Abstract
Activins and estrogens participate in regulating the breakdown of ovarian germ cell nests and follicle assembly in mammals. In 1994, our group reported elevated frequencies of abnormal, multioocytic ovarian follicles in 6 month old, environmental contaminant-exposed female alligators after gonadotropin challenge. Here, we investigated if maternal contribution of endocrine disrupting contaminants to the egg subsequently alters estrogen/inhibin/activin signaling in hatchling female offspring, putatively predisposing an increased frequency of multioocytic follicle formation. We quantified basal and exogenous gonadotropin-stimulated concentrations of circulating plasma steroid hormones and ovarian activin signaling factor mRNA abundance in hatchling alligators from the same contaminated (Lake Apopka) and reference (Lake Woodruff) Florida lakes, as examined in 1994. Basal circulating plasma estradiol and testosterone concentrations were greater in alligators from the contaminated environment, whereas activin/inhibin βA subunit and follistatin mRNA abundances were lower than values measured in ovaries from reference lake animals. Challenged, contaminant-exposed animals showed a more robust increase in plasma estradiol concentration following an acute follicle stimulating hormone (FSH) challenge compared with reference site alligators. Aromatase and follistatin mRNA levels increased in response to an extended FSH challenge in the reference site animals, but not in the contaminant-exposed animals. In hatchling alligators, ovarian follicles have not yet formed; therefore, these endocrine differences are likely to affect subsequent ovarian development, including ovarian follicle assembly.
Morphological malformations are often caused by underlying genetic, endocrine, or physiological abnormalities. Multioocytic follicles (MOFs, alternatively called polyovular follicles) are two or more oocytes surrounded by a common follicular envelope of granulosa cells. These malformed follicles are hypothesized to result from oogonial clusters that do not properly dissociate and remodel during normal ovarian follicle assembly (Iguchi and Takasugi, '86; Iguchi et al., '86; Kipp et al., 2007a; Mayo et al., 2007). Normally, this morphology is rare. However, increased frequencies of MOFs can be generated in animals by experimental or pharmaceutical prenatal and neonatal exposure to estrogens, such as the natural endogenous estrogen, estradiol-17β (E2) (Nakamura et al., 2008), pharmaceutical estrogens, such as diethylstilbestrol (DES) (Kim et al., 2009), or phytoestrogens (Jefferson et al., 2006). Additionally, transgenic modifications that lead to over expression of ovarian signaling factors, such as the inhibin α subunit (Inha), also can produce this pathology in rodents (McMullen et al., 2001). Therefore, MOFs can have multiple etiologies, but these factors could converge on a common signaling network regulating follicle assembly (Mayo et al., 2007).
Until a decade ago, research largely viewed increased frequencies of MOFs as a mammalian pathology resulting from improper estrogenic exposure. However, in 1994, our group reported MOFs at a very high frequency in female alligators exposed during embryonic development to environmental contaminants. Lake Apopka, Florida, is contaminated with various pesticides and anthropogenic nutrients (Heinz et al., '91; Guillette et al., '99; Rauschenberger et al., 2007), resulting in reproductive impairments to resident alligators (Woodward et al., '93; Fujisaki et al., 2007; Milnes and Guillette, 2008). Female hatchling alligators from Lake Apopka, after being administered a luteinizing hormone challenge, displayed MOFs (often 3–4 oocytes per follicle) and elevated plasma E2 concentrations, compared with a low (0–3% of the follicles in an ovary) frequency observed in reference females of a similar age within the same study (Guillette et al., '94b).
Studies of estrogenic exposures using another crocodilian species (Caiman latirostris) support our findings of altered gonadal morphology and endocrine physiology. Bisphenol A (BPA) is an industrial chemical shown to have estrogenic and antiandrogenic properties (Richter et al., 2007). Across vertebrates, BPA has the potential to induce a wide range of impacts on gonadal functions (Crain et al., 2007). In ovo exposure of developing caiman to E2 or BPA resulted in male to female sex reversal (Stoker et al., 2003). Therefore, as earlier observed in alligators (Crain et al., '97), embryonic crocodilian gonads are responsive to exogenous steroidal signaling through both endogenous ligands and endocrine disrupting contaminant exposures. Further, laboratory-raised female caiman exposed in ovo to E2 or BPA, during the beginning of sex differentiation, displayed elevated circulating E2 concentrations and higher proportions of advanced follicles as hatchlings. In animals incubated at female-producing temperatures and sex-reversed females incubated at male-producing temperatures, E2 treatment increased MOF frequencies in juvenile (3- and 12-months post-hatching) caiman (Stoker et al., 2008). Furthermore, BPA exposure increased MOF frequency in sex-reversed females compared with control sex-reversed animals. These findings support a hypothesis that in ovo exposure to endocrine disrupting contaminants can result in long-lasting impacts on reproductive endpoints.
At hatching, alligator ovaries do not possess follicles. Follicle assembly occurs slowly over many months post-hatching (Forbes, '40; Moore et al., 2010b, 2008), and the mechanisms regulating this process are being investigated. In mice, follicle assembly occurs over the first 3 days postnatal and some of the signaling mechanisms that regulate follicle assembly are becoming clear. Transforming growth factor β (TGF-β) superfamily signaling plays a vital role in follicle assembly and establishment of the ovarian follicle pool (Drummond, 2005; Trombly et al., 2009). Activins are transforming growth factor ligands that act as ovarian paracrine signals and regulate a variety of endpoints, including growth and cellular differentiation (Pangas et al., 2007; Onagbesan et al., 2009). Activin signaling plays a role in regulating follicle assembly in neonatal mice. Augmentation of activin levels in neonatal mice increases germ and granulosa cell proliferation and primordial follicle numbers in juvenile animals (Bristol-Gould et al., 2006).
TGF-β superfamily ligands form from large precursor proteins that are processed and assembled into mature dimers. Activin ligands are homo or heterodimers of two β subunits (Inhba and Inhbb). Activin ligands act as agonists, work through membrane-bound activin receptor complexes, stimulate Smad-mediated secondary messenger cascades, and ultimately modulate gene expression (Ethier and Findlay, 2001). Production of inhibins or follistatin (Fst) antagonizes activin signaling. Inhibin ligands, activin receptor binding and activation antagonists, are heterodimers of a β subunit and an α subunit (Inha), forming either inhibin A (Inhba+Inha) or inhibin B (Inhbb+Inha). Follistatin is a TGF-β ligand antagonist that binds and neutralizes activins (Nakamura et al., '92; Welt et al., 2002). We have demonstrated the expression of Inhba, Inhbb, Fst, and Inha mRNA in alligator gonads during the first 5 months post-hatching (Moore et al., 2010a). Furthermore, expression levels were sexually dimorphic, with testes expressing elevated levels of Inha and Inhbb mRNA and ovaries expressing elevated Fst mRNA levels.
During germ cell nest breakdown and subsequent follicle assembly, an activin-dominated signaling milieu could be critical (Mayo et al., 2007; Trombly et al., 2009). Activins participate in signaling crosstalk with steroid hormones. Estrogens suppress activin gene expression (Kipp et al., 2007a) while, in turn, activins induce the expression of estrogen receptors (Kipp et al., 2007b).
In rodents, it is hypothesized that high levels of maternal steroids impede follicle assembly and after birth, steroid levels fall and potentiate ovarian follicle assembly (Kezele and Skinner, 2003; Pepling, 2006; Chen et al., 2007), possibly via TGF-β superfamily signaling pathways (Mayo et al., 2007). Follicle stimulating hormone (FSH) may also play a role in regulating follicle assembly. When E2 levels fall in the postnatal mouse, serum FSH levels rise from birth to day 7. These changes encompass the period of primordial follicle formation. In vitro organ culture of mouse ovaries in the presence of low E2 (fetal level) allows FSH to upregulate the expression levels of FSH receptor (Fshr), activin βA subunit, and oocyte-specific transcription factors associated with primordial follicle formation (Lei et al., 2010). Furthermore, FSH facilitates the breakdown of germ cell nests and primordial follicle formation at both high (maternal) and low (fetal) E2 organ culture conditions. These results support a hypothesis that the regulation of ovarian follicle formation involves an integration of estrogen, activin, and FSH signaling.
In contrast to eutherian embryonic development, embryonic alligators do not maintain a direct maternal endocrine connection during embryonic development. However, alligator eggs are invested with a substantial, maternally derived yolk that supplies nutrient throughout in ovo and post-hatching development. Environmental contaminants, including pesticides, are passed from mother to yolk (Rauschenberger et al., 2007). Furthermore, Lake Apopka contaminants at concentrations observed in alligator eggs interact with alligator estrogen receptors in vitro (Vonier et al., '96) and produce male to female sex reversal after in ovo exposure of turtle (Trachemys scripta elegans) embryos (Willingham and Crews, '99). Thus, Lake Apopka is contaminated with a mix of chemicals that potentially impact multiple physiological systems; however, these results demonstrate that many Lake Apopka contaminants display estrogenic activity.
We have observed that alligators hatched from eggs removed from wild nests before sex determination and incubated under identical conditions with eggs from reference populations exhibited an increased frequency of MOFs. Therefore, factors that lead to an increased frequency of MOFs are not exogenous factors experienced during development in the nest, such as temperature or humidity, but more likely pass maternally to the embryo via the egg. We propose that in female alligators from Lake Apopka, the maternal contribution of contaminants to the egg yolk subsequently alters inhibin/activin signaling in female offspring putatively through alteration of estrogenic signaling and, therefore, predisposes increased frequency of MOF formation.
Here, we employ the paradigm of ovarian follicle assembly regulation through interactions between estrogen, activin, and FSH signaling. We examine both basal and FSH-stimulated levels of circulating E2 and testosterone (T) and gonadal mRNA expression levels of Inha, Inhba, Fst, aromatase (Cyp19a1), and Fshr. Comparatively, ovaries of both embryonic and hatchling chicken are responsive to exogenous FSH, both in vitro (Pedernera et al., '99) and in vivo (Gonzalez-Moran, '98; Mendez-Herrera et al., '98; Sanchez-Bringas et al., 2006), resulting in elevated circulating estradiol and ovarian cell proliferation. Furthermore, in cultured chicken granulosa, activin signaling is necessary to maintain morphological differentiation (Schmierer et al., 2003), whereas FSH increases expression of Inha, Fst, and Inhba mRNA (Davis et al., 2001; Safi et al., 2003). Differences in the levels of these factors between contaminant exposed and reference animals could differentially increase in a gonadotropin-challenged ovary, as occurred just before necropsy in the cohort described in our earlier work. We hypothesize that early exposure to abnormal estrogenic signaling alters both basal and stimulated levels of circulating steroid hormones and transcript levels of activin signaling factors.
MATERIALS AND METHODS
We collected American alligator (Alligator mississippiensis, Daudin, 1801) eggs from nests at Lake Woodruff National Wildlife Refuge and Lake Apopka on June 27 and 28, 2005, respectively (Permit #WX01310), before the period of temperature-dependent sex determination (Ferguson and Joanen, '83). Eggs were candled to assess viability at the University of Florida. Two of the eight clutches collected from Lake Apopka were entirely nonviable, whereas all Lake Woodruff clutches (n = 15) contained viable eggs. We used seven Lake Woodruff and six Lake Apopka clutches of eggs for this study (remaining clutches were assigned to other experiments). A subset of viable eggs from these clutches were systematically intermixed, placed into trays of damp sphagnum moss, and incubated at a female-producing temperature of 30°C. Daily rotation of trays minimized regional temperature effects within incubators.
Animal procedures conformed to an IACUC-approved protocol. Hatching animals were web tagged with numbered Monel tags and measured for body masses (BM), snout–vent lengths (SVL), and total lengths (TL). Alligators were co-housed in a temperature-controlled animal room in tanks (~20 neonates/0.7 m3) and experienced a 16:8 photoperiod with heat lamps for basking and daily water changes. Ambient room temperatures ranged from 27 to 31°C. We supplied no food during the experimental period because alligators subsist off the internalized yolk sac during this time (first 2 weeks post-hatching).
Hatching order systematically assigned animals to one of four experimental groups: necropsy at 1, 2, or 5 days after hatching or to a grow-out experiment not addressed in this article. Those animals examined either 2 or 5 days after hatching were part of similar FSH challenge studies of differing durations. Because reptile FSH preparations are not commercially available, we treated the animals with ovine FSH (Sigma-Aldrich #F8174-1VL). Earlier experimentation has shown robust hormonal and/or ovarian responsiveness to treatment in alligators (Lance and Vliet, '87; Edwards et al., 2004) and other reptiles (Jones et al., '75; Jones and Swain, 2000), and FSH directly modulates activin signaling in a variety of species (Knight, '96; Kumar et al., '97; Davis et al., 2001). Challenge study animals received either a sham needle insertion or IM injections of 0.8% sterile saline vehicle (isotonic to alligator blood), low dose (10 ng/g BM FSH) or high dose (50 ng/g BM FSH) to the base of the tail on a daily basis. We administered all treatments in an injection volume of 90 μL between 11:00 and 12:00 hr. Animals examined 2 days after hatching received one treatment on the day after hatching, whereas those examined 5 days after hatching received one treatment per day for the four consecutive post-hatching days.
Necropsies commenced at 12:00 hr on appointed days. Immediately before euthanasia, 1 mL of blood was collected from the supravertebral blood vessel, followed by a lethal dose (0.5 mg/g BM) of sodium pentobarbital (Sigma). Blood collected in a heparinized Vacutainer (BD Diagnostics, Franklin Lakes, NJ) was kept on ice until centrifugation at 1,500 g for 20 min at 4°C. Drawn-off plasma was stored at −80°C until radioimmunoassay. Plasma E2 and T concentrations were analyzed with a 96-well FlashPlate PLUS system (Perkin Elmer, Shelton, CT) earlier validated for A. mississippiensis. One ovary (from alternating sides) was dissected away and frozen in liquid nitrogen and stored at −80°C until RNA extraction. At hatching, the relatively translucent alligator ovary overlies and stands in contrast to the more vascularized mesonephric kidney and the more medially located, white colored adrenal tissues. Standard paraffin histology of the contralateral gonad confirmed sex.
Our standard RNA isolation and reverse transcription (RT) procedures have been earlier reported in detail (Milnes et al., 2008). Quantitative real-time PCR (Q-PCR) has been used to measure mRNA expression in American alligator tissues (Katsu et al., 2004; Gunderson et al., 2006; Kohno et al., 2008). Table 1 shows primer sequence information, annealing temperatures, and accession numbers. The MyiQ single color detection system (BioRad, Hercules, CA) performed Q-PCR following manufacturer's protocol using iQ SYBR Green Supermix (BioRad) in triplicate reaction volumes of 15 μL, with 0.6 μL of RT product and specific primer pairs. Reactions were performed with relative standard curves of serially diluted cDNA. Sample means were normalized using ribosomal protein L8 (Rpl8) expression (Kohno et al., 2008; Milnes et al., 2008).
Table 1.
Quantitative real-time PCR primers for alligator gonadal factors.
| Forward primer (5′–3′) | ||||
|---|---|---|---|---|
| Transcript | Reverse primer (5′–3′) | Anneal (°C) | Product (bp) | Accession |
| Ribosomal protein L8 (Rp18) | GGTGTGGCTATGAATCCTGT | 60.0 | 64 | Katsu et al., 2004 |
| ACGACGAGCAGCAATAAGAC | ||||
| Inhibin α (Inha) | ACAATCCACTTGTCCCAGCC | 70.0 | 68 | DQ010151 |
| CAACTGCCACCGCGC | ||||
| Activin βA (Inhba) | ACCCACAGGTTACCGTGCTAA | 63.8 | 67 | DQ101152 |
| GCCAGAGGTGCCCGCTATA | ||||
| Follistatin (Fst) | CGAGTGTGCCCTCCTCAAA | 66.5 | 65 | DQ010156 |
| TGCCCTGATACTGGACTTCAAGT | ||||
| Aromatase (Cyp19a1) | CAGCCAGTTGTGGACTTGATCA | 62.0 | 79 | AY029233 |
| TTGTCCCCTTTTTCACAGGATAG | ||||
| Follicle stimulating hormone receptor (Fshr) | GAAATTACCAAACGAGGTTTTTCAA | 60.0 | 81 | DQ010157 |
| GGGCAGGAAACTGATTCTTGTC |
JMP for windows version 7.0.2 (SAS Institute, Cary, NC) performed all statistical analyses. Morphometric data were log transformed and gene expression ratios were arcsin transformed to achieve homogeneous variances, as needed. Significance was set at P<0.05. Unpaired Student's t-tests compared BM, SVL, and TL measurement by lake of origin for all hatching alligators from the collected clutches and the subset of alligators systematically assigned to the experiments detailed in this article. Circulating steroid or gonadal mRNA expression levels were not different between sham and vehicle-treated animals (lowest observed P-value by independent t-tests; Fst mRNA P = 0.17). We combined these groups into a single control treatment group for further statistical analysis.
Two-way ANOVA followed by least square means Tukey–Kramer post-tests, when appropriate, compared steroid hormone concentrations and mRNA expression levels between control groups of differing ages (Fig. 1, ANOVA factors: age, lake of origin, or interaction) and between control and FSH treatment levels for animals in the 2 and 5 day old FSH challenge studies (Figs. 2, 3, respectively; ANOVA factors: treatment, lake of origin, or interaction). Statistical analysis between control groups of differing ages and FSH challenge experiments shared control animals of respective ages and lake of origin. We further investigated significant observed treatment effects revealed by two-way ANOVA with subsequent one-way ANOVA by individual lake of origin and Dunnett's post-tests, if appropriate, to quantify lake of origin differences in FSH responsiveness compared with controls within each age group.
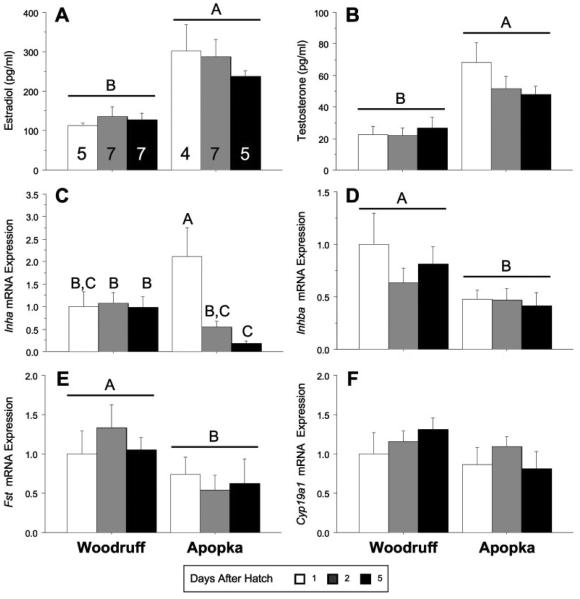
Figure 1.
Basal circulating steroids concentrations and ovarian mRNA expression levels, bars report means±SEM, in hatchling alligators: estradiol and sample sizes (A), testosterone (B), Inha (C), Inhba (D), Fst (E), and Cyp19a1 (F) in Lake Woodruff and Lake Apopka alligators. Days after hatching: white bars = one, gray bars = two, and black bars = five. All mRNA expression sample means are normalized using ribosomal protein L8 (Rpl8) expression and standardized for each endpoint to Lake Woodruff female, day 1 expression = one. Horizontal line above bars indicates statistical significance by lake of origin (P<0.05). Different letters above the bars indicate age by lake of origin statistical significances.
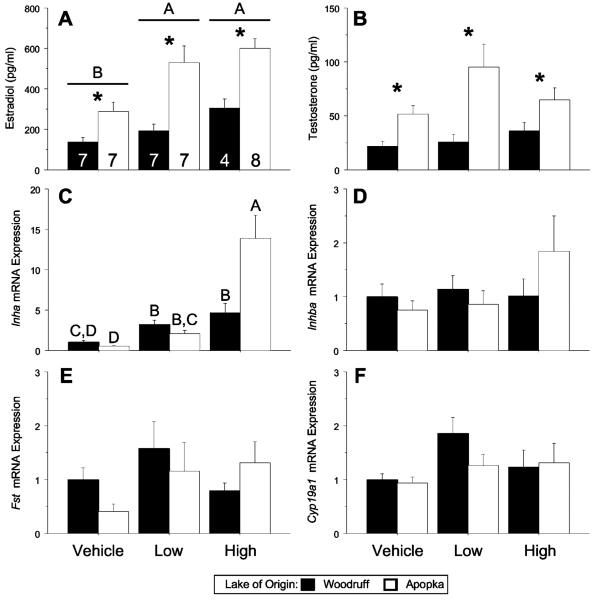
Figure 2.
Circulating steroid concentrations and ovarian mRNA expression levels in 2 day old FSH-challenged alligators. Bars report means±SEM: estradiol and sample sizes (A), testosterone (B), Inha (C), Inhba (D), Fst (E), and Cyp19a1 (F) in Lake Woodruff (black bars) and Lake Apopka (white bars) alligators. All mRNA expression sample means are normalized using ribosomal protein L8 (Rpl8) expression and standardized for each endpoint to Lake Woodruff female, vehicle treated expression = one. Horizontal lines above bars indicate statistical significance by treatment (P<0.05). Asterisks above bars indicate significant difference by lake of origin. Different letters above the bars indicate age by lake of origin statistical significances.
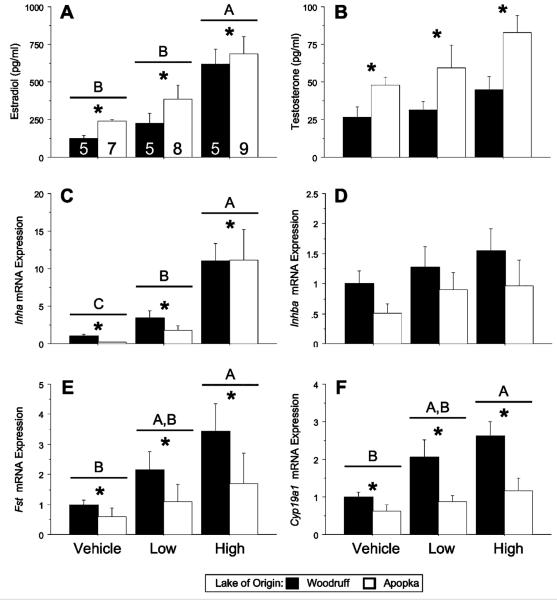
Figure 3.
Circulating steroid concentrations and ovarian mRNA expression levels in 5 day old FSH-challenged alligators. Bars report means±SEM: estradiol and sample sizes (A), testosterone (B), Inha (C), Inhba (D), Fst (E), and Cyp19a1 (F) in Lake Woodruff (black bars) and Lake Apopka (white bars) alligators. All mRNA expression sample means are normalized using ribosomal protein L8 (Rpl8) expression and standardized for each endpoint to Lake Woodruff female, vehicle treated expression = one. Horizontal lines above bars indicate statistical significance by treatment (P<0.05). Asterisks above bars indicate significant difference by lake of origin. Different letters above the bars indicate age by lake of origin statistical significances.
RESULTS
Upon collection, egg viability for the 13 clutches used for this study was 81% (237/291) from Lake Woodruff and 68% (181/267) from Lake Apopka. Of the subset of these eggs co-incubated, 86% (range by individual clutches: 67–100%) from Lake Woodruff (n = 125/145) and 59% (range by individual clutches: 18–92%) from Lake Apopka (n = 99/167) hatched. Of these, 54 Lake Woodruff and 37 Lake Apopka alligators were allocated to the hatchling experimental groups (Figs. 1–3 present sample sizes). At hatching, body morphometrics differed by lake or origin (Table 2). Average Lake Woodruff alligator body mass was greater than those measured in the Lake Apopka hatchling alligators between all hatchlings and the subset systematically assigned to the hatchling experimental groups.
Table 2.
Body morphometrics of hatchling alligators.
| Total hatchlings |
Experimental hatchlings |
|||
|---|---|---|---|---|
| Lake of origin | Woodruff | Apopka | Woodruff | Apopka |
| Body mass (g) | 61.9 ± 0.5 | 56.7 ± 0.6** | 62.0 ± 0.7 | 55.2 ± 1.2** |
| Snout–vent length (cm) | 11.7 ± 0.1 | 11.8 ± 0.8 | 11.7 ± 0.6 | 11.6 ± 0.9 |
| Total length (cm) | 24.5 ± 0.1 | 24.7 ± 0.1 | 24.5 ± 0.1 | 24.1 ± 0.2* |
Both gross anatomy at necropsy and gonadal histology observations confirmed that all hatchling animals were female. The ovarian cortex of all females contained oogonial clusters and meiotic germ cells showing varying degrees of physical interaction with somatic cells. However, ovaries from these neonates, whether 1, 2 or 5 days old from either lake, did not possess complete follicles. Ovarian expression levels of Rpl8 mRNA were not different between control groups or between FSH challenge study groups (P = 0.78, 0.92, and 0.41, respectively).
Comparing control group females from both lakes at 1, 2, or 5 days after hatching, Lake Apopka alligators had greater plasma concentrations of E2 (Fig. 1A; P<0.001) and T (Fig. 1B; P<0.001) compared with Lake Woodruff females. Gonadal Inha mRNA expression showed a lake by age effect (Fig. 1C; P = 0.009), with Lake Apopka expression levels greater than Lake Woodruff 1 day after hatching, but less than Lake Woodruff expression levels 5 days after hatching. Lake Woodruff alligators expressed greater gonadal mRNA levels of Inhba (Fig. 1D; P = 0.017) and Fst (Fig. 1E; P = 0.026) compared with those from Lake Apopka. Expression of Cyp19a1 (Fig. 1F; P = 0.33 or Fshr mRNA, data not shown; P = 0.25) did not significantly vary by age or lake of origin.
Within 2 days of birth, FSH-challenged animals, plasma E2 and T concentrations were greater in Lake Apopka alligators compared with Lake Woodruff (Fig. 2A, B; P<0.001 for each). Additionally, FSH treatment resulted in a significant elevation of plasma E2 concentration irrespective of lake (Fig. 2A; P = 0.008). Analysis of treatment effects by individual lake of origin showed that the circulating E2 concentrations for Lake Apopka animals responded to both low and high FSH doses (P = 0.027 and 0.015, respectively), whereas Lake Woodruff animals responded only to high dose FSH (P = 0.49 and 0.007, respectively). Gonadal expression of Inha mRNA showed a lake by treatment effect (Fig. 2C; P = 0.002), with Lake Apopka alligators showing a greater expression level response to the high FSH dose than Lake Woodruff alligators.
Further refinement of statistical analysis using Dunnett's post-test analysis of treatment effects by individual lake of origin showed that, both Lake Woodruff and Lake Apopka, 2 day old animals responded to both low and high dosages with elevated Inha levels (Woodruff: low P = 0.006, high P<0.001; Apopka: low P = 0.003, high P<0.001). Significant differences in Inhba, Fst, Cyp19a1, and Fshr mRNA expression levels were not observed (Fig. 2D–F; data not shown).
Within 5 days of birth, FSH-challenged study animals, plasma E2 and T concentrations were greater in Lake Apopka alligators compared with Lake Woodruff (Fig. 3A, B; P<0.005 and 0.001, respectively). Additionally, FSH treatment resulted in a significant elevation of plasma E2 concentration (Fig. 3A; P<0.001). Analysis of treatment effects by individual lake of origin showed circulating E2 concentrations of both Lake Apopka and Lake Woodruff animals responded only following high doses of FSH (P<0.001 and 0.005, respectively). Both lake of origin and treatment effects were observed in mRNA expression levels of Inha (Fig. 3C; lake of origin P<0.03, treatment P<0.001), Fst (Fig. 3E; lake of origin P = 0.003, treatment P = 0.03), Cyp19a1 (Fig. 3F; lake of origin P = 0.001, treatment P = 0.004), and Fshr (data not shown; lake of origin P = 0.006, treatment P = 0.002). Lake of origin effects showed that Lake Woodruff animals expressed greater levels of gonadal Inha, Fst, Cyp19a1, and Fshr mRNA than Lake Apopka animals.
Further refinement of statistical analysis using Dunnett's post-test analysis of treatment effects by individual lake of origin showed that both Lake Woodruff and Apopka 5 day old animals responded to both low and high dosages of FSH with elevated Inha levels (Woodruff: low P = 0.033, high P<0.001; Apopka: low P = 0.044, high P<0.001). In contrast, only Lake Woodruff animals responded to the FSH challenges with elevated mRNA levels of: Fst (Woodruff: low P = 0.09, high P = 0.022; Apopka: low P = 0.63, high P = 0.39), Cyp19a1 (Woodruff: low P = 0.033, high P<0.001; Apopka: low P = 0.42, high P = 0.42), and Fshr (Woodruff: low P = 0.47, high P = 0.006; Apopka: low P = 0.42, high P = 0.17).
DISCUSSION
Recent research in rodents has demonstrated that an interaction between activin and estrogen signaling participates in the breakdown of germ cell nests and the assembly of ovarian follicles. Here, we investigated the basal and gonadotropin stimulated levels of circulating hormones and gonadal mRNA expression of activin signaling factors in hatchling alligators from a contaminated and a reference environment. We observed evidence of elevated circulating steroid hormones (E2 and T) in alligators from the contaminated environment and, conversely, greater ovarian mRNA expression levels of activin signaling factors (Inhba and Fst) in alligators from the reference environment. Additionally, Inha mRNA expression levels in ovaries from Lake Apopka females changed substantially during the post-hatching period from greater than that measured in ovaries from Lake Woodruff females 1 day after hatching to lesser than those observed at 5 days after hatching. Hatchling alligator ovaries have germ cell nests that will develop into follicles over a period of several months; therefore, these endocrine differences are liable to affect subsequent ovarian development.
We observed these endocrine differences in animals that exhibited differing egg viability rates at the nest (Woodruff: 81%, Apopka: 68%), post-incubation hatch rates (Woodruff: 86%, Apopka: 59%), and average hatching BM (Woodruff: 62.0±0.7 g, Apopka: 55.2±1.2 g). Differences in hatching success continues observations of decreased egg viability and hatching success in Lake Apopka eggs observed for almost three decades (Fujisaki et al., 2007; Milnes and Guillette, 2008).
Although the role of FSH in ovarian nest breakdown and follicle assembly is unknown in alligators, we observed clear differences in response to a gonadotrophin challenge. Although alligators from both lakes showed responses to a single high dose FSH injection, only Lake Apopka animals showed a significant E2 increase to the low dose treatment, and ovarian Inha mRNA expression levels in Lake Apopka animals responded more robustly than ovaries from Lake Woodruff alligators to the high dose treatment. In 5 day old challenged alligators, we observed alterations in circulating E2 concentrations and ovarian Inha, Fst, Cyp19a1, and Fshr mRNA expression levels. Animals from both lakes responded with elevated plasma E2 concentrations to high dose treatments and increased Inha mRNA expression to both treatment levels. However, only Lake Woodruff animals responded to FSH with elevated Fst, Cyp19a1, and Fshr mRNA expressions. Therefore, Fst mRNA expression in Lake Woodruff animals is both greater at basal levels and is more inducible by FSH challenge than in Lake Apopka alligators. In mice, Wnt4 signaling suppresses male gonadal tissue differentiation during ovary development and regulates Fst expression (Yao et al., 2004). Because Fst expression also is critical for female germ cell survival, this Wnt4-Fst signaling cascade is both anti-testis and pro-ovary. Therefore, lower basal and stimulated Fst expression levels could be a robust sign of impaired reproductive health of Lake Apopka alligators.
Aromatase (translated from Cyp19a1 mRNA) is an enzyme that converts androgens to estrogens. Although we observed elevated circulating E2 concentrations in untreated Lake Apopka alligators and in FSH-stimulated alligators from both lakes, Cyp19a1 mRNA expression levels were not different between non-stimulated alligators from either lakes and the FSH challenge elevated expression levels only in 5 day old Lake Woodruff alligators. Circulating steroid hormone concentrations in an organism are the result of an integration of synthesis, plasma storage, hepatic biotransformation and clearance. Changes to these parameters can alter measured concentrations of any steroid. We observed elevated basal T concentrations in Lake Apopka animals, therefore, supplying an elevated level of available substrate for aromatization. Additionally, our laboratory group demonstrated that contaminant exposures can change expression levels of enzymes involved in hepatic steroid degradation (Gunderson et al., 2001). Finally, measured mRNA levels do not always predict rates of protein translation, length of mRNA stability, or length of enzymatic activity of the transcribed product. Therefore, numerous physiological processes can dissociate Cyp19a1 mRNA levels from circulating E2 concentrations.
Using reported means, standard errors, and samples sizes from our 1994 study, we calculated that the LH challenge significantly increased circulating concentrations of E2 in Lake Apopka alligators (P = 0.047), but not in Lake Woodruff animals (P = 0.74). Comparing this earlier work to data presented here, basal circulating E2 concentrations were greater in Lake Apopka than in Lake Woodruff alligators in both studies. Furthermore, Lake Apopka animals showed greater E2 responsiveness following treatment with either LH (Guillette et al., '94a) or FSH (this study). In ovo exposure of caiman embryos to exogenous E2 or BPA resulted in increased circulating E2 concentrations in 10 day old hatchlings, although this difference was no longer observed at 3–12 months post-hatching (Stoker et al., 2008). Taken together, these three studies strengthen a hypothesis that embryonic exposure to endocrine active substances can affect the endocrine milieu of hatching crocodilians, and likely other vertebrate species as well.
Although numerous studies have demonstrated increased MOF frequencies in estrogenically treated rodents (Iguchi and Takasugi, '86; Iguchi et al., '90; Nakamura et al., 2008; Kim et al., 2009;), only recently have studies reported the effects of similar treatments on activin signaling pathways. Treatments with E2 or DES of neonatal mice resulted in increased MOF frequencies and altered activin signaling 19 d postnatally (Kipp et al., 2007a). Alterations of activin signaling were observed as decreased Inha, Inhba, and Inhbb mRNA expressions and decreased Inhba and activin A ovarian protein levels. Treatments did not alter Fst mRNA levels. We observed consistently decreased Inhba and a rapid decrease in Inha mRNA expression levels in contaminant-exposed alligators over the observed period. Fst mRNA levels decreased in contaminant-exposed alligators, compared with no alteration observed in estrogenically treated mice. In contrast to the increased maturation of follicles observed in caiman treated in ovo with E2 (Stoker et al., 2008), neonatal E2 and DES treatment of mice resulted in fewer small antral follicles at day 19, as compared with controls. Therefore, although these exposure experiments share some similarity in molecular and physiological effects, these studies are difficult to compare given the different status of the follicle cohorts, before follicle formation in the alligator and caiman or at the time of follicle formation in mice.
In the mouse ovary, estrogen and activin signaling proteins colocalize in granulosa cells. Although the location of synthesis of these factors is unknown in the pre-follicular crocodilian ovary, we can compare spatial expression patterns in other vertebrates with morphologically similar ovaries (Moore et al., 2008) to formulate a hypothesis. Steroidogenic cells differentiate and migrate from the nephrogenous mesenchyme in chicken and mouse ovaries (Sekido and Lovell-Badge, 2007). In embryonic, pre-follicular chicken left ovaries, aromatase protein localizes to the medullary cords and not to the overlying cortex (Govoroun et al., 2004). Similarly, embryonic turtle ovaries express aromatase around the regressing medullary cords and at the cortex/medulla boundary after sex differentiation (Ramsey et al., 2007). In chicken ovaries 2 weeks after hatching, medullary steroidogenic cells migrate toward the cortex and incorporate into the thecal layers of developing follicles (Narbaitz and DeRobertis, '68; Pedernera et al., '88). Whereas in 5 week old chicken ovaries, aromatase is only detected in the thecal cells of developing follicles, granulosa cells do not express aromatase (Oreal et al., 2002; Govoroun et al., 2004). Furthermore, avian granulosa cells of the prehierarchical follicles exclusively express most activin signaling factors (Onagbesan et al., 2009). In light of the ovarian cortex possessing follicular cells that express activin, inhibin, and follistatin and an ovarian medulla containing the majority of steroidogenic cells before migration and differentiation into theca around small follicles, we hypothesize that in the pre-follicular alligator ovary, activin and estrogen signaling segregate spatially between cortex and medulla, respectively.
Embryonic chicken ovarian medullary cells bind FSH (Woods et al., '91) and this gonadotropin induces steroidogenesis (Gomez et al., 2001). At hatching, chickens treated with FSH in ovo respond with increased plasma E2 concentrations, thickening of the ovarian cortex and medullary cords (Gonzalez-Moran and Mancilla, '98), increased number of oogonia, and impeded meiosis observed as diminished numbers of oocytes (Gonzalez-Moran, '98). Furthermore, these researchers hypothesized that an FSH challenge does not directly augment germ or somatic cell numbers, direct somatic differentiation, or change entry into meiosis in the ovarian cortex, but rather produces a transient effect owing to altered medullary steroidogenesis. We expand this hypothesis to state that an FSH challenge alters cortical activin signaling by way of elevating plasma and tissue E2 concentrations that interact with activin signaling dynamics. Furthermore, our data presented in this study indicate that exposure to environmental contaminants can alter this response and interaction.
Amniote follicle formation is a function of initial germ cell proliferation followed by precipitous loss concomitant with complex interactions between the oocytes and somatic cells (Pepling and Spradling, 2001). The survival of oocytes depends on follicular assembly and those that fail to be surrounded by granulosa cells forming normal follicles undergo apoptosis (Pru and Tilly, 2001). A majority of the morphological processes described here are conserved among vertebrates (Pepling et al., '99; Matova and Cooley, 2001) and appear to share many of the molecular mechanisms underpinning gonadal maturation. As with the “canary in the coal mine,” we propose that if MOFs can be induced in wildlife by contaminant exposure with estrogenic activity, putatively through alteration in activin signaling, similar responses could also be observed in natural populations of mammals, including humans (Crain et al., 2008). Understanding and documenting these conserved mechanisms among vertebrates will strengthen causal and mechanistic relationships, allowing a better understanding of this phenomenon across vertebrates and allowing us to reduce or prevent these abnormalities in future populations.
ACKNOWLEDGMENT
Logistical support by the Florida Fish and Wildlife Conservation Commission made this study possible; specifically, we thank Allan Woodward for his continued assistance with fieldwork and permitting.
Grant Sponsor: NIH; Grant number: R21 ES014053-01; Grant Sponsor: Howard Hughes Medical Institute Professors program.
LITERATURE CITED
- Bristol-Gould SK, Kreeger PK, Selkirk CG, Kilen SM, Cook RW, Kipp JL, Shea LD, Mayo KE, Woodruff TK. Postnatal regulation of germ cells by activin: the establishment of the initial follicle pool. Dev Biol. 2006;298:132–148. doi: 10.1016/j.ydbio.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Chen Y, Jefferson WN, Newbold RR, Padilla-Banks E, Pepling ME. Estradiol, progesterone, and genistein inhibit oocyte nest breakdown and primordial follicle assembly in the neonatal mouse ovary in vitro and in vivo. Endocrinology. 2007;148:3580–3590. doi: 10.1210/en.2007-0088. [DOI] [PubMed] [Google Scholar]
- Crain DA, Guillette LJ, Rooney AA, Pickford DB. Alterations in steroidogenesis in alligators (Alligator mississippiensis) exposed naturally and experimentally to environmental contaminants. Environ Health Persp. 1997;105:528–533. doi: 10.1289/ehp.97105528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain DA, Eriksen M, Iguchi T, Jobling S, Laufer H, LeBlanc GA, Guillette LJ. An ecological assessment of bisphenol-A: evidence from comparative biology. Reprod Toxicol. 2007;24:225–239. doi: 10.1016/j.reprotox.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Crain DA, Janssen SJ, Edwards TM, Heindel J, Ho SM, Hunt P, Iguchi T, Juul A, McLachlan JA, Schwartz J, Skakkebaek N, Soto AM, Swan S, Walker C, Woodruff TK, Woodruff TJ, Giudice LC, Guillette LJ. Female reproductive disorders: the roles of endocrine-disrupting compounds and developmental timing. Fertil Steril. 2008;90:911–940. doi: 10.1016/j.fertnstert.2008.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AJ, Brooks CF, Johnson PA. Follicle-stimulating hormone regulation of inhibin alpha- and beta(B)-subunit and follistatin messenger ribonucleic acid in cultured avian granulosa cells. Biol Reprod. 2001;64:100–106. doi: 10.1095/biolreprod64.1.100. [DOI] [PubMed] [Google Scholar]
- Drummond AE. TGF beta signalling in the development of ovarian function. Cell Tissue Res. 2005;322:107–115. doi: 10.1007/s00441-005-1153-1. [DOI] [PubMed] [Google Scholar]
- Edwards TM, Gunderson MP, Milnes MR, Guillette LJ. Gonadotropin-induced testosterone response in peripubertal male alligators. Gen Comp Endocrinol. 2004;135:372–380. doi: 10.1016/j.ygcen.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Ethier JF, Findlay JK. Roles of activin and its signal transduction mechanisms in reproductive tissues. Reproduction. 2001;121:667–675. doi: 10.1530/rep.0.1210667. [DOI] [PubMed] [Google Scholar]
- Ferguson MWJ, Joanen T. Temperature-dependent sex determination in Alligator mississippiensis. J Zool. 1983;200:143–177. [Google Scholar]
- Forbes TR. Studies of the reproductive system of the alligator IV. Observations of the development of the gonad, the adrenal cortex, and the Mullerian duct. Contrib Embryol. 1940;174:131–154. [Google Scholar]
- Fujisaki I, Rice KG, Woodward AR, Percival HF. Possible generational effects of habitat degradation on alligator reproduction. J Wildlife Manage. 2007;71:2284–2289. [Google Scholar]
- Gomez Y, Velazquez PN, Peralta-Delgado I, Mendez MC, Vilchis F, Juarez-Oropeza MA, Pedernera E. Follicle-stimulating hormone regulates steroidogenic enzymes in cultured cells of the chick embryo ovary. Gen Comp Endocrinol. 2001;121:305–315. doi: 10.1006/gcen.2000.7600. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Moran G. Effect of follicle-stimulating hormone on different cell sub-populations in the ovary of newly hatched chicks treated during embryonic development. Br Poult Sci. 1998;39:128–132. doi: 10.1080/00071669889501. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Moran G, Mancilla C. Histomorphometric analysis in the ovary of newly hatched chicks treated with follicle-stimulating hormone during embryonic development. Eur J Morphol. 1998;36:11–18. doi: 10.1076/ejom.36.1.11.9027. [DOI] [PubMed] [Google Scholar]
- Govoroun MS, Pannetier M, Pailhoux E, Cocquet J, Brillard JP, Couty I, Batellier F, Cotinot C. Isolation of chicken homolog of the FOXL2 gene and comparison of its expression patterns with those of aromatase during ovarian development. Dev Dyn. 2004;231:859–870. doi: 10.1002/dvdy.20189. [DOI] [PubMed] [Google Scholar]
- Guillette LJ, Gross TS, Masson GR, Matter JM, Percival HF, Woodward AR. Developmental abnormalities of the gonad and abnormal sex-hormone concentrations in juvenile alligators from contaminated and control lakes in Florida. Environ Health Persp. 1994a;102:680–688. doi: 10.1289/ehp.94102680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillette LJ, Jr, Gross TS, Masson GR, Matter JM, Percival HF, Woodward AR. Developmental abnormalities of the gonad and abnormal sex hormone concentrations in juvenile alligators from contaminated and control lakes in Florida. Environ Health Persp. 1994b;102:680–688. doi: 10.1289/ehp.94102680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillette LJ, Brock JW, Rooney AA, Woodward AR. Serum concentrations of various environmental contaminants and their relationship to sex steroid concentrations and phallus size in juvenile American alligators. Arch Environ Contam Toxicol. 1999;36:447–455. doi: 10.1007/pl00006617. [DOI] [PubMed] [Google Scholar]
- Gunderson MP, LeBlanc GA, Guillette LJ. Alterations in sexually dimorphic biotransformation of testosterone in juvenile American alligators (Alligator mississippiensis) from contaminated lakes. Environ Health Persp. 2001;109:1257–1264. doi: 10.1289/ehp.011091257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunderson MP, Kohno S, Blumberg B, Iguchi T, Guillette LJ. Up-regulation of the alligator CYP3A77 gene by toxaphene and dexamethasone and its short term effect on plasma testosterone concentrations. Aquat Toxicol. 2006;78:272–283. doi: 10.1016/j.aquatox.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Heinz GH, Percival HF, Jennings ML. Contaminants in American alligator eggs from Lake Apopka, Lake Griffin, and Lake Okeechobee, Florida. Environ Monit Assess. 1991;16:277–285. doi: 10.1007/BF00397615. [DOI] [PubMed] [Google Scholar]
- Iguchi T, Takasugi N. Polyovular follicles in the ovary of immature mice exposed prenatally to diethylstilbestrol. Anat Embryol. 1986;175:53–55. doi: 10.1007/BF00315455. [DOI] [PubMed] [Google Scholar]
- Iguchi T, Takasugi N, Bern HA, Mills KT. Frequent occurrence of polyovular follicles in ovaries of mice exposed neonatally to diethylstilbestrol. Teratology. 1986;34:29–35. doi: 10.1002/tera.1420340105. [DOI] [PubMed] [Google Scholar]
- Iguchi T, Fukazawa Y, Uesugi Y, Takasugi N. Polyovular follicles in mouse ovaries exposed neonatally to diethylstilbestrol in vivo and in vitro. Biol Reprod. 1990;43:478–484. doi: 10.1095/biolreprod43.3.478. [DOI] [PubMed] [Google Scholar]
- Jefferson W, Newbold R, Padilla-Banks E, Pepling M. Neonatal genistein treatment alters ovarian differentiation in the mouse: inhibition of oocyte nest breakdown and increased oocyte survival. Biol Reprod. 2006;74:161–168. doi: 10.1095/biolreprod.105.045724. [DOI] [PubMed] [Google Scholar]
- Jones SM, Swain R. Effects of exogenous FSH on follicular recruitment in a viviparous lizard Niveoscincus metallicus (Scincidae) Comp Biochem Phys A. 2000;127:487–493. doi: 10.1016/s1095-6433(00)00279-8. [DOI] [PubMed] [Google Scholar]
- Jones RE, Tokarz RR, Lagreek FT. Endocrine control of clutch size in reptiles. 5. FSH-induced follicular formation and growth in immature ovaries of Anolis-Carolinensis. Gen Comp Endocrinol. 1975;26:354–367. [Google Scholar]
- Katsu Y, Bermudez DS, Braun EL, Helbing C, Miyagawa S, Gunderson MP, Kohno S, Bryan TA, Guillette LJ, Iguchi T. Molecular cloning of the estrogen and progesterone receptors of the American alligator. Gen Comp Endocrinol. 2004;136:122–133. doi: 10.1016/j.ygcen.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Kezele P, Skinner MK. Regulation of ovarian primordial follicle assembly and development by estrogen and progesterone: endocrine model of follicle assembly. Endocrinology. 2003;144:3329–3337. doi: 10.1210/en.2002-0131. [DOI] [PubMed] [Google Scholar]
- Kim H, Hayashi S, Chambon P, Watanabe H, Iguchi T, Sato T. Effects of diethylstilbestrol on ovarian follicle development in neonatal mice. Reprod Toxicol. 2009;27:55–62. doi: 10.1016/j.reprotox.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Kipp JL, Kilen SM, Bristol-Gould S, Woodruff TK, Mayo KE. Neonatal exposure to estrogens suppresses activin expression and signaling in the mouse ovary. Endocrinology. 2007a;148:1968–1976. doi: 10.1210/en.2006-1083. [DOI] [PubMed] [Google Scholar]
- Kipp JL, Kilen SM, Woodruff TK, Mayo KE. Activin regulates estrogen receptor gene expression in the mouse ovary. J Biol Chem. 2007b;282:36755–36765. doi: 10.1074/jbc.M705143200. [DOI] [PubMed] [Google Scholar]
- Knight PG. Roles of inhibins, activins, and follistatin in the female reproductive system. Front Neuroendocrinol. 1996;17:476–509. doi: 10.1006/frne.1996.0013. [DOI] [PubMed] [Google Scholar]
- Kohno S, Bermudez DS, Katsu Y, Iguchi T, Guillette LJ. Gene expression patterns in juvenile American alligators (Alligator mississippiensis) exposed to environmental contaminants. Aquat Toxicol. 2008;88:95–101. doi: 10.1016/j.aquatox.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Kumar TR, Wang Y, Lu NF, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15:201–204. doi: 10.1038/ng0297-201. [DOI] [PubMed] [Google Scholar]
- Lance VA, Vliet KA. Effect of mammalian gonadotropins on testosterone secretion in male alligators. J Exp Zool. 1987;241:91–94. doi: 10.1002/jez.1402410111. [DOI] [PubMed] [Google Scholar]
- Lei L, Jin S, Mayo KE, Woodruff TK. The interactions between the stimulatory effect of follicle-stimulating hormone and the inhibitory effect of estrogen on mouse primordial folliculogenesis. Biol Reprod. 2010;82:13–22. doi: 10.1095/biolreprod.109.077404. DOI:10.1095/biolreprod.109.077404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matova N, Cooley L. Comparative aspects of animal oogenesis. Dev Biol. 2001;231:291–320. doi: 10.1006/dbio.2000.0120. [DOI] [PubMed] [Google Scholar]
- Mayo K, Jameson L, Woodruff TK. Eggs in the nest. Endocrinology. 2007;148:3577–3579. doi: 10.1210/en.2007-0590. [DOI] [PubMed] [Google Scholar]
- McMullen ML, Cho BN, Yates CJ, Mayo KE. Gonadal pathologies in transgenic mice expressing the rat inhibin alpha-subunit. Endocrinology. 2001;142:5005–5014. doi: 10.1210/endo.142.11.8472. [DOI] [PubMed] [Google Scholar]
- Mendez-Herrera MC, Tamez L, Candido A, Reyes-Esparza JA, Pedernera E. Follicle stimulating hormone increases somatic and germ cell number in the ovary during chick embryo development. Gen Comp Endocrinol. 1998;111:207–215. doi: 10.1006/gcen.1998.7108. [DOI] [PubMed] [Google Scholar]
- Milnes MR, Guillette LJ. Alligator tales: new lessons about environmental contaminants from a sentinel species. Bioscience. 2008;58:1027–1036. [Google Scholar]
- Milnes MR, Bryan TA, Katsu Y, Kohno S, Moore BC, Iguchi T, Guillette LJ. Increased posthatching mortality and loss of sexually dimorphic gene expression in alligators (Alligator mississippiensis) from a contaminated environment. Biol Reprod. 2008;78:932–938. doi: 10.1095/biolreprod.107.064915. [DOI] [PubMed] [Google Scholar]
- Moore BC, Uribe-Aranzabal MC, Boggs ASP, Guillette LJ. Developmental morphology of the neonatal alligator (Alligator mississippiensis) ovary. J Morphol. 2008;269:302–312. doi: 10.1002/jmor.10583. [DOI] [PubMed] [Google Scholar]
- Moore BC, Hamlin HJ, Botteri NL, Guillette LJ. Gonadal mRNA expression levels of TGFβ signaling factors correspond with post hatching morphological development in American alligators. Sexual Dev. 2010a;4 doi: 10.1159/000277934. E-pub DOI: 10.1159/000277934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BC, Milnes MR, Kohno S, Katsu Y, Iguchi T, Guillette LJ. Influences of sex, incubation temperature, and environmental quality on gonadal estrogen and androgen steroid receptor mRNA expression in juvenile American alligators (Alligator mississippiensis) Biol Reprod. 2010b;82:194–201. doi: 10.1095/biolreprod.109.077305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Hasegawa Y, Sugino K, Kogawa K, Titani K, Sugino H. Follistatin inhibits activin-induced differentiation of rat follicular granulosa-cells in vitro. Biochim Biophys Acta. 1992;1135:103–109. doi: 10.1016/0167-4889(92)90173-9. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Katsu Y, Watanabe H, Iguchi T. Estrogen receptor subtypes selectively mediate female mouse reproductive abnormalities induced by neonatal exposure to estrogenic chemicals. Toxicology. 2008;253:117–124. doi: 10.1016/j.tox.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Narbaitz R, DeRobertis EM. Postnatal evolution Of steroidogenic cells in chick ovary. Histochemie. 1968;15:187–193. doi: 10.1007/BF00305882. [DOI] [PubMed] [Google Scholar]
- Onagbesan O, Bruggeman V, Decuypere E. Intra-ovarian growth factors regulating ovarian function in avian species: a review. Anim Reprod Sci. 2009;111:121–140. doi: 10.1016/j.anireprosci.2008.09.017. [DOI] [PubMed] [Google Scholar]
- Oreal E, Mazaud S, Picard JY, Magre S, Carre-Eusebe D. Different patterns of anti-Mullerian hormone expression, as related to DMRT1, SF-1, WT1, GATA-4, Wnt-4, and Lhx9 expression, in the chick differentiating gonads. Dev Dyn. 2002;225:221–232. doi: 10.1002/dvdy.10153. [DOI] [PubMed] [Google Scholar]
- Pangas SA, Jorgez CJ, Tran M, Agno J, Li XH, Brown CW, Kumar TR, Matzuk MM. Intraovarian activins are required for female fertility. Mol Endocrinol. 2007;21:2458–2471. doi: 10.1210/me.2007-0146. [DOI] [PubMed] [Google Scholar]
- Pedernera E, Gomez Y, Velazquez P, Juarezoropeza MA, Delpliego MG. Identification of steroidogenic cell subpopulations in the ovary of the newly hatched chicken. Gen Comp Endocrinol. 1988;71:153–162. doi: 10.1016/0016-6480(88)90306-1. [DOI] [PubMed] [Google Scholar]
- Pedernera E, Solis L, Peralta I, Velazquez PN. Proliferative and steroidogenic effects of follicle-stimulating hormone during chick embryo gonadal development. Gen Comp Endocrinol. 1999;116:213–220. doi: 10.1006/gcen.1999.7352. [DOI] [PubMed] [Google Scholar]
- Pepling ME. From primordial germ cell to primordial follicle: mammalian female germ cell development. Genesis. 2006;44:622–632. doi: 10.1002/dvg.20258. [DOI] [PubMed] [Google Scholar]
- Pepling ME, Spradling AC. Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev Biol. 2001;234:339–351. doi: 10.1006/dbio.2001.0269. [DOI] [PubMed] [Google Scholar]
- Pepling ME, de Cuevas M, Spradling AC. Germline cysts: a conserved phase of germ cell development? Trends Cell Biol. 1999;9:257–262. doi: 10.1016/s0962-8924(99)01594-9. [DOI] [PubMed] [Google Scholar]
- Pru JK, Tilly JL. Programmed cell death in the ovary: insights and future prospects using genetic technologies. Mol Endocrinol. 2001;15:845–853. doi: 10.1210/mend.15.6.0646. [DOI] [PubMed] [Google Scholar]
- Ramsey M, Shoemaker C, Crews D. Gonadal expression of SF1 and aromatase during sex determination in the red-eared slider turtle (Trachemys scripta), a reptile with temperature-dependent sex determination. Differentiation. 2007;75:978–991. doi: 10.1111/j.1432-0436.2007.00182.x. [DOI] [PubMed] [Google Scholar]
- Rauschenberger RH, Wiebe JJ, Sepulveda MS, Scarborough JE, Gross TS. Parental exposure to pesticides and poor clutch viability in American alligators. Environ Sci Technol. 2007;41:5559–5563. doi: 10.1021/es0628194. [DOI] [PubMed] [Google Scholar]
- Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, Vandenbergh JG, Walser-Kuntz DR, vom Saal FSV. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol. 2007;24:199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safi M, Onagbesan OM, Bruggeman V, Vleugels B, Volckaert G, Decuypere E. Regulation of inhibin alpha- and beta(A)-subunit messenger ribonucleic acid levels by gonadotropins and IGF-I in cultured chicken granulosa cells. Gen Comp Endocrinol. 2003;131:159–167. doi: 10.1016/s0016-6480(03)00007-8. [DOI] [PubMed] [Google Scholar]
- Sanchez-Bringas G, Salazar O, Pedernera E, Mendez C. Follicle-stimulating hormone treatment reverses the effect of hypophysectomy on cell proliferation in the chicken embryo ovary. Gen Comp Endocrinol. 2006;149:134–140. doi: 10.1016/j.ygcen.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Schmierer B, Schuster MK, Shkumatava A, Kuchler K. Activin and follicle-stimulating hormone signaling are required for long-term culture of functionally differentiated primary granulosa cells from the chicken ovary. Biol Reprod. 2003;68:620–627. doi: 10.1095/biolreprod.102.008714. [DOI] [PubMed] [Google Scholar]
- Sekido R, Lovell-Badge R. Mechanisms of gonadal morphogenesis are not conserved between chick and mouse. Dev Biol. 2007;302:132–142. doi: 10.1016/j.ydbio.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Stoker C, Rey F, Rodriguez H, Ramos JG, Sirosky P, Larriera A, Luque EH, Munoz-de-Toro M. Sex reversal effects on Caiman latirostris exposed to environmentally relevant doses of the xenoestrogen bisphenol A. Gen Comp Endocrinol. 2003;133:287–296. doi: 10.1016/s0016-6480(03)00199-0. [DOI] [PubMed] [Google Scholar]
- Stoker C, Beldomenico PM, Bosquiazzo VL, Zayas MA, Rey F, Rodriguez H, Munoz-de-Toro M, Luque EH. Developmental exposure to endocrine disruptor chemicals alters follicular dynamics and steroid levels in Caiman latirostris. Gen Comp Endocrinol. 2008;156:603–612. doi: 10.1016/j.ygcen.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Trombly DJ, Woodruff TK, Mayo KE. Roles for transforming growth factor beta superfamily proteins in early folliculogenesis. Semin Reprod Med. 2009;27:14–23. doi: 10.1055/s-0028-1108006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonier PM, Crain DA, McLachlan JA, Guillette LJ, Arnold SF. Interaction of environmental chemicals with the estrogen and progesterone receptors from the oviduct of the American alligator. Environ Health Persp. 1996;104:1318–1322. doi: 10.1289/ehp.961041318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welt C, Sidis Y, Keutmann H, Schneyer A. Activins, inhibins, and follistatins: from endocrinology to signaling. A paradigm for the new millennium. Exp Biol Med. 2002;227:724–752. doi: 10.1177/153537020222700905. [DOI] [PubMed] [Google Scholar]
- Willingham E, Crews D. Sex reversal effects of environmentally relevant xenobiotic concentrations on the red-eared slider turtle, a species with temperature-dependent sex determination. Gen Comp Endocrinol. 1999;113:429–435. doi: 10.1006/gcen.1998.7221. [DOI] [PubMed] [Google Scholar]
- Woods JE, Damianideskeenan M, Thommes RC. FSH-binding and TSH-binding cells in the ovary of the developing chick-embryo. Gen Comp Endocrinol. 1991;82:487–494. doi: 10.1016/0016-6480(91)90324-y. [DOI] [PubMed] [Google Scholar]
- Woodward AR, Jennings ML, Percival HF, Moore CT. Low clutch viability of American alligators on Lake Apopka. Fla Sci. 1993;56:52–63. [Google Scholar]
- Yao HHC, Matzuk MM, Jorgez CJ, Menke DB, Page DC, Swain A, Capel B. Follistatin operates downstream of Wnt4 in mammalian ovary organogenesis. Dev Dyn. 2004;230:210–215. doi: 10.1002/dvdy.20042. [DOI] [PMC free article] [PubMed] [Google Scholar]