Abstract
Background
The frequency of migraine headaches is higher in women than in men and in susceptible women attacks are related to changes in ovarian hormone levels. Intracranial mast cells (MCs) are likely to play a role in migraine headache genesis, and changes in the dural MC population might influence headache susceptibility. The present study thus tested the hypothesis that sex and ovarian hormones influence the density and phenotypic makeup of dural MCs.
Methods
Histochemistry combined with quantitative analyses was employed to investigate sex differences, estrous cycle and ovarian hormone influences on dural MCs density, phenotype and degranulation level in males and females rats.
Results
Our data show that in female rats, dural MC density fluctuates during the estrous cycle and is overall higher than in males. In ovariectomized rats, estradiol, but not progesterone, promoted an increase in dural MCs density. This effect was abolished by a splenectomy, suggesting estrogen-related recruitment of MCs from the spleen. Finally, our data suggest that the phenotypic make up of dural MCs, which represents the level of cellular maturity, is also governed by changes in estrogen levels.
Conclusions
Given the potential role of dural MCs in triggering headache, our data suggest that estrogen-related modulation of dural MC density and phenotypic makeup could play a role in mediating the higher frequency and severity of headaches, such as migraine, in women.
Introduction
The intracranial meninges are considered a key player in the genesis of headaches of intracranial origin (1–3). One major process that might contribute to headache triggering is sterile meningeal inflammation and the ensuing persistent activation of primary afferent nociceptive neurons that innervate the meninges and their related large blood vessels. The dural layer of the meninges is the one most heavily innervated by nociceptive afferents and is also highly populated by immune cells, including macrophages, dendritic cells and mast cells (MCs) (4, 5). Dural MCs are of particular interest because of their close proximity to dural nociceptors (6, 7). Given their pro-inflammatory properties, dural MCs have been suggested to play a role in headache precipitation (8–11). We have shown recently that degranulation of dural MCs can lead to persistent activation of dural nociceptors and the headache pain pathway (12), and that such response can lead to behavioral changes reminiscent of migraine headaches in humans (13). Taken together, dural MCs seem to be well positioned to serve as powerful mediators of the inflammatory cascade that leads to intracranial headaches. Given that MCs respond to a host of potential headache triggers (9, 10, 14–16), it is conceivable that endogenous or exogenous factors that influence the dural MC population might play a role in modulating headache susceptibility, frequency, or even the severity of attacks in subsets of headache sufferers.
Two related major factors that contribute to increased headache susceptibility are sex and changes in ovarian hormones. Headaches, in particular migraines, are more common in women than in men (17), and estrogen is thought to play a key role in mediating this susceptibility (18). The possibility that changes in dural MC density or maturation might influence headache susceptibility and the observation that in female rodents ovarian hormones influence MC populations, primarily within the reproductive system (19–21), has led us to explore the possibility that dural MCs are also subjected to such endocrine influence. In this work we thus tested in rats: 1) whether the density and phenotype of dural MC is sexually dimorphic 2) whether ovarian hormones, in particular estrogen influence the dural MC population and 3) whether the spleen might serve as a major source of MCs that are affected by estrogen and recruited to the intracranial dura mater.
Materials and methods
Animals
Adult, age-matched (60–70 days old) female (n=67) and male (n=7) Sprague-Dawley rats (Taconic) were kept in a climate-controlled room on a 12 h light/dark cycle with food and water available ad libitum. Handling, care, maintenance and testing of the animals were performed in accordance with the policies and recommendations of the International Association for the Study of Pain (IASP) and the National Institutes of Health guidelines for the handling and use of laboratory animals. The Animal Care and Use Committees of the Beth Israel Deaconess Medical Center and Harvard Medical School approved all experimental protocols. In normally cycling females, at least 2 weeks of daily vaginal smears were conducted to determine the estrous stage. Only those animals, which showed at least two consecutive regular 4-day estrous cycles were used. Cycling females were tested during proestrus, estrus, diestrus 1 and diestrus 2.
Surgeries and hormone administrations
A group of female rats underwent a bilateral ovariectomy (OVX) at 7–8 weeks of age (Performed by Taconic) and were treated with ovarian hormones 2 weeks later. Some female rats underwent an OVX concomitant with a splenectomy (SPLX). Vaginal smears were conducted to verify the lack of cyclical vaginal changes following OVX at the effect of hormone treatments. Post mortem autopsies were conducted to verify complete removals of the ovaries and spleen. To examine the effect of ovarian hormones, OVX females were injected S.C. with 0.1 ml of sesame oil (vehicle) containing 17-β-estradiol (E2, 5 μg) or progesterone (P4, 2.5 mg) + E2 (5 μg). Untreated animals were injected only with the vehicle. All injections were made between 8–9 AM.
Histology
Animals were deeply anesthetized (100 mg/kg Nembutal) and perfused through the ascending aorta with 0.1M phosphate-buffered saline (PBS) followed by 4% formalin solution in PBS. The dura mater was dissected as indicated previously (12). Briefly, a craniotomy was made to expose the supratentorial dura mater, which was then dissected free and cut along the superior sagittal sinus and one side of the transverse sinus. The supracerebellar dura remained attached to one of the dural specimen. Dural tissues were then mounted flat on slides and subjected to one of two histochemical staining protocols. To investigate potential changes in the density of the two major dural MC phenotypes, dural samples were subjected to the Alcian blue/Safranin histochemical method by incubating them for 40 min with acidified solutions containing 2% Alcian Blue (AB) followed by 5 min counterstaining with a 2% Safranin (SAF). Given that >95% of dural MCs were stained either only with SAF or with both SAF and AB (see also ref (22)) changes in the population stained only with AB is not reported herein. To investigate MC degranulation, the other dural half was stained for 1 minute with an acidified 0.1% solution of toluidine blue. Following dehydration in graded alcohols and xylene the slides were cover slipped. MC density was determined under X100 magnification by counting the number of red-stained cells (SAF+/AB− phenotype) or mixed red and blue staining (SAF+/AB+ phenotypes). The average MC density of each phenotype was based on MC counts from 15 different randomly chosen visual fields both rostral to the transverse sinus as well as caudal to it over in the supracerebellar tissue (7). Because the TB staining methods allows visualizing extruded MC granules, this method was used to investigate the degree of MC degranulation. To do so, TB-stained MCs were visualized under a X200 magnification in 10 random visual fields rostral and caudal to the transverse sinus. Cells were considered degranulated if there was an extensive dispersion of more than 15 extruded vesicles localized near the cell, or when there was an extensive loss of granule staining, giving the cell a “ghostly” look (12). MC counts, phenotype and degranulation level were conducted by a person blinded to the stage of the estrous cycle or treatment.
Statistical Analyses
Data is represented as means (± SEM) and was compared using non-parametric statistics. The Kruskal–Wallis one-way analysis of variance was used to compare differences between the densities of different MC phenotypes and overall MC densities as well as differences between the degrees of MC degranulation in the various groups. Two-group comparisons were conducted using the Mann-Whitney U test. All analyses were conducted using Statview (SAS institute). P ≤ 0.05 was considered statistically significant.
Results
Effects of estrous cycle, sex and OVX on dural MC density and phenotype
In normally cycling females (Figs 1, 2A), dural MC density fluctuated across the estrous cycle with significant changes observed for the SAF+/AB− (p<0.01) and SAF+/AB+ (p<0.05) phenotypes as well as for overall MC density (p<0.05). When compared to the diestrus 2 or proestrus phases, overall MC density was higher in estrus (p<0.01), which was largely due to an increase in the SAF+/AB+ phenotype (p<0.05). The increase in overall MC density was also evident during the next phase of the cycle, in diestrus 1 (p<0.01). At this stage of the cycle, there was also a major phenotypic change whereby the SAF+/AB− phenotype became the dominant phenotype (p<0.01, compared to all other estrous phases). This increase in MC density occurred concomitant with a decline in the density of the SAF+/AB+ phenotype. When comparing the overall density of dural MCs across the different phases of the estrous cycle to those observed in males, females had a higher MC density in all phases but proestrus (Fig 2A, p<0.05). When overall dural MC densities were averaged across the estrous cycle, values were significantly higher than those seen in the dura mater of males (Fig 2B, p<0.05). As Fig 2B further depicts, the sexual dimorphic difference in dural MC density was largely due to increased density of the SAF+/AB+ phenotype (p<0.05) in females. The density of dural MCs and their phenotypic makeup in 2 weeks OVX females was similar to that seen in males.
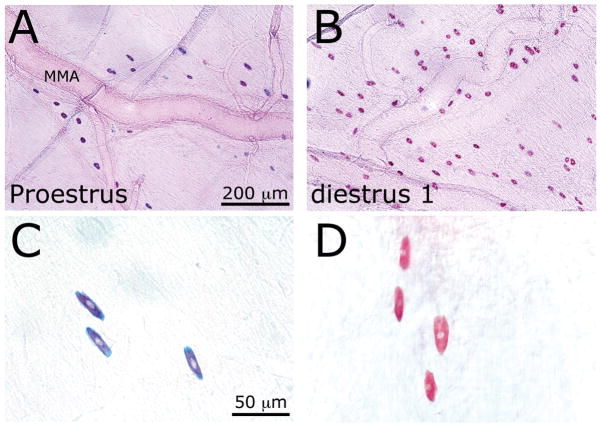
Figure 1.
(A) SAF/AB staining of dural MCs in the vicinity of the middle meningeal artery (MMA) in proestrus (a) and diestrus 1 (B) females. Note the higher MC density in diestrus 1. Also note the phenotypic difference between the two hormonal phases, with the SAF+/AB+) red and blue) phenotype in proestrus (c) and the prominent SAF+/AB− (red) phenotype in diestrus 1 (D).
Figure 2.
Dural MC density and phenotypic makeup in females and males. (A) Density and phenotypic makeup of dural MCs during the 4 stages of the estrous cycles, diestrus 2 (D2, n=6), proestrus (P, n=6), estrus (E, n=6) and diestrus 1 (D1, n=6). When compared to the P phase, statistical differences in the density of SAF+/AB+ phenotype are marked with the # sign (p<0.05), difference in the SAF+/AB-phenotype are marked with a ++ sign (p<0.01), and overall MC density with a ** sign (p<0.01). (B) Comparisons of the dural MC density of the two major phenotypes between males (n=7), females (n=24) and 2 weeks OVX females (n=7). When compared to males, intact females had overall higher MC density (*, p<0.05) that was primarily due to differences in the SAF+/AB+ population (#, p<0.05).
Effects of ovarian hormone treatments in OVX rats on dural MC density and phenotype
The administration of a single dose of E2 2 weeks after OVX gave rise to an increase in dural MC density. As Fig 3A demonstrates, when compared to vehicle-treated OVX females, 24 hrs following E2 treatment, there was an increase in the overall MC density (p<0.01), which was primarily due to an increase in the density of the SAF+/AB+ phenotype (p<0.05). At 48 hrs, the overall heightened density was maintained but the SAF+/AB− phenotype became the dominant phenotype (p<0.01). At 72 hrs post E2 administration, there was a decline in the densities of the SAF+/AB− and SAF+/AB+ phenotypes to a level that was no different than that seen in the vehicle-treated OVX females. Administration of P4 together with E2 blunted the increase in dural MCs density seen after E2 treatment alone, both at 24 hrs (not shown) and 48 hrs, with values similar to those seen in vehicle-treated OVX females. To investigate whether the spleen is a possible source of the MCs recruited to the dura mater under E2 influence, we tested whether E2 treatment leads to a similar dural MC hyperplasia in OVX and splenectomized (SPLX) animals. As Fig 3B depicts, in SPLX/OVX females the overall density or phenotypic makeup of dural MCs was not different when compared to OVX females with intact spleen. However, SPLX significantly inhibited the E2-mediated increases in the density of the SAF+/AB− MC phenotype (p<0.05) as well as overall MC density (p<0.05).
Figure 3.
Effects of acute treatment with ovarian hormones on overall MC density and their phenotypic makeup in 2 weeks OVX females and the effect of SPLX. (A) Administration of E2 (n=5/group) promoted a time-dependent increase in dural MC density and change in phenotype. Compared to vehicle-treated OVX females (OVX+V), the overall density of dural MCs increased at 24 and 48 hrs post treatment (**, p<0.01). Note the increases in the SAF+/AB+ phenotype at 24 hrs (#, p<0.05) and that of the SAF+/AB− phenotype at 48 hrs (++, p<0.01). Administration of E2+P4 (n=4) did not lead to an increase in dural MC density or phenotypic change. (B) A SPLX conducted together with OVX (n=4) significantly ameliorated the changes in the density of the SAF+/AB-phenotype a (+, p<0.05) as well as the overall dural MC density (*, p<0.05, vs OVX+E2) seen 48 hrs after E2 treatments. SPLX alone (n=4) did not affect dural MC density or phenotypic makeup in vehicle-treated OVX females.
Effect of the estrous cycle, OVX and ovarian hormone treatments on dural MC degranulation
As Fig 4 indicates, in cycling females there were no significant changes at the level of dural MC degranulation during the estrous cycle, although there was a trend for increased MC degranulation in proestrus. OVX or SPLX surgeries alone also did not influence dural MC degranulation level. Similarly, treatment of OVX female with E2 or vehicle did not affect the level of MC degranulation. However, 48 hrs following the treatment of OVX animals with E2+P4, the level of dural MC degranulation increased four-fold when compared to untreated OVX females (p<0.05).
Figure 4.

Level of dural MC degranulation in males, females during the estrus cycle and OVX females subjected to E2 or E2+P4 treatments. Only administration of E2+P4 led to an increase in the number of dural MCs showing signs of degranulation. *, p<0.05 compared to vehicle-treated OVX females.
Discussion
Studies in male rodents have indicated that under normal physiological conditions the population of mature MCs in connective tissues such as the dura (i.e. skin) is stable and has a relatively slow turnover (1–2 weeks) (23, 24). Studies in female rodents suggest however that in certain tissues, particularly the reproductive organs, MC density is subjected to fluctuations during the reproductive cycle. For example, the density of ovarian MCs increases during the phase of heightened estrogen levels (i.e. at proestrus) and declines when estrogen levels fall (25). In the uterus, however, MC density peaks two days after proestrus, during the diestrus 1 phase (26). In the brain, thalamic MCs also undergo rapid changes during the estrous cycle with increasing number of cells seen during the proestrus and estrus phases (27). The present study provides evidence that in female rats the density of dural MCs is also subjected to cyclical changes related to the 4-day estrous cycle, and that the increases in their density are similar to that seen in the uterus during the phases of estrus and diestrus 1.
Our study showed that in all but one phase of the estrous cycle (i.e. in proestrus), females exhibited higher dural MC densities than males. Sex differences in the density of rats’ thalamic MCs were also reported (28), although it is unclear whether all stages of the estrous cycle contributed equally to those findings. The sexual dimorphic differences observed in dural MC density may be related to the effect that hormonal fluctuations exert during the estrous cycle and/or due to chromosomal influences. The cyclical changes during the estrous cycle point to a role of ovarian hormones. The lower density of dural MCs in OVX females and the finding that acute administration of E2 to OVX rats led to a time-dependent increase in dural MCs density to levels seen in normally cycling females further suggest the influence of ovarian hormones. This latter finding together with the lack of changes seen in the E2+P4 treated group suggests that E2 is the main ovarian hormone that contributes to the increase in dural MC density and that P4 actions likely oppose this effect. The increased density of dural MCs during estrus and diestrus 1 and following E2 treatment in OVX females likely reflect the recruitment of MCs from one or more extradural sources. MCs are derived from immature hematopoietic progenitors that originate in the bone marrow. These progenitors are only minimally granulated and upon migration to tissues undergo granulation and final maturation in situ (29). Some MC progenitors may be retained within reservoirs, mainly in the spleen, (30, 31) and can further migrate to a specific tissue in response to chemotactic stimuli. The spleen has been implicated as the source of the leukocytes that infiltrate the ovaries during the night between proestrus and estrus (32). Our finding that a SPLX prevented the E2-related increase in dural MC density in OVX rats suggests that the spleen may be a major source of the MCs that are attracted to the dura under estrogenic influence. Although not assessed in this study due to the modulatory effect of SPLX on the recurrence of the estrous cycle in rats (33), it is plausible that the spleen serves as the source of the MCs that migrate to the dura during estrus and diestrus 1. The possibility that under estrogenic influence the spleen secretes molecules that enhance the migration and localization of MCs to the dura mater should also be considered.
The molecular mechanisms underlying the E2-related recruitment of MC to the dura mater as well as other tissues remains unclear. In vitro, E2 treatment can lead to upregulation of the chemokine receptors CCR3 and CCR5 in MCs of mice and CCR4 and CCR5 in human-derived MCs (21). These receptors could play a role in the migration of MCs towards various chemokines, including CCL5 (RANTES) (21, 34). The failure of dual treatment with E2 and P4 to increase MCs density in the dura is reminiscent of the inability of such treatment to increase MC numbers in the thalamus (27). This effect of P4 may be related to its ability to inhibit the expression of the CXCR4 receptor, another chemokine receptor that is necessary for the migration of MCs towards RANTES (35). It should be noted nonetheless that in the uterus P4 is not inhibitory when administered with E2 (21). Another possible action of P4 that could explain the lack of increase in the number of dural MCs following the combined E2 and P4 treatment is by enhancing dural MC degranulation. Such action might have caused a reduction in the number of cells amenable to the SAF/AB staining (which stains MC granules), thus resulting in a seemingly lower MC count. Because there was no significant increase in dural MCs degranulation during the estrous cycle, this P4-related MC degranulation is unlikely to have clinical relevance. The possibility that this effect was the result of a heightened physiological response related to the pharmacodynamics and potentially pharmacokinetics of the exogenously administered P4 should be considered.
We employed the Safranin and Alcian blue histochemical staining protocol to examine whether the estrous cycle and ovarian hormone replacement in OVX animals affect distinctively the density of the two major MC phenotypes found in the dura mater - the SAF+/AB− and SAF+/AB+ phenotypes (22, 36). These staining properties result from different proteglycan granule content - the SAF+/AB− phenotype contains primarily the proteoglycan heparin while the SAF+/AB+ phenotype contains a mix of heparin and non-heparin proteoglycan granules, mostly chondroitin sulphate E (37, 38). It has been suggested that the SAF+/AB− phenotype represents the fully mature form of connective tissue MCs while the SAF+/AB+ is regarded as a granulated but less mature form of connective tissue MCs (39, 40). In our study, we observed that the initial rise in dural MC density during the estrus phase reflected primarily an increase in the density of the SAF +/AB+ phenotype. However, the main phenotype seen during the peak phase, 24 hours later (i.e. diestrus 1), was the presumably more mature SAF+/AB− phenotype. Because this phenotypic switch occurred in the absence of a further increase in overall MC density, a likely mechanism is the differentiation of the SAF+/AB+ into the SAF+/AB− phenotype. Acute administration of E2 to OVX females had a similar effect on the phenotypic makeup of dural MCs - an initial increase in the density of the SAF+/AB+ phenotype followed by an increase in the SAF+/AB− phenotype a day later. These findings further indicate the possibility that E2-mediated MC recruitment to the dura mater is followed by a local maturation process. It is worth noting that the decrease in dural MC density seen a day following the attained peak, in both the normally cycling females and E2-treated OVX females, was not accompanied by increase in dural MC degranulation suggesting that such decrease was due to their migration outside the dura mater.
The phenotypic switch from the SAF+/AB+ to the SAF+/AB− phenotype is associated with increased granule heparin content. This change is also likely to be associated with increased granule content of the inflammatory mediators histamine, 5HT and proteases, which form complexes with this proteoglycan (41, 42). An increase in the content of these inflammatory mediators could potentially result in increased efficacy of the SAF+/AB− phenotype to mount an inflammatory response. In our study we observed that females, overall, exhibited higher density of the presumably less mature SAF+/AB+ phenotype when compared to males. It should be noted however that despite being considered less mature, dural MCs of the SAF+/AB+ phenotype can also become activated and release their granule contents (36). Given that the degree of the inflammatory response related to MC activation is likely determined by their local density as well as the repertoire and content of their inflammatory mediators, further functional electrophysiological or behavioral studies will be required to examine whether the higher density of the SAF+/AB+ phenotype seen in females in most estrous phases is associated with enhanced MC-related meningeal nociception, which could contribute to the sex difference in migraine susceptibility.
The finding that during diestrus 1, females had the highest density of the more mature SAF+/AB− MC phenotype is intriguing and suggests that at this hormonal stage a trigger leading to MC activation could promote a larger inflammatory response and presumably increased nociceptive response. While caution is needed when comparing the estrus cycle in rodents to the menstrual cycle in women, it is interesting to note that the hormonal milieu present during diestrus 1 (low E2 and P4 levels) is parallel to that of the late luteal phase in women (See Fig 1 in ref 43). Because the frequency of migraine attacks and the severity of the headache are known to increase during this hormonal phase in women, a condition known as menstrual migraine (18), it is tempting to speculate that the changes in density and phenotype of dural MCs we observed during the fall in the levels of ovarian hormones in rats might also be present in women during the premenstrual phase and thus may directly contribute to the development of menstrual migraine.
In summary, based on the ability of MCs to mount inflammation and promote intracranial nociception (10), we propose that factors capable of regulating dural MC density, maturity and their propensity to release inflammatory mediators could play a role in modulating headache susceptibility, frequency, or even the severity of an attack. Based on the present study, we suggest that estrogen-mediated changes in dural MCs density and phenotype could play a role in mediating the increase in migraine prevalence, frequency, and severity in women. Further studies are required to determine whether MC-related meningeal inflammation and activation of the headache pain pathway is enhanced during hormonal conditions that give rise to increased dural MC density and maturity. This process of estrogen-related dural MC migration and maturation might serve as a target for future migraine headache therapy.
Acknowledgments
Supported by grants from NIH (NINDS 061116), the National Headache Foundation and the American Headache Society.
References
- 1.Levy D. Migraine pain and nociceptor activation--where do we stand? Headache. 2010;50(2010):909–16. doi: 10.1111/j.1526-4610.2010.01670.x. [DOI] [PubMed] [Google Scholar]
- 2.Messlinger K. Migraine: where and how does the pain originate? Experimental brain research Experimentelle Hirnforschung. 2009;196(2009):179–93. doi: 10.1007/s00221-009-1756-y. [DOI] [PubMed] [Google Scholar]
- 3.Olesen J, Burstein R, Ashina M, Tfelt-Hansen P. Origin of pain in migraine: evidence for peripheral sensitisation. Lancet neurology. 2009;8(2009):679–90. doi: 10.1016/S1474-4422(09)70090-0. [DOI] [PubMed] [Google Scholar]
- 4.Zhang XC, Kainz V, Jakubowski M, Burstein R, Strassman A, Levy D. Localization of COX-1 and COX-2 in the intracranial dura mater of the rat. Neuroscience letters. 2009;452(2009):33–6. doi: 10.1016/j.neulet.2009.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McMenamin PG. Distribution and phenotype of dendritic cells and resident tissue macrophages in the dura mater, leptomeninges, and choroid plexus of the rat brain as demonstrated in wholemount preparations. The Journal of comparative neurology. 1999;405(1999):553–62. [PubMed] [Google Scholar]
- 6.Rozniecki JJ, Dimitriadou V, Lambracht-Hall M, Pang X, Theoharides TC. Morphological and functional demonstration of rat dura mater mast cell-neuron interactions in vitro and in vivo. Brain research. 1999;849(1999):1–15. doi: 10.1016/s0006-8993(99)01855-7. [DOI] [PubMed] [Google Scholar]
- 7.Strassman AM, Weissner W, Williams M, Ali S, Levy D. Axon diameters and intradural trajectories of the dural innervation in the rat. The Journal of comparative neurology. 2004;473(2004):364–76. doi: 10.1002/cne.20106. [DOI] [PubMed] [Google Scholar]
- 8.Sicuteri F. Mast cell and their active substances: Their role in the pathogenesis of migraine. Headache. 1963;3(1963):86. doi: 10.1111/j.1526-4610.1963.hed0303086.x. [DOI] [PubMed] [Google Scholar]
- 9.Theoharides TC. Mast cells and migraines. Perspect Biol Med. 1983;26(1983):672–5. doi: 10.1353/pbm.1983.0028. [DOI] [PubMed] [Google Scholar]
- 10.Levy D. Migraine pain, meningeal inflammation, and mast cells. Current pain and headache reports. 2009;13(2009):237–40. doi: 10.1007/s11916-009-0040-y. [DOI] [PubMed] [Google Scholar]
- 11.Smith JH, Butterfield JH, Cutrer FM. Primary headache syndromes in systemic mastocytosis. Cephalalgia : an international journal of headache. 2011;31(2011):1522–31. doi: 10.1177/0333102411421683. [DOI] [PubMed] [Google Scholar]
- 12.Levy D, Burstein R, Kainz V, Jakubowski M, Strassman AM. Mast cell degranulation activates a pain pathway underlying migraine headache. Pain. 2007;130(2007):166–76. doi: 10.1016/j.pain.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levy D, Kainz V, Burstein R, Strassman AM. Mast cell degranulation distinctly activates trigemino-cervical and lumbosacral pain pathways and elicits widespread tactile pain hypersensitivity. Brain Behav Immun. 2011;2011 doi: 10.1016/j.bbi.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baun M, Pedersen MH, Olesen J, Jansen-Olesen I. Dural mast cell degranulation is a putative mechanism for headache induced by PACAP-38. Cephalalgia : an international journal of headache. 2012;32(2012):337–45. doi: 10.1177/0333102412439354. [DOI] [PubMed] [Google Scholar]
- 15.Ottosson A, Edvinsson L. Release of histamine from dural mast cells by substance P and calcitonin gene-related peptide. Cephalalgia. 1997;17(1997):166–74. doi: 10.1046/j.1468-2982.1997.1703166.x. [DOI] [PubMed] [Google Scholar]
- 16.Reuter U, Bolay H, Jansen-Olesen I, Chiarugi A, Sanchez del Rio M, Letourneau R, et al. Delayed inflammation in rat meninges: implications for migraine pathophysiology. Brain. 2001;124(2001):2490–502. doi: 10.1093/brain/124.12.2490. [DOI] [PubMed] [Google Scholar]
- 17.Stovner L, Hagen K, Jensen R, Katsarava Z, Lipton R, Scher A, et al. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia. 2007;27(2007):193–210. doi: 10.1111/j.1468-2982.2007.01288.x. [DOI] [PubMed] [Google Scholar]
- 18.MacGregor EA. Menstrual migraine. Current opinion in neurology. 2008;21(2008):309–15. doi: 10.1097/WCO.0b013e3282fd185e. [DOI] [PubMed] [Google Scholar]
- 19.Harvey EB. Mast Cell Distribution in the Uterus of Cycling and Pregnant Hamsters. Anat Rec. 1964;148(1964):507–16. doi: 10.1002/ar.1091480309. [DOI] [PubMed] [Google Scholar]
- 20.Gibbons AF, Chang MC. Number of mast cells in the rat uterus with special reference to its relation to hormonal treatment and decidual response. Biol Reprod. 1972;6(1972):193–203. doi: 10.1093/biolreprod/6.2.193. [DOI] [PubMed] [Google Scholar]
- 21.Jensen F, Woudwyk M, Teles A, Woidacki K, Taran F, Costa S, et al. Estradiol and progesterone regulate the migration of mast cells from the periphery to the uterus and induce their maturation and degranulation. PloS one. 2010;5(2010):e14409. doi: 10.1371/journal.pone.0014409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michaloudi H, Batzios C, Chiotelli M, Papadopoulos GC. Developmental changes of mast cell populations in the cerebral meninges of the rat. Journal of anatomy. 2007;211(2007):556–66. doi: 10.1111/j.1469-7580.2007.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitamura Y, Shimada M, Hatanaka K, Miyano Y. Development of mast cells from grafted bone marrow cells in irradiated mice. Nature. 1977;268(1977):442–3. doi: 10.1038/268442a0. [DOI] [PubMed] [Google Scholar]
- 24.Fukuzumi T, Waki N, Kanakura Y, Nagoshi J, Hirota S, Yoshikawa K, Kitamura Y. Differences in irradiation susceptibility and turnover between mucosal and connective tissue-type mast cells of mice. Exp Hematol. 1990;18(1990):843–7. [PubMed] [Google Scholar]
- 25.Gaytan F, Aceitero J, Bellido C, Sanchez-Criado JE, Aguilar E. Estrous cycle-related changes in mast cell numbers in several ovarian compartments in the rat. Biol Reprod. 1991;45(1991):27–33. doi: 10.1095/biolreprod45.1.27. [DOI] [PubMed] [Google Scholar]
- 26.Maraspin LE, Bo WJ. Effects of hormones, pregnancy and pseudopregnancy on the mast cell count in the rat uterus. Life Sci I. 1971;10(1971):111–20. doi: 10.1016/0024-3205(71)90084-1. [DOI] [PubMed] [Google Scholar]
- 27.Kovacs KJ, Larson AA. Mast cells accumulate in the anogenital region of somatosensory thalamic nuclei during estrus in female mice. Brain research. 2006;1114(2006):85–97. doi: 10.1016/j.brainres.2006.07.100. [DOI] [PubMed] [Google Scholar]
- 28.Goldschmidt RC, Hough LB, Glick SD. Rat brain mast cells: contribution to brain histamine levels. J Neurochem. 1985;44(1985):1943–7. doi: 10.1111/j.1471-4159.1985.tb07191.x. [DOI] [PubMed] [Google Scholar]
- 29.Hallgren J, Gurish MF. Pathways of murine mast cell development and trafficking: tracking the roots and routes of the mast cell. Immunol Rev. 2007;217(2007):8–18. doi: 10.1111/j.1600-065X.2007.00502.x. [DOI] [PubMed] [Google Scholar]
- 30.Kitamura Y, Yokoyama M, Matsuda H, Ohno T, Mori KJ. Spleen colony-forming cell as common precursor for tissue mast cells and granulocytes. Nature. 1981;291(1981):159–60. doi: 10.1038/291159a0. [DOI] [PubMed] [Google Scholar]
- 31.Gurish MF, Boyce JA. Mast cells: Ontogeny, homing, and recruitment of a unique innate effector cell. J Allergy Clin Immunol. 2006;117(2006):1285–91. doi: 10.1016/j.jaci.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 32.Oakley OR, Kim H, El-Amouri I, Lin PC, Cho J, Bani-Ahmad M, Ko C. Periovulatory leukocyte infiltration in the rat ovary. Endocrinology. 2010;151(2010):4551–9. doi: 10.1210/en.2009-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsuyama S, Ohta M, Takahashi M. The critical period in which splenectomy causes functional disorder of the ovary in adult rats. Endocrinol Jpn. 1987;34(1987):849–55. doi: 10.1507/endocrj1954.34.849. [DOI] [PubMed] [Google Scholar]
- 34.Conti P, Reale M, Barbacane RC, Felaco M, Grilli A, Theoharides TC. Mast cell recruitment after subcutaneous injection of RANTES in the sole of the rat paw. Br J Haematol. 1998;103(1998):798–803. doi: 10.1046/j.1365-2141.1998.01060.x. [DOI] [PubMed] [Google Scholar]
- 35.Belot MP, Abdennebi-Najar L, Gaudin F, Lieberherr M, Godot V, Taieb J, et al. Progesterone reduces the migration of mast cells toward the chemokine stromal cell-derived factor-1/CXCL12 with an accompanying decrease in CXCR4 receptors. American journal of physiology Endocrinology and metabolism. 2007;292(2007):E1410–7. doi: 10.1152/ajpendo.00286.2006. [DOI] [PubMed] [Google Scholar]
- 36.Dimitriadou V, Buzzi MG, Moskowitz MA, Theoharides TC. Trigeminal sensory fiber stimulation induces morphological changes reflecting secretion in rat dura mater mast cells. Neuroscience. 1991;44(1991):97–112. doi: 10.1016/0306-4522(91)90253-k. [DOI] [PubMed] [Google Scholar]
- 37.Mayrhofer G. Fixation and staining of granules in mucosal mast cells and intraepithelial lymphocytes in the rat jejunum, with special reference to the relationship between the acid glycosaminoglycans in the two cell types. Histochem J. 1980;12(1980):513–26. doi: 10.1007/BF01011925. [DOI] [PubMed] [Google Scholar]
- 38.Seldin DC, Adelman S, Austen KF, Stevens RL, Hein A, Caulfield JP, Woodbury RG. Homology of the rat basophilic leukemia cell and the rat mucosal mast cell. Proceedings of the National Academy of Sciences of the United States of America. 1985;82(1985):3871–5. doi: 10.1073/pnas.82.11.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yong LC, Watkins S, Wilhelm DL. The mast cell: distribution and maturation in the peritoneal cavity of the adult rat. Pathology. 1975;7(1975):307–18. doi: 10.3109/00313027509081687. [DOI] [PubMed] [Google Scholar]
- 40.Feyerabend TB, Hausser H, Tietz A, Blum C, Hellman L, Straus AH, et al. Loss of histochemical identity in mast cells lacking carboxypeptidase A. Mol Cell Biol. 2005;25(2005):6199–210. doi: 10.1128/MCB.25.14.6199-6210.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pejler G, Maccarana M. Interaction of heparin with rat mast cell protease 1. The Journal of biological chemistry. 1994;269(1994):14451–6. [PubMed] [Google Scholar]
- 42.Forsberg E, Pejler G, Ringvall M, Lunderius C, Tomasini-Johansson B, Kusche-Gullberg M, et al. Abnormal mast cells in mice deficient in a heparin-synthesizing enzyme. Nature. 1999;400(1999):773–6. doi: 10.1038/23488. [DOI] [PubMed] [Google Scholar]
- 43.Staley K, Scharfman H. A woman’s prerogative. Nature neuroscience. 2005;8(2005):697–9. doi: 10.1038/nn0605-697. [DOI] [PubMed] [Google Scholar]