Abstract
Purpose.
Autophagy in photoreceptors and the RPE promotes homeostasis and survival. The purpose of this study is to determine the daily pattern of changes in autophagy and factors contributing to its regulation in the outer retina.
Methods.
Levels of autophagy markers in the retina and RPE were evaluated over a 24-hour period. To assess the role of phagocytosis in stimulating autophagy in the RPE, cultured RPE-J cells were incubated with isolated photoreceptor outer segments and levels of autophagy markers were measured. Electron microscopy was performed on retina sections and RPE-J cells to assess formation of double-membraned vesicles consistent with autophagosomes.
Results.
In wild-type C57BL/6 mice maintained under normal cycling light conditions, autophagy in photoreceptor cells and the RPE exhibited a bimodal pattern of activation. In photoreceptors, shifts between light and dark evoked a sharp decrease in autophagy that was followed by a time-dependent increase. In photoreceptors, translocation of transducin and arrestin from the outer to inner segment appeared to contribute to the light-dependent upregulation of autophagy. In contrast, the cyclic variations in RPE autophagy were independent of lighting conditions, and are triggered, at least in part, by ingestion of outer segments.
Conclusions.
Activation of autophagy in the outer retina exhibits a bimodal pattern that correlates with shifts in transduction proteins within the photoreceptor and by circadian ingestion of outer segments in the RPE. These dynamic shifts suggest a critical role for this pathway in maintaining homeostasis, with further study needed to define the mechanisms underlying the regulation of this phenomenon.
Keywords: autophagy, retina, retinal pigment epithelium, photoreceptors
Activation of autophagy in mouse outer retina exhibits a bimodal pattern that correlates with light/dark shifts in transduction proteins within the photoreceptor and by circadian ingestion of shed outer segments in the RPE.
Introduction
Autophagy has an important role in maintaining cellular homeostasis and regulating the response to environmental stressors.1 Autophagy is described classically as being activated in conditions of nutrient deprivation.2 Cytoplasmic materials are ingested by double-membrane structures called autophagosomes. The autophagosomes ultimately fuse with lysosomes, where the ingested material is degraded and released as core nutrients, such as amino acids, back into the cytoplasm.3 Autophagy also occurs within cells at a basal level, and helps maintain cellular homeostasis by removing toxic aggregates and damaged organelles.4,5 Dysregulation of autophagy has been implicated in many pathologic conditions, including cancer, infectious diseases and neurodegeneration.6,7 In the visual system, autophagy has been shown to have a role in regulating photoreceptor survival in animal models of hereditary retinal degeneration, oxidative stress, and retinal detachment.8–11
A daytime peak of autophagy in the rod photoreceptor inner segments was first documented by Reme and Suler,12 who observed increased formation of autophagosomes three hours after the peak period of disk-shedding (approximately five hours after the onset of the light period). Reme et al.13 concluded that autophagy in rat photoreceptors is regulated by circadian rhythm, as disc shedding and autophagy could be evoked by light pulses. Autophagy also has been shown to help regulate the response of the RPE to a variety of environmental stressors, such as light activation and oxidative stress.14–16 The RPE is a monolayer of cells located immediately adjacent to the photoreceptor layer of the neural retina. Although the retina has its own circulation, these vessels only serve the inner two-thirds of the retina. The photoreceptors are located on the outer surface of the retina, and obtain metabolic support from the RPE.17,18 In addition, the RPE is critical for the phagocytic clearance of the distal outer segments shed by the photoreceptors into the subretinal space.19 The ubiquitous presence of autophagic vacuoles in the RPE has been known for many years.20 Previous studies have examined the expression of autophagy proteins in the RPE, and the changes of autophagy activity with age and disease.16,21,22
The time course of daily fluctuations in the levels of autophagy occurring in the photoreceptors and RPE has not been characterized, and the mechanisms underlying the dynamic regulation of autophagy in these cells remains unknown. Thus, the goals of this study are to characterize the levels of autophagy in the photoreceptors and RPE over a 24-hour period, and to provide insight into the molecular mechanisms regulating these levels. Our results showed that basal autophagy in mouse photoreceptors and RPE follows a bimodal pattern with one light-period peak and one dark-period peak. We find that in photoreceptors, autophagy is controlled by the shift between dark and light, with translocation of phototransduction proteins acting as one potential regulator. In contrast, autophagy in the RPE is independent of changes in light environment, and appears to occur in response to ingestion of photoreceptor outer segments that are shed in a circadian manner.
Methods
Animals
Four strains of mice were used in the experiments: C57BL/6 and Sag−/− mice (knockout of Arrestin; Jackson Laboratories, Bar Harbor, ME, USA), Gnat1−/− mice (knockout of the α subunit of rod transducin),23 and GFP-LC3 mice24 (Riken Laboratories, Tsukuba, Japan). Mice were bred and housed in the University of Michigan, Kellogg Eye Center animal facility. All animal experiments were conducted in accordance with the Association for Research in Vision and Ophthalmology (ARVO) statement for the Use of Animals in Ophthalmic and Vision Research, and approved by the University Committee on the Use and Care of Animals of the University of Michigan. All mice were genotyped by established methods and only those negative for mutations in the Rd8 gene were used.25 Except as specified, mice were bred and reared under standard 12-hour light/12-hour dark conditions. At age 2 months, mice were euthanized and the retinas were collected from 3 groups of animals. For mice of all genotypes, Group 1 animals were euthanized every 3 hours over a 24-hour period. For Group 2, at 3 hours after the lights were turned on, animals were moved back to the dark, and euthanized at 1, 4, and 7 hours later. For Group 3, after the regular 12-hour dark period was complete the animals were kept in darkness and retinas were collected every 3 hours. At least 8 animals were used for each time point of each experimental group.
Tissue Collection
Retinal tissue and RPE-choroid complexes were isolated carefully at various time points. Briefly, mice were euthanized, and eyes were enucleated immediately and placed in a dish. The connective tissue, muscle, and optic nerve were removed from the back of the eye, and the cornea and lens were removed to form an eyecup. The retina was dissected off of the RPE, cut from the connection to the optic nerve head, and pulled away from the rest of the eye. The RPE/choroid was scraped carefully from the sclera with a pair of flat-top forceps. For the dark condition, the eyes were enucleated and tissue processed under far-red illumination. Though cross-contamination of the respective layers is a potential concern, we feel that this is reduced because the photoreceptor LC3 is localized primarily to the inner segments. Thus, the presence of any outer segments in the RPE prep would not be expected to affect the LC3 levels measured in the RPE significantly. Likewise, there easily can be some RPE contaminating the retina tissue prep; however, the amount of LC3 contributed by these few cells is dwarfed by the amount of protein coming from the photoreceptors.
RPE-J Cell Culture and Outer Segment Challenge
Rat RPE-J cells were maintained in Dulbecco's modified Eagle's medium, supplemented with 4% fetal bovine serum, and 1 mM nonessential amino acids at 33°C in 5% CO2. Cells were passaged, and then cultured for 3 to 6 days in six-well plates or on chamber slides, followed by incubation with photoreceptor outer segments (POS) isolated from bovine eyes26 at 2, 10, or 50 OS per RPE cell at 33°C for various periods of time (low, medium, or high doses, respectively). RPE-J cells were treated with the following inhibitors: 2 μM herbimycin for 16 hours before the addition of OS, blebbistatin (100 μM), and inactive-blebbistatin (100 μM; EMD Chemicals, Inc., Gibbstown, NJ, USA) for 30 minutes before the addition of outer segments and during incubation with OS for 7 hours at 33°C. Cells were harvested and prepared as described previously for Western analysis.27
Immunocytochemistry
The GFP-LC3 mice were euthanized at different time points as indicated in the Figure legends, and the eyes were enucleated and fixed by immersion in 4% paraformaldehyde at room temperature for 3 hours. The cornea and lens were removed and the eyecup was rinsed three times in PBS, transferred to 10% and then 20% sucrose in PBS for 2 hours each, before embedding in OCT (Tissue Tek; Sakura Finetek U.S.A., Inc., Torrance, CA, USA) mixed in a ratio of 1:1 with 20% sucrose. A cryostat was used to obtain serial sections of 10 μm thickness. Sections were washed in PBS, and blocked with 10% goat serum and 0.2% Triton-X 100 for 1 hour. After incubation overnight at 4°C with anti-GFP 488 (1:1000; Invitrogen, Carlsbad, CA, USA), sections were counterstained with ProLong Gold with 4′6-diamidino-2-phenylindole (DAPI; Invitrogen) to reveal cell nuclei.
For cultured RPE-J cells, confluent cultures on chamber slides were fixed in 4% paraformaldehyde (PFA) for 20 minutes at room temperature. Sections were washed in PBS, blocked with 10% goat serum and 0.2% Triton X-100 for 1 hour, and incubated with anti-LC3 antibody (LC3B 1:200; Cell Signaling, Danvers, MA, USA) overnight at 4°C. After incubation for 1 hour at room temperature with secondary antibodies, chamber slides were counterstained with ProLong Gold with DAPI (Invitrogen).
Images of the retinal sections and chamber slides of RPE-J cells were obtained using a confocal microscope (Leica SP5; Leica Corp., Wetzlar, Germany). Images were taken at the comparable area of the retina. All images in each individual experiment were acquired with a fixed detection gain.
Quantification of GFP-LC3 Puncta in Photoreceptor Inner Segment
Retinal images were obtained using confocal microscopy. Three representative sections (second, 10th, and 18th) crossing the optic nerve were used from each eye to avoid double counting. The number of LC3-GFP puncta in each section was counted in 5 nonoverlapping areas of 30 μm length of retina (containing approximately 80–90 inner segments). The counted areas comprised the inner segment region up to the outer limiting membrane. The perinuclear area and synaptic body were excluded. At least 5 animals were used for each time point of each experimental group.
Transmission Electron Microscopy
For transmission electron microscopy, the superior parts of the eye cups were dissected in the fixative (2.5% glutaraldehyde in 0.1 M Sorensen's buffer, pH 7.4) immediately after the eyes were enucleated and then were fixed overnight at 4°C. For analysis of RPE-J cultures, cells in the culture dish were washed twice with buffer at indicated times after POS-feeding, and then fixative was added and kept overnight at 4°C. After several buffer rinses, samples were postfixed in 1% osmium tetroxide in the same buffer. Samples were rinsed in double distilled water to remove phosphate salt and stained en bloc with aqueous 3% uranyl acetate for 1 hour. Eye cups and cells were dehydrated in ascending concentrations of ethanol. Eye cups were rinsed two times in propylene oxide, and RPE-J samples were rinsed two times in 100% ethanol (ETOH). Samples then were embedded in epoxy resin. The samples were ultrathin sectioned (70 nm in thickness), and poststained with uranyl acetate and lead citrate. The sections were examined using a Philips CM100 electron microscope at 60 kV. Images were recorded digitally using an Hamamatsu ORCA-HR digital camera system operated using AMT software (Advanced Microscopy Techniques Corp., Danvers, MA, USA).
Quantification of Autophagosomes in the RPE
The number of autophagosomes present in pigment epithelial cells was determined by counting directly on the electron microscope screen, at a magnification of ×10,500. Three representative thin sections crossing the optic nerve were used from each eye. In each section, two independent areas of 400 μm length of the RPE were counted.
Western Analysis
Retina and RPE/choroid samples were dissected from the experimental groups. Samples were homogenized in hypotonic buffer, and the proteins separated by SDS-PAGE (4%–15% Tris-HCl Ready Gels; Bio-Rad Laboratories, Hercules, CA, USA) and blotted on polyvinylidene fluoride membranes (Immobilon-P; Millipore, Billerica, MA, USA). The membranes were incubated overnight with primary antibodies according to the manufacturer's instructions. The following antibodies were used: LC3 and Atg12 (1:1000; Cell Signaling), Atg5 (1:1000; Abgent, San Diego, CA, USA), and GAPDH (1:30,000; Invitrogen). Secondary antibodies were from Dako (Dako, Glostrup, Denmark). Detection was by SuperSignal West Dura Substrate (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturers' protocols. Quantitative densitometry of the immunoblots was performed using ImageJ software (National Institutes of Health, Bethesda, MD, USA), and expressed as the mean density (±SD) from replicate animal groups. All experiments were performed a minimum of three times. Consistent outcomes were obtained and representative experiments are shown.
Statistical Analysis
Data representing the means ± SD for the results of at least three independent experiments were compared by the 1-way ANOVA test. Differences were considered significant at P < 0.05.
Results
Basal Autophagy in Mouse Retina and Photoreceptor Cells Exhibits a Bimodal Variation
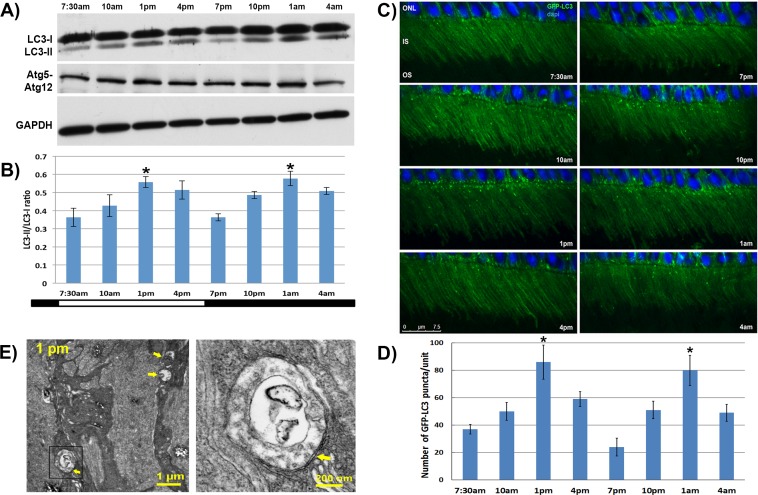
In experimental Group 1, C57BL/6 mice were bred and reared under standard 12-hour light/12-hour dark conditions (6 AM lights on, 6 PM lights off). At age 2 months, mice were euthanized and their retinas collected every 3 hours over a 24-hour period. Western blotting showed that there was a bimodal pattern of LC3-I conversion to LC3-II, a standard marker of autophagy activity, over the 24-hour time course (Figs. 1A, 1B). The two peaks of elevated autophagic activity occurred at 1 PM and 1 AM, corresponding to 7 hours after the onset of the light and dark periods, respectively. Formation of Atg5-Atg12 complex, a precursor to LC3-II formation, followed a similar bimodal pattern. The lowest levels of these autophagy markers were seen approximately 1 hour after the shift from dark to light (7 AM) or from light to dark (7 PM).
Figure 1.
Bimodal pattern of autophagy activation in mouse retinas. (A) Representative Western blots of retina samples from C57BL/6 mice taken at various time points over a 24-hour period. The upper panel was probed with an antibody recognizing the lipidated and nonlipidated forms of LC3, and the middle panel was probed for the Atg5-Atg12 complex. The GAPDH levels served as loading controls. (B) Ratio of LC3-II to LC3-I levels based on densitometry of the respective bands seen on the Western blot. The ratios at 1 PM and 1 AM were significantly higher than those of 7:30 AM or 7 PM (P < 0.05, n = 8 animals/time point). (C) Representative images of retinas from GFP-LC3 mice at various time points showing increased punctate pattern of LC3 (green). The LC3 localizes primarily to the photoreceptor inner segment (IS) with minimal amounts seen in other layers. The outer nuclear layer (ONL) is stained with DAPI (blue). (D) Quantification of the number of GFP-LC3 puncta as a function of time of day. (E) Electron micrograph of an inner segment at 1 PM showing a double-membrane structure consistent with an autophagosome (arrow). *The ratios at 1 PM and 1 AM were significantly higher than the 7 AM or 10 AM levels (P < 0.05, n = 8 animals per time point).
Immunohistochemical analysis of the distribution and staining pattern of the LC3 protein in retina sections showed localization primarily to the photoreceptor inner segments (Fig. 1C). Previous studies have shown that when low levels of autophagy activity are present in cells, staining for LC3 usually reveals a diffuse distribution of this protein, and that higher autophagy levels cause a shift in the LC3 staining pattern to a more punctate form consistent with the formation of autophagosomes.28 In our analysis, we found the formation of LC3-positive puncta paralleled the time course of peak LC3-I to LC3-II conversion seen on Western blots, with more punctate staining seen at the 1 PM and 1 AM time points at which LC3-II levels were increased (Fig. 1D). Transmission electron microscopy showed the presence of double-membrane vesicles within the photoreceptor inner segment, also consistent with autophagosome formation (Fig. 1E).
Autophagy Levels in Retina Are Dependent on Shifts in Light-Dark Exposure
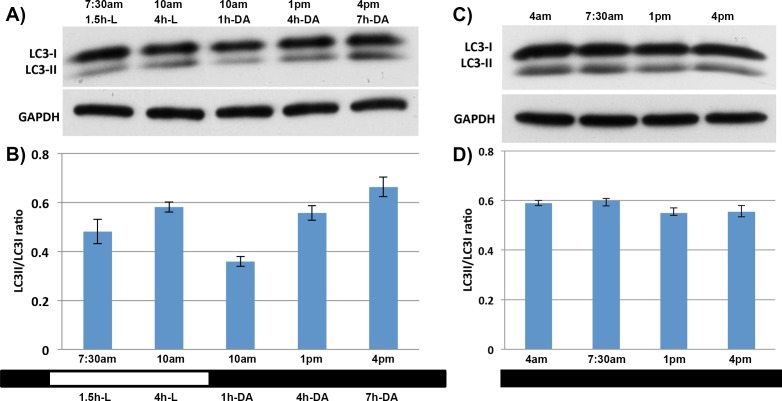
To evaluate whether the increases and decreases in LC3-II levels in mouse retina follow a circadian rhythm or are regulated by the changes in the environment, we investigated the effect of modifying their exposure to light/dark conditions. In experimental Group 2, at 3 hours after the lights were turned on, mice were moved back to the dark, and tissue harvested 1, 4, and 7 hours later. Retinas from mice in this group exhibited a sharp decrease in LC3-II levels shortly after being transferred from the light to the dark, with a subsequent time-dependent increase in the conversion of LC3-I to LC3-II the longer they were kept in the dark (Figs. 2A, 2B).
Figure 2.
Effect of varying light conditions on levels of LC3-I and LC3-II in mouse retinas. (A, B) Western blot, and quantification of LC3-I and LC3-II levels in the retinas of animals that were moved back to the dark at 9 AM, 3 hours after the lights were turned on. Retina samples were collected at various times after light (L) exposure or dark adaptation (DA). One set of animals was kept in the light as a control (10 AM, 4-hour L). (C, D) Western blot, and quantification of LC3-I and LC3-II levels in the retinas of animals in Group 3, in which animals were maintained in the dark without having the lights turned on at 6 AM (n = 8 animals per time point).
In Group 3, after the regular 12-hour dark period, instead of having the lights turn on as usual, mice were kept in continued darkness, and retina samples were collected every 3 hours. Western blotting showed that the conversion of LC3-I to LC3-II in retinas from this group remained unchanged (Figs. 2C, 2D) from the peak levels seen in the dark.
Autophagy Levels in the RPE Exhibit a Bimodal Circadian Rhythm Independent of Lighting Conditions
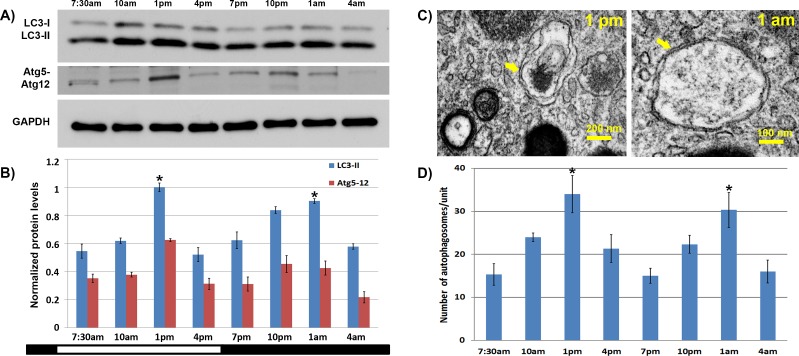
The RPE/choroid was collected from 2-month-old C57BL/6 mice bred and raised under standard 12-hour light/12-hour dark conditions. Tissues were obtained every 3 hours over a 24-hour period. Using Western analysis, we observed a bimodal pattern of LC3-II formation, with the one peak occurring approximately 7 hours after the onset of the light period, and a second peak occurring approximately 7 hours after the onset of the dark period (Figs. 3A, 3B). Quantification of the intensity of the Atg5 bands on the Western blot showed a similar trend, with peaks midcycle of the light and dark periods. Electron microscopy was used to confirm the formation of double membrane autophagosomes that were most predominant at the time points with the highest LC3-II levels (Figs. 3C, 3D).
Figure 3.
Bimodal pattern of autophagy activation in the mouse RPE. (A) Representative Western blot showing levels of LC3-I, LC3-II, and Atg5-Atg12 complex in the RPE of C57BL/6 mice maintained under normal 12-hour light/12-hour dark conditions. The GAPDH was used as a loading control. (B) Quantification of LC3-II to LC3-I ratio and Atg5-12 complex formation in the RPE. Ratios are normalized to the peak value seen at 1 PM. The ratios at 1 PM and 1 AM were higher than those of 7:30 AM, 7 PM, and 4 AM (n = 8 animals per time point). (C) Electron micrographs of RPE at 1 PM and 1 AM showing double membrane structures consistent with autophagosomes (arrows). (D) Quantification of the number of autophagosomes in the RPE as a function of time of day. *The ratios at 1 PM and 1 AM were significantly higher than the 7 AM or 10 AM levels (P < 0.05; n = 8 animals per time point).
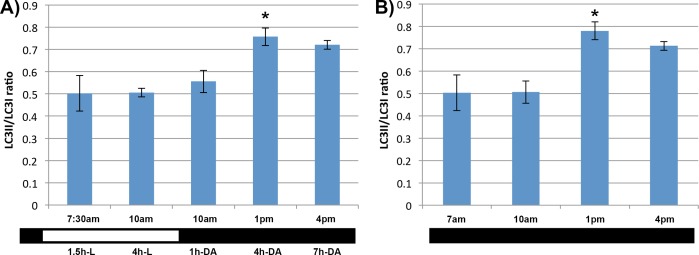
To determine whether the variation of LC3-II levels in the RPE follows a circadian rhythm or is regulated by the changes of the light-dark environment, we investigated LC3-II formation under two alternative scenarios. In experimental Group 2, at 3 hours after the lights were turned on, animals were moved to the dark, and RPE/choroid samples obtained 1, 4, and 7 hours later. Western blotting showed that the conversion of LC3-I to LC3-II in the RPE remained unchanged and peaked at the same time as compared to the animals in Group 1 (Fig. 4A). In experimental Group 3, after the regular 12-hour dark period, the lights remained off and the RPE/choroid samples were collected every 3 hours during this period of continued darkness. Western blotting showed that the conversion of LC3-I to LC3-II in the RPE of this group also remained unchanged (Fig. 4B).
Figure 4.
Effect of varying light conditions on levels of LC3-I and LC3-II in the RPE. (A) Quantification of LC3-I and LC3-II levels in the RPE of C57BL/6 mice in Group 2, in which the animals were moved back to the dark at 9 AM, 3 hours after the lights were turned on. One set of animals were kept in the light as a control (10 AM, 4-hour L). The RPE samples were collected at various times after L exposure or DA. The ratio at 1 PM (4-h DA) was significantly higher than the ratio at 7:30 AM or 10 AM kept in the light (4-h L, P < 0.05). (B) Quantification of LC3-I and LC3-II levels in the RPE of animals in Group 3, in which animals were maintained in the dark, as opposed to having the lights turned back on at 6 AM. *The ratio at 1 PM was significantly higher than the 7 AM or 10 AM levels (P < 0.05, n = 8 animals per time point).
Altered Levels of Autophagy Activation in Gnat1−/− and Sag−/− Mice
To investigate whether light-induced translocation of phototransduction proteins contributes to the diurnal variation in autophagy, we evaluated the time course of autophagy in retinas from knockout mice lacking the α subunit of rod transducin (Gnat1−/− mice). In the absence of transducin, retinal rods maintain their structure, but do not exhibit a light response. However, the translocation of arrestin in Gnat1−/− mice is not affected.29–32 We predicted that the peak of LC3-II levels seen in the light would be decreased in the Gnat1−/− mice, as this is when transducin accumulates in the inner segments, but that the increase in LC3-II levels seen in the dark still would occur in response to the accumulation of arrestin in the inner segment.
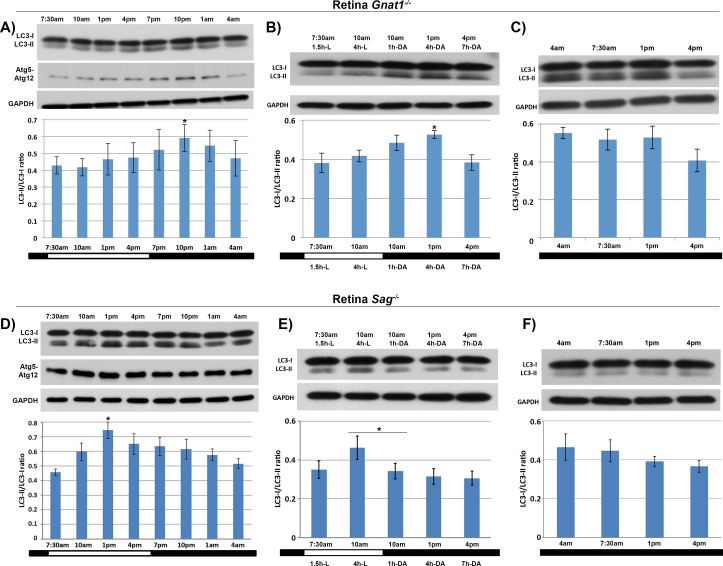
In contrast to the bimodal pattern of LC3-II formation observed in C57BL/6 mice, Figure 5A shows that Group 1 Gnat1−/− mice exhibited only one peak of LC3-II formation occurring during the dark period, with no increase in LC3-II formation observed during the light period. In Group 2 Gnat1−/− mice, at 3 hours after the lights were turned on, animals were moved to the dark, and retinas sampled 1, 4, and 7 hours later. Consistent with the autophagy profile in Gnat1−/− mice under Group 1 light conditions, we did not see a change in LC3-II formation from 7 AM to 10 AM; however, a significant increase in LC3-II formation was observed after the mice were put back in the dark (Fig. 5B). In contrast, in Group 3 Gnat1−/− mice that remained in the dark after the regular 12-hour dark period, and retina samples collected every 3 hours thereafter, the conversion of LC3-I to LC3-II remained unchanged (Fig. 5C).
Figure 5.
Autophagy activation in the retinas of Gnat1−/− and Sag−/− mouse strains. (A–C) Western blots of LC3 and quantification of LC3-I to LC3-II ratios in Group 1, Group 2, and Group 3 Gnat1−/− animals, respectively, as described in Methods. There is loss of the light-period peak of LC3-II and Atg5-Atg12 complex formation in Group 1 animals, with the ratio at 10 PM being significantly higher than that at 1 PM (P < 0.05, n = 8 animals per time point). Note that the dark-period peak occurs earlier in the Gnat1−/− mice as compared to wild-type controls. There is no loss of the dark-period peak, either during regular 12-hour light/12-hour dark cycle (A) or in Group 2 animals shifted from light back to dark at 9 AM, or after 3 hours in the light (B). (D–F) Western blots and quantification of LC3-I to LC3-II ratios in Group 1, Group 2, and Group 3 Sag−/− mice, respectively. There is loss of the dark-period peak of LC3-II and Atg5-Atg12 complex formation in Group 1 animals, with preservation of the light-period peak (P < 0.05, n = 8 animals per time point). Group 2 animals shifted from light back to dark at 9 AM, after 3 hours in the light, show a decrease in LC3-II levels, but no time-dependent regeneration of this isoform (E). Maintaining the animals in the dark resulted in a gradual decrease in LC3-II levels.
Our hypothesis predicted that the translocation of arrestin from the outer to the inner segment, which occurs when the animals are moved from the light to the dark, would stimulate autophagy. Thus, the absence of arrestin would be predicted to result in the loss of the dark-period peak of LC3-II activation. Analysis of arrestin knockout mice (Sag−/− mice) confirms the loss of the dark-period peak (Fig. 5D). Arrestin-deficient mice that are dark-adapted earlier than usual, Group 2 animals, show the expected drop in LC3-II levels, but then fail to display the expected time-dependent recovery of LC3-II in the dark (Fig. 5E). Maintaining these animals in the dark, Group 3 animals, shows a gradual time-dependent loss of the LC3-II signal (Fig. 5F).
Autophagy Activation in the RPE in Response to Outer Segment Phagocytosis
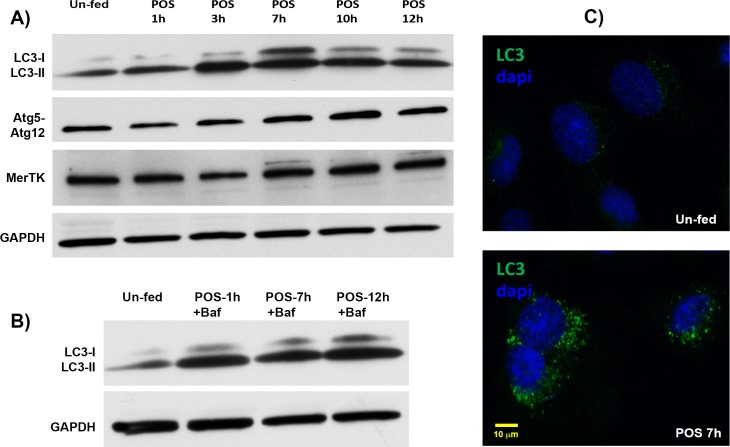
The circadian nature of autophagy activation in the RPE suggested that this was in response, at least in part, to the shedding of POS. To test this hypothesis, cultured RPE-J cells were fed bovine POS and the levels of autophagy activation were measured. We found that there was a time-dependent increase in the amount of LC3-II, as well as increased levels of Atg5-12 conjugate (Fig. 6A). Treatment with an inhibitor of autophagy, bafilomycin-A, prevented the decrease in LC3 levels seen at 12 hours postfeeding (Fig. 6B), consistent with the presence of increased autophagic flux.28 The increase in autophagy was preceded by a transient decrease in levels of MerTK, the receptor required for phagocytic uptake of shed POS by the RPE.19,27 Fed RPE-J cells exhibited a POS-dependent increase in punctate LC3 staining pattern (Fig. 6C).
Figure 6.
Autophagy activation in cultured RPE-J cells is increased by ingestion of POS. (A, B) Western blots of LC3 with quantification of LC3-I to LC3–II ratios, as well as Atg5-Atg12 complex formation, as a function of time after feeding cultured RPE-J cells POS. Coincubating with Bafilomycin-A, an inhibitor of autophagosome-lysosome fusion, showed that the decrease in LC3 levels after 7 hours was due to autophagy flux (i.e., catabolism of the LC3 protein by the autophagosome) and not a true decrease in autophagy. The Western blot for the Mer tyrosine kinase (MerTK) shows an early decrease consistent with phagocytic uptake of the POS. (C) Immunostaining of RPE-J cells before and after exposure to POS shows the shift in LC3 staining from a diffuse to punctate pattern, consistent with an increase in autophagosome formation.
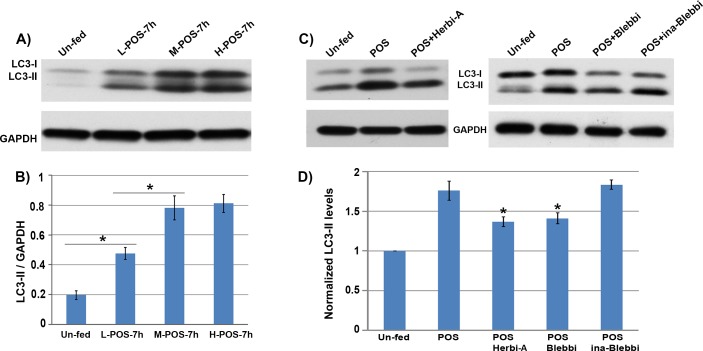
The increase in LC3-II formation was dependent on the amount of POS fed to the cells (Figs. 7A, 7B). To confirm the relationship between POS ingestion and LC3-II formation, we used two different inhibitors of phagocytosis: herbimycin A33 and blebbistatin.34 Treatment of RPE-J cells with either of these two agents reduced the amount of LC3-II formed (Figs. 7C, 7D).
Figure 7.
(A) Western blot of lysates from RPE-J cells exposed to various amounts of POS. L-, M-, and H- correspond to 2, 10, or 50 outer segments per RPE cell, respectively. (B) Quantification of the LC3-II levels based on the densitometry of the Western blots. The level of LC3-II appears to peak with the medium dose of 10 outer segments per RPE cell (P < 0.05). (C) Western blot of lysates from RPE-J cells fed POS with or without the addition of the phagocytosis inhibitors herbimycin-A (Herbi-A) or blebbistatin (Blebbi). As a control, an inactive form of blebbistatin (Ina-Blebbi) was used, and found to have no effect on LC3-II formation. (D) Quantification of the LC3-II levels based on the densitometry of the Western blots. The levels of LC3-II in the herbimycin-A and blebbistatin treated cells were significantly lower than control-treated cells (P < 0.05).
Discussion
The present study demonstrated the dynamic nature of autophagy activation, as measured by conversion of LC3-I to LC3-II, in the mouse retina. Our findings were consistent with previous studies showing a peak of autophagosome formation during the light period in rat rod photoreceptors.12 We now showed that a second peak of increased LC3-I to LC3-II conversion occurs during the dark period. In addition, we found that double-membrane structures are present in the photoreceptors at time points corresponding to peak LC3-II levels, supporting the interpretation that autophagy is occurring in these cells.
We found that fluctuations in the activation of autophagy in the retina are not entrained as a circadian rhythm, but are rapidly responsive to alterations in the light environment. Previous studies by Reme et al.13 found that autophagy in rat photoreceptors could be evoked by light pulses, and they attributed this phenomenon as being related to disc shedding. Although disc shedding may have a role in regulating autophagy, our results suggested that light-induced changes are a significant factor in this phenomenon. We found that when the 12-hour light/12-hour dark cycle is perturbed, the shift between light and dark evokes a sharp decrease, followed by a subsequent time-dependent increase, in LC3-I to LC3-II conversion in the retina. Shedding of rod photoreceptor discs follows a circadian rhythm that is not altered easily by acute changes in light condition.35 If disc shedding were the primary factor controlling autophagy levels in the photoreceptors, then we would expect the LC3-II levels to shift even when the animals were kept in the dark. Our finding of rapid increases in LC3-II levels when moving a light-adapted animal to the dark suggested an autophagy regulatory trigger other than disc shedding.
Several potential regulators of autophagy in photoreceptor cells have been suggested. Previous studies have shown that activation of autophagy is associated with lipofuscin accumulation and the degradation of opsin or rhodopsin.36,37 Loss of autophagy has been shown to increase susceptibility to light-induced photoreceptor degeneration, perhaps by increasing the sensitivity of cells to reactive oxygen species, such as all-trans retinal.38,39 In photoreceptors, light exposure results in the large-scale translocation of signal transduction proteins into and out of the inner and outer segments, with the presumed function of maintaining photoreceptor sensitivity in the face of changing ambient light levels.31 In other systems, autophagy has been shown to respond to shifts in intracellular protein concentrations.40 Our data, including that from analyses of Gnat1−/− and Sag−/− mice, suggested that the translocation of transducin and arrestin into the inner segment contributes to the light-period and dark-period peaks in autophagy, respectively. This explanation is consistent with the observation that autophagy levels changed after the shift from dark to light, rather than as an entrained circadian rhythm.
The translocation of arrestin and transducin from the inner to the outer segment occurs rapidly, with half-time to completion of approximately 5 minutes. This is much more rapid than the translocation of these proteins from the outer to the inner segment, a process that has a half-time of more than 1 hour for arrestin and 2.5 hours for transducin.41,42 This differential speed of translocation, depending on direction of protein movement, could help explain the rapid drop in autophagy levels upon shifting from dark to light, which would result in a rapid decrease of arrestin in the inner segments and a rapid drop in LC3-II levels. This then would be followed by the slower influx of the transducin into the inner segments and the more gradual re-elevation of LC3-II levels.
Our data also showed that the peak of autophagy in Gnat1−/− mice occurring during the dark-period is earlier than that of wild-type mice. A potential explanation for this could be a more rapid translocation of arrestin in Gnat1−/− mice, since in wild-type photoreceptors, arrestin and transducin are translocated, albeit in different directions, across the connecting cilium. In Gnat1−/− mice, arrestin does not have to compete with transducin, and, thus, may traverse the space more quickly. In wild-type mice, translocation of arrestin to the inner segment occurs only when the light reaches a specific threshold value.42 In Gnat1−/− mice, no threshold light level is required, and translocation can occur sooner and at lower light intensity.43 In the Sag−/− mice, the kinetics of the light-period peak were not shifted, presumably because the kinetics of transducin translocation, which is known to be slower than that of arrestin,41,42 was not necessarily accelerated by the absence of arrestin. The synthesis of transducin and arrestin is under circadian regulation, with peak production of the two proteins occurring just before lights turn on and off, respectively. In other words, there is a morning increase of transducin in the inner segments followed by an evening increase in arrestin.44–47 The fact that we did not see changes in autophagy levels when mice were kept in the dark suggested that the activation of autophagy is driven more by the translocation of protein from the outer segments into the inner segments than by de novo protein synthesis. The temporal separation of biosynthesis, which occurs within a small window of time, from the timing of translocation, further supports the conclusion that the movement of transduction proteins into and out of the inner segment contributes to the dynamic regulation of autophagy levels within the photoreceptor.
Autophagy has been known to occur in the RPE for many years,13 but the full time course of its activation over a 24-hour period has not been described previously to our knowledge. The function of autophagy in the RPE is not well understood; however, our data, and those of others,48 show that phagocytosis of shed photoreceptor outer segments is an important trigger for autophagy activation. It was shown recently that RPE-specific knockout of Atg5, an upstream activator of LC3-I to LC3-II conversion, results in decreased visual cycle function that was restored upon administration of exogenous chromophore.48 Based on their findings, the investigators suggest that autophagy functions to help in the processing of the ingested POS and redistribution of the digested components.
In the study of the RPE-specific knockout of the Atg5 gene, the investigators state that they did not find any double-membrane vesicles in the RPE of wild-type mice or in cultured RPE-J cells that were fed POS.48 It also was noted that only some of the autophagy-related machinery was required for LC3 localization to POS-containing phagosomes. These results were interpreted as evidence that the ingestion of POS triggered “noncanonical” autophagy, or LC3-associated phagocytosis (LAP).48,49 Our results showed that double membrane vesicles are present within the RPE of wild-type mice, especially at times with correspondingly high levels of LC3-II. We concluded that classic autophagy occurs in the RPE, and that it is triggered, at least in part, by the ingestion of shed outer segments. The time course of LC3-II formation was delayed when compared to the peak of photoreceptor shedding and phagocytosis.35 Though the reasons for this offset are not clear, the finding is consistent with the hypothesis that autophagy has a role in postphagocytosis processing of shed outer segments and reestablishment of RPE homeostasis, rather than with the early events related to phagocytic uptake. While our data did not exclude the presence of LAP in the RPE, we suspect that numerous aspects of autophagy function in the RPE remain to be elucidated as part of future studies.
Given the dynamic nature of autophagy activation in the outer retina, our data suggested that the effect an experimental condition must be assessed by the way in which it changes the bimodal peak of autophagy levels as well as the timing of the peaks. If autophagy levels are measured only at one time point, then one risks misinterpreting lower levels of autophagy markers as reduced autophagy, when the experimental condition could simply be altering the dynamics of autophagy activation—with some time points exhibiting higher and some time points exhibiting lower levels of autophagy. One risks missing this finding and its potential functional implications if autophagy levels are not evaluated at multiple time points.
In summary, basal levels of autophagy within the outer retina change in a dynamic nature during the course of the day and night. Shifts in levels of inner segment protein and uptake of shed outer segments appear to be triggers regulating the level of autophagy in the photoreceptors and RPE, respectively. Our data suggested that autophagy may be critical in maintaining normal outer retina homeostasis and photoreceptor cell function, with further study needed to define the molecular mechanisms underlying the dynamic regulation of autophagy in these cells.
Acknowledgments
The authors thank Jonathan Demb, Yale University (New Haven, CT, USA) for providing the Gnat−/− mouse.
Supported by the National Eye Institute R01-EY-020823 (DNZ), Foundation Fighting Blindness (DNZ, DAT), Research to Prevent Blindness, Inc. (DNZ; DAT is a Research to Prevent Blindness Senior Scientific Investigator), and University of Michigan Core Center for Vision Research (NEI-EY007003).
Disclosure: J. Yao, None; L. Jia, None; S.J. Shelby, None; A.M. Ganios, None; K. Feathers, None; D.A. Thompson, None; D.N. Zacks, None
References
- 1. Yang Z, Klionsky DJ. Mammalian autophagy: core molecular machinery and signaling regulation. Curr Opin Cell Biol. 2010; 22: 124–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mizushima N. The pleiotropic role of autophagy: from protein metabolism to bactericide. Cell Death Differ. 2005; 12: 1535–1541 [DOI] [PubMed] [Google Scholar]
- 3. Yoshimori T. Autophagy: a regulated bulk degradation process inside cells. Biochem Biophys Res Commun. 2004; 313: 453–458 [DOI] [PubMed] [Google Scholar]
- 4. Kuma A, Hatano M, Matsui M, et al. The role of autophagy during the early neonatal starvation period. Nature. 2004; 432: 1032–1036 [DOI] [PubMed] [Google Scholar]
- 5. Komatsu M, Waguri S, Ueno T, et al. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol. 2005; 169: 425–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boya P, González-Polo RA, Casares N, et al. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol. 2005; 25: 1025–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ravikumar B, Sarkar S, Davies JE, et al. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev. 2010; 90: 1383–1435 [DOI] [PubMed] [Google Scholar]
- 8. Kunchithapautham K, Rohrer B. Apoptosis and autophagy in photoreceptors exposed to oxidative stress. Autophagy. 2007; 3: 433–441 [DOI] [PubMed] [Google Scholar]
- 9. Kunchithapautham K, Rohrer B. Autophagy is one of the multiple mechanisms active in photoreceptor degeneration. Autophagy. 2007; 3: 65–66 [DOI] [PubMed] [Google Scholar]
- 10. Punzo C, Kornacker K, Cepko CL. Stimulation of the insulin/mTOR pathway delays cone death in a mouse model of retinitis pigmentosa. Nat Neurosci. 2009; 12: 44–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cagri CG, Chinskey ND, Zheng QD, Zacks DN. Autophagy activation in the injured photoreceptor inhibits fas-mediated apoptosis. Invest Ophthalmol Vis Sci. 2011; 52: 4193–4199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reme CE, Sulser M. Diurnal variation of autophagy in rod visual cells in the rat. Graefes Arch Clin Exp Ophthalmol. 1977; 203: 261–270 [DOI] [PubMed] [Google Scholar]
- 13. Reme CE, Aeberhard B, Schoch M. Circadian rhythms of autophagy and light responses of autophagy and disc shedding in the rat retina. J Comp Physiol A. 1985; 155: 669–678 [Google Scholar]
- 14. Kaarniranta K, Sinha D, Blasiak J, et al. Autophagy and heterophay dysregulation leads to retinal pigment epithelium dysfunction and development of age-related macular degeneration. Autophagy. 2013; 9: 973–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cho KS, Yoon YH, Choi JA, Lee SJ, Koh JY. Induction of autophagy and cell death by tamoxifen in cultured retinal pigment epithelial and photoreceptor cells. Invest Ophthalmol Vis Sci. 2012; 53: 5344–5353 [DOI] [PubMed] [Google Scholar]
- 16. Wang AL, Lukas TJ, Yuan M, Du N, Tso MO, Neufeld AH. Autophagy and exosomes in the aged retinal pigment epithelium: possible relevance to drusen formation and age-related macular degeneration. PLoS One. 2009; 4: e4160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Linsenmeier RA, Padnick-Silver L. Metabolic dependence of photoreceptor on the choroid in the normal and detached retina. Invest Ophthalmol Vis Sci. 2000; 41: 3117–3123 [PubMed] [Google Scholar]
- 18. Wangsa-Wirawan ND, Linsenmeier RA. Retinal oxygen: fundamental and clinical aspects. Arch Ophthalmol. 2003; 121: 547–557 [DOI] [PubMed] [Google Scholar]
- 19. Kevany BM, Palczewski K. Phagocytosis of retinal rod and cone photoreceptors. Physiology (Bethesda). 2010; 25: 8–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reme CE. Autophagy in visual cells and pigment epithelium. Invest Ophthalmol Vis Sci. 1977; 16: 807–814 [PubMed] [Google Scholar]
- 21. Krohne TU, Stratmann NK, Kopitz J, et al. Effects of lipid peroxidation products on lipofuscinogenesis and autophagy in human retinal pigment epithelial cells. Exp Eye Res. 2010; 90: 465–471 [DOI] [PubMed] [Google Scholar]
- 22. Viiri J, Hyttinen JM, Ryhanen T, et al. p62/sequestosome 1 as a regulator of proteasome inhibitor-induced autophagy in human retinal pigment epithelial cells. Mol Vis. 2010; 16: 1399–1314 [PMC free article] [PubMed] [Google Scholar]
- 23. Calvert PD, Krasnoperova NV, Lyubarsky AL, et al. Phototransduction in transgenic mice after targeted deletion of the rod transducin α-subunit. Proc Nat Acad Sci U S A. 2000; 97: 13913–13918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mizushima N, Kuma A. Autophagosomes in GFP-LC3 transgenic mice. Meth Mol Biol. 2008; 445: 119–124 [DOI] [PubMed] [Google Scholar]
- 25. Mattapallil MJ, Wawrousek EF, Chan CC, et al. The Rd8 mutation of the Crb1 gene is present in vendor lines of C57BL/6N mice and embryonic stem cells, and confounds ocular induced mutant phenotypes. Invest Ophthalmol Vis Sci. 2012; 53: 2921–2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Papermaster DS. Preparation of retinal rod outer segments. Methods Enzymol. 1982; 81: 48–52 [DOI] [PubMed] [Google Scholar]
- 27. Shelby SJ, Colwill K, Dhe-Paganon S, Pawson T, Thompson DA. MERTK interactions with SH2-domain proteins in the retinal pigment epithelium. PLoS One. 2013; 82: e53964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Klionsky DJ, Abdalla FC, Abeliovich H, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012; 8: 445–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brann MR, Cohen LV. Diurnal expression of transducin mRNA and translocation of transducin in rods of rat retina. Science. 1987; 235: 585–587 [DOI] [PubMed] [Google Scholar]
- 30. Broekhuyse RM, Tolhuizen EF, Janssen AP, Winkens HJ. Light induced shift and binding of S-antigen in retinal rods. Curr Eye Res. 1985; 4: 613–618 [DOI] [PubMed] [Google Scholar]
- 31. Calvert PD, Strissel KJ, Schiesser WE, Pugh EN Jr, Arshavsky VY. Light-driven translocation of signaling proteins in vertebrate photoreceptors. Trends Cell Biol. 2006; 16: 560–568 [DOI] [PubMed] [Google Scholar]
- 32. Mendez A, Lem J, Simon M, Chen J. Light-dependent translocation of arrestin in the absence of rhodopsin phosphorylation and transducin signaling. J Neurosci. 2003; 23: 3124–3129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Karl MO, Kroeger W, Wimmers S, et al. Endogenous Gas6 and Ca2+ channel activation modulates phagocytosis by retinal pigment epithelium. Cell Signal. 2008; 20: 1159–1168 [DOI] [PubMed] [Google Scholar]
- 34. Strick DJ, Feng W, Vollrath D. MerTK drives myosin II redistribution during retinal pigment epithelial phagocytosis. Invest Ophthalmol Vis Sci. 2009; 50: 2427–2435 [DOI] [PubMed] [Google Scholar]
- 35. LaVial MM. Rod outer segment disk shedding in rat retina: relationship to cyclic lighting. Science. 1976; 194: 1071–1074 [DOI] [PubMed] [Google Scholar]
- 36. Iwasaki M, Inomata H. Lipofuscin granules in human photoreceptor cells. Invest Ophthalmol Vis Sci. 1988; 29: 671–679 [PubMed] [Google Scholar]
- 37. Reme CE, Wolfrum U, Imsand C, Hafezi F, Williams TP. Photoreceptor autophagy: effects of light history on number and opsin content of degradative vacuoles. Invest Ophthalmol Vis Sci. 1999; 40: 2398–2404 [PubMed] [Google Scholar]
- 38. Chen Y, Sawada O, Kohno H, et al. Autophagy protects the retina from light-induced degeneration. J Biol Chem. 2013; 288: 7506–7518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rodriguez-Muela N, Koga H, Garcia-Ledo L, et al. Balance between autophagy pathways preserves retinal homeostasis. Aging Cell. 2013; 12: 478–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Boya P, Reggiori F, Codogno P. Emerging regulation and functions of autophagy. Nature Cell Biol. 2013; 15: 713–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sokolov M, Lyubarsky AL, Strissel KJ, et al. Massive light-driven translocation of transducin between the two major components of rod cells: a novel mechanism of light adaptation. Neuron. 2002; 33: 95–106 [DOI] [PubMed] [Google Scholar]
- 42. Strissel KJ, Sokolov M, Trieu LH, Arshavsky VY. Arrestin translocation is induced at a critical threshold of visual signaling and is superstoichiometric to bleached rhodopsin. J Neurosci. 2006; 26: 1146–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang H, Huang W, Zhang H, et al. Light-dependent redistribution of visual arrestin and transducin subunits in mice with defective phototransduction. Mol Vis. 2003; 9: 231–237 [PubMed] [Google Scholar]
- 44. Bowes C, van Veen T, Farber DB. Opsin, G-protein and 48-kDa protein in normal and rd mouse retinas: developmental expression of mRNAs and proteins and light/dark cycling of mRNAs. Exp Eye Res. 1988; 47: 369–390 [DOI] [PubMed] [Google Scholar]
- 45. Craft CM, Whitmore DH, Donoso LA. Differential expression of mRNA and protein encoding retinal and pineal S-antigen during the light/dark cycle. J Neurochem. 1990; 55: 1461–1473 [DOI] [PubMed] [Google Scholar]
- 46. McGinnis JF, Whelan JP, Donoso LA. Transient, cyclic changes in mouse visual cell gene products during the light-dark cycle. J Neurosci Res. 1992; 31: 584–590 [DOI] [PubMed] [Google Scholar]
- 47. Agarwal N, Nir I, Papermaster DS. Loss of diurnal arrestin gene expression in rds mutant mouse retinas. Exp Eye Res. 1994; 58: 1–8 [DOI] [PubMed] [Google Scholar]
- 48. Kim JY, Zhao H, Martinez J, et al. Noncanonical autophagy promotes the visual cycle. Cell. 2013; 154: 365–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sanjuan MA, Dillon CP, Tait SW, et al. Toll-like receptor signaling inmacrophages links the autophagy pathway to phagocytosis. Nature. 2007; 450: 1253–1257 [DOI] [PubMed] [Google Scholar]