Abstract
Objective
To determine viability and virulence of Streptococcus pneumoniae mutants deficient in pneumococcal surface protein A (PspA−) and pneumolysin (Ply−) in the chinchilla ear. Design: Bullae of chinchillas were inoculated bilaterally with wild-type, Ply−, PspA−, and Ply− /PspA− strains. Bacterial colony forming unit (CFU) levels in middle ear effusions were counted at 48 hours. CFUs of the PspA− group were also counted from 6 to 36 hours after inoculation. Temporal bone histopathology was compared.
Results
At 48 hours, CFUs in middle ears were increased for wild-type and Ply−/PspA− strains, but Ply− remained near inoculum level. No bacteria were detected in the PspA− group. CFUs of PspA− decreased over time to a very low level at 30–36 hours. In vitro, PspA− in Todd Hewitt Broth showed an increase in bacterial growth, of 2 logs, at 43 hours, indicating PspA− susceptibility to host defenses in vivo. PspA− and Ply− groups had less pathology than wild-type or Ply−/PspA− groups. Histopathological analysis showed significant differences in the number of bacteria in the scala tympani in the wild-type compared to the Ply−, PspA−, and Ply−/PspA− groups. The PspA− strain was least virulent.
Conclusion
The PspA− mutant was much less viable and less virulent in the ear than Ply−, Ply−/PspA− and wild-type strains. There was no significant attenuation in virulence and viability of the Ply−/PspA− compared to wild-type or single mutants. Viability and virulence of pneumococcal mutants appeared to be protein- and organ-specific.
INTRODUCTION
Otitis media is the most common indication for the prescription of antibiotics in the USA1, and pneumococci are responsible for 30 to 50% of otitis media cases in patients.2 Bacterial resistance to antibiotics has necessitated the search for alternative methods for the prevention and treatment of acute otitis media. Although there are currently pneumococcal capsular polysaccharide vaccines available, they have low efficacy against middle-ear pneumococcal infection and do not provide protection for all common Streptococcus pneumoniae serotypes that cause otitis media.3
Pneumococcal proteins, either alone or in combination, are being investigated as potential vaccine candidates.4 Pneumococcal surface protein A (PspA) is a surface bound protein that can bind to apolactoferrin to prevent its killing.5 It has been shown to interfere with complement deposition on pneumococci, reducing opsonization and clearance of bacteria by the host immune system.6 Pneumolysin (Ply) is a pore forming protein that is both cytotoxic and cytolytic.7 It can activate the classical complement pathway8 and prevent complement deposition on S. pneumoniae.9 Other activities of pneumolysin include: impairment of leukocyte function, promotion of inflammation, disruption of respiratory epithelial tight junctions, and inhibition of epithelial ciliary movement.10 Both PspA and Ply are expressed in the vast majority of pneumococcal serotypes, making them good vaccine candidates.11
We previously demonstrated that the pneumococcal mutant deficient in the PspA protein was less virulent in the middle ear than a mutant deficient in Ply protein.12–13 In this study, we compared the virulence/viability of the Ply− and PspA− single mutants, double Ply−/PspA− mutant, and their wild-type, parent strain D39 (serotype 2) in the middle and inner ears following their inoculation in the chinchilla middle ear, as well as in vivo bacterial growth to assess host immunity on these strains.
METHODS
Streptococcus pneumoniae serotype 2 strain D39 (NCTC 7466)14 was the wild-type strain used in our experiments and the parent strain for our mutants. This strain and its isogenic Ply−, PspA−, and Ply−/PspA− mutants have been described previously.14–17
Bacteria was grown in Todd Hewitt Broth (BD Diagnostics, Sparks, Maryland) containing 0.5 percent added yeast extract (BD Diagnostics, Sparks, Maryland) (THB), plated on sheep blood agar (SBA) plates, and stored in 10% glycerin solution at −80°C. Mutants were grown on SBA plates and in THB, both containing 0.3 µg/ml erythromycin. Bacteria were grown until they were in log phase. Their optical densities were measured at 660 nm on a spectrophotometer and they were diluted to the desired concentration in phosphate buffered saline. Ten-fold dilutions were plated, and viable cells were counted to confirm the actual concentration. The time-dependent viability of the PspA− mutant was also studied in THB at room temperature from 0 to 43 hours after inoculation. Strains were confirmed as S. pneumoniae by optochin sensitivity and production of serotype 2 capsule using specific antisera as described previously.18 The presence of PspA and Ply proteins in all strains were analyzed from lysate preparations by Western blotting using specific antisera as previously described.19
The care and use of the animals in these experiments were approved by the Institutional Animal Care and Use Committee of the University of Minnesota, Minneapolis. All animals were anesthetized prior to bacterial inoculation with 0.25 ml of a combination of ketamine hydrochloride (100 mg/kg) and acepromazine maleate (10 mg/kg). Chinchilla middle ears were inoculated with 0.5 ml of bacteria: 7 chinchillas with 1 × 106 CFU/ml wild-type; 7 with 6.6 × 105 CFU/ml Ply−; 6 with 3.5 × 106 CFU/ml PspA−; and 7 with 1.25 × 106 Ply−/PspA−. Bullae were removed and middle ear effusions (MEE) harvested for bacterial counts. Cochleae from all groups were embedded in epoxy resin, sectioned at a thickness of 1 µm and stained with toluidine blue. Round window membranes (RWM) were bisected and one side randomly selected for pathological evaluation. Images of the RWMs were taken at a magnification of 1,000× at the center of the sample and at 1 mm to the right and left of center. Images of the scala tympani were made immediately under adjacent areas of the RWM. Analysis of the RWM included thickness and number of inflammatory cells. Evaluation of the adjacent scala tympani included inflammatory cell infiltration, number of bacteria, number of bacteria within inflammatory cells, and number of bacteria free in the perilymph (per area). The measurements of these three selected areas of RWMs and scala tympani from each animal were averaged. The average of these numbers for wild-type and mutant groups were then used for statistical analysis, to compare all animal groups infected with the wild-type or its isogenic mutant strains. All results were expressed as mean ± SE. Differences between groups were analyzed with one-way analysis of variance (ANOVA) using SPSS 18 software (SPSS Inc, Chicago, IL). Differences were considered to be significant if P ≤ 0.05.
Viability in the chinchilla middle ear included 30 animals inoculated bilaterally with 0.5 ml of 2.7 × 106 CFU/ml of the PspA− mutant. Animals were killed at 6, 12, 18, 24, 30, and 36 hours after inoculation for bacterial counts of MEE and histological analysis.
RESULTS
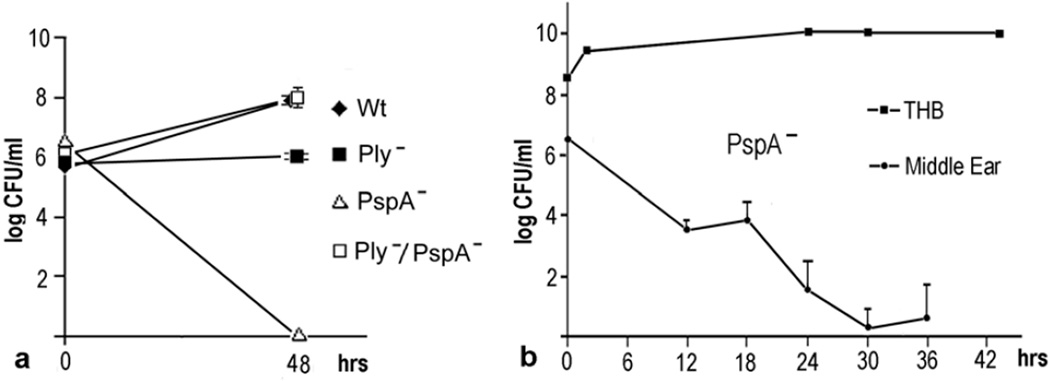
At 48 hours after inoculation, CFU counts in middle ear effusions (Fig. 1a) were lower than the inoculum for the PspA− mutants and very near the inoculum level for the Ply− mutants. Counts for the wild-type (Wt) and the Ply−/PspA− strains were highly increased compared to the inoculum. Significant differences (P < 0.001) in CFU counts were seen between Ply− and PspA− mutants and between each of the single mutants compared to the Wt or Ply−/PspA− strains. No significant difference was found between Wt and Ply−/PspA− strains. The PspA− mutant had no detectable bacteria.
Fig. 1.
Effects of mutations in PspA, and Ply on bacterial growth. a) At 48 hours after inoculation, CFUs in middle ear effusions were significantly lower (P < 0.001) for the Ply− and PspA− mutants compared to the wild-type (Wt) and Ply−/PspA− strains. Significant differences (P < 0.001) in CFU counts were seen between Ply− and PspA− mutants. Counts for the Wt and the Ply−/PspA− strains were highly increased while for the Ply− remained near the initial inoculum levels. There were no detectable CFU levels in the PspA− group. b) In middle ear effusions, CFUs of the PspA− mutant decreased steadily overtime with only a few remaining at 30 hours after inoculation. After 43 hours in Todd Hewitt broth (THB), however, there was an increase of about 2 logs.
Because the PspA− mutant was not viable in vivo at 48 hours, a time course study was done using 30 chinchillas inoculated with 2.7 × 106 CFU/ml of PspA−. Animals were killed and effusion harvested at 6, 12, 18, 24, 30 and 36 hours after bacterial inoculation (Fig 1b). In middle ear effusions, CFUs of the PspA− mutant decreased steadily over time with very few CFU remaining by 30 hours of inoculation. In addition, in vitro, CFU counts for the PspA− strain grown in THB were done at 0, 2, 24, 30 and 43 hours to study the effect of host defenses on bacterial growth (Fig. 1b). After 43 hours in THB, however, there was an increase of about 2 logs, indicating that the loss of PspA− viability in vivo was probably due to the host immune system.
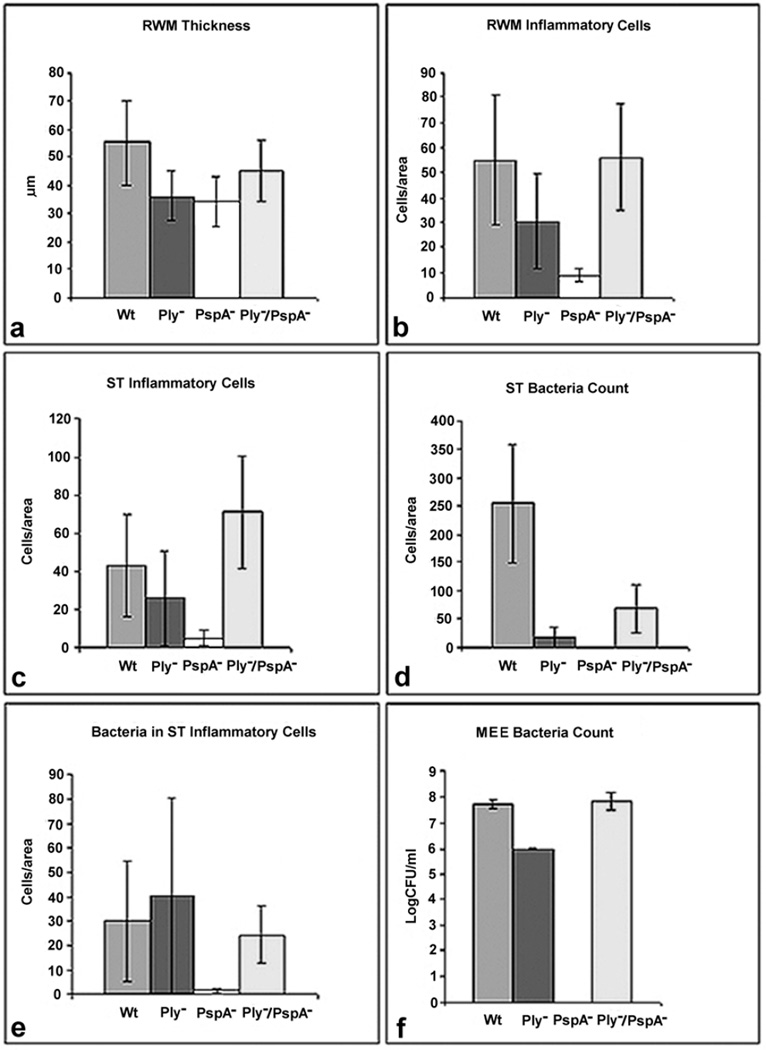
At 48 hours after inoculation, histologic data from the bullae from the wild-type, Ply−, PspA−, and Ply−/PspA− groups were examined to compare thickness and number of inflammatory cells in the round window membrane (RWM), and number of inflammatory cells and bacteria in the scala tympani (Fig. 2). Although not significant, the RWM of the PspA− and Ply− mutant groups tended to be less thick than the wild-type or double mutant groups (Fig. 2a). There was no significant difference among groups in the number of inflammatory cells in the RWM (Fig. 2b), however, the PspA− group tended to have less inflammation. There were no significant differences in the number of inflammatory cells in the scala tympani of the inner ear (Fig. 2c) among groups, but the PspA− mutant had the least number of cells. There was a significant difference in the number of free floating bacteria in the scala tympani (Fig. 2d) between the Wt and the Ply− (P = 0.009), Wt and PspA− (P = 0.006), and Wt and Ply−/PspA− (P = 0.028) groups. No bacteria were seen in the PspA− group. There was no significant difference in the number of phagocitized bacteria in inflammatory cells in the scala tympani (Fig. 2e), but fewer were seen in the PspA− group. The absence of free-floating bacteria in the scala tympani is consistent with no detectable CFU level in the middle ear effusions for the PspA− group, 48 hours after inoculation (Fig. 2f).
Fig. 2.
Disease progression and pathology at the round window and scala tympani 48 hours post inoculation in the middle ear with approximately 1×106 CFU/ml of the indicated strains. a) Although not significant, the round window membrane (RWM) of the single mutant groups tended to be less thick than the wild-type or double mutant groups. b) There was no significant difference among groups in the number of inflammatory cells; however, the PspA− mutant had the least number of cells in the RWM and c) scala tympani (ST). d) There was a significant difference in the number of free-floating bacteria/per area counted by light microscopy in tissue sections stained with toluidine blue in the ST between the wild-type (Wt) and the Ply− (P = 0.009), Wt and PspA− (P = 0.006), and Wt and Ply−/PspA− (P = 0.028) groups. No bacteria were seen in the PspA− group. e) There were no significant differences in the numbers of phagocytized bacteria in inflammatory cells in the ST, but fewer were seen in the PspA− group. f) Light microscopic analysis of the ST for the PspA− group found no detectable bacteria at 48 hours, consistent with CFU counts in middle ear effusions (MEE), 48 hours after infection.
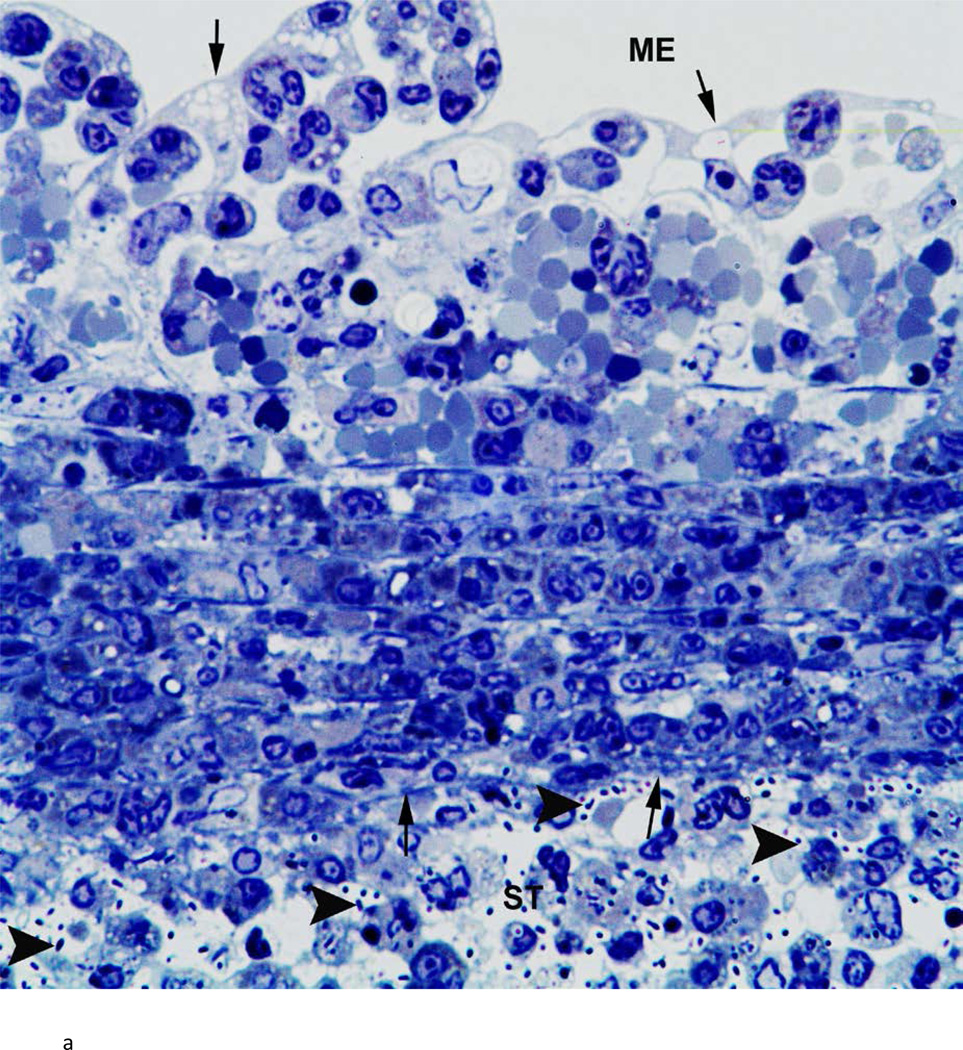
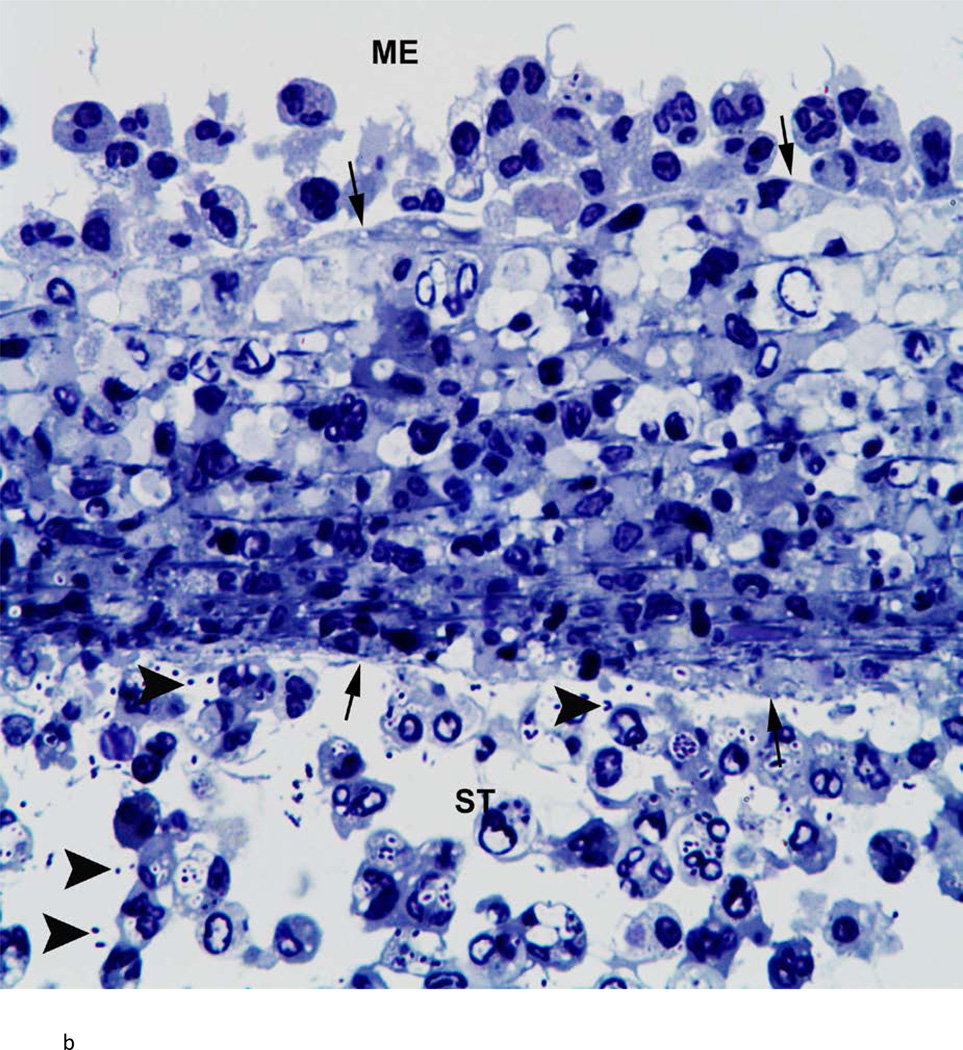
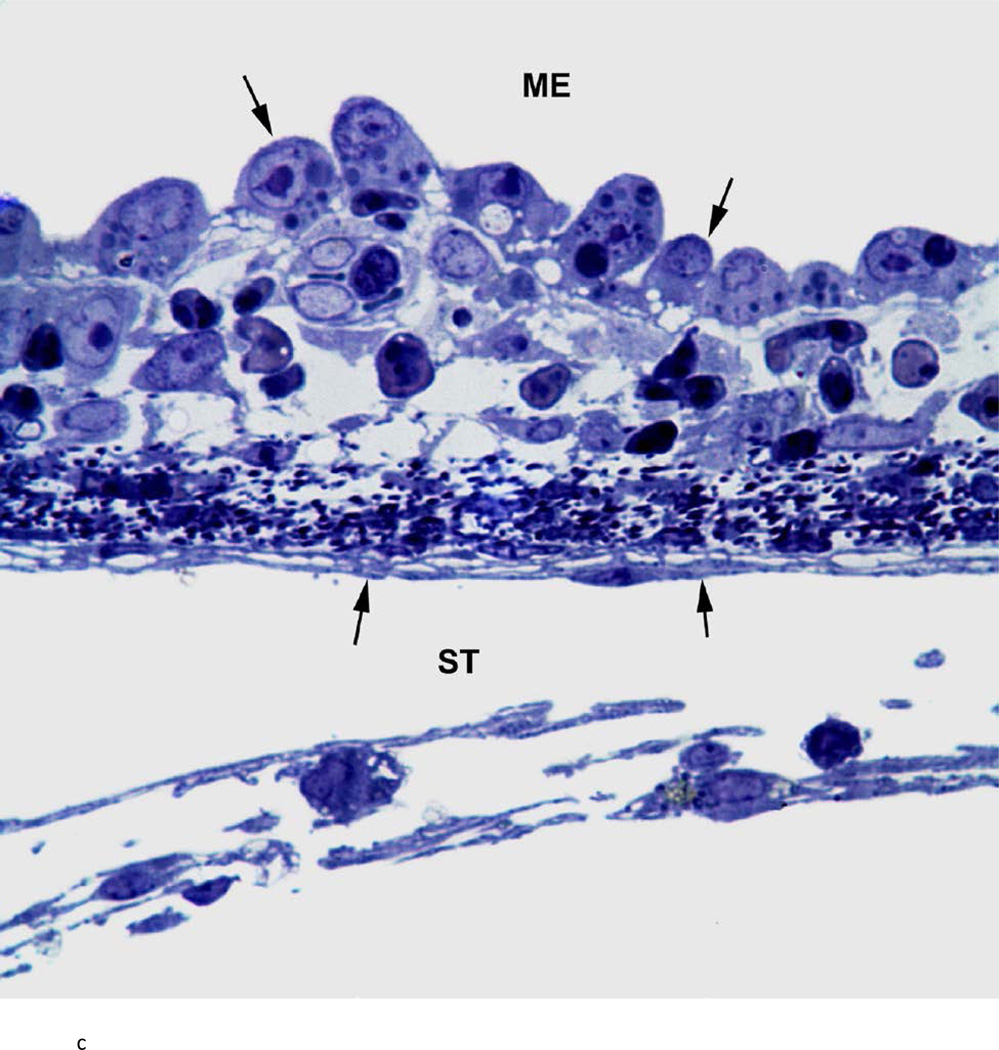
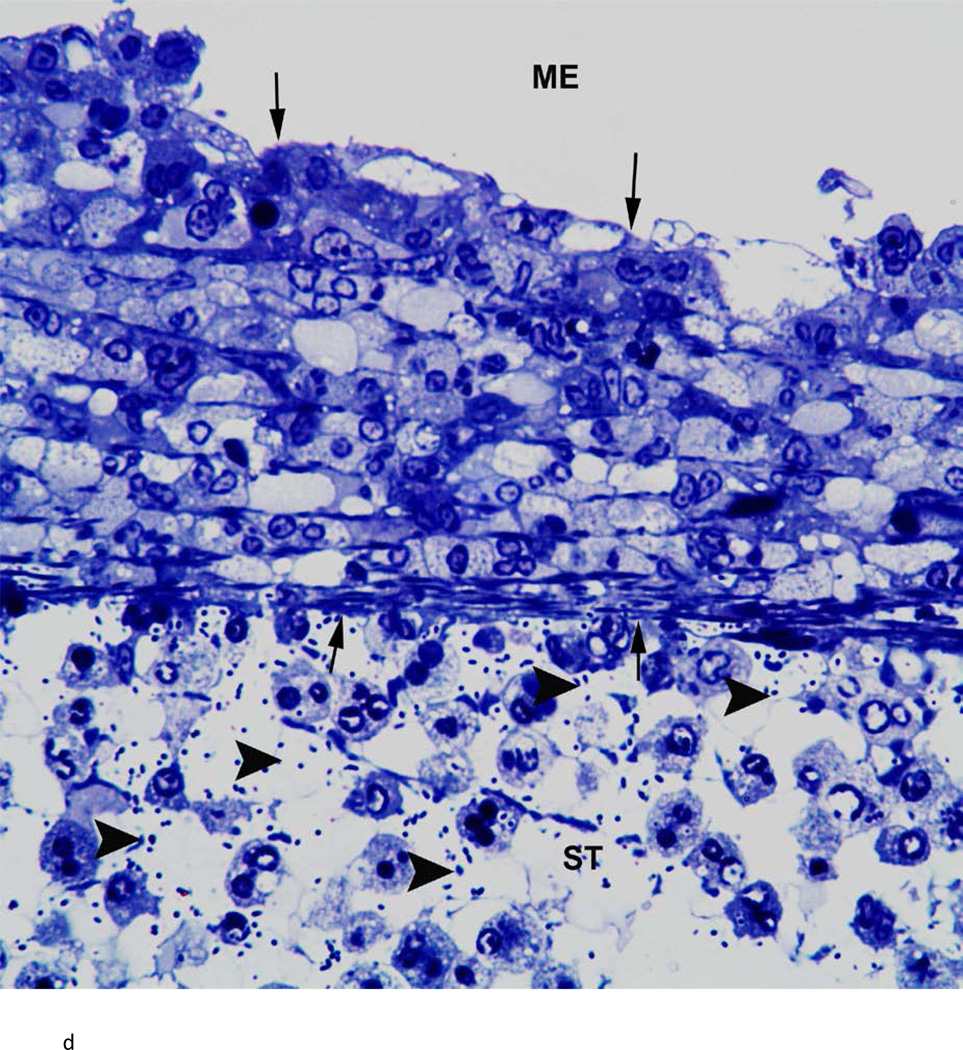
There was variability between animals in each infected group. Figure 3 represents the most severe pathology of the RWM and adjacent areas in each group. Assessment of these groups was as follows: the RWM from a chinchilla inoculated with the wild-type strain (Fig. 3a) was thickened from inflammatory cell infiltration. There were bacteria in the scala tympani both free floating and within inflammatory cells. After inoculation with the Ply− mutant, histopathology of the RWM (Fig. 3b) was similar to the wild-type strain, with infiltration of inflammatory cells and bacteria in the RWM and scala tympani. The RWM from the animal inoculated with the PspA− mutant (Fig. 3c) was not as thick as the other groups, and there was no penetration of bacteria into the scala tympani. There was no attenuation in the pathology of the Ply−/PspA− strain compared to that of the wild-type or single mutant strains (Fig. 3d).
Fig. 3.
Histologic analysis of the round window membrane (RWM). a) This RWM from a chinchilla inoculated with the wild-type strain is thickened from inflammatory cell infiltration. Bacteria (arrowheads) in the scala tympani (ST) can be seen within inflammatory cells and free floating. ME = middle ear, arrows = boundary of RWM. Original magnification 1,000×, Toluidine blue stain. b) After inoculation with the Ply− mutant, histopathology of the RWM was similar to the wild-type and double mutant strains, with infiltration of inflammatory cells and bacteria (arrowheads) within the ST. ME = middle ear, arrows = boundary of RWM. Original magnification 1,000×, Toluidine blue stain. c) RWM from this animal inoculated with the PspA− mutant was not as thick as the other groups, and did not have bacterial penetration into the ST. ME = middle ear, arrows = boundary of RWM. Original magnification 1,000×, Toluidine blue stain. d) There was no attenuation in the pathology of the Ply−/PspA− strain compared to that of the wild-type or single mutant strains. ME = middle ear, ST = scala tympani, arrowheads = bacteria, arrows = boundary of the round window membrane. Original magnification 1,000×, Toluidine blue stain.
DISCUSSION
Streptococcus pneumoniae continues to be one of the most commonly cultured organisms from middle ears of children with otitis media. Due to the increased antibiotic resistance of pneumococci, interest has focused on a vaccine to prevent pneumococcal otitis media. The current polysaccharide-based vaccines are successful in some populations, against invasive pneumococcal diseases (bacteremia and meningitis); however, the vaccines are serotype-specific and have low-efficacy against otitis media.20 This suggests the need for a different vaccine approach. There is currently an interest in producing a vaccine based on one or more of the pneumococcal proteins.21 This requires a thorough understanding of the effect of these proteins on the viability and virulence of pneumococci within the middle and inner ears, to select the most suitable vaccine candidates. Two potential candidates are pneumolysin (Ply) and pneumococcal surface protein A (PspA). Both proteins are expressed in virtually all pneumococcal serotypes, and immunization with these antigens can provide serotype-independent protection against pneumococci.
Both of the single mutants, Ply− and PspA−, in our study were less virulent (induced less pathological changes to the round window membrane, compared to the wild-type and double mutant strains). PspA− strain was the least virulent. The decreased virulence of PspA− mutant may be due, at least in part, to the decreased viability of PspA− in the middle ear. Berry and Paton17 reported similar results that colonization of the nasopharynx decreased 30 fold, from day 1 to 4, in mice inoculated with the PspA− mutant. In our study, the wild-type and other mutant strains were still viable 48 hours after middle ear inoculation; however, the CFU counts of the PspA− mutant decreased over time with very low counts at 30 hours and no detectable levels at 48 hours. The in vitro experiment with the PspA− mutant in Todd Hewitt Broth, however, showed an expected increase of about 2 logs at 43 hours, suggesting that the PspA− mutant may be more susceptible to host factors in the ear. This is presumably due to killing of bacteria by apolactoferrin5 and/or sensitivity to complement deposition.22
An intriguing finding of our study was that viability and virulence of the Ply−/PspA− double mutant was not attenuated compared to the single mutants, but were similar to the wild-type, parent strain. When this was observed, we re-confirmed the type 2 capsule expression and the lack of expression of pneumolysin and PspA by this strain. Comparison of studies of the streptococcal proteins is confounded by many factors; such as, mode of infection, colonization versus survival, and different anatomic targets. Nevertheless, there are some variables that can be compared. Berry and Paton17 found an additive attenuation of the double Ply−/PspA− mutant compared to either of the single mutants, based on survival of sepsis induced by intranasal challenge. Ogunniyi et al23, also found increased survival of mice after infection with the double Ply−/PspA− mutant compared to the single mutants, following intraperitoneal inoculation. Interestingly, however, after intranasal inoculation, they found an increase in colonization of the nasopharynx with the double Ply−/PspA− mutant compared to the Ply− or PspA− mutants. The single mutant deficient in PspA decreased from day 1 to 4, but levels of the double Ply−/PspA− mutant at day 4 were the same as days 1 and 2, although CFU counts in the lungs were decreased. The increased colonization of the nasopharynx with the double mutant reported by Ogunniyi et al23 is similar to our findings in the middle ear with this strain.
Both the nasopharynx and the middle ear are composed of respiratory epithelium. Respiratory epithelium is a complex active epithelium involved in inflammation and host defense. It provides mucociliary clearance for the physical removal of bacteria, recognition of microbial exposure by pattern recognition receptors expressed on epithelial cells for detection of pathogen-associated molecular patterns, secretion of a variety of pro- and anti-inflammatory mediators, and secretion of a variety of antimicrobial substances including antimicrobial peptides.24 It is not surprising that different organs and different epithelial mucosa might respond with immunologically diverse mechanisms to microbial infection with S. pneumoniae. Oggioni et al25 observed two patterns of in vivo gene expression by S. pneumoniae proteins. One pattern was characteristic of the pneumococci in the brain and lungs, and the other in the bloodstream. Based on the above findings, the expression of pneumococcal proteins appears to be organ specific. It is difficult, however, to explain the lack of attenuation in the double mutant compared to its single mutant counter-parts. One possibility may be pathogen associated molecular patterns (PAMPs) are in part responsible for the unexpected result with the double mutant. The innate immune response relies on recognition of PAMPs. These are conserved molecules unique to groups of related microorganisms and are not associated with human cells. PAMPs are diverse and include nucleic acids, proteins, peptides, glycoconjugates or lipids,26 and are recognized by host pattern-recognition receptors (PRRs). There are four main classes of PRRs - Toll-like receptors, NOD-like receptors, RIG-like receptors and C-type lectin receptors. Upon PAMP recognition, PRRs signal to the host, activating a multitude of intracellular signaling pathways, including adaptor molecules, kinases, and transcription factors, triggering proinflammatory and antimicrobial responses27 that result in the activation of gene expression and synthesis of a broad range of molecules, including cytokines, chemokines, cell adhesion molecules, and immunoreceptors.28 One possible explanation for the lack of attenuation of the double mutant might be that at least one of these two pneumococcal proteins needs to be present for PAMP recognition and clearance.
It is not in the scope of this study to determine the mechanism for the lack of attenuation of the double Ply−/PspA− mutant in the ear: whether it could be related to complement, pathogen-associated molecular pattern recognition, a more active role of other pneumococal virulence factors in the absence of the Ply and PspA proteins, or some other mechanism. It does appear, however, that pneumococcal proteins have diverse organ-specific effects on the viability and virulence of pneumococci. Further study is needed to better understand the potential of these proteins and their combinations as vaccine candidates against different diseases in diverse anatomic locations caused by pneumococci.
Acknowledgements
We would like to acknowledge James C. Paton for providing the two mutants lacking pneumolysin for use in these studies, and Janice King and Pat Coan for verifying the identify of the D39 mutants.
This work was supported in part by: NIH/NIDCD R01 DC006452, NIH/NIDCD 3U24 DC008558-03S1, NIH/NIAID R01 A1021548, The International Hearing Foundation, The Starkey Foundation, and 5 M Lions International.
Footnotes
This work was presented at the 35th Meeting of Association for Research in Otolaryngology, February 25–29, 2012, San Diego, CA
REFERENCES
- 1.American Academy of Pediatrics Subcommittee on Management of Acute Otitis Media. Diagnosis and management of acute otitis media. Pediatrics. 2004;113(5):1451–1465. doi: 10.1542/peds.113.5.1451. [DOI] [PubMed] [Google Scholar]
- 2.Bogaert D, Hermans PW, Adrian PV, Rümke HC, de Groot R. Pneumococcal vaccines: an update on current strategies. Review. Vaccine. 2004;22(17–18):2209–2220. doi: 10.1016/j.vaccine.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 3.Eskola J, Kilpi T, Palmu A, et al. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med. 2001;344(6):403–409. doi: 10.1056/NEJM200102083440602. [DOI] [PubMed] [Google Scholar]
- 4.Briles DE, Hollingshead SK, Nabors GS, Paton JC, Brooks-Walter A. The potential for using protein vaccines to protect against otitis media caused by Streptococcus pneumoniae. Vaccine. 2001;19(1):S87–S95. doi: 10.1016/s0264-410x(00)00285-1. [DOI] [PubMed] [Google Scholar]
- 5.Shaper M, Hollingshead SK, Benjamin WH, Briles DE. PspA protects Streptococcus pneumoniae from killing by apolactoferrin, and antibody to PspA enhances killing of pneumococci by apolactoferrin. Infect Immun. 2004;72(9):5031–5040. doi: 10.1128/IAI.72.9.5031-5040.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ren B, Szalai AJ, Thomas O, Hollingshead SK, Briles DE. Both family 1 and family 2 PspA proteins can inhibit complement deposition and confer virulence to a capsular serotype 3 strain of Streptococcus pneumoniae. Infect Immun. 2003;71(1):75–85. doi: 10.1128/IAI.71.1.75-85.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jedrzejas MJ. Pneumococcal virulence factors: structure and function. Microbiol Mol Biol Rev. 2001;65(2):187–207. doi: 10.1128/MMBR.65.2.187-207.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paton JC, Rowan-Kelly B, Ferrante A. Activation of human complement by the pneumococcal toxin pneumolysin. Infect Immun. 1984;43(3):1085–1087. doi: 10.1128/iai.43.3.1085-1087.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuste J, Botto M, Paton JC, Holden DW, Brown JS. Additive inhibition of complement deposition by pneumolysin and PspA facilitates Streptococcus pneumoniae septicemia. J Immunol. 2005;175(3):1813–1819. doi: 10.4049/jimmunol.175.3.1813. [DOI] [PubMed] [Google Scholar]
- 10.Rayner CF, Jackson AD, Rutman A, et al. Interaction of pneumolysin-sufficient and - deficient isogenic variants of Streptococcus pneumoniae with human respiratory mucosa. Infect Immun. 1995;63(2):442–447. doi: 10.1128/iai.63.2.442-447.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Briles DE, Paton JC, Hollingshead SK, Boslego JW. Pneumococcal common proteins and other vaccine strategies. In: Levine MM, Dougan G, Good MF, et al., editors. New Generation Vaccines. New York: Marcel Dekker, Inc.; 2010. pp. 482–488. [Google Scholar]
- 12.Schachern P, Tsuprun V, Cureoglu S, et al. The round window membrane in otitis media: effect of pneumococcal proteins. Arch Otolaryngol Head Neck Surg. 2008;134(6):658–662. doi: 10.1001/archotol.134.6.658. [DOI] [PubMed] [Google Scholar]
- 13.Schachern PA, Tsuprun V, Cureoglu S, et al. Virulence of pneumococcal proteins on the inner ear. Arch Otolaryngol Head Neck Surg. 2009;135(7):657–661. doi: 10.1001/archoto.2009.72. [DOI] [PubMed] [Google Scholar]
- 14.Avery OT, MacLeod CM, McCarty M. Studies on the chemical nature of the substance inducing transformation of pneumococcal types. Induction of transformation by a desoxyribonucleic acid fraction isolated from pneumococcus type III. J Exp Med. 1944;79:137–158. doi: 10.1084/jem.79.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yother J, Handsome GL, Briles DE. Truncated forms of PspA that are secreted from Streptococcus pneumoniae and their use in functional studies and cloning of the pspA gene. J Bacteriol. 1992;174(2):610–618. doi: 10.1128/jb.174.2.610-618.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berry AM, Yother J, Briles DE, Hansman D, Paton JC. Reduced virulence of a defined pneumolysin-negative mutant of Streptococcus pneumoniae. Infect Immun. 1989;57(7):2037–2042. doi: 10.1128/iai.57.7.2037-2042.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berry AM, Paton JC. Additive attenuation of virulence of Streptococcus pneumoniae by mutation of the genes encoding pneumolysin and other putative pneumococcal virulence proteins. Infect Immun. 2000;68(1):133–140. doi: 10.1128/iai.68.1.133-140.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gray BM, Converse GM, 3rd, Dillon HC., Jr Serotypes of Streptococcus pneumoniae causing disease. J Infect Dis. 1979;140(6):979–983. doi: 10.1093/infdis/140.6.979. [DOI] [PubMed] [Google Scholar]
- 19.Waltman WD, McDaniel LS, Gray BM, Briles DE. Variation in the molecular weight of PspA (pneumococcal surface protein A) among Streptococcus pneumoniae. Microb Pathog. 1990;8(1):61–69. doi: 10.1016/0882-4010(90)90008-e. [DOI] [PubMed] [Google Scholar]
- 20.Peltola H, Booy R, Schmitt HJ. What can children gain from pneumococcal conjugate vaccines? Eur J Pediatr. 2004;163:509–516. doi: 10.1007/s00431-004-1430-0. [DOI] [PubMed] [Google Scholar]
- 21.Tai SS. Streptococcus pneumoniae protein vaccine candidates: properties, activities and animal studies. Crit Rev Microbiol. 2006;32:139–153. doi: 10.1080/10408410600822942. [DOI] [PubMed] [Google Scholar]
- 22.Ren B, Li J, Genschmer K, Hollingshead SK, Briles DE. The absence of PspA or presence of antibody to PspA facilitates the complement-dependent phagocytosis of pneumococci in vitro. Clin Vaccine Immunol. 2012;19(10):1574–1582. doi: 10.1128/CVI.00393-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogunniyi AD, LeMessurier KS, Graham RMA, et al. Contributions of pneumolysin, pneumococcal surface protein A (PspA), and PspC to pathogenicity of Streptococcus pneumoniae D39 in a mouse model. Infect Immun. 2007;75(4):1843–1851. doi: 10.1128/IAI.01384-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hiemstra PS, Bals R. Series introduction: innate host defense of the respiratory epithelium. J Leukoc Biol. 2004;75:3–4. doi: 10.1189/jlb.0903410. [DOI] [PubMed] [Google Scholar]
- 25.Oggioni MR, Trappetti C, Kadioglu A, et al. Switch from planktonic to sessile life: a major event in pneumococcal pathogenesis. Mol Microbiol. 2006;61(5):1196–1210. doi: 10.1111/j.1365-2958.2006.05310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ray A, Cot M, Puzo G, Gilleron M, Nigou J. Bacterial cell wall macroamphiphiles: Pathogen-/microbe-associated molecular patterns detected by mammalian innate immune system. Biochimie. 2013;95(1):33–42. doi: 10.1016/j.biochi.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 27.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 28.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]