The challenge of restenosis has been with us since the dawn of intravascular percutaneous interventions. Much effort has been spent on developing strategies designed to effectively treat it. These ranged from mechanical means such as minimal plaque removal with an idea of not “disturbing” the media (directional atherectomy), to extensive debulking (rotational atherectomy), to creating the largest possible lumen and then preventing the recoil (stenting), to systemic and local use of numerous drug classes including antithrombotic, anti-platelet, antiproliferative drugs, calcium channel blockers, statins, steroids, and other antiinflammatory agents among the others.
While most of these failed, as frequently happens in medicine, the effective therapy was arrived at much earlier than any glimmer of understanding of the biology of the process that was treated became apparent. Bare metal stents offered a major reduction in restenosis, largely by preventing arterial recoil despite a markedly increased stress on the media and unappreciated at the time damage to the adventitia. Interestingly, whereas angiographically defined restenosis was substantially reduced, intimal proliferation was actually increased,1 emphasizing that bare metal stenting was a purely mechanical means for dealing with the problem and that the fundamental biology was not affected. The first truly significant reduction in intimal formation was achieved using drug eluting stents with either paclitaxel- or sirolimus-like compounds. The effect was ascribed to their antiproliferative effect on smooth muscle cells (SMC) even though multiple other antiproliferative drugs showed no comparable activity, suggesting there was something special about these 2 classes of medications. Nevertheless, the matter seemed settled until a belated realization of increased late stent thrombosis of drug-eluting stents attributable to delayed endothelial coverage of stented surfaces brought realization of the need for alternative approaches and better understanding of biology.
In parallel with mechanical efforts to limit restenosis, biological approaches have focused on understanding the processes driving neointimal development and means of stopping them. Early studies have suggested that the loss of endothelial coverage after an intraarterial injury exposed media to circulating growth factors thereby stimulating SMC proliferation and migration. Therefore, restoring endothelial coverage was seen as the means to terminate neointimal growth by removing the stimulus. Indeed, several studies by Isner and colleagues using plasmid-mediated VEGF gene transfer suggested that VEGF-driven reendothelialization reduces restenosis.2 However, later studies failed to confirm these findings and suggested that VEGF treatment increased neointimal formation thus promoting restenosis.3,4 A clinical trial of local VEGF delivery for restenosis failed to show any benefit.5 Furthermore, additional investigations have shown that neointima formation could be induced in the absence of any damage to the endothelium, eg, with an intact endothelial layer, further questioning the link between the luminal endothelial loss and restenosis.6 At the same time it became increasingly clear that an inflammatory response in the adventitia, defined by the presence of blood-derived mononuclear cells7 and accompanied by adventitial angiogenesis,8 may significantly impact neointimal formation. Indeed, stimulation of adventitial angiogenesis with VEGF, FGF2, or PR39 led to a profound restenotic response in vessels with intact endothelial coverage, whereas suppression of angiogenesis using VEGF traps or inhibitors of FGF signaling greatly reduced it.6
Several other lines of evidence have further linked adventitial angiogenesis to restenosis, including a direct demonstration of a correlation between micro-CT–determined extent of adventitial angiogenesis and the extent of neointima,9–11 the correlation between the magnitude of the arterial stretch and the extent of neointimal formation,1 and an equally well-established correlation between the inflammatory response and angiogenesis.12 Finally, one frequently forgotten aspect of restenosis biology is the extensive vascularization of the neointima,13 with adventitial vasa vasorum being the primary sources of this “intraarterial” vasculature.14 Therefore it looked plausible that VEGF, by promoting adventitial angiogenesis, would indeed promote restenosis. However, although these potential prorestenotic effects of VEGF became apparent, the mechanism of its action remained unresolved.
The paper by Koga et al in this issue of Arteriosclerosis, Thrombosis, and Vascular Biology adds important information to our understanding of this biology.15 The authors have used a knock-in mouse line with a VEGF receptor 1 (VEGF-R1 or Flt-1) replaced by a truncated form of the receptor that does not have tyrosine kinase activity. The mutant receptor is still expressed on the cell surface and is able to bind VEGF with high affinity but intracellular signaling is abolished. Flt-1 has a broad distribution with expression detectable not only in endothelial cells but in blood mononuclear cells among a number of other cell types. The Flt-1 mutant mice demonstrate the same extent of neointimal formation after arterial injury as the wild-type mice and exhibited the same extent of mononuclear cell infiltration. Blockade of VEGF signaling using a VEGF trap abolished neointimal formation in both Flt-1 mutant and control mice, consistent with previous observations. Thus, the entire process of VEGF-driven neointimal formation appears to be independent of Flt signaling.
This is unexpected as the ability of VEGF to induce monocyte accumulation at the site of injury via Flt-1 has long been considered the sine qua non of its biology. Instead, the mechanism apparently involves VEGF-induced stimulation of MCP1 expression by SMCs via SMC VEGF-R2. The activated SMCs then secrete MCP-1 thereby inducing monocyte accumulation. The presence of both VEGF receptors on SMCs has been well accepted16 although most VEGF effects on SMCs, including induction of SMC migration, have been thought to occur via Flt-1.17 The role of MCP-1 in restenosis18 and indeed in angiogenesis12 has also been long established, but the VEGF connection is new. Furthermore, although the role of endogenous VEGF in prorestenotic activity of MCP-1 has been hinted at previously,19 the Koga study suggests a much more compelling mechanism of action.
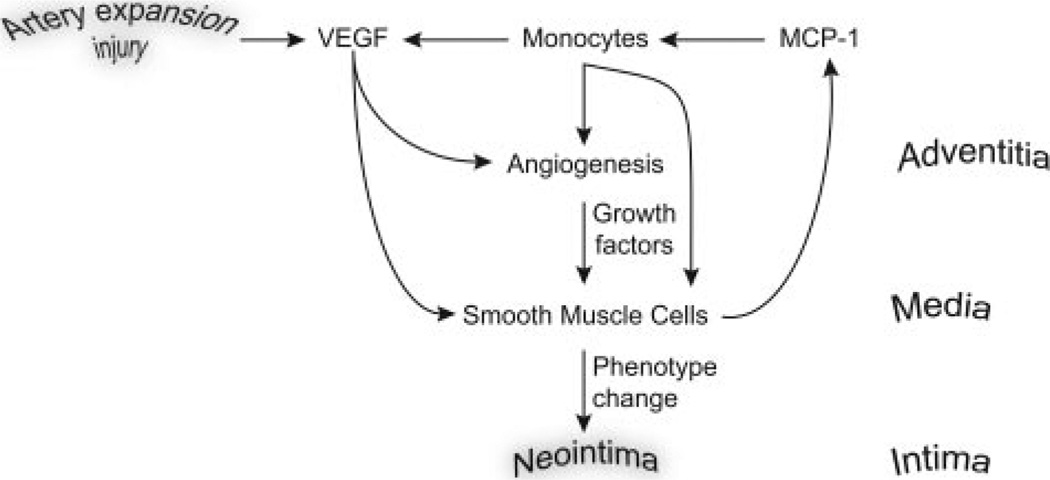
Taken together with the evolving body of knowledge on the biology of restenosis, the plausible sequence of events is illustrated in the Figure. The damage to the adventitia by a balloon stretch/stent expansion leads to the initiation of a local inflammatory response and production of VEGF which, in turn, induces MCP-1 expression in media SMCs while also promoting adventitial angiogenesis. MCP-1 production by SMCs leads to accumulation of monocytes in the adventitia that secrete additional VEGF thereby leading to a positive amplification of the cascade. Together, mitogenic SMC stimulation by MCP-1, VEGF, and other growth factors present in this niche such as FGF2, leads to SMC phenotypic modulation that allows migration and formation of neointima supported by adventitia-derived vasa vasorum.
Figure.
Figure legend text.
The key points of this paradigm are that processes controlling neointimal formation occur in the adventitia, and that the state of endothelial coverage of the luminal segment of the artery is less important in terms of restenosis than previously thought. Ultimately, the effective management of stented arteries will require inhibition of adventitial inflammation and angiogenesis (and in this context it is interesting to note antiangiogenic activity of paclitaxel) or the ability to prevent SMC phenotypic modulation (a newly discovered effect of rapamycin20) to prevent neointima formation and restenosis and promotion of reendothelialization of the luminal surface to reduce thrombosis.
Footnotes
Disclosures
None.
References
- 1.Kuntz RE, Gibson CM, Nobuyoshi M, Baim DS. Generalized model of restenosis after conventional balloon angioplasty, stenting and directional atherectomy. J Am Coll Cardiol. 1993;21:15–25. doi: 10.1016/0735-1097(93)90712-a. [DOI] [PubMed] [Google Scholar]
- 2.Asahara T, Chen D, Tsurumi Y, Kearney M, Rossow S, Passeri J, Symes JF, Isner JM. Accelerated restitution of endothelial integrity and endothelium-dependent function after phVEGF165 gene transfer. Circulation. 1996;94:3291–3302. doi: 10.1161/01.cir.94.12.3291. [DOI] [PubMed] [Google Scholar]
- 3.Bhardwaj S, Roy H, Heikura T, Yla-Herttuala S. VEGF-A, VEGF-D and VEGF-D(DeltaNDeltaC) induced intimal hyperplasia in carotid arteries. Eur J Clin Invest. 2005;35:669–676. doi: 10.1111/j.1365-2362.2005.01555.x. [DOI] [PubMed] [Google Scholar]
- 4.Shiojima I, Walsh K. The role of vascular endothelial growth factor in restenosis: the controversy continues. Circulation. 2004;110:2283–2286. doi: 10.1161/01.CIR.0000146723.23523.47. [DOI] [PubMed] [Google Scholar]
- 5.Hedman M, Hartikainen J, Syvanne M, Stjernvall J, Hedman A, Kivela A, Vanninen E, Mussalo H, Kauppila E, Simula S, Narvanen O, Rantala A, Peuhkurinen K, Nieminen MS, Laakso M, Yla-Herttuala S. Safety and feasibility of catheter-based local intracoronary vascular endothelial growth factor gene transfer in the prevention of postangioplasty and in-stent restenosis and in the treatment of chronic myocardial ischemia: phase II results of the Kuopio Angiogenesis Trial (KAT) Circulation. 2003;107:2677–2683. doi: 10.1161/01.CIR.0000070540.80780.92. [DOI] [PubMed] [Google Scholar]
- 6.Khurana R, Zhuang Z, Bhardwaj S, Murakami M, De Muinck E, Yla-Herttuala S, Ferrara N, Martin JF, Zachary I, Simons M. Angiogenesis-dependent and independent phases of intimal hyperplasia. Circulation. 2004;110:2436–2443. doi: 10.1161/01.CIR.0000145138.25577.F1. [DOI] [PubMed] [Google Scholar]
- 7.Ohtani K, Egashira K, Hiasa K, Zhao Q, Kitamoto S, Ishibashi M, Usui M, Inoue S, Yonemitsu Y, Sueishi K, Sata M, Shibuya M, Sunagawa K. Blockade of vascular endothelial growth factor suppresses experimental restenosis after intraluminal injury by inhibiting recruitment of monocyte lineage cells. Circulation. 2004;110:2444–2452. doi: 10.1161/01.CIR.0000145123.85083.66. [DOI] [PubMed] [Google Scholar]
- 8.Khurana R, Simons M, Martin JF, Zachary IC. Role of angiogenesis in cardio-vascular disease: a critical appraisal. Circulation. 2005;112:1813–1824. doi: 10.1161/CIRCULATIONAHA.105.535294. [DOI] [PubMed] [Google Scholar]
- 9.Kwon HM, Sangiorgi G, Ritman EL, Lerman A, McKenna C, Virmani R, Edwards WD, Holmes DR, Schwartz RS. Adventitial vasa vasorum in balloon-injured coronary arteries: visualization and quantitation by a microscopic three-dimensional computed tomography technique. J Am Coll Cardiol. 1998;32:2072–2079. doi: 10.1016/s0735-1097(98)00482-3. [DOI] [PubMed] [Google Scholar]
- 10.Kantor B, Mohlenkamp S. Imaging of myocardial microvasculature using fast computed tomography and three-dimensional microscopic computed tomography. Cardiol Clin. 2003;21:587–605. ix. doi: 10.1016/s0733-8651(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 11.Cheema AN, Hong T, Nili N, Segev A, Moffat JG, Lipson KE, Howlett AR, Holdsworth DW, Cole MJ, Qiang B, Kolodgie F, Virmani R, Stewart DJ, Strauss BH. Adventitial microvessel formation after coronary stenting and the effects of SU11218, a tyrosine kinase inhibitor. J Am Coll Cardiol. 2006;47:1067–1075. doi: 10.1016/j.jacc.2005.08.076. [DOI] [PubMed] [Google Scholar]
- 12.Simons M. Angiogenesis: where do we stand now? Circulation. 2005;111:1556–1566. doi: 10.1161/01.CIR.0000159345.00591.8F. [DOI] [PubMed] [Google Scholar]
- 13.Brasen JH, Kivela A, Roser K, Rissanen TT, Niemi M, Luft FC, Donath K, Yla-Herttuala S. Angiogenesis, vascular endothelial growth factor and platelet-derived growth factor-BB expression, iron deposition, and oxidation-specific epitopes in stented human coronary arteries. Arterioscler Thromb Vasc Biol. 2001;21:1720–1726. doi: 10.1161/hq1101.098230. [DOI] [PubMed] [Google Scholar]
- 14.Drinane M, Mollmark J, Zagorchev L, Moodie K, Sun B, Hall A, Shipman S, Morganelli P, Simons M, Mulligan-Kehoe MJ. The antiangiogenic activity of rPAI-123 inhibits vasa vasorum and growth of atherosclerotic plaque. Circ Res. doi: 10.1161/CIRCRESAHA.108.184622. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koga J, Matoba T, Egashira K, Kubo M, Miyagawa M, Iwata E, Sueishi K, Shibuya M, Sunagawa K. Soluble Flt-1 gene transfer ameliorates neointima formation after wire injury in flt-1 tyrosine kinase deficient mice. Arterioscler Thromb Vasc Biol. 2009;29:458–464. doi: 10.1161/ATVBAHA.109.183772. [DOI] [PubMed] [Google Scholar]
- 16.Ishida A, Murray J, Saito Y, Kanthou C, Benzakour O, Shibuya M, Wijelath ES. Expression of vascular endothelial growth factor receptors in smooth muscle cells. J Cell Physiol. 2001;188:359–368. doi: 10.1002/jcp.1121. [DOI] [PubMed] [Google Scholar]
- 17.Banerjee S, Mehta S, Haque I, Sengupta K, Dhar K, Kambhampati S, Van Veldhuizen PJ, Banerjee SK. VEGF-A165 induces human aortic smooth muscle cell migration by activating neuropilin-1-VEGFR1-PI3K axis. Biochemistry. 2008;47:3345–3351. doi: 10.1021/bi8000352. [DOI] [PubMed] [Google Scholar]
- 18.Egashira K, Zhao Q, Kataoka C, Ohtani K, Usui M, Charo IF, Nishida K, Inoue S, Katoh M, Ichiki T, Takeshita A. Importance of monocyte chemoattractant protein-1 pathway in neointimal hyperplasia after periarterial injury in mice and monkeys. Circ Res. 2002;90:1167–1172. doi: 10.1161/01.res.0000020561.03244.7e. [DOI] [PubMed] [Google Scholar]
- 19.Parenti A, Bellik L, Brogelli L, Filippi S, Ledda F. Endogenous VEGF-A is responsible for mitogenic effects of MCP-1 on vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2004;286:H1978–H1984. doi: 10.1152/ajpheart.00414.2003. [DOI] [PubMed] [Google Scholar]
- 20.Martin KA, Merenick BL, Ding M, Fetalvero KM, Rzucidlo EM, Kozul CD, Brown DJ, Chiu HY, Shyu M, Drapeau BL, Wagner RJ, Powell RJ. Rapamycin promotes vascular smooth muscle cell differentiation through insulin receptor substrate-1/phosphatidylinositol 3-kinase/Akt2 feedback signaling. J Biol Chem. 2007;282:36112–36120. doi: 10.1074/jbc.M703914200. [DOI] [PubMed] [Google Scholar]