Abstract
Background
Alterations in 5-hydroxytryptamine (HT) signaling in inflamed gut may contribute to pathogenesis of inflammatory bowel diseases. Adenosine 5′-triphosphate (ATP) regulates mucosal-mechanosensory reflexes and ATP receptors are sensitive to mucosal inflammation. Yet, it remains unknown whether ATP can modulate 5-HT signaling in enterochromaffin cells (EC). We tested the novel purinergic hypothesis that ATP is a critical autocrine regulator of EC mechanosensitivity and whether EC expression of ATP-gated P2X3-ion channels is altered in inflammatory bowel diseases.
Methods
Laser confocal (fluo-4) Ca2+ imaging was performed in 1947 BON cells. Chemical stimulation or mechanical stimulation (MS) was used to study 5-HT or ATP release in human BON or surgical mucosal specimens, and purine receptors by reverse transcription-polymerase chain reaction, Western Blot, or P2X3-immunoreactivity in BON or 5-HT+ human EC (hEC) in 11 control and 10 severely inflamed ulcerative colitis (UC) cases.
Results
ATP or MS triggered Ca2+-transients or 5-HT release in BON. ATP or adenosine diphosphate increased 5-HT release 5-fold. MS caused ATP release, detected after 5′ecto-ATPase inhibition by ARL67156. ARL67156 augmented and apyrase blocked Ca2+/5-HT mechanosensitive responses. 2-Methyl-thio-adenosine diphosphate 5′-monophosphate-evoked (P2Y1,12) or mechanically-evoked responses were blocked or augmented by a P2Y1,12 antagonist, MRS2179, in different cells or inhibited by U73122. A P2Y12 antagonist, 2MeSAMP, augmented responses. A P2X1,3 agonist, α,β-MeATP, triggered Ca2+ responses, whereas a P2X1,2/3,3 antagonist, 2′,3′-O-(2,4,6-trinitrophenyl)-ATP, blocked mechanical responses or cell-surface 5′ATP-TR labeling. In hEC, α,β-MeATP stimulated 5-HT release. In UC, P2X3-immunoreactivity decreased from 15% to 0.2% of 5-HT+hECs. Human mucosa and BON expressed P2X1, P2X3, P2X4, P2X5, P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, and P2Y12R-messenger RNA transcripts.
Conclusions
ATP is a critical determinant of mechanosensation and 5-HT release via autocrine activation of slow P2Y1-phospholipase C/inositol-1,4,5-triphosphate-Ca2+ or inhibitory P2Y12-purinergic pathways, and fast ATP-gated P2X3-channels. UC downregulation of P2X3-channels (or A2B) is postulated to mediate abnormal 5-HT signaling.
Keywords: ATP, mechanosensitivity, 5-HT release, ulcerative colitis, ATP-gated P2X3 channels
The enterochromaffin cell (EC) synthesizes and releases 5-hydroxytryptamine (HT) to initiate enteric neural reflexes and transmit information about visceral pain sensation.1–8 Alterations in 5-HT release, content, or reuptake mechanisms may contribute to the pathogenesis of inflammatory bowel diseases (IBDs), diarrhea with bacterial toxin enterocolitis, and irritable bowel syndrome (IBS)9–14; associations also exist with diverticular disease, colorectal cancer, and celiac disease.15 However, the basic mechanisms regulating 5-HT release in human enterochromaffin cells (hECs) remain poorly understood. Understanding how 5-HT release is regulated at cellular and molecular levels is a necessity for understanding the basis of these disorders.16,17
ECs have chemo- and mechanosensitive elements that detect changes in force or contents of the intestinal lumen.2 The human carcinoid BON cells is an established hEC line shown to be a suitable model to study both chemo- and mechanosensation, in addition to receptor regulation, postreceptor signaling pathways, and physiological regulation of 5-HT release.1,2,18–21 We found that a major component of mechanosensation is activation of the Gαq/phospholipase C (PLC)/Ca2+ signaling pathway to increase 5-HT release. Because 5-HT release is a Ca2+-dependent pathway, study of intracellular free Ca2+ levels in BON cells is a suitable target to study regulation of 5-HT release mechanisms at a single cell level.
Adenosine 5′-triphosphate (ATP) is a major player in mechanosensory reflexes, but its role in modulating 5-HT release was unknown.18,22,23 We could show that mechanical stimulation of the mucosa in rodents releases ATP that is required for triggering secretomotor reflexes,22,23 and we speculate that ATP, in part, acts on ECs to facilitate 5-HT release. In fact, in the studies in BON cells, we found that adenosine, a metabolite of ATP, is an important autoregulatory modulator of Ca2+-dependent 5-HT release.19
The concept of purinergic signaling stems from studies that were designed to identify the nonadrenergic noncholinergic inhibitory neurotransmitter in the gut.24 Purine receptors are classified as P1 for nucleosides (adenosine) and P2 for nucleotides (ATP). The P2 family can be subdivided into ion channel P2X1–7 and metabotropic P2Y1,2,4,6,11–14 receptor families.25–27 ATP is a purinergic transmitter in the enteric nervous system, is involved in neuron-to-glial communication28,29 and gliotransmission,30 and is known to act at all levels of gut secretory and motility reflexes.31,32 ATP is released by mechanical stimulation from organ structures that form tubes and sacs to participate in mechanosensory reflexes.32 In the gastrointestinal (GI) tract, ATP release from epithelial cells by mechanical stimulation can initiate gut reflexes22,23,33 or activate P2X3 receptors on local afferent nerve endings to transmit pain sensation to the brain in animal models of IBS or IBD.34
We sought to test the novel purinergic hypothesis that ATP is a critical autocrine regulator of mechanosensitive 5-HT release. Purinergic pathways are very sensitive to inflammation leading to alterations in expression or function of purinergic targets (receptors or enzymes).35–39 Gut inflammation causes discrete alterations in various purine messenger RNA (mRNA) gene transcripts in colitis models36 or mucosal biopsies from Crohn’s disease (CD) or ulcerative colitis (UC).40 However, it is unknown if the expression of any receptor is altered in hEC from intact human gut in IBD. In preliminary experiments, we identified mRNA transcripts P2X1–P2X6 in hECs from intact gut mucosa. Transcripts for P2X1–6 receptors are expressed in hEC. Because the ATP-gated P2X3 ion channel is upregulated in enteric neurons in human IBD41 and it can transmit pain sensation,34,42 we tested whether P2X3 expression is also altered in hEC of UC patients. Our strategy was to target a receptor that is linked to mechanosensitivity in hECs.
MATERIALS AND METHODS
Surgical Specimens for 5-HT Release
Surgical tissue from 8 patients were collected after patient consent and included 2 sigmoid colons from patients with polyposis, 4 jejunums from Roux-en-Y bypass procedures, 1 rectal colon, and 1 transverse colon from a colectomy. Tissue was placed in Krebs’/oxygenated buffer at 4°C and transported to the laboratory within 15 minutes. The 5-HT release was monitored in microdissected mucosa–submucosa tissues (~1 cm2 tissues) floating in Krebs’ solution oxygenated at 37°C. Tissues were equilibrated for 30 minutes before exposure to drugs for 30 minutes. Supernatants were collected, and a 5-HT enzyme-linked immunosorbent assay (ELISA) kit was used to determine the concentration of 5-HT release in supernatants (serotonin ELISA, BAE5900; Rocky Mountain Diagnostics, Inc., Colorado Springs, CO) using a Wallac-Victor3 plate reader. Sensitivity of the assay is 0.005 ng/mL × c (correction for dilution).
Surgical Specimens for P2X3-immunoreactivity in hEC
A trained clinical GI pathologist screened 70 specimens from different patients to identify and select 10 control diverticulitis (noninflamed portions) specimens and 11 UC specimens (inflamed) from the sigmoid colon. Three sections/specimen were analyzed and scored according to Geboes et al.43 Unpaired t test was used to calculate the difference in the grading score of inflammation (0–5.4 scale).
BON Cells in Culture
BON cells were a gift from C.M. Townsend Jr (University of Texas, Galveston, TX). Clone No. 7 was highly enriched with 5-HT. The cells were seeded on No. 0 cover slips (MatTek, Corp., Ashland, MA) at a density of 105 cells for touch experiments and at a density of 106 cells for shaking experiments. Cells were grown in Dulbecco-modified Eagle medium–nutrient mixture F-12 (1:1), supplemented with 10% fetal calf serum, 100 IU/mL penicillin, and 100 µg/mL streptomycin (Life Technologies, Grand Island, NY). Cells were grown in a humidified atmosphere of 95% air and 5% CO2 at 37°C without reaching confluence for touch experiments and reaching confluence for shaking experiments.2
Mechanical Stimulation of a Single BON Cell
BON cells were loaded with 5 µM Fluo-4/AM in Dulbecco-modified Eagle medium–nutrient mixture F-12 (Life Technologies) for 20 minutes in a 95% air and 5% CO2 incubator at 37°C for touch Ca2+ experiments using a modified-Zeiss LSCM 410/REN laser scanning confocal imaging system19 described in Supplemental Methods, Supplemental Digital Content 1, http://links.lww.com/IBD/A230, and Figure 4.
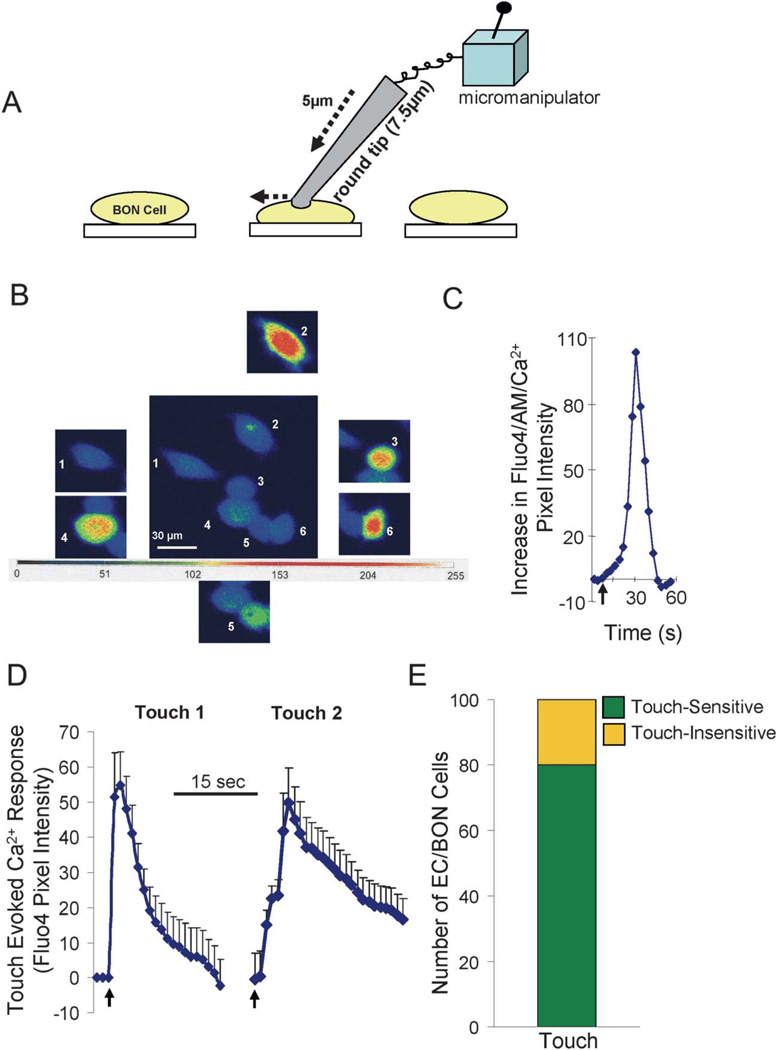
FIGURE 4.
Touch/stretch of single BON cells causes a Ca2+ response that can be quantified and is reproducible. A, Schematic of the touch/stretch technique—a piezo-micromanipulator is used to stretch the membrane of a single cell using a fire-polished glass tip (5–7.5 µm diameter). B, Touch of each BON cell individually elicited a Ca2+ response in cells 2 to 6 but not cell 1. Cells are pseudo-colored based on a gray scale of intensity ranging from 0 to 255. C, Kinetic Ca2+ response to touch in a single cell. D, The touch-evoked Ca2+ response indicated by arrows is reproducible if repeated after a 10-minute interval between trials (touch 1 and touch 2; n = 80 cells individually touched). E, Eighty percent of BON cells are touch sensitive with a Ca2+ response.
Mechanical Stimulation of BON Cell Monolayers to Release 5-HT
Another mechanical stimulus was a mild rotational shaking at 80 rpm to measure 5-HT release from the population of cells, as we reported previously.2,19 Briefly, the cells were exposed to mechanical stimulation on a shaker (Lab-Line, Melrose Park, IL), which rotated the culture plates containing the cells. The supernatants were collected as described previously (2) and frozen at -80C until the 5-HT assay was carried out.
ATP Quantification in Supernatants of BON Cell Monolayers After Rotational Shaking
To measure ATP release from BON cell monolayers, ATP release was obtained by using the luciferin/luciferase assay44,45 and assay glow kits according to the manufacturer’s instructions.
Statistical Analysis
Mean values ± standard error of the mean are reported. SPSS 17.0, GraphPad Prism 3.02, Stat-View 54.51, and laser scanning confocal microscope (LSM) Zeiss software were used for analysis. Dose-response curves were analyzed by analysis of variance (ANOVA) followed by Newman–Keuls multiple comparison test. For other parameters, simple unpaired/2-tailed t tests were used. Differences were considered statistically significant at P < 0.05.
Ethical Considerations
Patient consent was obtained for the procurement of surgical tissue that would otherwise be discarded following pathologic examination. This is outlined in our approved institutional review board protocols 2004H0165 and 2012H0231 through the ethics committee at The Ohio State University Office of Responsible Research Practices.
RESULTS
Purinergic Ca2+ Responses in BON Cells
Ca2+ transients were analyzed in 1947 single BON cells—mechanical stimulation (touch) experiments were done in 1490 cells. Purinergic receptor agonists were tested in 457 cells. Additional experiments were done on 5-HT or ATP release. Our findings are summarized in Figures 1 to 11, Figures in Supplemental Digital Content 2 and 3, http://links.lww.com/IBD/A231 and http://links.lww.com/IBD/A232, respectively, Tables 1 and 2 and Table in Supplemental Digital Content 4, http://links.lww. com/IBD/A233.
FIGURE 1.
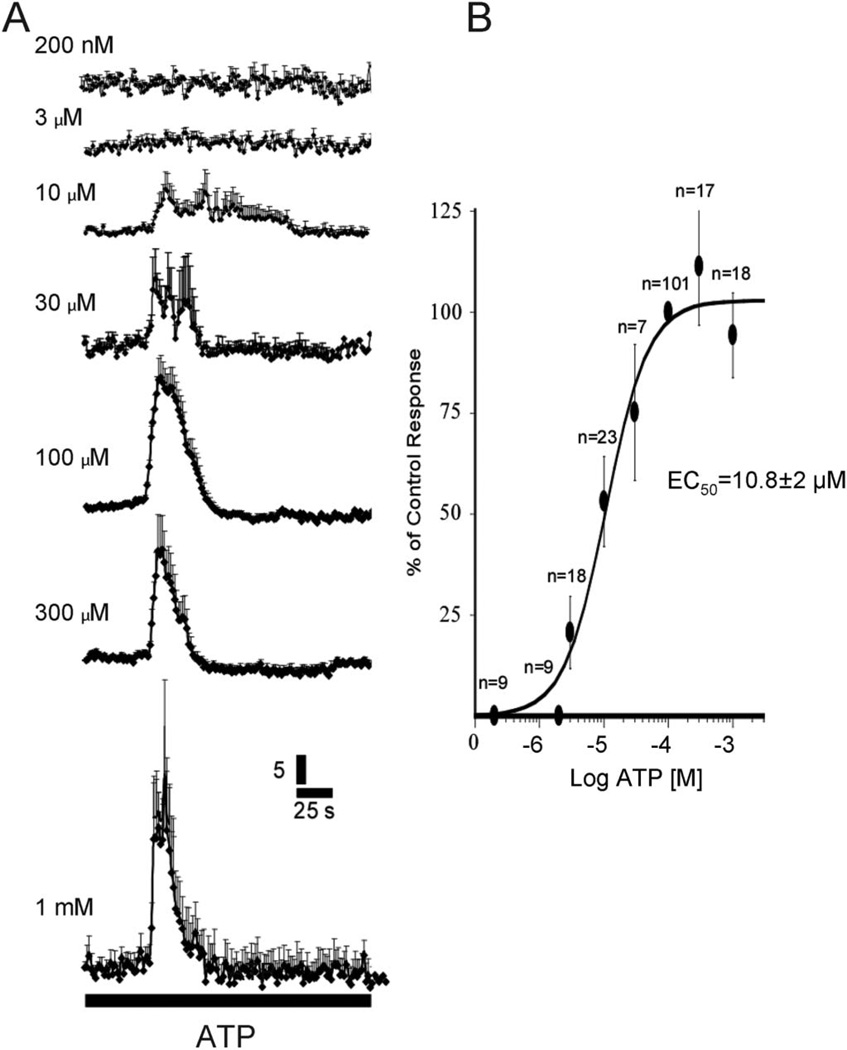
ATP Ca2+ transients in BON cells. A, Ca2+ responses at different concentrations of ATP. B, ATP caused a concentration-dependent Ca2+ response with an EC50 at 10.8 ± 2 µM. n, number of cells; 100 µM ATP was used as the peak response for normalizing data. ANOVA, P < 0.001.
FIGURE 11.
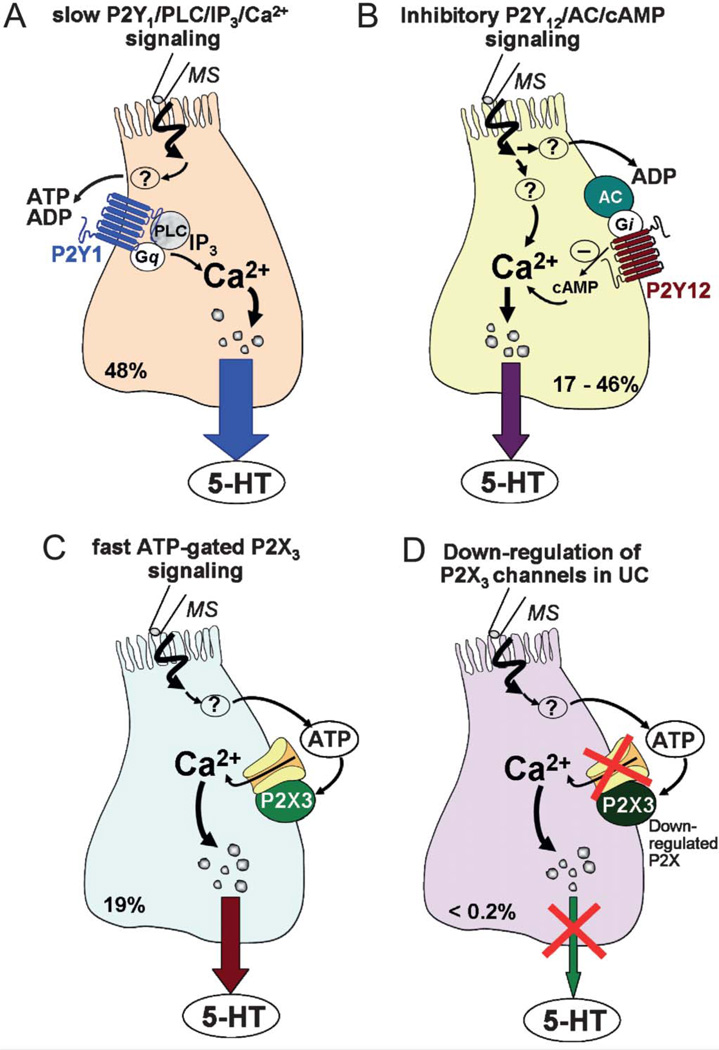
Purinergic autoregulation model of mechanosensitivity in human ECs. In a majority of hEC, mechanically evoked 5-HT release is tightly autoregulated by corelease of ATP via 3 distinct purinergic signaling mechanisms. In the remainder of cells, either autoregulation does not occur or it involves nonpurinergic mechanisms. A, Mechanical stimulation releases ATP (or ADP) to activate a slow P2Y1-PLC/IP3-Ca2+-dependent mechanism in 48% cells, leading to stimulation of 5-HT release. B, In a distinct subset of hEC, a slow P2Y12AC/cAMP signaling pathway is activated by ATP (or ADP) release leading to attenuation of 5-HT release. C, ATP release in 19% cells can alternatively activate a fast ATP-gated P2X3 ion channel to stimulate 5-HT release. D, Purinergic pathways are very sensitive to inflammation, and severe downregulation of P2X3 channels occurring in UC is expected to alter 5-HT release and contribute to abnormal signaling, physiology, and visceral sensation. In this model, purinergic autoregulation does not occur in all hEC. 5-HT release evoked by mechanical stimulation is not regulated by ATP in about 25% to 30% of hEC. Overall, mechanical stimulation coreleases 5-HT, ATP/other purines (ADP and adenosine), or other mediators (prostaglandins and PACAP19). 5-HT, ATP, and perhaps other mediators can trigger gut motor reflexes and visceral sensation, and any disruption in purinergic autoregulation and fine tune modulation of 5-HT release could have important consequences in IBD.
TABLE 1.
Summary of the BON Cell Population Responses to Purinergic Drug/Manipulations for Touch or Purinergic Agonist-Evoked Ca2+ Transients
| Ca2+ Responses to Nucleotides/Analogs (Percentage of Cell Population*) | |||
|---|---|---|---|
| Treatment | Stimulate | No Effect | n |
| ATP | 65 | 35 | 80 |
| UTP | 48 | 52 | 69 |
| α,β-MeATP | 19 | 81 | 152 |
| 2MeSADP | 54 | 46 | 156 |
| Microejection (1 mM) or perfusion (10 and 100 µM) | |||
| Ca2+ Responses to Touch/Stretch (Percentage of Cell Population**) | ||||
|---|---|---|---|---|
| Antagonist/Blocker/Inhibitor | Block/Abolish Response | Augment Response | No Effect | n |
| Apyrase (5 U/mL) | 77 | 0 | 23 | 67 |
| ARL67156 (10 µM) | 0 | 63 | 37 | 65 |
| PPADS (10 µM) | 52 | 0 | 48 | 100 |
| MRS2179 (10 µM) | 48 | —— | —— | 100 |
| 17 (reduced) | 35 | |||
| TNP-ATP (20 µM) | 27 | 18 | 55 | 73 |
| 2MeSAMP | 0 | 46 | 54 | 81 |
| Piroxicam | 0 | 73 | 27 | 41 |
| Ca2+-free medium (0.5 mM Ca2+, 10 µM EGTA) | 3.6 | —— | 96.4 | 84 |
| U73343 | 0 | —— | 100 | 100 |
| U73122 | 57 | —— | 43 | 152 |
Data are expressed as *percentage of cell population responding to the drug for a single drug concentration or **touch/stretch.. Four different effects were analyzed including percentage of cells in which response was abolished, percentage of cells in which response was augmented, percentage with no effect, or percentage with partial inhibition of the response. Concentrations were chosen from concentration-response curves generated for touch-Ca2+ responses. The concentration of each drug used in most cases is >EC80 concentration. For MRS2179, an EC50 concentration is used. Antagonists were perfused for 10 to 20 minutes and agonists for 3 to 5 minutes.
TABLE 2.
Discrete Alterations in P2X3 and A2B Purine Receptor Expression in hEC, Epithelia, and Lamina Propria From Sigmoid Colon of UC Surgical Cases Compared With Controls
| Δ in UC Compared With Noninflamed Diverticulitis Controls in Sigmoid Colon | |||||||
|---|---|---|---|---|---|---|---|
| Purinergic Receptor/ Other |
Percentage of 5-HT+hECs (1.2% Cells) |
Percentage of Ganglia |
Percentage of Epithelial Cells |
hEC/5-HT+ Cells (Δ in Expression) |
Epithelial Cells | Lamina Propria/ Immune Cells (Δ in Number) |
ENS (SMP) s100 +/HuC/D+ (Δ in +ve Ganglia) |
| 5-HT | NA | NA | NA | ↓ 50% (P < 0.0005) | NA | NA | NA |
| P2X3 | 15 | 13 | 0.43 | ↓ 98% (P = 0.025) | ↓ 74% (P < 0.005) | ↑ +++ | Ø |
| A2B | 53 | 46 | 5.7* | ↓ 68% (P = 0.001) | ↓ 66% (P = 0.011) | ↑ +++ | ↓ 57% (P = 0.029) |
Percentage of cells/A2B+ crypt; percentage reduction is calculated on the basis of the numbers of fewer cells expressing the receptor in UC compared with control.
NA, not applicable; Ø, no change; Δ, change.
ATP Responses
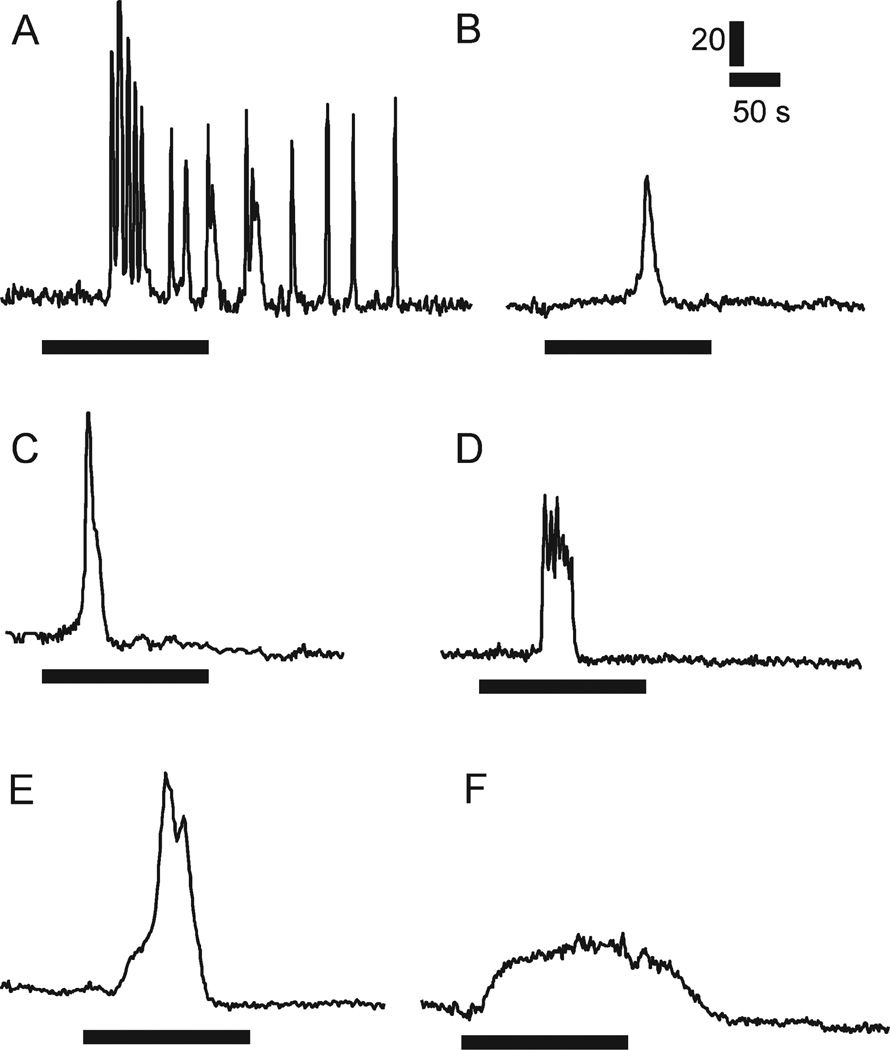
ATP (Tocris/R&D Systems, Minneapolis, MN) elicits a concentration-dependent increase in intracellular-free Ca2+ levels ([Ca2+]i] in BON cells with a 50% effective concentration (EC50) of 10.8 ± 2.0 µM (Fig. 1). ATP can elicit several types of ATP Ca2+ transients or responses that can be distinguished by their kinetic profiles as shown in Figure 2: ATP can evoke a Ca2+ wave in about 30% to 50% of responsive cells (Fig. 2A) associated with Ca2+ oscillations. The frequency of oscillations ranges from 2 to 30 oscillations. Some Ca2+ transients have simple kinetic profiles (Fig. 2B, C). More complex multiphasic responses also occur in some cells (Fig. 2D, E) without oscillations in Ca2+ responses. Rarely, ATP evokes a slow rising Ca2+ response that persists for 200 to 300 seconds (Fig. 2F).
FIGURE 2.
ATP Ca2+ transients in BON cells with distinct kinetic-profiles: (A) Ca2+ waves or oscillations; (B,C) simple monophasic Ca2+ transients; (D,E). complex multiphasic responses with a burst of Ca2+—spikes or multiple peaks; (F) rare Ca2+ response with very slow rising and falling phases. All responses were evoked with 100 µM ATP. Horizontal bar, duration of ATP perfusion. Vertical calibration, pixel intensity.
P2X and P2Y Responses and mRNA Transcripts in BON Cells and Human Intestinal Surgical Tissues
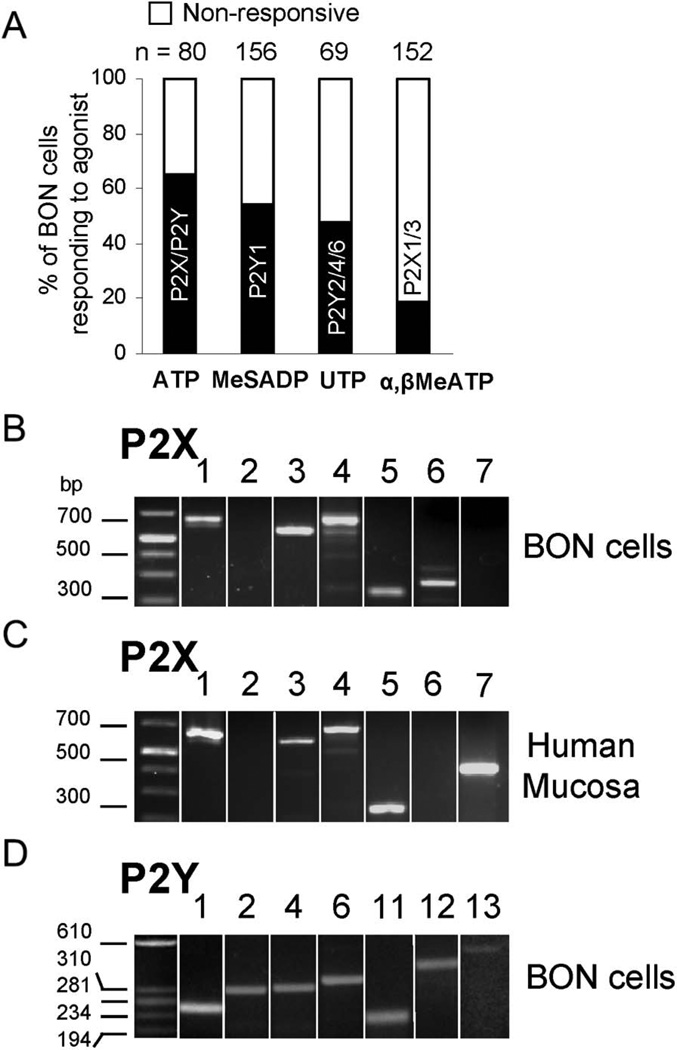
Laser confocal Ca2+ imaging reveals responses to ATP, the P2Y1,12 agonist 2-methyl-thio-adenosine diphosphate 5′-monophosphate (2MeSADP; Tocris/R&D Systems), the P2Y2,4,6 agonist uridine triphosphate (UTP; Sigma Aldrich, St. Louis, MO), and the P2X1,3 agonist α,β-MeATP (Tocris/R&D Systems) in different proportions of BON cells (Fig. 3A). Various P2X and P2Y transcripts are revealed by reverse transcription-polymerase chain reaction (RT-PCR) as shown in Figure 3B–D. Primer sequences for P2X and P2Y receptor transcripts (Table, Supplemental Digital Content 4, http://links.lww.com/IBD/A233) and additional description of methodology are described in the Supplemental Methods, Supplemental Digital Content 1, http://links.lww.com/IBD/A230
FIGURE 3.
Expression of different P2X and P2Y receptor subtypes in BON cells/EC. A, Calcium imaging reveals multiple functional receptors. ATP, a P2Y1,12 agonist 2-MeSADP, a preferential P2Y2,4,6 agonist UTP, or a P2X1,3 agonist α,β-MeATP caused a Ca2+ response. B, mRNA transcripts for P2X receptors in BON cells. C, mRNA transcripts for P2X receptors in human mucosa. D, mRNA transcripts for P2Y receptors in BON cells.
Touch Evoked Ca2+ Response
Touch/stretch of a single BON cell causes a transient Ca2+ response that can be quantified and is reproducible (Fig. 4).19 It is interesting that the second transient lasts longer on average than the first one—the mechanism for this “phenomenon” remains unknown. Eighty percent of BON cells are touch sensitive with a Ca2+ response. Touch causes a peak increase in [Ca2+]i corresponding to a fluorescence pixel intensity of 56.3 ± 2.7 (n = 80). Low Ca2+ – Krebs’ solution does not affect touch Ca2+ responses (Table 1).
Effect of ARL67156 and Apyrase on Touch Ca2+ Responses
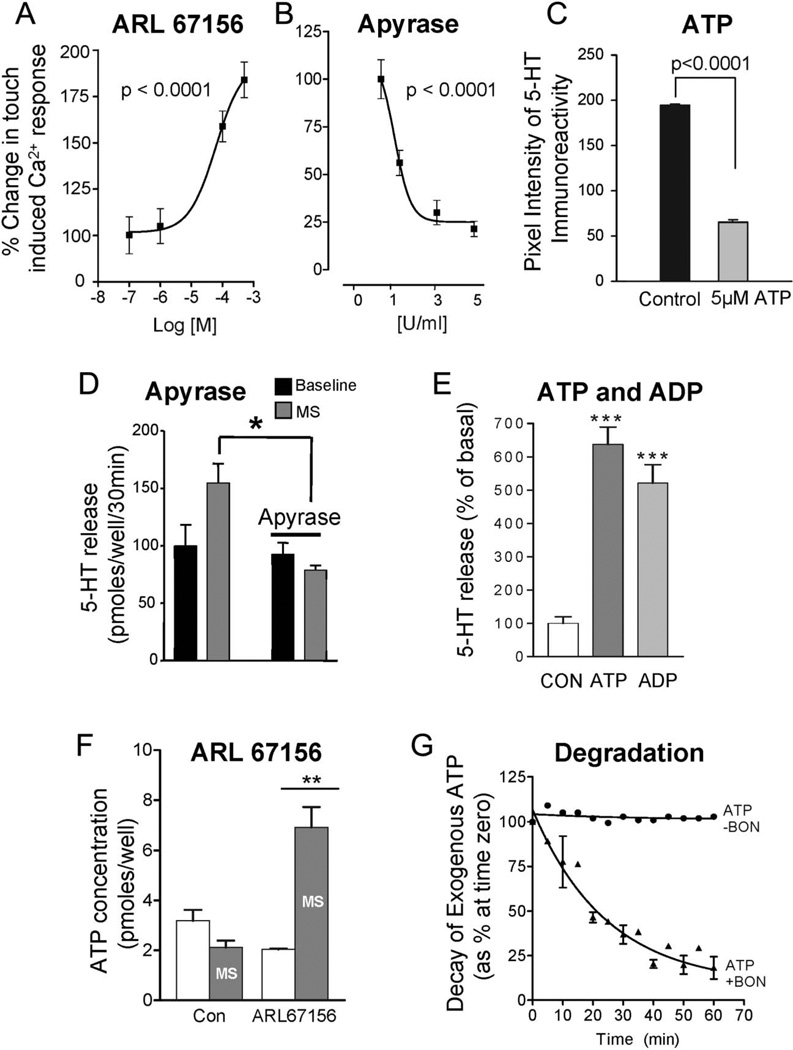
ARL67156 (Sigma-Aldrich) is an inhibitor of 5′-ectonucleotidases that is used to protect against breakdown of nucleotides such as ATP. It induced a concentration-dependent augmentation in the peak touch-Ca2+ response in 63% cells (Fig. 5A; n = 102, P < 0.0001). The EC50 for ARL67156 was 6.1 × 10−5 M. Apyrase (Sigma-Aldrich) reduced the touch-Ca2+ response by 79% ± 4.0 (n = 67) at concentrations between 0.5 and 5 U/mL (n = 256, ANOVA P < 0.0001). The EC50 for apyrase was 1 U/mL (Fig. 5B).
FIGURE 5.
Endogenous nucleotides are involved in the mechanically evoked Ca2+ response or 5-HT release in BON cells. A, The 5″-ectonucleotidase inhibitor ARL67156 augments touch-evoked Ca2+ responses. B, The ecto-ATPase inhibitor apyrase inhibits touch-Ca2+ responses. C, Exogenous ATP can deplete the 5-HT content in single BON cells as determined by quantitative analysis of 5-HT ir. 5-HT ir is quantified according to intensity levels using a Zeiss LSM/REN 410 imaging software and the same setting on the confocal (pinhole, contrast, brightness, power of laser, 40×/1.3 N.A objective). Cells were exposed to ATP for 20 minutes at 378C in oxygenated Krebs’ buffer. D, Rotational shaking evokes 5-HT release that is abolished by apyrase. E, Exogenous ATP or ADP causes a dramatic increase in 5-HT release from BON cells (N = 7, P < 0.0001); 106/well BON cells were seeded in a 12-well plate and incubated for 40 hours; cells were washed with OptiMEM solution (Life Technologies) and equilibrated for 30 minutes; 10−5 M ATP or 10−5 M ADP was incubated for 20 minutes, and supernatant was collected for 5-HT analysis by ELISA kit. Basal 5-HT = 1.12 ± 0.2 pmol/well/20 min. F, ATP release from BON cells in response to rotational shaking is only revealed if 10 µM ARL67156 is used to protect against degradation of endogenous ATP release; 106/well BON cells were incubated 40 hours, and cells were treated with 10−5 M ARL67156. Static incubation of cells was done at 37°C or mechanical stimulation was done by rotation shaking at 80 rpm and ATP detection in supernatants was done using an ATPlite kit using firefly luciferinluciferase; Rlu luminescence was measured in a Victor3 (Perkin Elmer, Waltham, MA). *P < 0.05; **P < 0.005;*** P < 0.0001. G, Decay kinetics of 100 nM exogenous ATP by BON cells; the enzyme 5′-ectonucleotidase reduces the ATP-concentration over time by causing metabolic degradation.
Depletion of 5-HT Content by ATP Stimulation
Exogenous ATP can cause depletion of 5-HT content in single BON cells as determined by quantitative analysis of 5-HT immunoreactivity (ir; Fig. 5C, P < 0.0001, n = 200).
Effect of Apyrase and ARL67156 on 5-HT Release Evoked by Mechanical Stimulation
In BON monolayers, apyrase (5 U/mL, 20 min) inhibited mechanically evoked 5-HT release (Fig. 5D, N = 4 dishes, P < 0.05). Incubation of BON cells with 10−5 M ATP or 10−5 M adenosine diphosphate (ADP; Sigma-Aldrich) caused a 4- to 5-fold increase in 5-HT release (Fig. 5E; N = 7, P < 0.0001).
ATP Release by Mechanical Stimulation Is Revealed by ARL67156
To test whether ATP was released by mechanical stimulation, BON cell monolayers were stimulated for 30 minutes by rotational shaking at 80 rpm. Mechanical stimulation increased ATP by 3.3-fold compared with baseline ATP (2.15 ± 0.4 pmol/well/30 min, N = 3; Fig. 5F). To evaluate if enzymes in BON cells are capable of degrading endogenous ATP release during mechanical stimulation, we determined the effect of ARL67156 on ATP release from BON cells in response to rotational shaking. As shown in Figure 5F, detectable levels of ATP release are revealed only if 1 × 10−5 M ARL67156 is used to protect against degradation of endogenous ATP release (P < 0.005). The activity of endogenous 5′-ectonucleotidases is confirmed by showing that exogenous ATP can be degraded by BON cells (Fig. 5G).
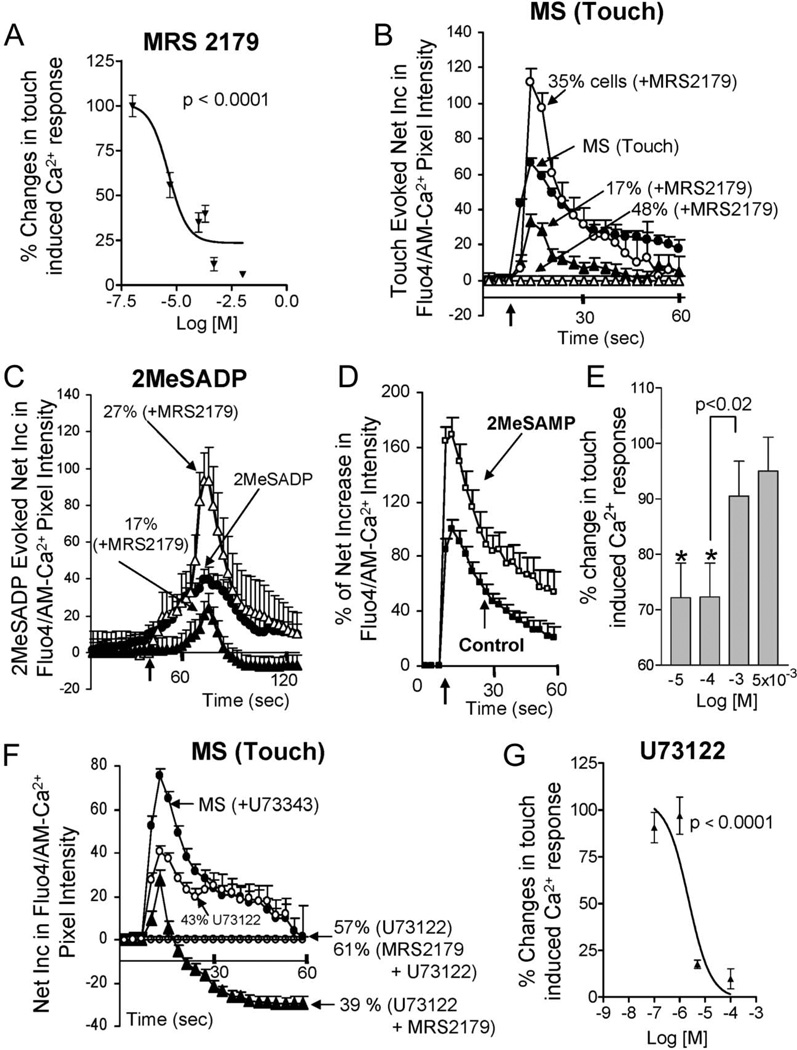
ADP is the preferred mediator for both P2Y1 and P2Y12 receptors, and MRS2179 (Tocris/R&D Systems) targets both of them. Therefore, we tested whether a P2Y12 antagonist 2MeSAMP (Sigma-Aldrich) could influence mechanical stimulation. 2MeSAMP mimics only one component of the MRS2179 response by only augmenting touch-Ca2+ responses (Fig. 6D, E).
FIGURE 6.
P2Y1 receptors, PLC signaling, and mechanosensitivity in BON cells. A, The prototypical P2Y1 antagonist MRS2179 blocks the touch-evoked Ca2+ response in a concentration-dependent manner (n = 228). B, MRS2179 prevents or reduces touch-evoked Ca2+ responses in a majority of BON cells. In a different subset of cells (35%), MRS2179 augments the response. C, A P2Y1 agonist 2MeSADP elicits a Ca2+ response in BON cells that is sensitive to MRS2179 (n = 152). Similar to touch responses, MRS2179 either blocks or augments the 2-MeSADP-induced Ca2+ response in different cells. D, The P2Y12 antagonist 2MeSAMP augments the touch-Ca2+ response. E, Concentration-dependent response to 2MeSAMP. F, The PLC inhibitor U73122 abolishes the touch Ca2+ response in 57% of cells or reduces it in the remaining 43% of cells (n = 150). Interactions between MRS2179 and U73122 on touch-Ca2+ responses occur. U73122+MRS2179 can abolish touch Ca2+ responses and further reduce basal Ca2+ levels below the baseline. G, Concentration-dependent effect of the PLC inhibitor U73122 on touch-Ca2+ responses in sensitive cells (n = 247).
The P2Y1 receptor is linked to the Gαq/PLC/inositol-1,4,5-triphosphate (IP3)-Ca2+ signaling pathway, and the PLC inhibitor U73122 could abolish the touch-Ca2+ response in 57% of touch-sensitive cells (0.1 µM–10 µM, n = 150), in contrast to the inactive analog U73343 (Tocris/R&D Systems) (Table 1). In the remaining 43% cells, U73122 (Tocris/R&D Systems) caused modest reduction in response, indicating a Gαq/PLC/IP3-insensitive mechanism. Additional interactions occur between MRS2179 and U73122 causing further reduction in basal Ca2+ levels (Fig. 6F). The threshold concentration was 1 µM for U73122. The 50% inhibitory concentration for U73122 was 2.3 × 10−6 M (Fig. 6G, n = 247).
Relationship Between 2MeSADP Sensitivity and Touch Sensitivity
In another experiment, “micropuff” application of 2Me-SADP (1 mM, 200 ms) increased [Ca2+]i in ~54% of cells (79/156). 2MeSADP-sensitive cells (58/59 cells) responded with a Ca2+ transient to the touch stimulus. Therefore, a 1:1 relationship exists between 2MeSADP and touch sensitivity. Mechanically evoked or 2MeSADP-induced Ca2+ or 5-HT release responses are blocked by MRS2179 (Figure, Supplemental Digital Content 2, http://links.lww.com/IBD/A231).
Functional P2X Receptors in BON Cells
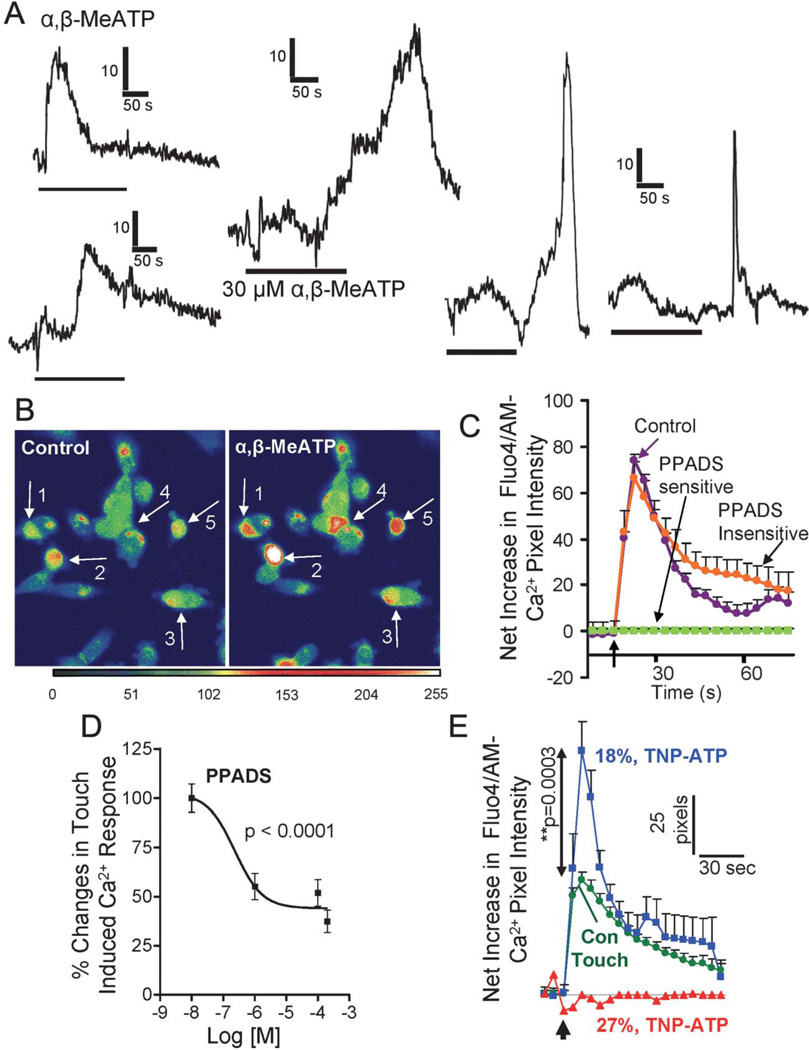
The P2X1,3 agonist α,β-MeATP triggered various types of Ca2+ responses in BON cells (Fig. 7A, B). In 52% BON cells, touch-evoked Ca2+ responses were blocked by a P2X antagonist pyridoxal-phosphate-6-azophenyl-2′-4′-disulphonic acid (PPADS, 10 µM [R&D System, Minneapolis, MN], Fig. 7C) in a concentration-dependent manner. The apparent 50% inhibitory concentration for PPADS is 5 × 10−7 M (Fig. 7D). A selective P2X1,3,2/3 receptor antagonist 2′,3′-O-(2,4,6-trinitrophenyl)-ATP (TNP-ATP; Sigma-Aldrich) prevented touch-Ca2+ responses in 27% BON cells. In another 18% cells, TNP-ATP augmented peak Ca2+ responses instead of blocking them, suggesting a different mechanism (Fig. 7E). Approximately 19% cells had P2X1,3 agonist responses to α,β-MeATP (Table 1). A 100-millisecond micropuff of 5′ATP-Texas Red (5′ATP-TR) caused cell membrane labeling and Ca2+ transients in BON cells (Fig. 8). The P2X antagonist PPADS could also block the 5′ATP-TR response.
FIGURE 7.
P2X receptors are linked to mechanosensitivity in BON cells. A, Shapes of Ca2+ transients in response to perfusion of the P2X1/3 agonist α,β-MeATP. B, Visualization of Ca2+ responses in BON cells exposed to α,β-MeATP; pseudocolor: images of Ca2+ responses obtained in 5 cells loaded with fluo-4/AM; image with α,β-MeATP taken after 20-second exposure to 1 mM puff of drug. C, In 52% BON cells, touch-evoked Ca2+ responses are blocked by a P2X antagonist PPADS (10 µM, PPADS-sensitive cells). In the remaining 48% cells, PPADS has no effect on mechanical stimulation. D, PPADS blocks touch-Ca2+ responses in a concentration-dependent manner (n = 100). E, A selective P2X1,3,2/3 receptor antagonist TNP-ATP blocks touch-Ca2+ responses in 27% cells. In an additional 18% cells, TNP-ATP augments the peak Ca2+ response.
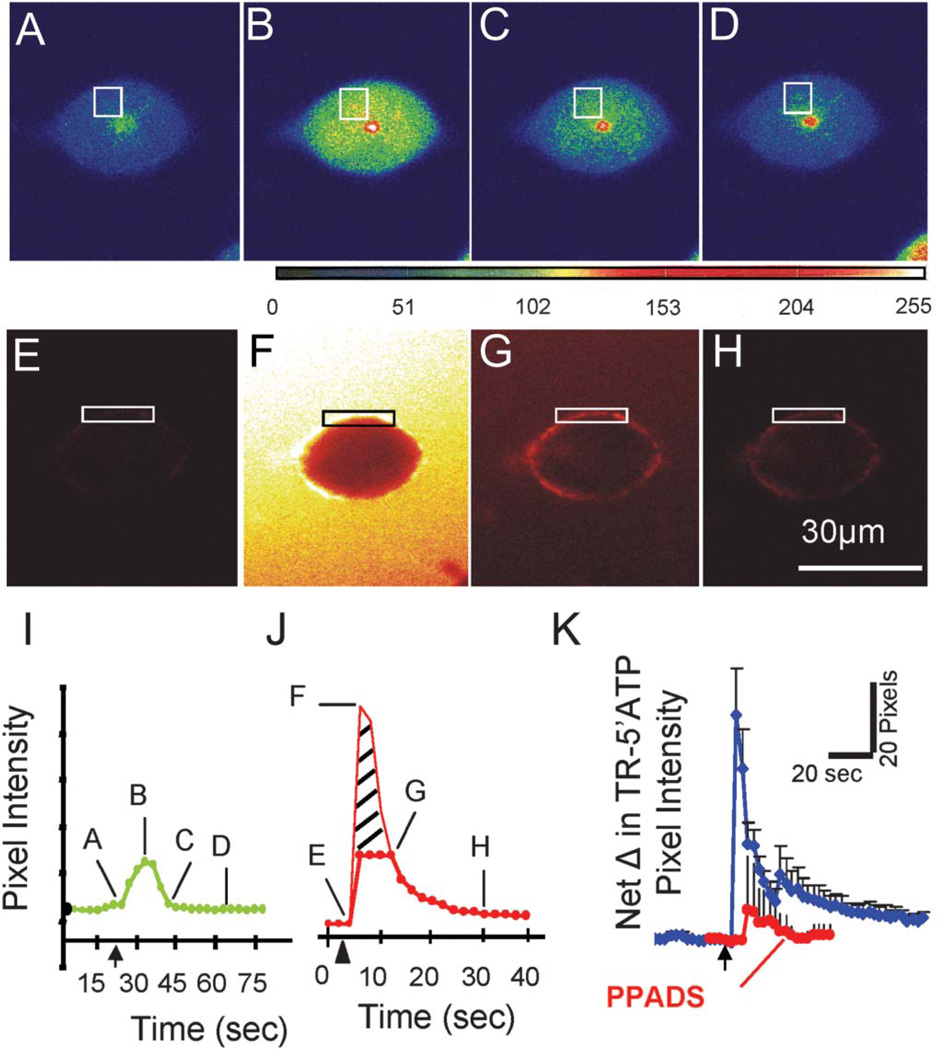
FIGURE 8.
Visualization of 5′ATP-TR membrane binding in a live BON cell that is touch sensitive. A–D, The touch-sensitive Ca2+ response in a BON cell is revealed by laser confocal imaging; 10 × 10 pixels used in analysis. E–H, Fast puff application (100 ms) of 5′ATP-TR causes cell membrane labeling of P2X sites. I, Touch-evoked Ca2+ transient of cell in A–D. J, 5′ATP-TR fluorescence labeling of cell in E–H. F, entire cell is transiently visible. G, Specific membrane binding to P2X receptors at 12 seconds after the puff. K, Puff of 5′ATP-TR membrane labeling is repeatable at 10-minutes intervals (not shown), and exposure to a P2X antagonist 10 mM PPADS blocks 5′ATP membrane binding in response to a puff (P < 0.0001, n = 79).
Dual Purinergic Modulation of 5-HT Release in hEC of Surgical Specimens
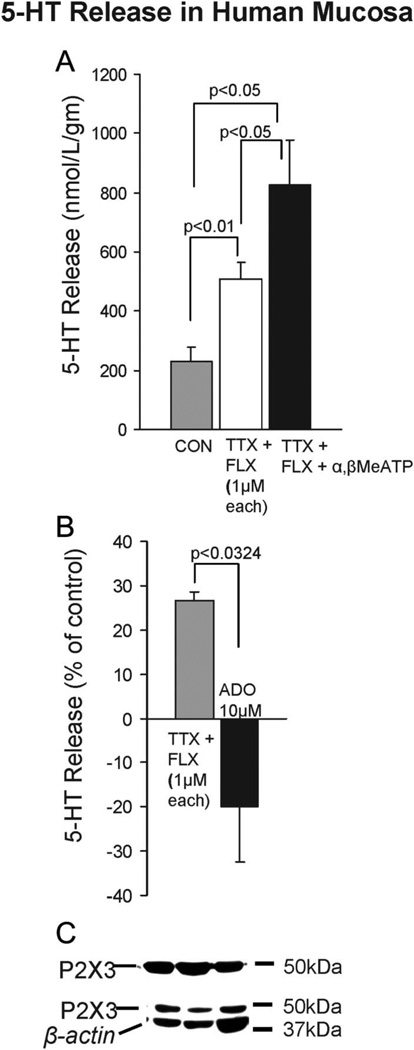
Data obtained in 8 surgical specimens from colon or jejunum illustrate dual modulation of 5-HT release by a P2X1,3 agonist α, β-MeATP (Fig. 9A) and a metabolite of ATP, adenosine (Fig. 9B). Tetrodotoxin (Tocris/R&D Systems) was used to block nerve conduction. Fluoxetine (R&D Systems) increases 5-HT release (overflow) by blocking serotonin transporter-uptake of 5-HT. Western Blot (WB) identified a P2X3 receptor in intact human mucosa (Fig. 9C), and immunofluorescent labeling identified P2X3-ir in hECs (Fig. 10). Clear expression of P2X1 or P2X2 in mucosa was not observed in WB (not shown). P2X3 expression by WB was done in specimens from the colon (N = 3). Whether regional differences exist in the receptor expressions was not evaluated.
FIGURE 9.
Dual modulation of 5-HT release by α,β-MeATP and adenosine in human intestinal mucosa. A, P2X1,3 receptor activation with 10 µM α,β-MeATP causes an increase in the 5-HT release from hECs collected in the supernatants. Inhibition of serotonin transporter-uptake of 5-HT by fluoxetine elevates 5-HT release (N = 6 each); experiments are done in tetrodotoxin to block nerve conduction. B, Adenosine (10 µM) can abolish basal 5-HT release occurring in the presence of fluoxetine and tetrodotoxin (N = 4). C, WB indicates expression of P2X3 in human sigmoid colon; surgical specimens included 2 sigmoid colons, 4 jejunums, 1 rectal tissue, and 1 transverse colon, and data were pooled since release occurred in each case with α,β-MeATP; ELISA measured 5-HT release in supernatants during 30 minutes incubation at 37°C in oxygenated Krebs’ buffer.
FIGURE 10.
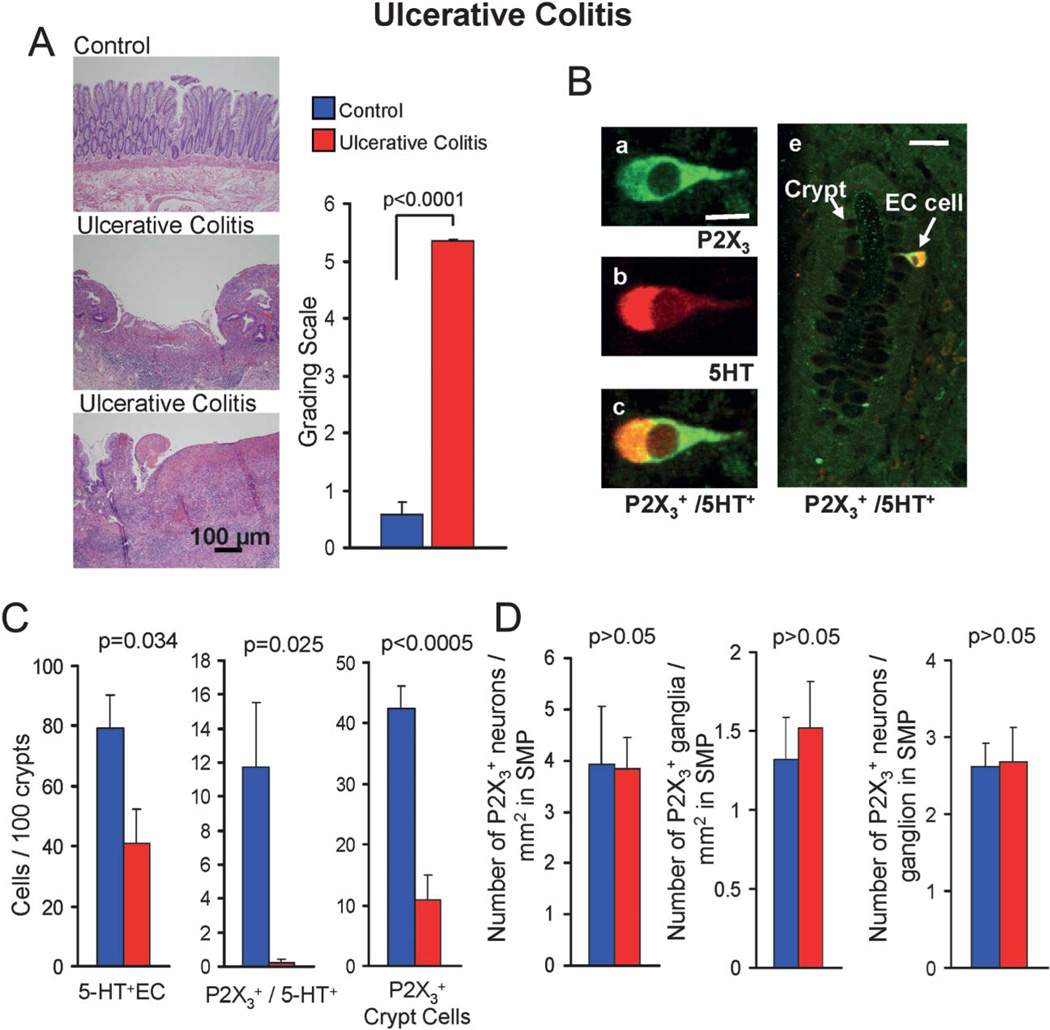
Downregulation of P2X3 receptor expression in hECs from human sigmoid colon in UC. A, Hematoxylin and eosin staining of tissue showing areas with severe crypt cell damage, inflammatory cell infiltration, and ulcers in UC. Control tissue appears fairly normal without inflammation. The clinical score for UC is significantly higher than control. Histopathologic assessment of inflammation in UC and controls was done according to Geboes et al.43 Quantification of numbers of cells expressing receptors was done in areas with intact mucosa and crypt cells. B, P2X3-ir is colocalized in 5-HT+hECs in the human sigmoid colon. Top panel, single hEC in a crypt colabeled for P2X3-ir and 5-HT-ir. Overlay images for P2X3-ir (green, fluorescein isothiocyanate-conjugated secondary antibody) and 5-HT-ir (red, TR-conjugated secondary antibody) to show colocalization. This approach was used to quantify the numbers of hECs expressing P2X3-ir. D, In UC there is a reduction in the proportion of 5-HT+hECs in comparison with controls. There is also a dramatic reduction in hECs or crypt cells expressing P2X3-ir. P2X3 + hECs were reduced by <98% in UC. E, In submucous ganglia, P2X3-ir expression is not altered.
Alterations in P2X3 Receptor Expression in hECs From Surgical Cases of UC
Quantification of hEC expression of P2X3 receptors was done in a pilot study of surgical specimens from 11 control cases (diverticulitis and noninflamed regions) and 10 inflamed cases (UC; Fig. 10A). Inflammation was confirmed by a blinded GI pathologist in UC (score = 5.4) compared with control (score = 0.65, P < 0.0001); 19 of 21 cases were sigmoid colon and 2 cases rectum. Control tissue is without inflammation or low level inflammation; in 5 of 11 cases, the score is 0.0 (no inflammation); in 3 cases, the score is 1.1 (low inflammation); and in 2 cases, the score is 1.3.
P2X3-ir is colocalized in 5-HT+hECs in the human sigmoid colon (Fig. 10B). P2X3-ir is expressed in 15% 5-HT+hECs, 0.43% epithelia, and 13% submucous ganglia. The number of 5-HT+ cells is reduced in UC by 50%. In UC, P2X3-ir is reduced by 98% to <0.2% of 5-HT+hECs in crypts (P = 0.025) and did not change in submucous neurons. P2X3-ir is abundant in inflammatory cells infiltrating the lamina propria in UC. The A2B receptor is expressed in 53% hEC, 46% ganglia, and 5.7% epithelia. In contrast to P2X3, in UC, A2B expression is reduced by 68% in hEC, 66% in epithelia, and 57% in neurons (Table 2; Fig. 10; Figure, Supplemental Digital Content 3, http://links.lww.com/IBD/A232).
DISCUSSION
The EC is a major player in initiating enteric and visceral nociceptive reflexes.5–8 Alterations in 5-HT release or handling mechanisms may contribute to the pathogenesis of IBD, diarrhea, and IBS.9–14 However, basic mechanisms regulating 5-HT release in hEC are poorly understood.16,17 ATP is a key purinergic mediator in mechanosensory reflexes,22,23 and purinergic pathways are sensitive to inflammation, but its role in modulating 5-HT release was unclear. Our study focused on BON cells and hECs. BON cells display an EC phenotype2,5–7,18–21,46 relevant to hECs and are the only hEC model shown to be suitable for mechanosensitivity studies in single cells.1,2,8,19 Our study proved that ATP release is a critical regulator of mechanosensitivity, Ca2+ signaling, and 5-HT release. Furthermore, in UC patients, ATP-gated P2X channels on hECs are very sensitive to inflammation.
Ca2+ responses to ATP in BON cells vary greatly in shape, size, and kinetics, and ATP can also trigger Ca2+ oscillations (or wave forms), suggesting multiple purinergic receptor mechanisms. Ca2+ waves are not involved in touch/stretch-induced responses, because mechanical stimulation could not trigger a Ca2+ wave in a single cell from ~1500 cells touched. Additional proof for multiple receptors is the finding that different proportions of cells respond to agonists (ATP, 2-MeSADP, UTP, and α,β-MeATP) or antagonists (MRS2179, 2MeSAMP, PPADS, and TNP-ATP) with selectivity for different receptors. Ten of 12 purine receptors are expressed in both BON and human mucosa—including P2X1, P2X3, P2X4, P2X5, P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, and P2Y12. The cognate mediators for these receptors are ATP, ADP, UTP, uridine diphosphate, ADP, AMP, or β-NAD.47
ATP or ADP could stimulate a 5-fold increase in 5-HT release from BON cells and mechanical stimulation caused both ATP and 5-HT release. The ATP released is quickly inactivated by 5′ecto- ATPase and NTPDase enzymes.48,49 ATP release could be revealed only after enzyme activity was inhibited by ARL67156, because these enzymes are very active in BON as shown in ATP degradation experiments. Exposure to purinergic antagonists (PPADS, TNP-ATP, MRS2179, and 2MeSAMP) or an enzyme that degrades nucleotides (apyrase) could block touch-Ca2+ responses or 5-HT release, whereas ARL67176 could augment responses. Eighty percent of cells are touch sensitive, and effects with apyrase or ARL67156 were evident in 77% and 63%, respectively, in touch-sensitive cells. We forward the concept that purinergic autoregulation via ATP is a primary mechanism in transducing of mechanical stimuli into intracellular Ca2+ signals leading to release of 5-HT to trigger mucosal reflexes. ATP release by mechanical stimulation from epithelial cells that make up 99% cells in the mucosa is also involved in mechanosensory reflexes,31as is ATP.22 Therefore, it is likely that purines provide a local mechanism for fine-tuning autocrine modulation of 5-HT release that triggers gut reflexes.
Touch deforms the cell membrane of single cells and generates forces such as strain, tactile stimulation, stretch, and pressure. Turbulent shear stress, tactile forces, centrifugal forces, or changes in hydrostatic pressure are some of the forces generated by rotational shaking that lead to the release of 5-HT from the entire population of BON cells.2 These forces are generated in the intestines during various patterns of motility. A recent study on fluorescence-activated cell sorting (FACS)-sorted hEC applied stretch–relaxation using a vacuum-operated Flexercell strain unit (FX-4000; Hillsborough, NC) and confirmed our earlier findings19 that adenosine release acts via A2B receptors to stimulate 5-HT release.50 Our results show that the forces that comprise touch (stretch) and light shaking trigger a series of biological events leading to 5-HT release that is tightly regulated by ATP release.
Our study revealed 3 distinct purinergic signaling pathways in hEC that are linked to mechanosensitivity. First, P2Y1 receptors are known to be linked to Gαq/PLC/IP3-Ca2+ pathway.51 The P2Y1 receptor is coupled to this Ca2+ pathway and a P2Y1 antagonist (MRS2179) prevents or reduces responses in 65% cells, and all are sensitive to PLC inhibition (U73122). Furthermore, complex interactions occur between P2Y1 and the PLC signaling pathway (Fig. 6F). ADP is the cognate agonist for P2Y1 receptors, and a more potent, stable ADP analog 2MeSADP triggers Ca2+ responses and 5-HT release, and these responses are sensitive to blockade with MRS2179. All cells that responded to 2MeSADP were touch sensitive. It is concluded that a slow P2Y1-Gαq/PLC/IP3-Ca2+ pathway is a major mechanism involved in autocrine regulation of mechanosensitivity in hEC.
However, ADP is also the cognate agonist for P2Y12 (AC-coupled receptor), and data show that 2MeSADP (ADP analog) or touch-induced Ca2+ responses are dually modulated by MRS2179 in different cells. In a separate hEC population from those expressing a slow P2Y1-Gαq/PLC/IP3-Ca2+ pathway, MRS2179 augments rather than block the Ca2+ response to 2MeSADP or mechanical stimulation. This suggests that MRS2179 is blocking ongoing inhibition mediated via P2Y12 receptors resulting in augmentation of the response. This is likely the case, because a P2Y12 antagonist 2MeSAMP could only augment touch-Ca2+ responses. Therefore, in a distinct population of hECs ADP activation of a P2Y12/AC/cAMP signaling pathway provides ongoing inhibitory modulation of Ca2+ signals and hence 5-HT release.
A third purinergic mechanism regulating mechanosensitivity is activation of ATP-gated P2X ion channels in hECs. In human mucosa, blocking reuptake of 5-HT by serotonin transporter-inhibition elevates 5-HT release, and under these conditions, the P2X1,3 agonist α,β-MeATP could then stimulate 5-HT release. In contrast, a metabolite of ATP, adenosine, inhibits 5-HT release. A P2X1,3 agonist α,β-MeATP triggers Ca2+ responses, whereas a P2X (PPADS)52 or P2X1,2,3,2/3 antagonist (TNP-ATP)53,54 blocks mechanical stimulation-Ca2+ responses or cell-surface P2X-labeling to ATP-TR. of touch-Ca2+ responses with TNP-ATP suggests a different as yet unknown mechanism. However, reverse transcription-polymerase chain reaction identified P2X1 and P2X3 transcripts in BON and human mucosa, and P2X3 protein in human mucosa; P2X2 transcripts were absent in BON or human mucosa. Therefore, P2X2 or P2X2/3 heterodimers are unlikely to be involved. P2X3 and P2X1 channels are candidates for modulation of the mechanosensory signaling pathway in hEC leading to 5-HT release.
P2X3 channels represent a therapeutic target for pain/analgesia.34,42 ATP release from hEC (or epithelial cells) can activate ATP-gated P2X3 channels on local afferent nerve endings to transmit pain sensation to the brain in IBD or IBS models.32,34 The hEC is another target for P2X3 modulation of 5-HT release that can act on intrinsic and extrinsic nerve endings of sensory neurons to modulate peristalsis and initiate nociception. In IBD and IBS models, P2X3 upregulation is deemed pathologic in pain transmission. In human sigmoid colon, we found that P2X3-ir is expressed in 15% of 5-HT+hECs compared with a majority of hECs expressing A2B receptors. In UC, discrete alterations occur in the expression of P2X3 channels in hEC and other cell types. The most striking effect of mucosal inflammation in UC is on P2X3 downregulation in hEC. Detectable P2X3-ir is reduced from 15% to <0.2% of cells in 5-HT+hEC, whereas expression did not change in neurons and is highly expressed in inflammatory cells. Downregulation of P2X3 in UC may represent an adoptive response to attenuate reflexes and visceral pain. In IBD, there is also diminished P2X-purinergic vasoconstriction.55 P2X expression is upregulated after injury to dorsal root ganglia,56 in IBD and IBS models.42 In CD, P2X3 upregulation occurs in myenteric neurons,41 which is expected to lead to abnormal motility. A new study is necessary to determine functional consequences of downregulation of P2X3 in hEC from UC. A study evaluating P2X3-expression in hEC in relation to visceral hyperalgesia in IBD (or IBS) patients could further evaluate the clinical importance of our findings. In our study, UC caused downregulation of A2B-ir and was undetectable in 68% hECs in fixed intestinal sections, whereas in FACS-sorted hEC from CD patients, it was shown to cause upregulation of A2B50; our study did not evaluate whether, in cells still expressing the receptor (e.g., 32%) in UC specimens, it was upregulated. A2B receptors in biopsy were shown to be upregulated in UC and CD.40 Differences between studies could also be because of the different diseases, severity, chronicity, or treatment of disease, EC handling during isolation, A2B expression in cells other than hEC, or different ways to analyze expression.
CONCLUSIONS
Endogenous purines are critical determinants of 5-HT release evoked by mechanical stimulation, and our working model is depicted in Figure 11. Most cells are sensitive to mechanical forces and respond by releasing ~0.5% to 10% of intracellular pool of nucleotides. Mechanical stimulation activates ATP to diffuse from the cells into the extracellular compartment to bind to receptors on the surface of the cell and activate the mechanosensitive signaling pathway.21 We forward the novel hypothesis that purines provide a mechanism for finetuning modulation of 5-HT release in triggering gut mechanical intrinsic and extrinsic reflexes. ATP could also act directly to activate the reflex.
In hEC, positive feedback modulation via a P2X channel or P2Y1 would allow cells to translate small changes in mechanical stimulation into large changes in 5-HT release—P2X channels may provide speed and amplification whereas P2Y could provide slower/more sustained effects on reflexes. In a majority of hEC mechanically-evoked 5-HT release is tightly autoregulated by co-release of ATP via three distinct purinergic signaling mechanisms.
Mechanical stimulation releases ATP (or ADP) to activate a slow P2Y1-PLC/IP3-Ca2+dependent 5-HT release. In distinct subsets of hEC, ongoing activation of a slow P2Y12-AC/cAMP signaling pathway by ADP attenuates 5-HT release to dampen reflexes. ATP release in a minority of hEC in intact mucosa (<20%) can also activate a fast ATP-gated P2X3 (or P2X1) channel to stimulate 5-HT release. Overall, mechanical stimulation coreleases 5-HT, ATP, other purines, prostaglandins, PACAP, or other mediators20 that influence or trigger reflexes or activate visceral sensory afferents.
Purinergic pathways are very sensitive to inflammation and severe downregulation of ATP-gated P2X3 channels or A2B receptors occurs in UC, and we postulate that this would alter 5-HT release and contribute to abnormal signaling, physiology, and visceral sensation—purinergic signaling pathways offer new potential therapeutic targets. Novel approaches to monitor 5-HT release by electrochemical detection will permit such studies in intact human mucosa or single hEC.57
Supplementary Material
ACKNOWLEDGMENTS
Supported by the National Institutes of Health on NIH DK093499-01, DK044179-15, and NCRR S10RR11434 to F.L.C. and 5K08 DK078035-05 to K.W.
Appreciation to Dr. Courtney Townsend, University of Texas, Galveston, for providing BON cells.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.ibdjournal.org).
REFERENCES
- 1.Kim M, Cooke HJ, Javed NH, et al. D-glucose releases 5-hydroxytryptamine from human BON cells as a model of enterochromaffin cells. Gastroenterology. 2001;121:1400–1406. doi: 10.1053/gast.2001.29567. [DOI] [PubMed] [Google Scholar]
- 2.Kim M, Javed NH, Yu JG, et al. Mechanical stimulation activates Galphaq signaling pathways and 5-hydroxytryptamine release from human carcinoid BON cells. J Clin Invest. 2001;108:1051–1059. doi: 10.1172/JCI12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kellum JM, Albuquerque FC, Stoner MC, et al. Stroking human jejunal mucosa induces 5-HT release and Cl-secretion via afferent neurons and 5-HT4 receptors. Am J Physiol. 1999;277:G515–G520. doi: 10.1152/ajpgi.1999.277.3.G515. [DOI] [PubMed] [Google Scholar]
- 4.Fang X, Liu S, Wang XY, et al. Neurogastroenterology of tegaserod (HTF 919) in the submucosal division of the guinea-pig and human enteric nervous system. Neurogastroenterol Motil. 2008;20:80–93. doi: 10.1111/j.1365-2982.2007.00983.x. [DOI] [PubMed] [Google Scholar]
- 5.Christofi FL. Purinergic receptors and gastrointestinal secretomotor function. Purinergic Signal. 2008;4:213–236. doi: 10.1007/s11302-008-9104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooke HJ, Christofi FL. Enteric neural regulation of mucosal secretion. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. 4th ed. New York: Academic Press; 2006. pp. 737–762. [Google Scholar]
- 7.Raybould HE, Cooke HJ, Christofi FL. Sensory mechanisms: transmitters, modulators and reflexes. Neurogastroenterol Motil. 2004;16:60–63. doi: 10.1111/j.1743-3150.2004.00477.x. [DOI] [PubMed] [Google Scholar]
- 8.Bozarov A, Wang YZ, Yu JG, et al. Activation of adenosine low-affinity A3 receptors inhibits the enteric short interplexus neural circuit triggered by histamine. Am J Physiol Gastrointest Liver Physiol. 2009;297:G1147–G1162. doi: 10.1152/ajpgi.00295.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galligan JJ. 5-hydroxytryptamine, ulcerative colitis, and irritable bowel syndrome: molecular connections. Gastroenterology. 2004;126:1897–1899. doi: 10.1053/j.gastro.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 10.Linden DR, Chen JX, Gershon MD, et al. Serotonin availability is increased in mucosa of guinea pigs with TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2003;285:G207–G216. doi: 10.1152/ajpgi.00488.2002. [DOI] [PubMed] [Google Scholar]
- 11.O’Hara JR, Ho W, Linden DR, et al. Enteroendocrine cells and 5-HT availability are altered in mucosa of guinea pigs with TNBS ileitis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G998–G1007. doi: 10.1152/ajpgi.00090.2004. [DOI] [PubMed] [Google Scholar]
- 12.Gershon MD. Review article: serotonin receptors and transporters—roles in normal and abnormal gastrointestinal motility. Aliment Pharmacol Ther. 2004;20(suppl 7):3–14. doi: 10.1111/j.1365-2036.2004.02180.x. [DOI] [PubMed] [Google Scholar]
- 13.Kordasti S, Sjovall H, Lundgren O, et al. Serotonin and vasoactive intestinal peptide antagonists attenuate rotavirus diarrhoea. Gut. 2004;53:952–957. doi: 10.1136/gut.2003.033563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crowell MD. Role of serotonin in the pathophysiology of the irritable bowel syndrome. Br J Pharmacol. 2004;141:1285–1293. doi: 10.1038/sj.bjp.0705762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manocha M, Khan WI. Serotonin and GI disorders: an update on clinical and experimental studies. Clin Transl Gastroenterol. 2012;3:e13. doi: 10.1038/ctg.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Racke K, Schworer H, Agoston DV, et al. Evidence that neuronally released vasoactive intestinal polypeptide inhibits the release of serotonin from enterochromaffin cells of the guinea pig small intestine. Acta Endocrinol (Copenh) 1991;124:203–207. doi: 10.1530/acta.0.1240203. [DOI] [PubMed] [Google Scholar]
- 17.Racke K, Reimann A, Schworer H, et al. Regulation of 5-HT release from enterochromaffin cells. Behav Brain Res. . 1996;73:83–87. doi: 10.1016/0166-4328(96)00075-7. [DOI] [PubMed] [Google Scholar]
- 18.Cooke HJ, Wunderlich J, Christofi FL. “The Force Be with You”: ATP in the gut mechanosensory transduction. News Physiol Sci. 2003;18:43–49. doi: 10.1152/nips.01411.2002. [DOI] [PubMed] [Google Scholar]
- 19.Christofi FL, Kim M, Wunderlich JE, et al. Endogenous adenosine differentially modulates 5-hydroxytryptamine release from a human enterochromaffin cell model. Gastroenterology. 2004;127:188–202. doi: 10.1053/j.gastro.2004.04.070. [DOI] [PubMed] [Google Scholar]
- 20.Germano PM, Lieu SN, Xue J, et al. PACAP induces signaling and stimulation of 5-hydroxytryptamine release and growth in neuroendocrine tumor cells. J Mol Neurosci. 2009;39:391–401. doi: 10.1007/s12031-009-9283-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim M, Christofi FL, Xue J, et al. Mechanically evoked 5-hydroxytryptamine release is mediated by caveolin-associated cholesterol rich membrane domains. Neurogastroenterol Motil. 2007;19:309–317. doi: 10.1111/j.1365-2982.2007.00912.x. [DOI] [PubMed] [Google Scholar]
- 22.Cooke HJ, Xue J, Yu JG, et al. Mechanical stimulation releases nucleotides that activate P2Y1 receptors to trigger neural reflex chloride secretion in guinea pig distal colon. J Comp Neurol. 2004;469:1–15. doi: 10.1002/cne.10960. [DOI] [PubMed] [Google Scholar]
- 23.Christofi FL, Wunderlich J, Yu JG, et al. Mechanically evoked reflex electrogenic chloride secretion in rat distal colon is triggered by endogenous nucleotides acting at P2Y1, P2Y2, and P2Y4 receptors. J Comp Neurol. 2004;469:16–36. doi: 10.1002/cne.10961. [DOI] [PubMed] [Google Scholar]
- 24.Burnstock G, Satchell DG, Smythe A. A comparison of the excitatory and inhibitory effects of non-adrenergic, non-cholinergic nerve stimulation and exogenously applied ATP on a variety of smooth muscle preparations from different vertebrate species. Br J Pharmacol. 1972;46:234–242. doi: 10.1111/j.1476-5381.1972.tb06868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khakh BS, Burnstock G, Kennedy C, et al. International union of pharmacology. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmacol Rev. 2001;53:107–118. [PubMed] [Google Scholar]
- 26.Burnstock G. Purinergic signalling—an overview. Novartis Found Symp. 2006;276:26–48. discussion 48–57, 275–281. [PubMed] [Google Scholar]
- 27.von Kügelgen I. Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Ther. 2006;110:415–432. doi: 10.1016/j.pharmthera.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 28.Gulbransen BD, Sharkey KA. Purinergic neuron-to-glia signaling in the enteric nervous system. Gastroenterology. 2009;136:1349–1358. doi: 10.1053/j.gastro.2008.12.058. [DOI] [PubMed] [Google Scholar]
- 29.Wunderlich JE, Grants I, Needleman B, et al. Glial transmission in human enteric nervous system. Gastroenterology. 2012;142(suppl 1):S–45. [Google Scholar]
- 30.Gulbransen BD, Sharkey KA. Novel functional roles for enteric glia in the gastrointestinal tract. Nat Rev Gastroenterol Hepatol. 2012;9:625–632. doi: 10.1038/nrgastro.2012.138. [DOI] [PubMed] [Google Scholar]
- 31.Burnstock G. Purinergic mechanosensory transduction and visceral pain. Mol Pain. 2009;5:69. doi: 10.1186/1744-8069-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burnstock G. The journey to establish purinergic signaling in the gut. Neurogastroenterol Motil. 2008;20:8–19. doi: 10.1111/j.1365-2982.2008.01107.x. [DOI] [PubMed] [Google Scholar]
- 33.Bertrand PP. ATP and sensory transduction in the enteric nervous system. Neuroscientist. 2003;9:243–260. doi: 10.1177/1073858403253768. [DOI] [PubMed] [Google Scholar]
- 34.Burnstock G. Purinergic receptors as future targets for treatment of functional GI disorders. Gut. 2008;57:1193–1194. doi: 10.1136/gut.2008.151134. [DOI] [PubMed] [Google Scholar]
- 35.Antonioli L, Fornai M, Colucci R, et al. Regulation of enteric functions by adenosine: pathophysiological and pharmacological implications. Pharmacol Ther. 2008;120:233–253. doi: 10.1016/j.pharmthera.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 36.Guzman J, Yu JG, Suntres Z, et al. ADOA3R as a therapeutic target in experimental colitis: proof by validated high-density oligonucleotide microarray analysis. Inflamm Bowel Dis. 2006;12:766–789. doi: 10.1097/00054725-200608000-00014. [DOI] [PubMed] [Google Scholar]
- 37.Kolachala V, Asamoah V, Wang L, et al. TNF-alpha upregulates adenosine 2b (A2b) receptor expression and signaling in intestinal epithelial cells: a basis for A2bR overexpression in colitis. Cell Mol Life Sci. 2005;62:2647–2657. doi: 10.1007/s00018-005-5328-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kolachala VL, Vijay-Kumar M, Dalmasso G, et al. A2B adenosine receptor gene deletion attenuates murine colitis. Gastroenterology. 2008;135:861–870. doi: 10.1053/j.gastro.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ren T, Grants I, Alhaj M, et al. Impact of disrupting adenosine A3 receptors (A3−/−AR) on colonic motility or progression of colitis in the mouse. Inflamm Bowel Dis. 2011;17:1698–1713. doi: 10.1002/ibd.21553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rybaczyk L, Rozmiarek A, Circle K, et al. New bioinformatics approach to analyze gene expressions and signaling pathways reveals unique purine gene dysregulation profiles that distinguish between CD and UC. Inflamm Bowel Dis. 2009;15:971–984. doi: 10.1002/ibd.20893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yiangou Y, Facer P, Baecker PA, et al. ATP-gated ion channel P2X(3) is increased in human inflammatory bowel disease. Neurogastroenterol Motil. 2001;13:365–369. doi: 10.1046/j.1365-2982.2001.00276.x. [DOI] [PubMed] [Google Scholar]
- 42.Xu GY, Shenoy M, Winston JH, et al. P2X receptor-mediated visceral hyperalgesia in a rat model of chronic visceral hypersensitivity. Gut. 2008;57:1230–1237. doi: 10.1136/gut.2007.134221. [DOI] [PubMed] [Google Scholar]
- 43.Geboes K, Riddell R, Ost A, et al. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut. 2000;47:404–409. doi: 10.1136/gut.47.3.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sorensen CE, Novak I. Visualization of ATP release in pancreatic acini in response to cholinergic stimulus. Use of fluorescent probes and confocal microscopy. J Biol Chem. 2001;276:32925–32932. doi: 10.1074/jbc.M103313200. [DOI] [PubMed] [Google Scholar]
- 45.Taylor AL, Kudlow BA, Marrs KL, et al. Bioluminescence detection of ATP release mechanisms in epithelia. Am J Physiol. 1998;275:C1391–C1406. doi: 10.1152/ajpcell.1998.275.5.C1391. [DOI] [PubMed] [Google Scholar]
- 46.Jacques-Silva MC, Correa-Medina M, Cabrera O, et al. ATP-gated P2X3 receptors constitute a positive autocrine signal for insulin release in the human pancreatic beta cell. Proc Natl Acad Sci U S A. 2010;107:6465–6470. doi: 10.1073/pnas.0908935107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Durnin L, Sanders KM, Mutafova-Yambolieva VN. Differential release of β-NAD+ and ATP upon activation of enteric motor neurons in primates and murine colons. Neurogastroenterol Motil. 2013;25:e194–e204. doi: 10.1111/nmo.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alvarado-Castillo C, Harden TK, Boyer JL. Regulation of P2Y1 receptor-mediated signaling by the ectonucleoside triphosphate diphosphohydrolase isozymes NTPDase1 and NTPDase2. Mol Pharmacol. 2005;67:114–122. doi: 10.1124/mol.104.006908. [DOI] [PubMed] [Google Scholar]
- 49.Kukulski F, Komoszyński M. Purification and characterization of NTPDase1 (ecto-apyrase) and NTPDase2 (ecto-ATPase) from porcine brain cortex synaptosomes. Eur J Biochem. 2003;270:3447–3454. doi: 10.1046/j.1432-1033.2003.03734.x. [DOI] [PubMed] [Google Scholar]
- 50.Chin A, Svejda B, Gustafsson BI, et al. The role of mechanical forces and adenosine in the regulation of intestinal enterochromaffin cell serotonin secretion. Am J Physiol Gastrointest Liver Physiol. 2012;302:G397–G405. doi: 10.1152/ajpgi.00087.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wunderlich JE, Needleman BJ, Chen Z, et al. Dual purinergic synaptic transmission in the human enteric nervous system. Am J Physiol Gastrointest Liver Physiol. 2008;294:G554–G566. doi: 10.1152/ajpgi.00500.2007. [DOI] [PubMed] [Google Scholar]
- 52.Flores-Soto E, Reyes-Garcia J, Sommer B, et al. PPADS, a P2X receptor antagonist, as a novel inhibitor of the reverse mode of the Na+/Ca2+ exchanger in guinea pig airway smooth muscle. Eur J Pharmacol. 2012;674:439–444. doi: 10.1016/j.ejphar.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 53.Burgard EC, Niforatos W, van Biesen T, et al. Competitive antagonism of recombinant P2X(2/3) receptors by 2′, 3′-O-(2,4,6-trinitrophenyl) adenosine 5′-triphosphate (TNP-ATP) Mol Pharmacol. 2000;58:1502–1510. doi: 10.1124/mol.58.6.1502. [DOI] [PubMed] [Google Scholar]
- 54.Coddou C, Yan Z, Obsil T, et al. Activation and regulation of purinergic P2X receptor channels. Pharmacol Rev. 2011;63:641–683. doi: 10.1124/pr.110.003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neshat S, deVries M, Barajas-Espinosa AR, et al. Loss of purinergic vascular regulation in the colon during colitis is associated with upregulation of CD39. Am J Physiol Gastrointest Liver Physiol. 2009;296:G399–G405. doi: 10.1152/ajpgi.90450.2008. [DOI] [PubMed] [Google Scholar]
- 56.Xiang Z, Xiong Y, Yan N, et al. Functional up-regulation of P2X3 receptors in the chronically compressed dorsal root ganglion. Pain. 2008;140:23–34. doi: 10.1016/j.pain.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao H, Bian X, Galligan JJ, et al. Electrochemical measurements of serotonin (5-HT) release from the guinea pig mucosa using continuous amperometry with a boron-doped diamond electrode. Diam Relat Mater. 2010;19:182–185. doi: 10.1016/j.diamond.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.