receptor activator of nuclear factor κB ligand by osteoblasts and thereby drives the formation of osteoclasts particularly in pathological conditions. Thus, cellular effects of OSM on bone metabolism include direct and indirect actions mediated by two related receptor/ligand complexes. OSM therefore provides an example of paracrine and endocrine control mechanisms that regulate bone mass by controlling both bone formation and resorption.
Introduction: oncostatin M (OSM)
To design new therapies to improve bone health, we need to understand the key mechanisms that influence bone metabolism in both physiological and pathological conditions. Here we focus on OSM, a member of the interleukin (IL)-6 family of cytokines, as this hormone strongly influences both bone formation and resorption.
OSM is a pleiotropic cytokine first identified by Zarling et al.1 as a secreted product of macrophage-like cells that inhibited the proliferation of melanoma-, neuroblastoma- and lung cancer-derived cell lines. In contrast to these early roles, OSM also has stimulatory roles in a number of human cancers including Kaposi's sarcoma2 and breast cancer.3 Frequently acting in conjunction with inflammatory factors or other gp130-dependent cytokines, OSM has been implicated in diverse disorders including pulmonary tissue fibrosis,4 cardiac disease and repair,5 prostate cancer,6 asthma,7 periodontal disease8 and both rheumatoid arthritis and osteoarthritis.9 In the context of skeletal biology, OSM is of particular interest as it displays anabolic effects on both cortical and trabecular bone (which would be clinically desirable to emulate) while at the same time driving osteoclast formation; notably, both influences are at least in part mediated by OSM action on the osteoblast lineage. It is also becoming clear that OSM has numerous strong influences on other less well-characterized cell types within the bone microenvironment. The existence of multiple pathways of influence raises questions about which cellular mechanisms are most critical in influencing bone anabolism.
Multiple cellular interactions regulate bone structure
Bone mass in health and disease is determined by the actions of two unique cell types: myelomonocyte-derived multinucleated osteoclasts that resorb bone, and mesenchymal-derived osteoblasts that form bone matrix and regulate its mineralization. The coordinated actions of these two cell types are central to the life-long process of skeletal renewal, termed bone remodeling. Their actions are also coordinated during fetal development, bone growth, fracture repair, changes of bone structure in response to mechanical loading and in non-mechanical functions such as serum calcium and phosphate maintenance. Any disruption in the balance of their activities may result in skeletal disease.
Precise osteoclast–osteoblast coordination is a particular requirement for bone remodeling. This process maintains bone strength and quality throughout life by osteoclastic resorption of areas of old or damaged bone and replacement with a similar amount of new bone at the same site by osteoblasts.10 Although early studies suggested that such coordination between osteoclast and osteoblast activity occurred in relative isolation in the basic multicellular unit, it is now clear that several important local accessory cell types influence bone metabolism through their paracrine secretions,11 and may mediate many of the effects of drugs and cytokines on bone metabolism. These accessory cells include osteocytes,12 bone marrow stromal cells and osteal macrophages;13 some less well-delineated influences include those from local endothelial cells,14 nerve cells15,16 and cell populations of the bone marrow cavity such as lymphocytes, as discussed below.
OSM is produced by several cell types found in the bone microenvironment, and it influences the skeleton both by direct actions on the osteoblast lineage and by indirect actions through other accessory cells. These will be discussed below with reference to their contributions to normal physiology, pathological bone disorders and possibilities for therapeutic application of this knowledge.
The influences of OSM on the cells of bone: osteoclasts, osteoblasts and osteocytes
OSM stimulates osteoclast formation via osteoblastic receptor activator of nuclear factor κB ligand (RANKL) expression. OSM probably does not affect osteoclasts or their progenitors directly, but its action does strongly increase osteoclast formation both in vivo and in hematopoietic populations cocultured with osteoblasts.17 The latter is due to an OSM-induced increase in osteoblastic expression of RANKL.18 This action is mediated by the OSM receptor (OSMR):gp130 receptor complex and downstream initiation of JAK/STAT signaling (principally STAT3 activation19) in osteoblastic cells.20 OSM induces RANKL transcription by promoting STAT3 and RNA polymerase II binding to a subset21 of 5 distal enhancer regions of the RANKL gene also utilized by parathyroid hormone (PTH) and 1,25-dihydroxyvitamin-D3,22 and a third more distal enhancer region.23 Notably, genetic deletion of the distal control region led to a significant increase in bone mass owing to a low level of RANKL expression and limited bone resorption,24 a phenotype strikingly similar to that of OSMR-null mice.25
The influence of OSM in driving osteoclast formation was the focus of much early work in osteoclast biology, particularly in the context of osteolytic bone disease. Indeed, OSM participates significantly in osteoclast formation in the context of breast cancer invasion of bone,26 and may also be involved in the increased bone destruction associated with inflammatory arthritis.27,28 The low osteoclast numbers observed in the OSMR-null mice also suggested a role for OSM in normal physiological levels of resorption in the process of bone remodeling.25 However, osteoclast generation from OSMR-deficient marrow cells is similar to that of wild-type bone marrow. This indicates that changes in osteoclast numbers are not caused by the OSMR-null hematopoietic phenotype, and probably does not reflect a change in osteoclast progenitor number or responsiveness to RANKL.29 Rather, our studies using osteoblast/bone marrow cocultures have indicated that osteoclast differentiation is defective when it is supported by osteoblasts that lack the OSMR. This is the case not only when the hormonal stimulus inducing osteoblastic RANKL is OSM, but also when it is 1,25-dihydroxyvitamin-D3; this points to a previously unsuspected influence of OSMR in osteoblast RANKL production that may be critical to osteoclast formation and bone metabolism.25 As neither osteocyte-specific nor osteoblast-lineage-specific gp130-null mice exhibit any alteration in osteoclast numbers,30 the reduced osteoclast formation observed in OSMR-null mice may reflect an influence mediated by early osteoblast progenitors rather than by mature osteoblasts or osteocytes.
The influence of OSMR in the action of PTH
Although the above observations indicate that the presence of OSMR increases the osteoblastic capacity to produce RANKL, we also made an observation that strikingly deviated from this pattern. This was seen when OSMR-null osteoblasts or mice were treated with PTH.
Treatment with PTH injection (which results in intermittently high PTH serum levels) has two particular effects in vivo: it stimulates RANKL production in osteoblasts and stimulates bone formation by promoting proliferation and differentiation of osteoblasts while inhibiting their apoptosis.31 As PTH treatment also induces OSMR expression, we suggested that some PTH actions may depend on OSMR signaling.32 However, when OSMR-deficient osteoblasts in vitro were stimulated with PTH, their support of osteoclast formation was much greater than that of wild-type osteoblasts.32 Increased osteoclast formation was also seen in vivo when OSMR-null mice were treated with PTH to the degree that a PTH injection regimen that showed anabolic effects in control mice was actually catabolic in OSMR-null mice. Anabolic effects of intermittent PTH treatment are normally associated with only a short and transient induction of RANKL31 and a similarly transient increase in osteoclast activity,33 two actions that probably contribute to an anabolic action by the release of osteoclast-derived coupling factors.34,35 In contrast, persistently high circulating PTH, induced by PTH infusion or other means, is catabolic,36 owing to a corresponding persistently high level of RANKL31 that increases osteoclast formation. In OSMR-null osteoblasts, an unusual persistently high RANKL mRNA response to PTH injection was observed; this could thus explain the conversion of a normally PTH anabolic treatment to catabolic response in the OSMR-null mouse.32 The reason why OSMR-null osteoblasts have a persistent RANKL response to PTH (and not to other osteolytic hormones) remains unclear. If these catabolic effects of PTH in OSMR-null mice in vivo are wholly osteoblast mediated, it suggests that under some circumstances OSMR signals can result in both procatabolic actions (via increased RANKL levels) and anticatabolic actions. It is possible that OSMR induction by PTH provides negative feedback that limits the duration of RANKL response. Indeed, recent work has indicated that OSM stimulates the production of Wnt16, an osteoblast-derived stimulus of OPG production, suggesting that OSMR signaling may restrain osteoclast formation through this pathway.37 As PTH treatment induces many gene responses in osteoblasts,38 including increased expression of many cytokines, OSMR has much scope to modify PTH action.
It should be noted that OSMR also associates with the gp130-like receptor IL-31RA to form a receptor for the Th2 cytokine IL-31; another consequence of OSMR deletion is a lack of IL-31 response.39 Although we could find no effect of IL-31 on osteoblast or osteoclast differentiation in vitro,25 IL-31RA deletion has been noted to increase hepatic cytokine response to OSM.40 This suggests that OSMR deletion may increase responses to other gp130-dependent cytokines by increasing the availability of other receptor components.
OSM stimulates osteoblast differentiation and bone formation by actions on both osteocytes and osteoblast progenitors
OSM suppresses osteocytic sclerostin
Osteocytes have recently emerged from their relative obscurity to be recognized as major regulatory cells, with a critical role in regulating bone mass through their production of an antianabolic inhibitor of osteoblast activity, sclerostin,41 and (like all osteoblast-lineage cells) their production of RANKL.42,43 In mice, injected OSM has a strongly anabolic effect on bone, and this is associated with a strong suppression of sclerostin in vivo, which is also observed in cultured cells.25 However, unlike OSM stimulation of RANKL, suppression of sclerostin is not mediated by the classical OSMR:gp130 heterodimer but is mediated by the leukemia inhibitory factor receptor (LIFR), presumably in a LIFR:gp130 heterodimer.25 Notably, injected OSM is still anabolic in mice that lack OSMR.25 The degree to which the suppression of osteocyte sclerostin mediates the anabolic OSM influence is unclear, as it has yet to be examined in sclerostin-null animals. Nevertheless, since the anabolic action of OSM is lost in mice that lack the gp130 signaling unit specifically in osteocytes,30 it is clear that the anabolic action of OSM is mediated by its action in the osteocyte.
One technical problem with interpreting many of the early studies of OSM and bone has been the tendency of such studies to use human OSM and murine OSM somewhat interchangeably. This has complicated matters, as human OSM has equal affinity for both human LIFR and human OSMR,44 whereas in murine cells human OSM acts exclusively through mouse LIFR.45 Different again is murine OSM, which binds at a much higher affinity to murine OSMR than to murine LIFR.45 It is notable that, in rodent cells, suppression of sclerostin levels is a property that other cytokines that act through the LIFR:gp130 heterodimer also possess; this includes human OSM, cardiotrophin-1 and leukemia inhibitory factor (LIF).25 The suppression of sclerostin by IL-11, a cytokine that does not recruit LIFR, is far less potent.25 As sclerostin is the only target of LIFR:gp130 thus far identified, it is possible that the use of this receptor complex is specific to osteocytes, and it may depend on other factors such as scaffolding proteins, present in these cells, or altered receptor availability.
OSM directs stromal cell commitment to osteoblastogenesis
The action of OSM on osteoblast differentiation is probably not restricted to its effects on sclerostin. As OSMR is expressed by mesenchymal stem cells (MSCs), it is likely that direct effects of OSM stimulate osteoblast differentiation and activity in both murine and human MSCs while reducing adipocyte formation in the same populations.25,46,47 Some of the influences of OSM can be attributed to suppression of sclerostin as noted above; however, enhanced differentiation of osteoblasts and impaired adipogenesis occurs in primary osteoblast cultures and osteoblastic cell lines even when little sclerostin is detectable. This suggests some direct action on immature osteoblastic cells, as indicated by rapid effects on the expression of C/EBPα and PPARγ.25 Furthermore, it should be noted that all bone formation depends to some degree on the recruitment of new, primitive osteoblast progenitors. Indeed, OSM shares a number of direct gene targets with PTH in osteoblasts; these include RANKL,48 IL-3349 and the transcription factor Zfp467, a factor that regulates osteoblast/adipocyte commitment.50 This is consistent with the similarity of OSM and PTH effects on bone even though their physiological roles otherwise seem to be quite different.
Although OSM and LIF have similar effects in vitro on osteoblast and adipocyte differentiation,25,51 it should be noted that both LIF- and OSMR-null mice demonstrate reduced osteoblast differentiation and enhanced adipogenesis in vivo,25,51 indicating that the roles of LIF:LIFR and OSM:OSMR signaling that regulate stromal cell differentiation to osteoblasts are not redundant in normal physiology. It remains unknown whether OSM:gp130:LIFR and LIF:gp130:LIFR complex formations elicit redundant or distinct influences on the undifferentiated stromal cell, osteoblast or osteocyte. If there are differences, this would suggest altered receptor conformation and/or recruitment of different accessory proteins, but the structures of the OSM:gp130:LIFR and LIF:gp130:LIFR complexes remain unresolved. Furthermore, it is not yet known whether the phenotypes of the OSMR-null mice and mice lacking OSM (and therefore lacking both OSM:gp130:LIFR and OSM:gp130:OSMR signaling) are similar. These experiments would resolve the lingering question about redundancy between LIF and OSM signaling.
The source of OSM: osteoblasts, macrophages, T cells and malignant cells
OSM as a paracrine factor within the osteoblast lineage
Significant levels of OSM mRNA and protein have been detected at all stages of differentiation in the osteoblast lineage, including bone lining cells, matrix-producing osteoblasts and osteocytes in murine bone.25 In human bone, OSM mRNA has been detected in bone marrow stromal cells and osteoblasts from normal and arthritic specimens, although this has yet to be confirmed at the protein level.52 Osteoblastic cells express all three receptor subunits than can be used by OSM (gp130, OSMR and LIFR),25 and their expression of OSM, gp130 and OSMR is strongly stimulated by PTH and PTH related protein (PTHrP).32 Furthermore, in whole bones subjected to mechanical loading, OSM and OSMR are both significantly upregulated.53 The regulation of both osteoblastic and osteocytic genes by OSM indicate functions for OSM at multiple stages of osteoblast differentiation to regulate bone metabolism in response to anabolic and catabolic stimuli, as described above.
Macrophage-derived OSM supports osteoblast differentiation
Macrophages come in many varieties capable of distinct (but often as yet poorly defined) activities and secretory profiles. They are highly responsive to many influences, notably emanating from the immune system, that exert strong and malign effects in osteolytic bone diseases associated with chronic inflammation, particularly rheumatoid arthritis. Macrophages also have a wide secretory repertoire, and inflammatory macrophages produce factors such as tumor necrosis factor and dickkopf WNT signaling pathway inhibitor 1 (Dkk1) that have strong negative influences on osteoblast activity,54,55,56,57 but proanabolic influences involving IL-6 family cytokines, notably OSM, are also beginning to emerge. Resident osteal macrophages are present at or near the bone surface in intimate proximity to osteoblasts and bone lining cells,13 and their paracrine secretions are likely to influence bone formation. Close cooperative interactions between resident macrophages and mesenchymally derived cells are seen in many tissues, such as joint synovial membranes, but such interactions in bone are now receiving renewed attention. It is not clear whether osteal macrophages differ from other local mature macrophages, as they cannot be studied in isolation. However, bone-derived macrophages can enhance mineralization of osteoblasts in vitro,13 and specific deletion of macrophages (c-fms-expressing cells) greatly reduces bone mass and is catastrophic for the process of fracture repair,58 pointing to a significant role of macrophages in stimulating bone formation.
Three research groups, Guihard et al., Nicolaidou et al. and ourselves, have observed that monocyte-derived macrophages strongly stimulate the osteoblastic commitment of human MSCs.59,60,61 Guihard et al.59 observed that this depended on a diffusible factor released by M1 (classically) activated adult macrophages. Work with neutralizing antibodies showed that this was principally dependent upon their production of OSM, although IL-6 and LIF also contributed to the pro-osteoblastic actions. As detailed above, OSM had indeed previously been demonstrated to drive human and murine MSC commitment to mineralizing osteoblasts,25,46,47 and LIF has similar effects.51
Although an increase in osteoblast progenitor maturation induced by M1-activated macrophages may be surprising, given their production of tumor necrosis factor, this may shed some light on the patterns of abnormal bone formation seen in inflammatory bone diseases such as ankylosing spondylitis and in osteophyte formation. However, OSM can clearly be produced by macrophages under other stimulatory conditions, including in rheumatoid arthritis.55,62,63 Nicolaidou et al.60 observed a similarly strong OSM-dependent osteoblastic influence of monocyte-derived macrophages not with activation but when stimulated by contact with MSCs themselves. This might point to a very localized paracrine control of MSC maturation by macrophages in close proximity; the activity of bone resorption itself may attract MSCs64 that interact with local OSM-producing osteal macrophages, and this could lead to osteoblastic formation by such MSCs at or near the bone resorption site. Our own work identified that immature proliferative macrophages (probably naïve or M2 polarized), nevertheless, stimulated osteoblast maturation in MSCs in an OSM-dependent manner.61 Collectively, these observations suggest that macrophages of various types can be persuaded to provide an OSM-dependent stimulus of osteoblastic maturation of MSCs.
These studies emphasize the importance of MSCs themselves in bone formation, and their potential, in addition to mature osteoblasts and osteocytes, as targets for anabolic therapies. As MSCs are attracted to sites of bone resorption,64 it is plausible that they could participate in the bone formation that occurs in response to osteoclast-derived coupling factors in remodeling. Osteoclast formation at a site of resorption involves the recruitment of osteoclast progenitors (immature macrophages), but it remains to be seen whether these also influence MSCs.
T cells as a source of OSM
T cells also have the capacity to greatly influence bone metabolism, although the degree of their influence is controversial. T cells both produce and respond to OSM, which, for example, stimulates extrathymic T-cell development.65 In rheumatoid arthritis, both activated T cells and macrophages produce OSM, which, as described above, could thereby locally stimulate both focal bone destruction, by stimulating osteoclastogenesis, and periosteal bone formation, through actions on the osteoblast lineage. Although there is clear evidence that T cells might influence bone formation and resorption in inflammation, their influence in normal bone physiology is less certain given their relative paucity in the bone microenvironment and bone marrow cavity in normal physiological conditions. Activated T cells produce RANKL, which can drive osteoclast formation,66 and indeed ablation of RANKL in T cells mildly increases trabecular bone mass.67 However, as T cells do not express the OSMR subunit,68 it is unlikely that T-cell expression of RANKL is regulated by OSM.
Concluding statements
OSM is a multifactorial cytokine, expressed by a range of cells within the bone microenvironment (Figure 1), which thereby exert both anabolic and catabolic effects on bone. It appears that both these effects are mediated through the osteoblast lineage. OSM action via two different receptor complexes that activate JAK-STAT signaling (OSMR/gp130 and LIFR/gp130) give different cellular outcomes via different target cells, affecting osteoblast/stromal cell RANKL and osteocyte sclerostin, respectively, in vivo and in vitro.25 Its actions on bone have a number of parallels with those of PTH, which also exerts its actions via several different cellular targets,69 including OSMR and gp130. This suggests that the careful dissection of intracellular pathways elicited by OSM (and related cytokines) and evaluating the roles of its target cells in bone could lead to important insights into the design of novel anabolic therapies for bone.
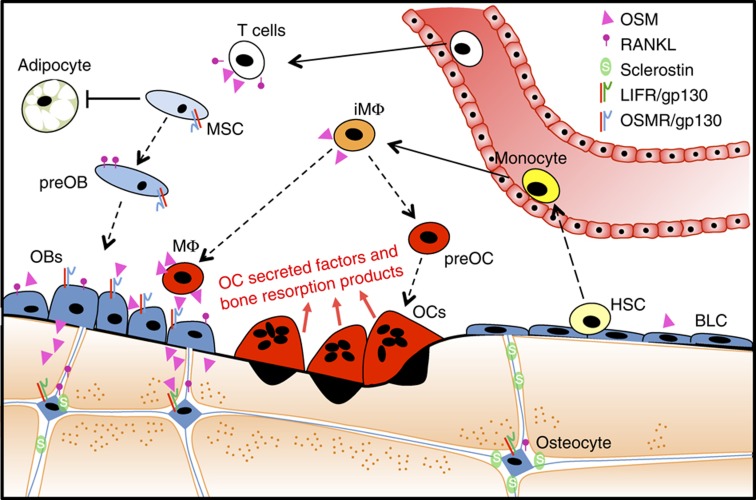
Figure 1.
Oncostatin M (OSM) is produced both by cells derived from hemopoietic stem cells (HSCs,; including T cells, immature macrophages (iMΦ), mature macrophages (MΦ)) and by the osteoblast lineage, including osteoblasts on the bone surface (OBs), bone lining cells (BLCs) and osteocytes. When preosteoblasts (preOBs), OBs and osteocytes are stimulated by OSM through the OSMR:gp130 complex, their differentiation (dotted lines) from mesenchymal stem cells (MSCs) is stimulated, and adipogenesis is inhibited. In addition, RANKL production is increased in these cells with OSM response through OSMR:gp130; T cells also produce soluble RANKL when activated, but do not express OSMR. RANKL stimulates the differentiation of osteoclast precursors (preOC) to mature osteoclasts (OCs) that resorb the bone matrix. OCs release factors (by secretion and through breakdown of bone matrix) that also stimulate osteoblast differentiation. In contrast to its action on RANKL mediated by OSMR:gp130, OSM acts on osteocytes through LIFR:gp130 to inhibit sclerostin production. This action results in increased bone formation by OBs on the bone surface.
Footnotes
The authors declare no conflict of interest.
References
- Zarling JM, Shoyab M, Marquardt H, Hanson MB, Lioubin MN, Todaro GJ et al. Oncostatin M: a growth regulator produced by differentiated histiocytic lymphoma cells. Proc Natl Acad Sci USA 1986;83:9739–9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair BC, DeVico AL, Nakamura S, Copeland TD, Chen Y, Patel A et al. Identification of a major growth factor for AIDS-Kaposi's sarcoma cells as oncostatin M. Science 1992;255:1430–1432. [DOI] [PubMed] [Google Scholar]
- Queen MM, Ryan RE, Holzer RG, Keller-Peck CR, Jorcyk CL. Breast cancer cells stimulate neutrophils to produce oncostatin M: potential implications for tumor progression. Cancer Res 2005;65:8896–8904. [DOI] [PubMed] [Google Scholar]
- Mozaffarian A, Brewer AW, Trueblood ES, Luzina IG, Todd NW, Atamas SP et al. Mechanisms of oncostatin M-induced pulmonary inflammation and fibrosis. J Immunol 2008;181:7243–7253. [DOI] [PubMed] [Google Scholar]
- Poling J, Gajawada P, Lorchner H, Polyakova V, Szibor M, Bottger T et al. The Janus face of OSM-mediated cardiomyocyte dedifferentiation during cardiac repair and disease. Cell Cycle 2012;11:439–445. [DOI] [PubMed] [Google Scholar]
- Taniya T, Tanaka S, Yamaguchi-Kabata Y, Hanaoka H, Yamasaki C, Maekawa H et al. A prioritization analysis of disease association by data-mining of functional annotation of human genes. Genomics 2012;99:1–9. [DOI] [PubMed] [Google Scholar]
- Simpson JL, Baines KJ, Boyle MJ, Scott RJ, Gibson PG. Oncostatin M (OSM) is increased in asthma with incompletely reversible airflow obstruction. Exp Lung Res 2009;35:781–794. [DOI] [PubMed] [Google Scholar]
- Pradeep AR, S TM, Garima G, Raju A. Serum levels of oncostatin M (a gp 130 cytokine): an inflammatory biomarker in periodontal disease. Biomarkers 15:277–282. [DOI] [PubMed] [Google Scholar]
- Sims NA, Walsh NC. GP130 cytokines and bone remodelling in health and disease. BMB Rep 2010;43:513–523. [DOI] [PubMed] [Google Scholar]
- Frost HM. Dynamics of bone remodeling. In: Frost HM (ed). Bone Biodynamics. Little, Brown & Co.: Boston, 1964, p315–333. [Google Scholar]
- Sims NA, Martin TJ. Coupling the activities of bone formation and resorption: a multitude of signals within the basic multicellular unit. BoneKEy Rep 2014;3:481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso L, Herman BC, Verborgt O, Laudier D, Majeska RJ, Schaffler MB. Osteocyte apoptosis controls activation of intracortical resorption in response to bone fatigue. J Bone Miner Res 2009;24:597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MK, Raggatt LJ, Alexander KA, Kuliwaba JS, Fazzalari NL, Schroder K et al. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J Immunol 2008;181:1232–1244. [DOI] [PubMed] [Google Scholar]
- Collin-Osdoby P, Rothe L, Anderson F, Nelson M, Maloney W, Osdoby P. Receptor activator of NF-kappa B and osteoprotegerin expression by human microvascular endothelial cells, regulation by inflammatory cytokines, and role in human osteoclastogenesis. J Biol Chem 2001;276:20659–20672. [DOI] [PubMed] [Google Scholar]
- Elefteriou F, Campbell P, Ma Y. Control of bone remodeling by the peripheral sympathetic nervous system. Calcif Tissue Int 2014;94:140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi YC, Baldock PA. Central and peripheral mechanisms of the NPY system in the regulation of bone and adipose tissue. Bone 2012;50:430–436. [DOI] [PubMed] [Google Scholar]
- Tamura T, Udagawa N, Takahashi N, Miyaura C, Tanaka S, Yamada Y et al. Soluble interleukin-6 receptor triggers osteoclast formation by interleukin 6. Proc Natl Acad Sci USA 1993;90:11924–11928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmqvist P, Persson E, Conaway HH, Lerner UH. IL-6, leukemia inhibitory factor, and oncostatin M stimulate bone resorption and regulate the expression of receptor activator of NF-kappa B ligand, osteoprotegerin, and receptor activator of NF-kappa B in mouse calvariae. J Immunol 2002;169:3353–3362. [DOI] [PubMed] [Google Scholar]
- O'Brien CA, Gubrij I, Lin SC, Saylors RL, Manolagas SC. STAT3 activation in stromal/osteoblastic cells is required for induction of the receptor activator of NF-kappaB ligand and stimulation of osteoclastogenesis by gp130-utilizing cytokines or interleukin-1 but not 1,25-dihydroxyvitamin D3 or parathyroid hormone. J Biol Chem 1999;274:19301–19308. [DOI] [PubMed] [Google Scholar]
- Levy JB, Schindler C, Raz R, Levy DE, Baron R, Horowitz MC. Activation of the JAK-STAT signal transduction pathway by oncostatin-M cultured human and mouse osteoblastic cells. Endocrinology 1996;137:1159–1165. [DOI] [PubMed] [Google Scholar]
- Kim S, Yamazaki M, Shevde NK, Pike JW. Transcriptional control of receptor activator of nuclear factor-kappaB ligand by the protein kinase A activator forskolin and the transmembrane glycoprotein 130-activating cytokine, oncostatin M, is exerted through multiple distal enhancers. Mol Endocrinol 2007;21:197–214. [DOI] [PubMed] [Google Scholar]
- Fu Q, Manolagas SC, O'Brien CA. Parathyroid hormone controls receptor activator of NF-kappaB ligand gene expression via a distant transcriptional enhancer. Mol Cell Biol 2006;26:6453–6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop KA, Meyer MB, Pike JW. A novel distal enhancer mediates cytokine induction of mouse RANKl gene expression. Mol Endocrinol 2009;23:2095–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli C, Zella LA, Fretz JA, Fu Q, Pike JW, Weinstein RS et al. Targeted deletion of a distant transcriptional enhancer of the receptor activator of nuclear factor-kappaB ligand gene reduces bone remodeling and increases bone mass. Endocrinology 2008;149:146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EC, McGregor NE, Poulton IJ, Solano M, Pompolo S, Fernandes TJ et al. Oncostatin M promotes bone formation independently of resorption when signaling through leukemia inhibitory factor receptor in mice. J Clin Invest 2010;120:582–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolin C, Tawara K, Sutherland C, Redshaw J, Aranda P, Moselhy J et al. Oncostatin m promotes mammary tumor metastasis to bone and osteolytic bone degradation. Genes Cancer 2012;3:117–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui W, Cawston TE, Richards CD, Rowan AD. A model of inflammatory arthritis highlights a role for oncostatin M in pro-inflammatory cytokine-induced bone destruction via RANK/RANKL. Arthritis Res Ther 2005;7:R57–R64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong PK, Quinn JM, Sims NA, van Nieuwenhuijze A, Campbell IK, Wicks IP. Interleukin-6 modulates production of T lymphocyte-derived cytokines in antigen-induced arthritis and drives inflammation-induced osteoclastogenesis. Arthritis Rheum 2006;54:158–168. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Hirabayashi Y, Sekiguchi T, Inoue T, Katsuki M, Miyajima A. Targeted disruption of oncostatin M receptor results in altered hematopoiesis. Blood 2003;102:3154–3162. [DOI] [PubMed] [Google Scholar]
- Johnson RW, Brennan HJ, Vrahnas C, Poulton IJ, McGregor NE, Standal T et al. The Primary Function of gp130 Signaling in Osteoblasts is to Maintain bone Formation and Strength, Rather than Promote Osteoclast Formation. J Bone Mineral Res (e-pub ahead of print 11 December 2013; 10.1002/jbmr.2159). [DOI] [PubMed] [Google Scholar]
- Ma YL, Cain RL, Halladay DL, Yang X, Zeng Q, Miles RR et al. Catabolic effects of continuous human PTH (1--38) in vivo is associated with sustained stimulation of RANKL and inhibition of osteoprotegerin and gene-associated bone formation. Endocrinology 2001;142:4047–4054. [DOI] [PubMed] [Google Scholar]
- Walker EC, Poulton IJ, McGregor NE, Ho PW, Allan EH, Quach JM et al. Sustained RANKL response to parathyroid hormone in oncostatin M receptor-deficient osteoblasts converts anabolic treatment to a catabolic effect in vivo. J Bone Miner Res 2012;27:902–912. [DOI] [PubMed] [Google Scholar]
- Holtrop ME, King GJ, Cox KA, Reit B. Time-related changes in the ultrastructure of osteoclasts after injection of parathyroid hormone in young rats. Calcif Tissue Int 1979;27:129–135. [DOI] [PubMed] [Google Scholar]
- Martin TJ, Sims NA. Osteoclast-derived activity in the coupling of bone formation to resorption. Trends Mol Med 2005;11:76–81. [DOI] [PubMed] [Google Scholar]
- Gooi JH, Pompolo S, Karsdal MA, Kulkarni NH, Kalajzic I, McAhren SH et al. Calcitonin impairs the anabolic effect of PTH in young rats and stimulates expression of sclerostin by osteocytes. Bone 2010;46:1486–1497. [DOI] [PubMed] [Google Scholar]
- Frolik CA, Black EC, Cain RL, Satterwhite JH, Brown-Augsburger PL, Sato M et al. Anabolic and catabolic bone effects of human parathyroid hormone (1–34) are predicted by duration of hormone exposure. Bone 2003;33:372–379. [DOI] [PubMed] [Google Scholar]
- Moverare Skrtic S, Henning P, Borjesson A, Sjogren K, Windahl S, Isaksson H et al. WNT16 is a novel osteoblast-derived paracrine regulator of osteoclastogenesis, cortical bone mass and fracture susceptibility. J Bone Miner Res 2013;28(Suppl 1): Available at http://www.asbmr.org/asbmr-2013-abstract-detail?aid=7082a5e7-f969-4126-9a02-950c97db185c Accessed October 30, 2013. [Google Scholar]
- Allan EH, Hausler KD, Wei T, Gooi JH, Quinn JM, Crimeen-Irwin B et al. EphrinB2 regulation by PTH and PTHrP revealed by molecular profiling in differentiating osteoblasts. J Bone Miner Res 2008;23:1170–1181. [DOI] [PubMed] [Google Scholar]
- Cornelissen C, Luscher-Firzlaff J, Baron JM, Luscher B. Signaling by IL-31 and functional consequences. Eur J Cell Biol 2012;91:552–566. [DOI] [PubMed] [Google Scholar]
- Bilsborough J, Mudri S, Chadwick E, Harder B, Dillon SR. IL-31 receptor (IL-31RA) knockout mice exhibit elevated responsiveness to oncostatin M. J Immunol 2010;185:6023–6030. [DOI] [PubMed] [Google Scholar]
- van Bezooijen RL, ten Dijke P, Papapoulos SE, Lowik CW. SOST/sclerostin, an osteocyte-derived negative regulator of bone formation. Cytokine Growth Factor Rev 2005;16:319–327. [DOI] [PubMed] [Google Scholar]
- Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med 2011;17:1231–1234. [DOI] [PubMed] [Google Scholar]
- Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O'Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med 2011;17:1235–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosley B, De Imus C, Friend D, Boiani N, Thoma B, Park LS et al. Dual oncostatin M (OSM) receptors. Cloning and characterization of an alternative signaling subunit conferring OSM-specific receptor activation. J Biol Chem 1996;271:32635–32643. [DOI] [PubMed] [Google Scholar]
- Ichihara M, Hara T, Kim H, Murate T, Miyajima A, Oncostatin M. and leukemia inhibitory factor do not use the same functional receptor in mice. Blood 1997;90:165–173. [PubMed] [Google Scholar]
- Gimble JM, Wanker F, Wang CS, Bass H, Wu X, Kelly K et al. Regulation of bone marrow stromal cell differentiation by cytokines whose receptors share the gp130 protein. J Cell Biochem 1994;54:122–133. [DOI] [PubMed] [Google Scholar]
- Song HY, Jeon ES, Kim JI, Jung JS, Kim JH. Oncostatin M promotes osteogenesis and suppresses adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells. J Cell Biochem 2007;101:1238–1251. [DOI] [PubMed] [Google Scholar]
- Fu Q, Jilka RL, Manolagas SC, O'Brien CA. Parathyroid hormone stimulates receptor activator of NFkappa B ligand and inhibits osteoprotegerin expression via protein kinase A activation of cAMP-response element-binding protein. J Biol Chem 2002;277:48868–48875. [DOI] [PubMed] [Google Scholar]
- Saleh H, Eeles D, Hodge JM, Nicholson GC, Gu R, Pompolo S et al. Interleukin-33, a target of parathyroid hormone and oncostatin m, increases osteoblastic matrix mineral deposition and inhibits osteoclast formation in vitro. Endocrinology 2011;152:1911–1922. [DOI] [PubMed] [Google Scholar]
- Quach JM, Walker EC, Allan E, Solano M, Yokoyama A, Kato S et al. Zinc finger protein 467 is a novel regulator of osteoblast and adipocyte commitment. J Biol Chem 2011;286:4186–4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulton IJ, McGregor NE, Pompolo S, Walker EC, Sims NA. Contrasting roles of leukemia inhibitory factor in murine bone development and remodeling involve region-specific changes in vascularization. J Bone Miner Res 2012;27:586–595. [DOI] [PubMed] [Google Scholar]
- Lisignoli G, Piacentini A, Toneguzzi S, Grassi F, Cocchini B, Ferruzzi A et al. Osteoblasts and stromal cells isolated from femora in rheumatoid arthritis (RA) and osteoarthritis (OA) patients express IL-11, leukaemia inhibitory factor and oncostatin M. Clin Exp Immunol 2000;119:346–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantila Roosa SM, Liu Y, Turner CH. Gene expression patterns in bone following mechanical loading. J Bone Miner Res 2011;26:100–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiland GR, Zwerina K, Baum W, Kireva T, Distler JH, Grisanti M et al. Neutralisation of Dkk-1 protects from systemic bone loss during inflammation and reduces sclerostin expression. Ann Rheumatic Dis 2010;69:2152–2159. [DOI] [PubMed] [Google Scholar]
- Gilbert LC, Chen H, Lu X, Nanes MS. Chronic low dose tumor necrosis factor-alpha (TNF) suppresses early bone accrual in young mice by inhibiting osteoblasts without affecting osteoclasts. Bone 2013;56:174–183. [DOI] [PubMed] [Google Scholar]
- Bohm C, Derer A, Axmann R, Hillienhoff U, Zaiss MM, Luther J et al. RSK2 protects mice against TNF-induced bone loss. J Cell Sci 2012;125:2160–2171. [DOI] [PubMed] [Google Scholar]
- Walsh NC, Gravallese EM. Bone remodelling in rheumatic diseases: a question of balance. Immunol Rev 2010;233:301–312. [DOI] [PubMed] [Google Scholar]
- Alexander KA, Chang MK, Maylin ER, Kohler T, Muller R, Wu AC et al. Osteal macrophages promote in vivo intramembranous bone healing in a mouse tibial injury model. J Bone Miner Res 26:1517–1532. [DOI] [PubMed] [Google Scholar]
- Guihard P, Danger Y, Brounais B, David E, Brion R, Delecrin J et al. Induction of osteogenesis in mesenchymal stem cells by activated monocytes/macrophages depends on oncostatin M signaling. Stem Cells 2012;30:762–772. [DOI] [PubMed] [Google Scholar]
- Nicolaidou V, Wong MM, Redpath AN, Ersek A, Baban DF, Williams LM et al. Monocytes induce STAT3 activation in human mesenchymal stem cells to promote osteoblast formation. PLoS ONE 2012;7:e39871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes TJ, Hodge JM, Singh PP, Eeles DG, Collier FM, Holten I et al. Cord blood-derived macrophage-lineage cells rapidly stimulate osteoblastic maturation in mesenchymal stem cells in a glycoprotein-130 dependent manner. PLoS ONE 2013;8:e73266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H, Yamamura M, Morita Y, Harada S, Makino H, Ota Z. The synovial expression and serum levels of interleukin-6, interleukin-11, leukemia inhibitory factor, and oncostatin M in rheumatoid arthritis. Arthritis Rheum 1997;40:1096–1105. [DOI] [PubMed] [Google Scholar]
- Shrestha S, Tang J, Kaslow RA. Gene copy number: learning to count past two. Nat Med 2009;15:1127–1129. [DOI] [PubMed] [Google Scholar]
- Tang Y, Wu X, Lei W, Pang L, Wan C, Shi Z et al. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med 2009;15:757–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg CH, Rulffes JT, Wallace PM, Haugen HS. Regulation of an extrathymic T-cell development pathway by oncostatin M. Nature 1996;384:261–263. [DOI] [PubMed] [Google Scholar]
- Horwood NJ, Elliott J, Martin TJ, Gillespie MT. Osteotropic agents regulate the expression of osteoclast differentiation factor and osteoprotegerin in osteoblastic stromal cells. Endocrinology 1998;139:4743–4746. [DOI] [PubMed] [Google Scholar]
- Fumoto T, Takeshita S, Ito M, Ikeda K. Physiological functions of osteoblast lineage and T cell-derived RANKL in bone homeostasis. J Bone Miner Res 2013;29:830–842. [DOI] [PubMed] [Google Scholar]
- Tamura S, Morikawa Y, Tanaka M, Miyajima A, Senba E. Developmental expression pattern of oncostatin M receptor beta in mice. Mech Dev 2002;115:127–131. [DOI] [PubMed] [Google Scholar]
- Sims NA. Building bone with a SOST-PTH partnership. J Bone Miner Res 2010;25:175–177. [DOI] [PubMed] [Google Scholar]