Abstract
Many neurodegenerative disorders involve the abnormal accumulation of proteins. In addition to the well-known findings of neurofibrillary tangles and β-amyloid plaques in Alzheimer’s disease, here we show that abnormal accumulations of gephyrin, an inhibitory receptor anchoring protein, are highly correlated with the neuropathologic diagnosis of AD (odds ratio of 72.7; p = 6.844 × 10−6 by Fisher’s exact test, n = 17 AD and n = 14 control cases). Furthermore, the gephyrin accumulations are specific for AD and not seen in other neurodegenerative diseases. Gephyrin accumulations overlap with β-amyloid plaques and, more rarely, neurofibrillary tangles. Follow-up biochemical and proteomic studies suggest alterations in the gephyrin solubility and reveal elevated levels of gephyrin lower-molecular-weight species in the AD insoluble fraction. Since gephyrin is involved in synaptic organization and synaptic dysfunction is an early event in AD, these findings point to a possible role for gephyrin in AD pathogenesis.
Keywords: gephyrin, Alzheimer’s disease, β-amyloid, presenilin, synapse, GABA
INTRODUCTION
Alzheimer’s disease (AD) is a devastating neurodegenerative disease that affects approximately 13% of individuals over the age of 65 years (1). Pathologically, it is characterized by the accumulation of β-amyloid as extracellular neuritic plaques and phosphorylated tau as neurofibrillary tangles (2). The accumulation of β-amyloid is important in AD pathogenesis since all known AD-causing mutations lead to overproduction of β-amyloid, and results of recent genome-wide association studies implicate genes involved in processing β-amyloid (3, 4). The exact physiologic roles amyloid precursor protein and β-amyloid play is not clear, but they may be involved in maintaining synapses and synaptic plasticity (5, 6).
Multiple lines of evidence suggest synaptic loss is a prominent early feature of AD pathogenesis in humans (7–9). Elevated excitatory activity is seen in transgenic animal models of AD (10, 11). This is also presumably at work in patients with AD as some AD patients may develop seizures during the course of their illness and one of the main symptomatic therapies for AD, memantine, may work by antagonizing aberrant excitatory activity (12). Interestingly, recent work by Limon et al. also implicates loss of functional GABA receptors, a major inhibitory receptor in the brain, as being partially responsible (13). Their data suggest that in AD, there is a loss of GABA receptor association with gephyrin, a main organizer of the inhibitory synapse. Similarly, Agarwal and colleagues suggest gephyrin expression is reduced in AD (14). These results implicate proteins that maintain the integrity of the inhibitory synapse, like gephyrin, in AD pathogenesis. If true, we expect those proteins should be mislocalized in AD and apparent in the brains of affected individuals. Here we sought to test that hypothesis for gephyrin.
A number of synaptic and extra-synaptic functions have been identified for gephyrin. Gephyrin is a 93-kDa protein originally identified as a glycine receptor subunit that is present throughout the brain and spinal cord (15). Gephyrin knockout or knockdown experiments reveal deficiencies in glycine and GABA clustering at synapses (16–18). Glycine receptors bind directly to the C-terminal tail of gephyrin, and localization studies demonstrate a strong association between GABA receptors and gephyrin (19, 20). Distinct from its ability to associate with GABA receptors is gephyrin’s ability to catalyze the synthesis of molybdenum cofactor, an essential protein for clearing toxic metabolites throughout the cell (21, 22). Recent data also show a direct interaction between gephyrin and the eukaryotic translation initiation factor 3 complex (23). In conjunction with another binding partner, collybistin, gephyrin has also been implicated in regulating synaptic protein synthesis (23).
Here we provide evidence for abnormal accumulations of gephyrin immunoreactivity in AD postmortem tissues that are specific for AD. In AD, we found gephyrin immunoreactivity resembles neuritic plaques, and these gephyrin plaques colocalize with amyloid plaques and are only rarely associated with neurofibrillary tangles. We also observed an overall loss of normally distributed gephyrin immunoreactivity in AD. Finally, biochemical studies suggest that gephyrin cleavage products or lower-molecular-weight isoforms accumulate, whereas full-length gephyrin is reduced in AD insoluble fractions following ultracentrifugation. These findings further support the involvement of gephyrin in AD.
MATERIALS AND METHODS
Human tissue
Fixed, cryopreserved free-floating 50-µm-thick sections of frontal cortex (Brodaman’s area 9) from control, AD, PD, FTD, and CBD cases were used (specimens obtained from the Emory Alzheimer’s Disease Research Center Neuropathology Core, Marla Gearing, PhD, Atlanta, Georgia and University of Washington Alzheimer’s Disease Center Neuropathology Core, Thomas Montine MD, PhD, Seattle, Washington). Tissues from the Emory ADRC were fixed in 4% paraformaldehyde (Sigma, St. Louis, MO) for one to two weeks before being transferred to cryopreservative. The Washington tissues were older and hence fixed and stored in 3–10% formaldehyde. The proteomics experiments utilized fresh frozen frontal cortex (Brodman’s area 8) from the Baltimore Longitudinal Study of Aging (BLSA) provided by Juan Troncoso MD in the Johns Hopkins University Brain Resource Center (See Table, Supplemental Digital Content 1 which shows individual case characteristics)(24).
All except two of the AD cases (both sporadic, one used for immunohistochemistry and one for biochemistry) met NIH-Reagan criteria for high-likelihood that the clinical dementia was due to AD. Controls used for immunohistochemistry had no history of neurological disease. Only three of these (all of whom had tested cognitively normal within a year of death) had significant neocortical neuritic plaques (i.e., widespread moderate or frequent neuritic plaques), and none of the three had neocortical neurofibrillary tangles.
Antibodies and reagents
We used paired helical filament-tau (AT8, Pierce-MN1020, Thermo Fisher Scientific, Rockford, IL; 0.1µg/ml), gephyrin (Abcam, Cambridge, MA, ab32206/antibody 1/carboxy-terminus epitope 0.15µg/ml and BD Biosciences, San Jose, CA, 610584/antibody 2/internal epitope 0.6µg/ml), and β-amyloid (in-house monoclonal to recognize amino acids 1–16 of β-amyloid; clone 87-5H8; concentrated tissue culture medium; used at 1:1000) in these studies. Fluorescent conjugated cyanine 3 donkey anti-mouse (Jackson Immunoresearch, West Grove, PA, 715-165-150; 1:200 dilution) was used as a secondary antibody. A gephyrin C-terminal peptide (Abcam, Cambridge, MA, ab32205) and recombinant gephryin (aa294-736, Synaptic Systems, Goettingen, Germany, 147–111) were used in antibody blocking experiments. The plasmid for gephyrin overexpression was purchased from Origene (RC205986, Rockville, MD)
Immunohistochemistry (IHC)
IHC was performed as previously described (25) on free-floating cryopreserved sections. Briefly, sections were washed (5 times, 3 minutes each; same for all subsequent wash steps) in 0.1M phosphate buffer (PB, EMD Millipore, Billerica, MA), incubated with 1% hydrogen peroxide (diluted in 0.1M-PB, 10–15 minutes, Sigma, St. Louis, MO), washed with 0.1M-PB, and blocked for 1 hour at 4°C (tris-buffered saline (TBS, Fisher Scientific, Pittsburgh, PA), 8% goat serum (Life Technologies, Grand Island, NY), 0.1% triton X-100 (Sigma, St. Louis, MO), and Avidin D 10µg/ml (Vector Laboratories, Burlingame, CA, A-2000)). Sections were then washed (TBS) and incubated with primary antibody (in TBS, 2% goat serum and biotin (50µg/ml), Sigma, St. Louis, MO, B0301; 24–48 hours at 4°C). For antibody blocking experiments, primary antibodies were incubated with peptide or recombinant protein for 1 hour at room temperature prior to incubation with tissue sections. Sections were washed (TBS) and incubated with biotinylated secondary antibody (in TBS with 2% goat serum). Sections were washed (TBS), incubated with Vectastain Elite ABC amplification reagent (Vector Laboratories, Burlingame, CA, PK-6200) for 1 hour at 4°C, and washed (TBS). Proteins were visualized with 3,3-diaminobenzidine solution (DAB, Sigma, St. Louis, MO, D4418) or FITC- or cy3-tyramide amplification (Perkin-Elmer, Waltham, MA). Fluorescently conjugated secondary antibodies were also used as indicated. DAB-labeled tissues were visualized with an Olympus light microscope (Olympus, Center Valley, PA). The presence of abnormal gephyrin immunoreactivity was assessed by direct visual scanning of 2–3 cm2 pieces of frontal cortex by 2 blind raters. Immunofluorescence images were obtained using a Zeiss LSM-510 confocal microscope (Zeiss, Oberkochen, Germany, Emory NIH Imaging Core) with attached two-photon laser. Immunofluorescence images presented are maximum intensity projections from 20 µm z-stack images (0.5µm step). Grayscale images are presented as they provide the best visual representation for immunoreactivity. Brighter white intensity represents stronger immunoreactivity. Color overlay images provide a visual to demonstrate overlap of staining from different primary antibodies.
Immunoblotting
Brain homogenates were prepared as follows from 5 control and 5 AD cases from the BLSA (24) cohort. Approximately 200 mg of fresh-frozen postmortem human frontal cortex was homogenized in low-salt sucrose buffer (sucrose 250mM, HEPES pH 7.0 50mM, EDTA 1mM, NaCl 25mM (Sigma, St. Louis, MO)) with complete protease inhibitor cocktail (Roche, San Francisco, CA, 13006200) on ice. NaCl concentration was increased to 1M, and N-lauroylsarcosine (Sigma, St. Louis, MO L-9150) to a final 1% was added. Samples were sonicated (3 × 5-second pulse at 30% amplitude in cold room,), and then centrifuged for 30 minutes at 200,000G at 4°C. The supernatant was removed and stored on ice. The insoluble pellet was washed with N-lauroylsarcosine high-salt buffer and centrifuged another 30 minutes. The insoluble pellet was resuspended in 8M urea (Sigma, St. Louis, MO). SDS sample buffer with β-mercaptoethanol (MP Biomedicals, Solon, OH) was added to 50 µg of protein from each supernatant or insoluble pellet and loaded onto 10% SDS denaturing gels. Western blots were performed per standard protocols (26). An Odyssey Scanner (LI-COR, Lincoln, NE) was used to visualize proteins labeled with fluorescent secondary antibodies. This technique provides improved sensitivity and detection capabilities as compared to standard chemiluminescent techniques (see http://www.licor.com/bio/PDF/IRquant.pdf on the LI-COR website for further details). Gel bands were quantified via pixel intensity using ImageJ (Analyze drop-down menu then Gels menu to identify bands highlighted with the rectangular selection tool).
Proteomic Analysis
50 µg of protein from the insoluble pellet of 5 control and 5 AD cases were subject to dual mass-spectrometry analysis as previously described (26) (See Table, Supplemental Digital Content 1 which shows individual case characteristics). Briefly, 50 µg from the insoluble pellet was resolved via SDS-PAGE. Lanes were divided into 5 molecular weight regions, and then subject to in-gel digestion with trypsin prior to mass-spectrometry analysis and identification of peptides that are quantitatively represented as spectral counts. Synaptic proteins demonstrating significant expression level differences between control and AD cases were initially identified using extracted ion intensities (XIC, normalized protein intensities based on raw signal to noise ratio) as described previously (27). Synaptic proteins that also demonstrated statistical differences (Student’s Ttest) in spectral counts between control and AD cases are presented.
RESULTS
Gephyrin Immunoreactivity
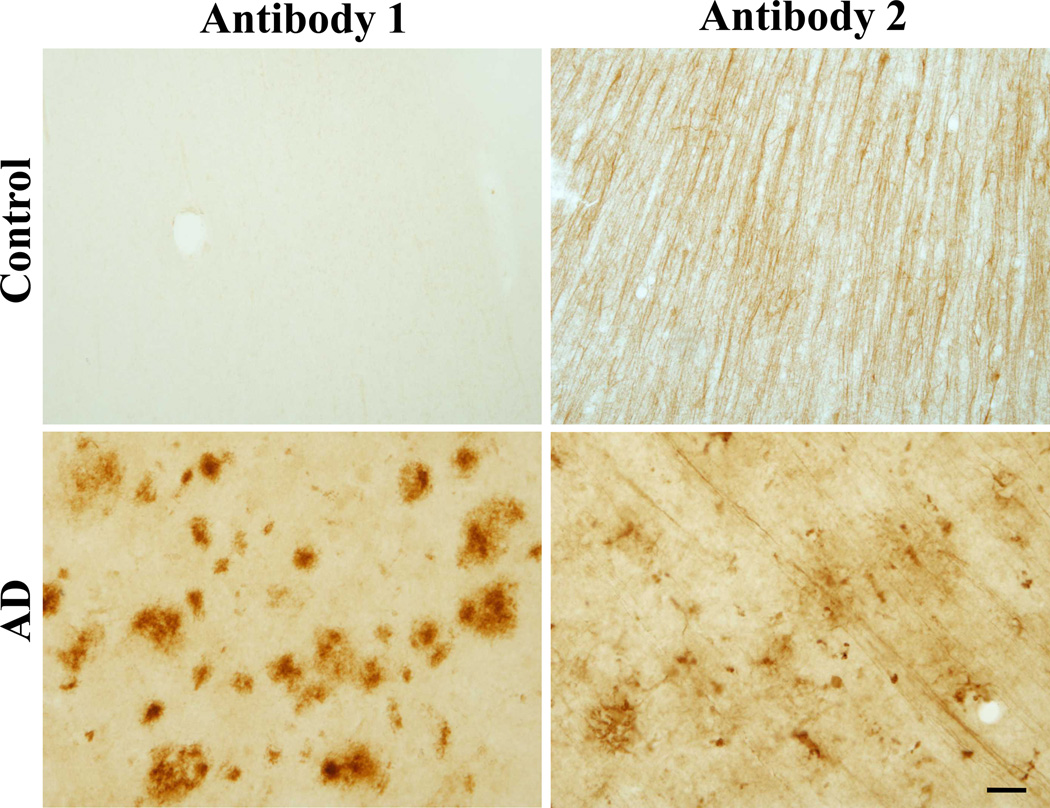
Demographic information on the cases studied is provided in Table 1. We stained free-floating fixed tissue to test for the presence or absence of abnormal gephyrin immunoreactivity in the frontal cortex of 14 control and 17 sporadic AD cases. In AD cases, we found gephyrin immunoreactivity was present in plaque-like structures throughout all cortical layers (Figure 1). The gephyrin plaques were present in only one control case (Table 2). Abnormal gephyrin accumulations were highly correlated with the neuropathologic diagnosis of AD (odds ratio of 72.7; p = 6.844 × 10−6 by Fisher’s exact test). Immunohistochemistry staining with a second gephyrin antibody in the same control and AD cases also revealed plaque-like structures in AD cases and showed significant correlation with Antibody 1 (p=0.001412 by Pearson’s product-moment; Figure 1). Using the second gephyrin antibody, we also observed more normal gephyrin immunoreactivity in both AD and control cases; however, the density of neuronal processes was dramatically reduced in AD cases (Figure 1). Gephyrin immunoreactivity was abolished with antibody preabsorption for both gephyrin antibodies (Figure, Supplemental Digital Content 2 that shows antibody preabsorption and antibody epitopes).
Table 1.
Demographics
| Alzheimer’s Disease | Control | |
|---|---|---|
| (n = 17) | (n = 14) | |
| Female | 53% | 50% |
| White | 82% | 79% |
| Median age-at-onset, range (years) | 74.3, (60–84) | NA |
| Median age-at-death, range (years)1 | 83.5, (72–93) | 61.2, (20–87) |
| Median postmortem interval, range (hours) | 8.25, 2.5–30.5 | 11, 3–31 |
| ApoE E4 carrier | 82% | 50% |
| APOE Genotype | ||
| 2–3 | 0 | 2 |
| 3–3 | 3 | 6 |
| 3–4 | 9 | 3 |
| 4–4 | 5 | 3 |
Significant difference between AD and controls; p=0.0006484 by t-test.
Figure 1. Gephyrin plaques in Alzheimer’s disease postmortem tissue.
Representative immunhistochemistry DAB staining with gephyrin antibody 1 and gephyrin antibody 2 of cryopreserved frontal cortex free-floating 50-µm sections from control and AD cases. Scale bar: 25 µm.
Table 2.
Abnormal plaque-like gephyrin immunoreactivity
| Cases | Normal | Abnormal |
|---|---|---|
| Control | 13 | 1 |
| AD | 2 | 15 |
| PD | 3 | 0 |
| CBD | 3 | 0 |
| FTD | 3 | 0 |
To test whether the staining of gephyrin immunoreactiviy was specific for AD, we assessed other neurodegenerative diseases. We found no gephyrin accumulations in the frontal cortices of patients with Parkinson’s disease (n = 3), corticobasal degeneration (n = 3), or TDP-43-positive frontotemporal lobar degeneration (n = 3) (Table 2).
Gephyrin in Familial Alzheimer’s Disease
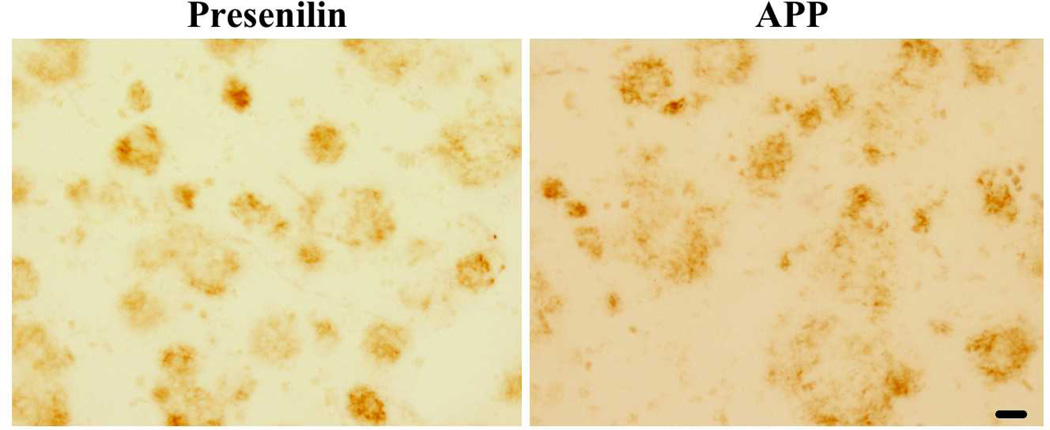
Approximately 10% of early-onset Alzheimer’s disease (i.e., onset on or before age 60 years) is caused by autosomal dominant mutations in presenilin 1, presenilin 2, or amyloid precursor protein (28). Studies of these genes have yielded much of the foundational work to support the amyloid hypothesis of AD. There are similar pathological findings in familial and sporadic forms of AD suggesting a common pathway. We therefore investigated the localization of gephyrin in the frontal cortex of 7 carriers of a presenilin 1 mutation and 2 carriers of an amyloid precursor protein mutation. We saw robust gephyrin plaque staining in 4 presenilin 1 mutation cases and 1 APP mutation (representative image shown in Figure 2). It is unclear if the absence of gephyrin plaques in the 3 other presenilin carriers was due to a lack of pathology or a result of over-fixation as these familial cases had been fixed and stored in formaldehyde for years.
Figure 2. Gephyrin plaques in familial AD.
Representative example of frontal cortex free-floating 50-µm section from research subjects with either a presenilin or an APP familial mutation and clinical history of AD. Scale bar is 25 µm. Images acquired with Olympus light microscope.
Relation of Abnormal Gephyrin Immunoreactivity to Neuritic Plaques and Neurofibrillary Tangles
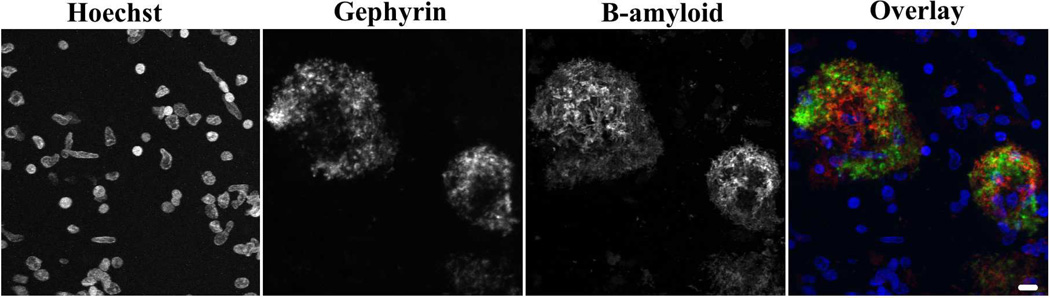
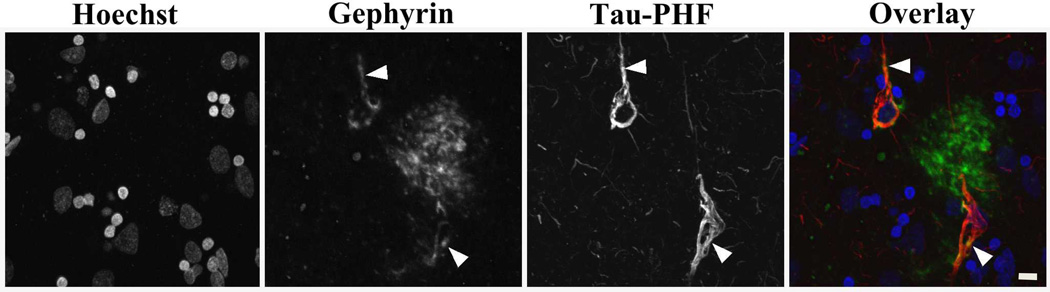
We next investigated the localization of gephyrin with known AD pathologic changes. Immunofluorescence was used to double label 50-µm free-floating tissue sections with either gephyrin and β-amyloid or gephyrin and tau paired helical filament. Gephyrin accumulations occurred in or around regions of abnormal β-amyloid immunoreactivity. A representative example is shown in Figure 3. There were occasional gephyrin-negative β-amyloid-positive plaques (large white arrowheads) or gephyrin-positive β-amyloid-negative plaques (small arrows, Figure, Supplemental Digital Content 3 that shows non-overlapping gephyrin and β-amyloid plaques); however, this may be due to differences in antibody penetration or sensitivity rather than true differences in plaque composition. We found that diffuse and neuritic amyloid plaques showed gephyrin immunoreactivity; however, dense cores had more limited gephyrin staining. In addition, there were rare tau-positive neurofibrillary tangles with gephyrin immunoreactivity but other tau positive neurites did not show any significant gephyrin immunoreactivity (Figure 4).
Figure 3. Gephyrin plaques colocalize with β-amyloid plaques.
Immunofluorescence image from AD post-mortem tissue with Hoechst-labeled nuclei, FITC-tyramide-labeled gephyrin (antibody 1), and cy3-labeled β-amyloid with overlay image combining the 3 images (blue-Hoechst, green-gephyrin, red-β-amyloid). Grayscale images are shown for individual channels to improve visualization of all areas of immunoreactivity, including those areas with less intense staining. Scale bar: 10 µm.
Figure 4. Rare gephyrin and neurofibrillary tangle colocalization.
Immunofluorescence image from AD post-mortem tissue with Hoechst-labeled nuclei, FITC-tyramide-labeled gephyrin (antibody 1), and cy3-labeled tau with overlay image combining the 3 images (blue-Hoechst, green-gephyrin, red-tau/paired helical filament (PHF)). Grayscale images are shown for individual channels to improve visualization of all areas of immunoreactivity, including those areas with less intense staining. White arrows indicate gephyrin and tau colocalization. Scale bar is 10 µm.
Gephyrin in the Insoluble Fraction of AD Cases
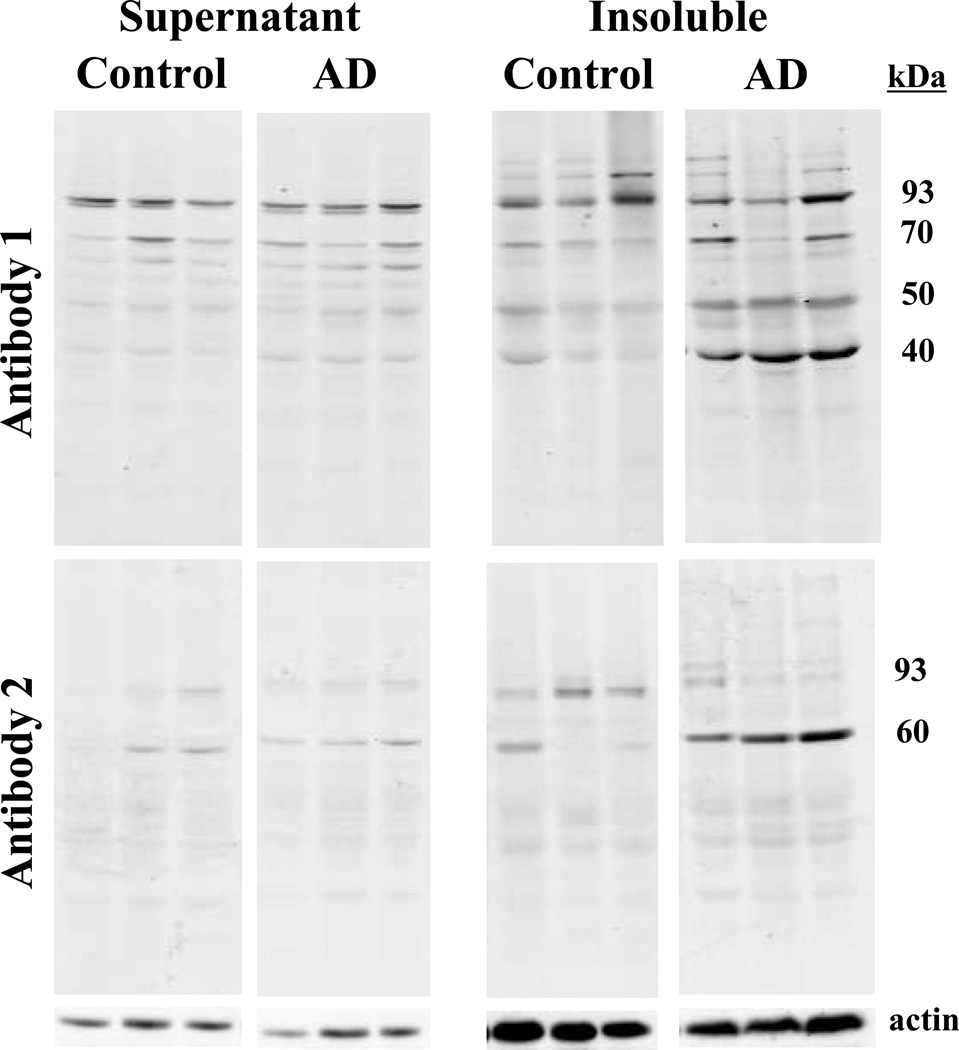
Given the abnormal accumulations of gephyrin in AD brains, we assessed whether the gephyrin immunoreactivity was associated with a shift in gephyrin solubility in AD. To do this, we examined the solubility of gephyrin in control and AD cases using frontal cortex homogenate of 5 control and 5 AD. Gephyrin antibody 1 (C-terminus epitope), which showed the most striking difference between cases and controls in immunohistochemistry, revealed a significant reduction of full-length gephyrin in the soluble fraction of AD cases (Table 3, Figure 5). As a result, we expected to see a reciprocal increase of gephyrin in the insoluble fraction; however, we found no statistical difference for full-length gephyrin between AD and control cases. Interestingly, this antibody labeled 3 other major lower-molecular-weight gephyrin products: 40-kDa, 50-kDa, and 70-kDa (Table 3, Figure 5). All 3 lower-molecular-weight products showed a statistically significant increase in the AD insoluble fraction compared to controls. The gephyrin bands preabsorbed with excess epitope peptide (Figure, Supplemental Digital Content 4 that demonstrates antibody specificity on protein blots). Using the gephyrin antibody 2 (internal epitope), we also found elevated levels of a lower-molecular-weight species (Table 3, Figure 5)
Table 3.
Gephyrin quantitation from protein blots
| Supernatant: | Insoluble pellet: | |||||
|---|---|---|---|---|---|---|
| Antibody 1 | Antibody 1 | |||||
| Control | AD | p-value | Control | AD | p-value | |
| 93-kDa band | 1237+/−383 | 770+/−321 | 0.035* | 1717+/−1194 | 2158+/−1378 | 0.302 |
| 70-kDa band | 1579+/−1010 | 977+/−751 | 0.158 | 737+/−217 | 1704+/−1023 | 0.036* |
| 50-kDa band | 1487+/−276 | 1614+/−514 | 0.318 | 900+/−376 | 2666+/−1160 | 0.006* |
| 40-kDa band | 2371+/−1446 | 1581+/−615 | 0.146 | 207+/−179 | 2376+/−1416 | 0.005* |
| Antibody 2 | Antibody 2 | |||||
| Control | AD | p-value | Control | AD | p-value | |
| 93-kDa band | 2253+/−1388 | 1861+/−1005 | 0.312 | 2855+/−908 | 1474+/−1486 | 0.057 |
| 60-kDa band | 1996+/−855 | 1374+/−1053 | 0.168 | 1108+/−1266 | 3306+/−1508 | 0.019* |
Denotes statistical significance with p-value <0.05 when comparing control (n=5) to AD (n=5), Student’s t-test.
Values given are arbitrary pixel intensity units +/− standard deviation.
Figure 5. Lower-molecular weight gephyrin products are enriched in the AD insoluble fraction.
Fresh frozen post-mortem brains from AD and control subjects were homogenized and fractionated at 180,000G in an ultracentrifuge. 50µg of supernatant and insoluble pellet (resuspended in 8M urea) were immunoblotted with gephyrin antibody 1 and gephyrin antibody 2. Actin is provided as a loading control below. 3 AD and 3 controls are shown. Densitometry analysis provided in Table 3.
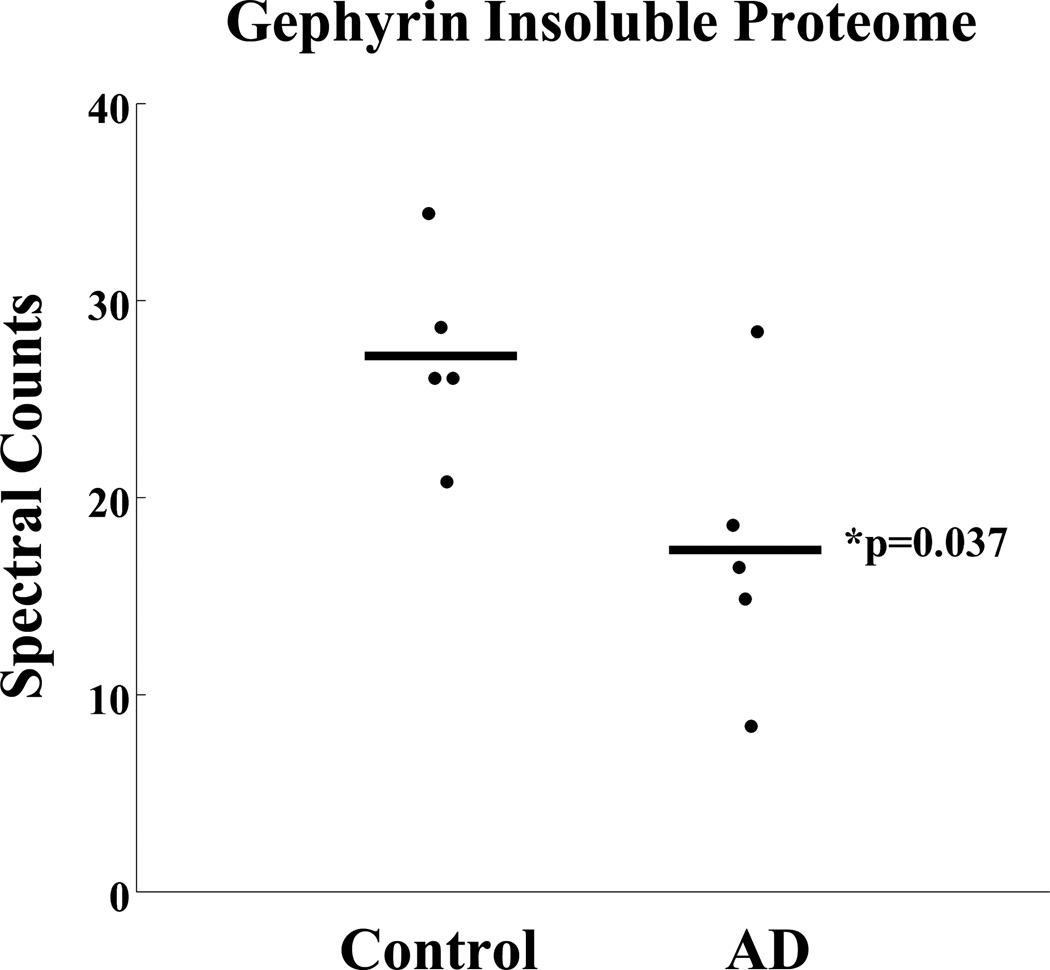
To further understand whether abnormal gephyrin immunoreactivity reflects a shift in gephryin solubility, we used a proteomics approach on control and AD insoluble preparations. We used in-gel digestion, followed by tandem mass spectrometry and quantitative proteomics. Interestingly, in AD we saw a statistically significant reduction in full-length insoluble gephyrin rather than an expected increase (Figure 6) as peptides isolated were from the molecular weight region expected to contain full-length gephyrin. Of the 154 total synaptic proteins identified in the AD insoluble fraction, 6 others also demonstrated a statistical difference when comparing control and AD insoluble fractions (Figure, Supplemental Digital Content 5 that shows other synaptic proteins with significant changes in the AD insoluble fractions.). No other gephyrin or inhibitory synapse associated proteins were significantly enriched or depleted in AD in this analysis. Two of the synaptic proteins identified, VAMP2 and MT3, have previously been associated with Alzheimer’s disease (29, 30). Unfortunately, despite repeated experiments, we were unable to detect gephyrin peptides from lower-molecular-weight regions thus limiting our ability to draw further conclusions about lower-molecular-weight gephyrin isoforms, cleavage products or solubility shifts. Deeper sequencing may be possible in the future with improved proteomic technologies.
Figure 6. Full-length gephyrin expression is reduced in AD insoluble proteome.
The insoluble fraction (urea soluble) from 5 control and 5 AD cases (including cases in Figure 5) were subject to mass spectrometry analysis. Samples were resolved on an acrylamide gel and lanes were divided into 5 different molecular weight regions. Gephyrin peptides were identified in the molecular region consistent with full-length gephyrin. Spectral counts (number of gephyrin peptides sequenced) were normalized following removal of system background. The AD mean is significantly reduced compared to controls (p-value <0.05).
DISCUSSION
The sequence of events that leads to Alzheimer’s disease is complex and not completely understood. Here we provide evidence for a novel association between gephyrin and neuritic plaques. Our study demonstrates that gephyrin protein levels are diminished in the brains of AD patients and suggest this may be due to a shift in solubility. We also identified several lower-molecular-weight species of gephyrin that generally appear enriched in AD and may account for immunohistochemistry findings, but whose physiologic role is uncertain.
What is the relevance of gephyrin abnormalities in AD? Since gephyrin is responsible for clustering and anchoring inhibitory receptors at their respective synapses, subtle changes in gephyrin solubility may lead to changes in synaptic activity and other downstream effects. Gephyrin also plays a role in local synaptic protein translation (23), and disruptions in this process could contribute to a loss of functional synapses. Loss of gephyrin’s molybdenum cofactor biosynthesis properties (31) could also reduce the ability of cells to manage certain toxic metabolites and/or respond to oxidative stress.
Previous reports have shown reduced gephyrin expression in AD (14), although direct comparison to our study is difficult because of differences in brain homogenate preparation. The choice of Agarwal et al. to use a non-detergent-extracted method with low-speed spin likely led to a reduction in the soluble protein analyzed, whereas in our work, a reduction in gephyrin expression is supported by a loss of gephyrin immunoreactivity in neuronal processes (Figure 1) and by quantitative proteomic analysis. Epitope accessibility is likely responsible for immunostaining variability noted between the c-terminal antibody 1 and more internal antibody 2. The loss of gephyrin expression may in part reflect the substantial synaptic loss that occurs in AD. Similarly, loss of normal gephyrin staining in AD (Figure 1, Antibody 2) is likely secondary to the reduction in full-length gephryin. More abundant molecules and a larger number of unrelated proteins in the lower molecular weight regions are likely confounding our efficiency to detect lower molecular weight gephyrin fragments via quantitative proteomics. These protein findings are not in agreement with recent gephyrin transcript quantitation, which suggests no major differences between control and AD (13). The discrepancy between transcriptome and proteome, however, is not unexpected since transcript levels may not always be predictive of protein expression (32).
Other molecules in plaques, like β-amyloid, typically migrate into the insoluble fraction as disease progresses (26). Since IHC demonstrated gephyrin plaque accumulations, we expected to see an increase in gephyrin insolubility. With our proteomics experiment, we actually saw the opposite with a significant decrease of gephyrin in the insoluble fraction in AD. Significant changes were also noted in several other synaptic proteins, including AD related proteins MT3 and VAMP2. Interestingly, many other inhibitory synapse proteins, like GABA receptors, did not differ in the control and AD insoluble fractions thereby suggesting that gephyrin may be selectively affected at the inhibitory synapse. Higher case numbers may yield other inhibitory synaptic proteins with more subtle changes. There were proteins, like the gephyrin clustering protein neuroligin-2, that demonstrated a near significant trend similar to that of gephyrin. Lower-molecular-weight gephyrin products in the AD insoluble fraction, as noted on immunoblotting, were also unanticipated but may help to reconcile the quandary. Gephyrin may indeed have increased insolubility however it may exist as fragments or other isoforms in the diseased state. Since proteins that aggregate in neurodegeneration are often insoluble and because we have two different antibodies labeling plaque-like structures, the abnormal accumulations of gephyrin (fragment or full-length) are likely insoluble. Initial proteomic attempts to clarify this issue failed to demonstrate gephyrin peptides in the lower molecular weight regions. More in-depth proteomic sequencing, laser capture microdissection, and immunoprecipitations could provide further details in future studies.
Since gephyrin plaques are present in familial AD cases, gephyrin accumulation is likely influenced by events in the amyloid cascade. Rodent models of aberrant amyloid processing may therefore prove useful in further clarifying the role of gephyrin in AD pathogenesis. The variability in staining between familial cases may be related to regional sampling differences or more likely fixation issues since 6 of the 7 familial presenilin cases were fixed in formaldehyde for years. Future studies should help establish the temporal presentation of gephyrin plaques in relation to amyloid deposition. Moreover, such studies could also address whether presenilin, which is known to cleave multiple intracellular substrates, also cleaves gephyrin.
In summary, we found a specific association of plaque-like accumulations of gephyrin in AD. The functional relevance of this association remains unclear and merits future study. This new pathological association in addition to our identification of gephyrin low molecular weight species gives us a foundation for exploring gephyrin as a new marker for AD that should be evaluated in spinal fluid and in pre-symptomatic cases of AD.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported in part by the Department of Veterans Affairs and the NIA intramural Research Program of the National Institutes of Health. This work does not represent the views of the Department of Veterans Affairs or the United States Government.
Funding: C.M.H.-American Brain Foundation Clinical Research Training Fellowship, A.I.L-Emory Alzheimer’s Disease Research Center-NIA-AG025688, J.J.L.-NIAP01AG014449, Emory Neuroscience NINDS Core Facility-P30NS055077, J. C. T-John’s Hopkins University Alzheimer’s Disease Research Center-NIA-AG005146 and Alzheimer’s Association-IIRG-09-134090.-T.S.W.-The Department of Veteran Affairs-CDA1-002-09F and 1IK2BX001820.
Footnotes
Author Contributions: C.M.H., J.J.L, A.I.L, and T.S.W conceived and supervised this project. C.M.H and H.R. performed immunohistochemistry. C. M. H. performed immunofluorescence and immunoblotting. E.B.D, N.T.S, and D.M.D. performed proteomics and analyzed data. M.G., T.J.M, M.T. and J.C.T. collected human samples. C.M.H. and T.S.W. wrote the paper.
References
- 1.Hebert LE, Scherr PA, Bienias JL, et al. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003 Aug;60(8):1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 2.Serrano-Pozo A, Frosch MP, Masliah E, et al. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med. 2011 Sep;1(1):a006189. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lambert JC, Heath S, Even G, et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer's disease. Nat Genet. 2009 Oct;41(10):1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- 4.Naj AC, Jun G, Beecham GW, et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer's disease. Nature Genetics. [Meta-Analysis Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] 2011 May;43(5):436–441. doi: 10.1038/ng.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamenetz F, Tomita T, Hsieh H, et al. APP processing and synaptic function. Neuron. 2003 Mar 27;37(6):925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 6.Priller C, Bauer T, Mitteregger G, et al. Synapse formation and function is modulated by the amyloid precursor protein. J Neurosci. 2006 Jul 5;26(27):7212–7221. doi: 10.1523/JNEUROSCI.1450-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mosconi L, Sorbi S, de Leon MJ, et al. Hypometabolism exceeds atrophy in presymptomatic early-onset familial Alzheimer's disease. J Nucl Med. 2006 Nov;47(11):1778–1786. [PubMed] [Google Scholar]
- 8.Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002 Oct 25;298(5594):789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 9.Scheff SW, Price DA, Schmitt FA, et al. Hippocampal synaptic loss in early Alzheimer's disease and mild cognitive impairment. Neurobiol Aging. 2006 Oct;27(10):1372–1384. doi: 10.1016/j.neurobiolaging.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Palop JJ, Chin J, Roberson ED, et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron. 2007 Sep 6;55(5):697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oddo S, Caccamo A, Shepherd JD, et al. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003 Jul 31;39(3):409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 12.Reisberg B, Doody R, Stoffler A, et al. Memantine in moderate-to-severe Alzheimer's disease. N Engl J Med. 2003 Apr 3;348(14):1333–1341. doi: 10.1056/NEJMoa013128. [DOI] [PubMed] [Google Scholar]
- 13.Limon A, Reyes-Ruiz JM, Miledi R. Loss of functional GABA(A) receptors in the Alzheimer diseased brain. Proc Natl Acad Sci U S A. 2012 Jun 19;109(25):10071–10076. doi: 10.1073/pnas.1204606109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agarwal S, Tannenberg RK, Dodd PR. Reduced expression of the inhibitory synapse scaffolding protein gephyrin in Alzheimer's disease. J Alzheimers Dis. 2008 Jul;14(3):313–321. doi: 10.3233/jad-2008-14305. [DOI] [PubMed] [Google Scholar]
- 15.Schmitt B, Knaus P, Becker CM, et al. The Mr 93,000 polypeptide of the postsynaptic glycine receptor complex is a peripheral membrane protein. Biochemistry. 1987 Feb 10;26(3):805–811. doi: 10.1021/bi00377a022. [DOI] [PubMed] [Google Scholar]
- 16.Kirsch J, Malosio ML, Wolters I, et al. Distribution of gephyrin transcripts in the adult and developing rat brain. Eur J Neurosci. 1993 Sep 1;5(9):1109–1117. doi: 10.1111/j.1460-9568.1993.tb00965.x. [DOI] [PubMed] [Google Scholar]
- 17.Feng G, Tintrup H, Kirsch J, et al. Dual requirement for gephyrin in glycine receptor clustering and molybdoenzyme activity. Science. 1998 Nov 13;282(5392):1321–1324. doi: 10.1126/science.282.5392.1321. [DOI] [PubMed] [Google Scholar]
- 18.Kneussel M, Brandstatter JH, Laube B, et al. Loss of postsynaptic GABA(A) receptor clustering in gephyrin-deficient mice. J Neurosci. 1999 Nov 1;19(21):9289–9297. doi: 10.1523/JNEUROSCI.19-21-09289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kneussel M, Betz H. Receptors, gephyrin and gephyrin-associated proteins: novel insights into the assembly of inhibitory postsynaptic membrane specializations. J Physiol. 2000 May 15;525(Pt 1):1–9. doi: 10.1111/j.1469-7793.2000.t01-4-00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schrader N, Kim EY, Winking J, et al. Biochemical characterization of the high affinity binding between the glycine receptor and gephyrin. J Biol Chem. 2004 Apr 30;279(18):18733–18741. doi: 10.1074/jbc.M311245200. [DOI] [PubMed] [Google Scholar]
- 21.Reiss J, Johnson JL. Mutations in the molybdenum cofactor biosynthetic genes MOCS1, MOCS2, and GEPH. Hum Mutat. 2003 Jun;21(6):569–576. doi: 10.1002/humu.10223. [DOI] [PubMed] [Google Scholar]
- 22.Smolinsky B, Eichler SA, Buchmeier S, et al. Splice-specific functions of gephyrin in molybdenum cofactor biosynthesis. J Biol Chem. 2008 Jun 20;283(25):17370–17379. doi: 10.1074/jbc.M800985200. [DOI] [PubMed] [Google Scholar]
- 23.Sertie AL, de Alencastro G, De Paula VJ, et al. Collybistin and gephyrin are novel components of the eukaryotic translation initiation factor 3 complex. BMC Res Notes. 2010;3:242. doi: 10.1186/1756-0500-3-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shock NW, Greulich RC, Andres R, et al. NIH Publication No 84-2450. Washington, DC: Government Printing Office; 1984. Normal Human Aging: The Baltimore Longitudinal Study of Aging. [Google Scholar]
- 25.Sager KL, Wuu J, Leurgans SE, et al. Neuronal LR11/sorLA expression is reduced in mild cognitive impairment. Ann Neurol. 2007 Dec;62(6):640–647. doi: 10.1002/ana.21190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gozal YM, Duong DM, Gearing M, et al. Proteomics analysis reveals novel components in the detergent-insoluble subproteome in Alzheimer's disease. J Proteome Res. 2009 Nov;8(11):5069–5079. doi: 10.1021/pr900474t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dammer EB, Fallini C, Gozal YM, et al. Coaggregation of RNA-binding proteins in a model of TDP-43 proteinopathy with selective RGG motif methylation and a role for RRM1 ubiquitination. PLoS One. 2012;7(6):e38658. doi: 10.1371/journal.pone.0038658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campion D, Dumanchin C, Hannequin D, et al. Early-onset autosomal dominant Alzheimer disease: prevalence, genetic heterogeneity, and mutation spectrum. Am J Hum Genet. 1999 Sep;65(3):664–670. doi: 10.1086/302553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu WH, Lukiw WJ, Bergeron C, et al. Metallothionein III is reduced in Alzheimer's disease. Brain Res. 2001 Mar 9;894(1):37–45. doi: 10.1016/s0006-8993(00)03196-6. [DOI] [PubMed] [Google Scholar]
- 30.Pham E, Crews L, Ubhi K, et al. Progressive accumulation of amyloid-beta oligomers in Alzheimer's disease and in amyloid precursor protein transgenic mice is accompanied by selective alterations in synaptic scaffold proteins. FEBS J. 2010 Jul;277(14):3051–3067. doi: 10.1111/j.1742-4658.2010.07719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pratico D. Evidence of oxidative stress in Alzheimer's disease brain and antioxidant therapy: lights and shadows. Ann N Y Acad Sci. 2008 Dec;1147:70–78. doi: 10.1196/annals.1427.010. [DOI] [PubMed] [Google Scholar]
- 32.Pradet-Balade B, Boulme F, Beug H, et al. Translation control: bridging the gap between genomics and proteomics? Trends Biochem Sci. 2001 Apr;26(4):225–229. doi: 10.1016/s0968-0004(00)01776-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.