Abstract
Introduction:
Dermatophytosis is defined as the fungal infection of the skin, hair and nails by a group of keratinophillic fungi known as dermatophytes.
Aims and Objectives:
This study is an attempt to find out various species of dermatophytes in clinically suspected cases of dermatophytosis.
Materials and Methods:
One hundred samples were subjected to direct microscopy by potassium hydroxide wet mount (KOH) and isolation on culture with Sabourauds dextrose agar.
Results:
Out of these 80 (80%) samples were KOH positive while 20 (20%) were KOH negative. Overall culture positivity rate was 68%. Dermatophytosis was more common in males, the M:F ratio was 4:1.
Conclusion:
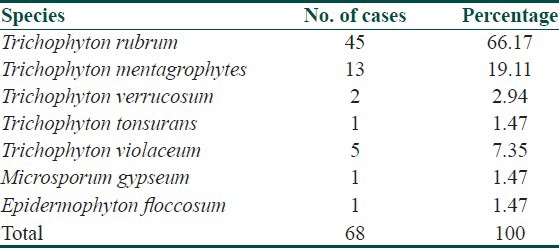
Total seven species were isolated on culture. Trichophyton rubrum (66.17%) was the commonest isolate followed by Trichophyton mentagrophytes (19.11%), Trichophyton violaceum (7.35%), Trichophyton tonsurans (2.94%) and one isolate each of Epidermophyton floccosum and Microsporum gypseum (1.47%).
Keywords: Dermatophytosis, Epidermophyton, Microsporum, Trichophyton
Introduction
What was known?
Dermatophytes are responsible for a variety of clinical manifestations in man, Trichophyton rubrum being the commonest isolate worldwide.
Fungal infections are very common in man. They are assuming greater significance both in developed and developing countries due to illnesses like diabetes, HIV infection and the use of immunosuppressive drugs. Overall ringworm infections account for 10% of the total cases who attend the skin outpatient department.[1] Dermatophytosis has a wide geographical distribution no part being completely free from it. Some species have a cosmopolitan distribution whereas others are geographically restricted. Five to six species are prevalent globally, Trichophyton rubrum being the commonest.[2] The Indian subcontinent has a varied topography and the hot and humid climate which is more conducive to the acquisition of fungal infections. The nature of dermatophytosis may change with the passage of time, living conditions and evolution of preventive measures and hygienic conditions in society. These variations inspired us to study the mycological pattern of dermatophytosis in this region.
Materials and Methods
The study was conducted in the department of Microbiology and Dermatovenerology at IGMC Shimla over a period of one year from May 2008 to April 2009. The study group comprised of hundred clinically suspected cases of dermatophytosis. A detailed clinical history including age, sex, duration and the clinical presentation were recorded. As per the involvement of the anatomical site they were grouped into various clinical types. Various samples i.e. skin, nails and hair were collected in sterile petri dishes. The samples were divided into two parts. Direct microscopy using 10% potassium hydroxide (KOH) for skin scrapings and epilated hairs and 40% KOH for nail specimens was done. The KOH wet mounts were screened under low power (×10) and then at high power (×40) for visualization of the fungal hyphae. Septate hyphae with or without branching and a diameter of 2-4 μm, or hyphae with arthroconidia were taken as a criterion to label the slide as KOH positive. The second part of the sample was inoculated on two sets of Sabourauds dextrose agar containing chloramphenicol (0.05 mg/ml) and cyclohexamide (0.1-0.4 mg/ml) and incubated at 25 and 37°C. The cultures were examined twice a week and if no growth was obtained till 4 weeks they were declared negative. The culture isolates were further identified by studying the colony morphology and microscopic examination of the lactophenol cotton blue mounts. Further special tests like hair perforation, urease production and slide culture were performed wherever necessary according to standard techniques.[3]
Results
Peak incidence of dermatophytosis was seen in the third decade of life (28%). Males were commonly affected than females and the M:F ratio was 4:1. Out of 100 cases 77 (77%) presented with a single site involvement while 23 (23%) had affliction of two or more anatomical sites. Tinea corporis was the commonest clinical presentation (27.7%) followed by tinea cruris (22%). However tinea unguium was seen in 19.48% and tinea pedis in 10.38% cases only. Association of tinea corporis with other clinical types was present in 12 out of 23 (52.17%) cases. Combination of tinea unguium with tinea pedis and tinea mannum was seen in 11 (47.8%) and 4 (17.3%) out of 23 cases respectively. On KOH wet mount examination 80 out of 100 (80%) clinically suspected cases were KOH positive. Out of these only 57 samples was culture positive. There were 11 KOH negative samples that yielded growth on culture. Overall, 68% samples were culture positive,taking into consideration both KOH positive and negative specimens [Table 1].
Table 1.
Correlation between clinical type, KOH and Culture
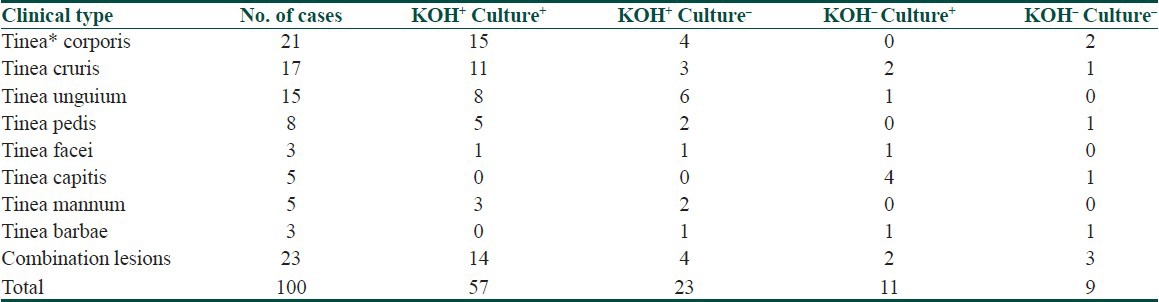
Culture positivity was highest in tinea corporis (80.95%) and lowest in cases of tinea barbae (33.33%) as depicted in Table 2. Trichophyton was the most common genus in 66 out of 68 (97.05%) culture isolates. There was one isolate each of genus Epidermophyton and Microsporum. Total seven species of dermatophytes were isolated on culture (Figs. Plate). Trichophyton rubrum was the commonest species (66.17%) followed by Trichophyton mentagrophytes (19.11%), Trichophyton violaceum (7.35%), Trichophyton verrucosum (2.94%). There was one isolate each of Trichopyton tonsurans (1.47%) and Microporum gypseum respectively [Table 3].
Table 2.
Correlation of clinical type and total culture positivity
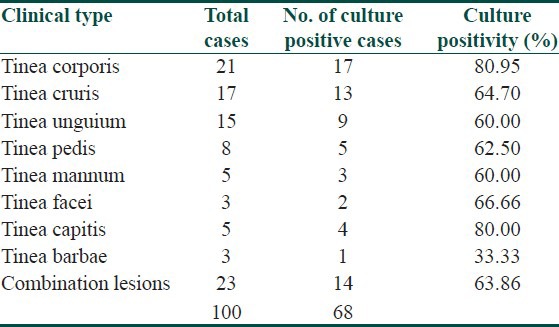
Table 3.
Isolation rate of various species
Discussion
Maximum number of cases were seen in the age group of 21-30 years (28%), followed by 31-40 years (24%) and males were affected more than females. Similar peak in this age group has been observed by various workers.[4,5] It can be attributed to the fact that the males may be more prone because of prolonged vigorous outdoor activity and increased perspiration which creates an environment for the development of tinea infection. In the present study tinea corporis was the commonest (27.27%) clinical entity. This pattern of clinical preponderance has been observed in other studies.[6,7] On the contrary reports from Manipal and Aurangabad show 77 and 40% cases respectively.[8,9] Perhaps these areas are hot and humid as compared to our region which has a temperate climate which accounts for the difference. Tinea corporis was the second commonest clinical manifestation. Some reports quote similar rates however others have reported a larger proportion of cases with this clinical type.[10,11] This could be attributed to the difference in the personal hygiene and habits of the people. Tinea unguium constituted 19.48% cases. Similar findings have been observed in previous studies conducted in North India.[6,12]
On KOH wet mount examination positivity rates ranging from 23.8% to as high as 91.2% have been reported.[9,13] In our study 80% samples were KOH positive. Selection criteria of cases and skill involved in sampling techniques perhaps accounts for the difference. Thus all KOH negative samples should be cultured. In a study conducted in Ludhiana 13.2% of KOH negative samples exhibited growth on culture which coincides with our observations.[14] Non visualization of hyphae on direct microscopy could possibly be due to a severe inflammatory reaction which obscures them.[15] This finding elucidates the importance of culture to establish diagnosis. The culture positive rate in our study was 68%. A variability in culture isolation ranging from 44.6% to70.7% has been found in the Indian sub-continent.[10,16] In our study Trichophyton rubrum was the predominant species which concurs with other reports.[17] This anthropophillic species has become well adapted to the human skin as climatic and crowded living conditions enhance its prevalence. The second commonest species was Trichophyton mentagrophytes. Trichophyton violaceum was isolated from cases of tinea capitis in children in the prepubertal age group. This species accounted for 7.35% of total culture positive cases. However in a study conducted in Hyderabad higher isolation rates upto 14.68% have been observed.[18] This could be attributed to the predominant muslim population who wear caps and thus are more prone to the acquisition of superficial fungal infections. One isolate each of Trichophyton verrucosum and Microsporum gypseum were obtained from cases of tinea facei. Trichophyton verrucosum is a zoophillic dermatophyte whereas Microsporum gypseum is geophillic. Both these species were isolated from children with a rural background so a probable transmission of infection could have occurred from above sources.[19] Isolation of Microsporum gypseum from one case is of special interest in the present study as this genus is generally less commonly encountered in India. One isolate each of Trichophyton violaceum and Trichopyton tonsurans were recovered from nail specimens on culture. Similar association of ringworm of the nails has been observed.[20] The present study has given us a clear insight regarding the mycological pattern of dermatophytosis in this region. The overall findings suggest that the study is comparable to those from different parts of India.
What is new?
Rare dermatophyte species are emerging rapidly, mimicking other clinical diseases. Their evaluation is critical to establish the diagnosis.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Mohapatra LN. A study of medical mycology in India: An overview. Indian J Med Res. 1989;89:351–2. [PubMed] [Google Scholar]
- 2.Aly R. Ecology and epidemiology of dermatophyte infections. Med Mycol. 2000;38(Suppl 1):183–8. [PubMed] [Google Scholar]
- 3.Roberts GD. Laboratory methods in basic mycology. In: Ellen Baron JO, Peterson LR, Finegold SM., editors. Bailey and Scott's Diagnostic Microbiology. 9th ed. St. Louis: CV Mosby Company, Ltd; 1990. pp. 715–24. [Google Scholar]
- 4.Rani U, Saigal RK, Kanta S, Krishan R. Study of dermatophytosis in Punjabi population. Indian J Pathol Microbiol. 1983;26:243–7. [PubMed] [Google Scholar]
- 5.Mohanty JC, Mohanty SK, Sahoo RC, Sahoo A, Praharaj CN. Incidence of dermatophytosis in Orissa. Indian J Med Microbiol. 1988;76:78. [Google Scholar]
- 6.Sharma NL, Gupta ML, Sharma RC, Singh P, Gupta N. Superficial mycoses in Shimla. Indian J Dermatol Venerol Leprol. 1983;49:266–9. [PubMed] [Google Scholar]
- 7.Huda MM, Chakarborty N, Sharma JN. A clinico-mycological study of superficial mycoses in upper Assam. Indian J Dermatol Venerol Leprol. 1995;61:329–32. [PubMed] [Google Scholar]
- 8.Patwardhan N, Dave R. Dermatomycosis in and around Aurangabad. Indian J Pathol Microbiol. 1999;42:455–62. [PubMed] [Google Scholar]
- 9.Stephen S, Rao KN. Superficial mycoses in Manipal. Indian J Dermatol Venereol Leprol. 1975;41:106–10. [Google Scholar]
- 10.Singh S, Beena PM. Profile of dermatophyte infections in Baroda. Indian J Dermatol Venereol Leprol. 2003;69:281–3. [PubMed] [Google Scholar]
- 11.Khare KA, Singh G, Pandey SS, Sharma BM, Kaur P. Pattern of dermatophytosis in and around Varanasi. Indian J Dermatol Venereol Leprol. 1985;51:328–31. [PubMed] [Google Scholar]
- 12.Mohan U, Jindal N, Devi P. Dermatophytosis in Amritsar. Indian J Med Microbiol. 1997;15:46. [Google Scholar]
- 13.Nath P, Aggarwal PK. Clinical manifestations in dermatophytosis. Indian J Med Res. 1972;60:1148–55. [PubMed] [Google Scholar]
- 14.Gupta BK, Kumar S, Kumar R, Khurana S. Mycological aspects of dermatophytosis in Ludhiana. Indian J Pathol Microbiol. 1993;36:233. [PubMed] [Google Scholar]
- 15.Rook, Wilkinson, Ebling . Superficial and cutaneous mycoses. In: Champion RH, Barton JL, Burns DA, Breatnack SM, editors. A Text Book of Dermatology. 6th ed. Vol. 2. 1998. pp. 1301–14. [Google Scholar]
- 16.Rangnathan S, Menon S, Selvi S, Kamalam A. Effect of socioeconomic status on the prevalence of dermatophytosis in Madras. Indian J Dermatol Venereol Leprol. 1995;61:16–8. [PubMed] [Google Scholar]
- 17.Sen SS, Rasul ES. Dermatophytosis in Assam. Indian J Med Microbiol. 2006;24:76–7. doi: 10.4103/0255-0857.19907. [DOI] [PubMed] [Google Scholar]
- 18.Naghabhushnam P, Tirumalrao D, Patnaik R. Tineacapitis in Hyderabad area. Indian J Dermatol Venereol Leprol. 1972;38:56. [Google Scholar]
- 19.Rippon JW, editor. Medical Mycology. The pathogenic fungi and the actinomycetes. 3rd ed. Philadelphia: WB Saunders; 1988. Cutaneous Infections. Dermatophytosis and dermatomycosis; p. 194. [Google Scholar]
- 20.Singh UK, Nath P. Fungal flora in the superficial infections of the skin and nails in Lucknow. Indian J Pathol Microbiol. 1977;20:189–93. [PubMed] [Google Scholar]


