Abstract
Background:
Isotretinoin is indicated for moderate to severe cases of acne which are unresponsive to conventional therapy. The classical recommended dose is 0.5 to 1.0 mg/kg/day. As the side effects are dose related, low-dose isotretinoin therapy for acne is an attractive option; however, but little data exists on the safety and efficacy of this strategy.
Materials and Methods:
In this prospective, non-comparative study, 50 participants, both male and female, having moderate to severe acne vulgaris were enrolled and treated with isotretinoin at a dose of 20 mg/day (approximately 0.3-0.4 mg/kg/day), for a period of 3 months. Participants were evaluated by means of clinical and laboratory investigations before starting isotretinoin. Investigations were repeated at the end of the first and third months following completion of treatment, and participants were followed up for 6 months to look for any relapse.
Results:
At the end of the treatment, very good results were observed in 90% of participants. Cheilitis was the most common among the side effects observed and was seen in 98% of the participants. One participant developed vitiligo as a side effect, which is a new finding, and has not reported in literature before. Elevated serum lipid levels were observed in 6% of the participants, and relapse occurred in 4% of the participants over a 6 month follow up period.
Conclusion:
Three months of treatment with low-dose isotretinoin (20 mg/day) was found to be effective in the treatment of moderate to severe acne vulgaris, with a low incidence of serious side effects. This dose also was more economical than the higher doses.
Keywords: Acne, low-dose isotretinoin, safety and efficacy
Introduction
What was known?
Isotretinoin is known to cause most of its side effects at a higher dose(0.5-1 mg/kg/day) which is the classical recommended dose. As the side effects are dose related, low-dose (0.3-0.4 mg/kg/day) of isotretinoin seems to be an attractive option, but little data exists on the safety and efficacy of this strategy.
Acne is a chronic inflammatory disease of the pilosebaceous units, and is primarily seen in adolescents. It can have a significant psychological and social impact. Most cases of acne present with a pleomorphic variety of lesions consisting of comedones, papules, pustules and nodules, and in some cases are accompanied by scarring.[1] Retinoids are a key component of anti-acne therapy. The introduction of isotretinoin, a first generation synthetic retinoid, for the treatment of patients with moderate to severe acne vulgaris is regarded as a major therapeutic advance in dermatology.[2]
Isotretinoin is indicated for severe acne and moderate cases of acne that are unresponsive to conventional therapy. The classical recommended dose is 0.5 to 1.0 mg/kg/day for 4 to 8 months. As the side effects are dose related, the idea of low-dose isotretinoin therapy for acne is attractive; however, little data exists on the safety and efficacy of this strategy in Indian participants.[3] We have undertaken this study to evaluate the safety and efficacy of low-dose isotretinoin in the treatment of moderate to severe acne vulgaris, and thus determine an effective modality of treatment with a low incidence of severe side effects, in a more cost effective manner.
Materials and Methods
Source of data
A total of 50 participants diagnosed as having moderate to severe acne vulgaris, and attending the dermatology Out Patient Department (OPD) at Fr. Muller Medical college Hospital, Mangalore, were studied. The participants were recruited over a period of two years, from September 2009 to September 2011, and were followed up for three months to know the safety and efficacy of low-dose isotretinoin in the treatment of moderate to severe acne vulgaris.
Methods of collection of data
In this prospective non-comparative study, 50 participants having moderate to severe acne vulgaris were selected after taking an informed written consent using purposive sampling technique. The participants’ diagnostic and clinical data including age, sex, site of acne, duration and severity of acne and indications for isotretinoin therapy was noted down. The initial assessment of acne was done using the global acne grading system (GAGS) [Table 1].[4] This system divides the face, chest and back into six areas (forehead, each cheek, nose, chin, chest and back) and assigns a factor to each area on the basis of size. Baseline investigations including complete blood cell counts, liver function tests, lipid profile and urine pregnancy test (for female participants of child bearing potential) were done before starting the participant on isotretinoin, and follow up investigations were carried out at the end of first and third month following completion of the treatment. Female participants were started on treatment only after their next menstrual period to ensure that they are not pregnant at the time of initiation of the treatment. The participants were also followed up for 6 months after completion of treatment to look for any relapse. The dose of isotretinoin used was 20 mg/day over a period of 3 months. The response to treatment was evaluated at end of 1 month and at the end of 3 months following completion of treatment and, was assessed on scales 0-4.
Table 1.
Global acne grading system
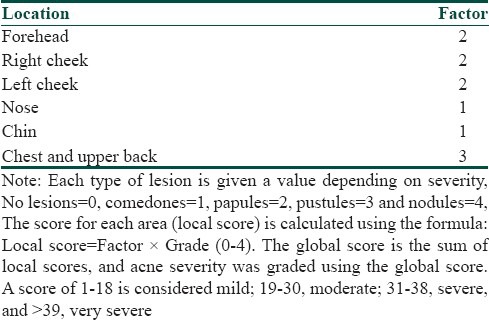
Scale definition
0 – completely cleared 100% lesion clearance
1 – marked improvement > 75% lesion clearance
2 – moderate improvement 50-75% lesion clearance
3 – mild improvement 25-50% lesion clearance
4 – insignificant improvement < 25% lesion clearance
The efficacy of the drug was assessed by lesion counts at each visit. The participants were also evaluated for safety and efficacy of the drug on each follow up visit.
Data analysis
Data was analyzed by the Friedman test and t-test and Statistical Package for Social Sciences (SPSS)-version 13 software was used to generate the analysis.
Inclusion criteria
Age above 12 years and of both the sexes
Participants having moderate to severe facial acne vulgaris
Participants willing to undergo treatment and follow ups
Exclusion criteria
Age less than 12 years
Participants having mild facial acne vulgaris
Pregnant women and women who intend to become pregnant during the course of treatment
Participants who intend to consume alcohol during the treatment course
Presence of any renal or hepatic compromise or any pre-existing hyperlipidemia
Participants not willing for the necessary investigations
Results
Out of the total number of 50 participants, 38 participants (76%) were males and 12 participants (24%) were females. There was an overall male preponderance, the male to female ratio being 3.1:1. Majority of the participants, 35 in number (70%), were in the age group of 21-30 years, the mean age calculated as 26.36 years (Standard Deviation, S.D-5.90). Nodulocystic acne was the most common indication for treatment and was seen in 22 participants (44%), followed by the reason of acne being resistant to treatment in 20 participants (40%), and frequent relapses in 8 participants (16%). On cutaneous assessment, it was found that at week 0, 6 participants (12%) had grade II acne, 28 participants (56%) had grade III acne and 16 participants (32%) had grade IV acne. At the end of 12 weeks, that is, on the completion of treatment, 3 participants (6%) had grade 0 acne, 45 participants (90%) had grade I acne and 2 participants (4%) had grade II acne. During the follow up after 6 months, it was found that 3 participants (6%) had grade 0 acne, 44 participants (88%) had grade I acne, 2 participants (4%) had grade II acne and 1 participant (2%) had grade III acne. Relapse was seen in 2 participants (4%); 1 participant falling in the range of grade I to grade II acne, and 2nd participant in the range of grade II to grade III acne [Table 2]. A significant reduction in the grade of acne was found at the end of treatment, and the reduction in the grade of acne was found to be statistically significant (Friedman Test, χ2 = 146.23, P < 0.0001, Highly Significant) [Table 3]. At the end of 12 weeks post treatment, 3 participants (6%) had 100% lesion clearance (scale 0), 45 participants (90%) had > 75% lesion clearance (scale 1) and 2 participants (4%) had 50-75% lesion clearance (scale 2) [Table 4] [Figures 1a,b and 2a,b].
Table 2.
Grade of acne in the patients in the study
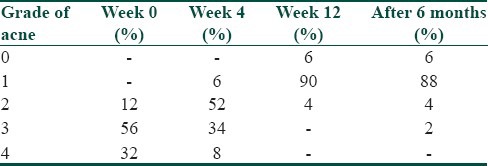
Table 3.
Results-grade of acne in the patients in the study at the end of treatment
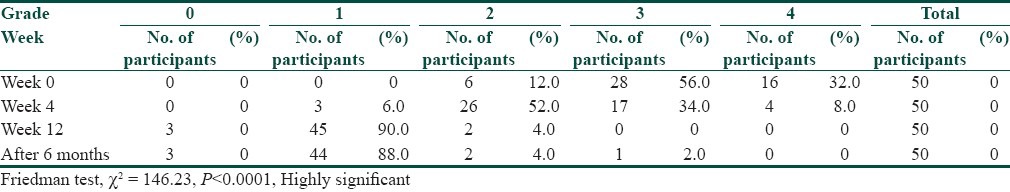
Table 4.
Post treatment-scale of acne in the patients in the study
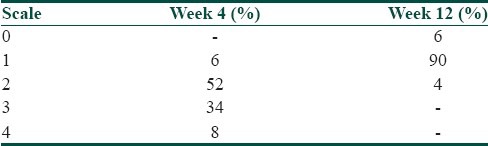
Figure 1.
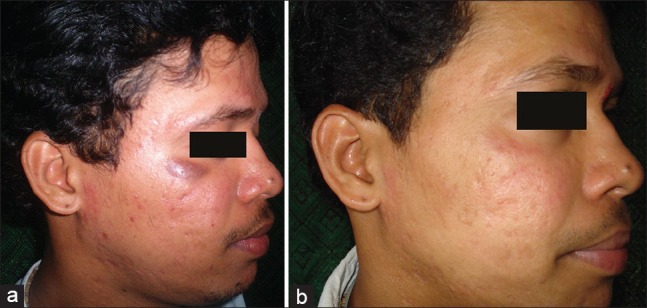
(a) Pretreatment photograph (b) Post treatment photograph
Figure 2.
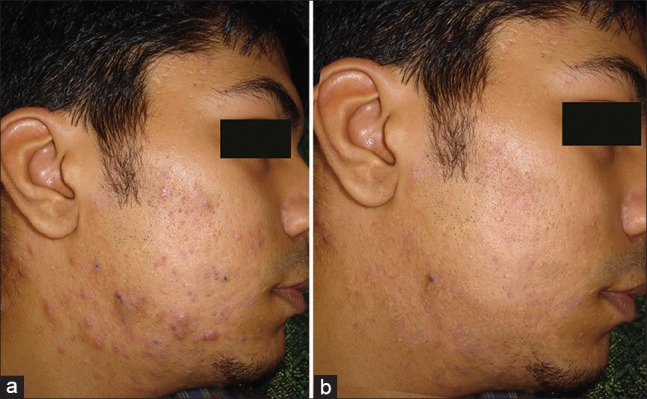
(a) Pretreatment photograph (b) Post treatment photograph
The adverse effects observed on treatment with isotretinoin were as follows:
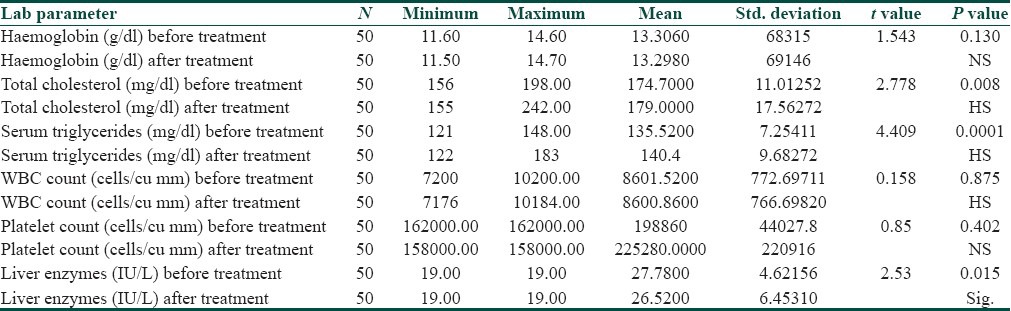
Cheilitis was the most common among the adverse effects observed, and was seen in 49 participants (98%) [Figure 3a], followed by Xerosis in 42 participants (84%), dandruff in 6 participants (12%), erythema of the face [Figure 3b] and hair rough and dry in 4 participants each (8%), dryness of mouth, abnormal levels of total cholesterol and serum triglycerides, abnormal levels of liver enzymes in 3 participants each (6%). Hair loss and headache were observed in 2 participants (4%). One participant (2%) developed lesions of vitiligo. [Figure 3c and Table 5]. When the lab investigations were performed, a significant increase was seen in the level of total cholesterol and serum triglycerides, and a raise above normal values was seen in 3 participants (6%). There was a significant increase in the level of liver enzymes before and after treatment and a raise above normal values was seen in 3 participants (6%). However, no significant changes were observed in the levels of hemoglobin, white blood cells count and platelet count before and after treatment [Table 6].
Figure 3.
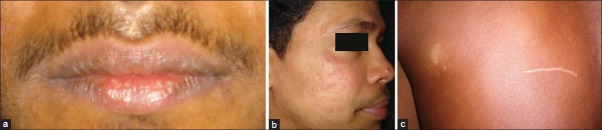
(a) Side effect: Cheilitis (b) Side effect: Erythema of the face (c) Side effect: Development of vitiligo in a patient in the study
Table 5.
Side effects observed in the patients in the study
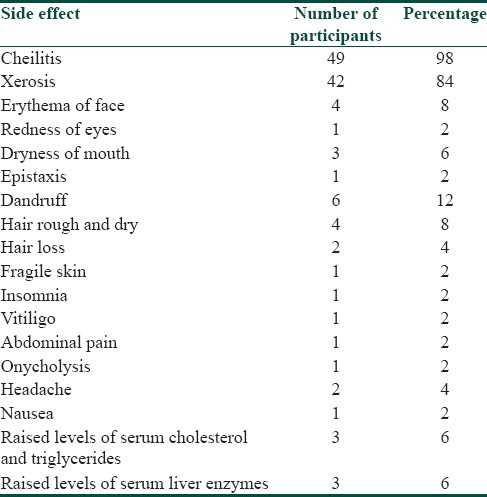
Table 6.
Results of Lab investigations of the patients in the study
Discussion
Several studies have been conducted to study the safety and efficacy of low-dose isotretinoin in the treatment of moderate to severe acne vulgaris.[3,5,6,7,8,9,10] In a study conducted by Amichai et al.,[3] good results were seen in 93% of the participants and relapse was seen in 4% of the participants. Cheilitis was the most commonly observed side effect and was seen in 91% of the participants. Abnormal values of liver enzymes were observed in 4.8% of the participants and abnormal serum lipid values were observed in 4.2% of the participants. The results obtained in our study was similar to the study done by Amichai et al.[3] In our study, excellent results were seen in 6% of the participants, very good results were seen in 90% of the participants and relapse was observed in 4% of the participants. Chelitis was the most common among the side effects observed, and was seen in 98% of the participants, with abnormal values of liver enzymes in 6% of the participants and abnormal values of serum lipids in 6% of the participants. An analysis of the studies revealed that due to the intrinsic superlative efficacy of isotretinoin in acne, the efficacy was as high as 88.52%, and a mean of 90% efficacy was maintained.[11,12,13] Thus, the comparative analysis can be logically based on the dose of isotretinoin discounting the varying protocols. In conclusion, the efficacy of the drug ranged from 69 to 99%.[5,6,7,8,9,10,11,12,13,14] There was no difference between the weekly and the daily/alternate day regimens.[8,9] In our study, the efficacy of isotretinoin in the treatment of moderate to severe acne vulgaris was found to be 90%.
Hermes et al.,[6] (8.3 months; 10 mg/d up to 0.43 mg/kg) reported very good results in 62.8% and good results in 31.9% of the participants. Mandekou-Lefaki et al.,[10] achieved excellent results in 68% and fair to good results in 31.2% (dose of 0.15-0.4 mg/kg/d; 8 months). In a study from India,[9] a low-dose isotretinoin treatment (0.15-0.28 mg/kg/d) lead to clinically significant results in 87.54% of the participants, including 68.20% very good and 19.34% of good results. However in our study, excellent results were seen in 6% of the participants, and very good results were seen in 90% of the participants (20 mg/d up to 3 months).
Goulden et al.[15] followed their participants for four months and stated that 85% were free of acne during this period. Nonetheless, two points of the isotretinoin therapy cannot be ignored: 1) Slow response to treatment, and 2) Flare-up of the lesions. Some researchers believe that macrocomedones and nodules are the most common factors showing the slow response and flare ups. The flare-up (defined as the worsening of lesions, mandating prescription of prednisolone) rate was found to be zero in the study. Similarly, in our study there was no flare up of acne following treatment with low-dose isotretinoin.
Our study demonstrated that the adverse effects of isotretinoin were not serious and could be treated by conservative measures in most instances. In a study done by Zane et al.,[16] it was observed that among the side effects, Cheilitis was the most common and occurred in 76% participants, followed by xerosis in 24% participants. Laboratory parameters had little alterations with limited rise in total cholesterol, serum triglyceride and liver enzyme levels. Abnormalities in hematological tests were not seen. These results were similar to the results obtained in our study, where cheilitis was the most common side effect observed in 98% of the participants, followed by xerosis in 84% of the participants. Abnormal values of liver enzymes and serum lipids were seen in 6% of the participants for each category and no abnormalities in the hematological tests were seen. One participant in our study developed lesions of vitiligo during the course of treatment with isotretinoin. This was a new finding which has not been reported in any of the previous studies with low-dose isotretinoin.
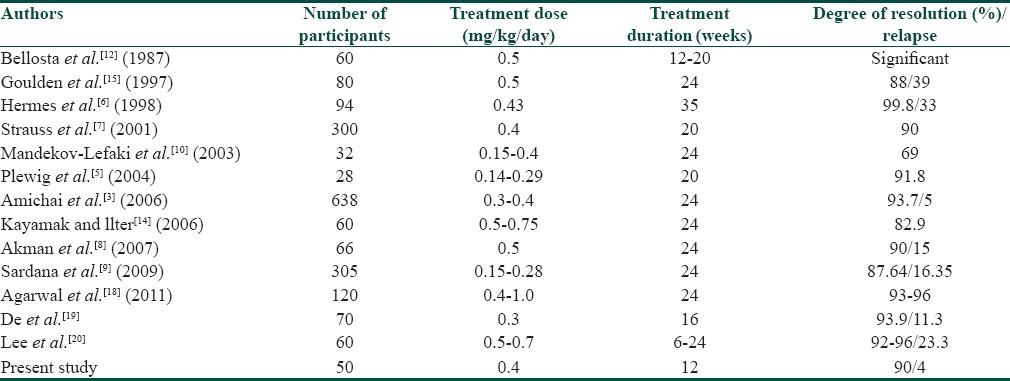
Previous studies have shown that treatment with isotretinoin can cause many adverse psychiatric effects including depression, psychosis, mood swings, violent behavior, suicide and suicide attempts.[17] However, in our study no such adverse psychiatric effects were observed. On comparison with previous studies of low-dose isotretinoin in acne, it was observed that the efficacy of low-dose isotretinoin in the treatment of acne, varied from 69% to 99%, and the relapse rate varied from 5% to 39% [Table 7].[3,5,6,7,8,9,10,12,14,15] However in the present study, the efficacy of low-dose isotretinoin in the treatment of moderate to severe acne vulgaris was found to be 90%, and the relapse rate was found to be 4% over a follow up period of 6 months.
Table 7.
Comparison of previous studies carried out with low-dose isotretinoin
Very few studies which highlight the safety and efficacy of low-dose isotretinoin in acne have been published in Indian literature. In an Indian study published by Sardana et al. the in Journal of European Academy of Dermatology Venereology and Leprosy, a fixed dose of 20 mg isotretinoin was administered for a duration of 24 weeks along with topical clindamycin.[9] In two other studies, different doses of isotretinoin and addition of azithromycin were used, respectively.[18,19] In our study the drug was administered daily for a duration of 12 weeks without any topical medication. We also observed vitiligo in one patient as a side effect during the treatment, which is a new observation and hasn’t been reported before.
Conclusion
Three months of treatment with low-dose isotretinoin (20 mg/day) was found to be effective in the treatment of moderate to severe acne vulgaris. The benefits accrued to the society from using isotretinoin outweigh the risks, and thus low-dose isotretinoin can be used in the treatment of moderate to severe acne vulgaris as an effective modality of treatment, with a low incidence of severe side effects and at a lesser cost. We recommend judicious use of low-dose isotretinoin in patients with moderate to severe acne, because acne not only scars the face, but also the mind and the heart.
What is new?
Three months of treatment with low-dose isotretinoin (20 mg/day) was found to be effective in the treatment of moderate to severe acne vulgaris, with a low incidence of serious side effects. This dose also was more economical than the higher doses.
Footnotes
Source of Support: Nil
Conflict of Interest: Paper was presented at the World Congress of Dermatology (WCD) 2011, Seoul. WCD 2011 Scholarship Awardee
References
- 1.Zaenglein AL, Graber EM, Thiboutut DM, Strauss JS. Acne vulgaris and Acneiform eruptions. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Fitzpatrick's dermatology in general medicine. 7th ed. New York: McGraw-Hill; 2008. pp. 690–700. [Google Scholar]
- 2.Shahidullah M, Tham SN, Goh CL. Isotretinoin therapy in acne vulgaris: A 10 year retrospective study in Singapore. Int J Dermatol. 1994;33:60–3. doi: 10.1111/j.1365-4362.1994.tb01500.x. [DOI] [PubMed] [Google Scholar]
- 3.Amichai B, Shemer A, Grunwald MH. Low-dose isotretinoin in the treatment of acne vulgaris. J Am Acad Dermatol. 2006;54:644–6. doi: 10.1016/j.jaad.2005.11.1061. [DOI] [PubMed] [Google Scholar]
- 4.Doshi A, Zaheer A, Stiller MJ. A comparison of current acne grading systems and proposal of a novel system. Int J Dermatol. 1997;36:416–8. doi: 10.1046/j.1365-4362.1997.00099.x. [DOI] [PubMed] [Google Scholar]
- 5.Plewig G, Dressel H, Pfleger M, Michelsen S, Kligman AM. Low-dose isotretinoin combined with tretinoin is effective to correct abnormalities of acne. J Dtsch Dermatol Ges. 2004;2:31–45. doi: 10.1046/j.1439-0353.2004.03739.x. [DOI] [PubMed] [Google Scholar]
- 6.Hermes B, Praetel C, Henz BM. Medium dose isotretinoin for the treatment of acne. J Eur Acad Dermatol Venereol. 1998;11:117–21. [PubMed] [Google Scholar]
- 7.Strauss JS, Leyden JJ, Lucky AW, Lookingbill DP, Drake LA, Hanifin JM, et al. A randomized trial of the efficacy of a new micronized formulation versus a standard formulation of isotretinoin in participants with severe recalcitrant nodular acne. J Am Acad Dermatol. 2001;45:187–95. doi: 10.1067/mjd.2001.115965. [DOI] [PubMed] [Google Scholar]
- 8.Akman A, Durusoy C, Senturk M, Koc CK, Soyturk D, Alpsoy E. Treatment of acne with intermittent and conventional isotretinoin: A randomized, controlled multicenter study. Arch Dermatol Res. 2007;299:467–73. doi: 10.1007/s00403-007-0777-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sardana K, Garg VK, Sehgal VN, Mahajan S, Bhushan P. Efficacy of fixed low-dose isotretinoin (20 mg, alternate days) with topical clindamycin gel in moderately severe acne vulgaris. J Eur Acad Dermatol Venereol. 2009;23:556–60. doi: 10.1111/j.1468-3083.2008.03022.x. [DOI] [PubMed] [Google Scholar]
- 10.Mandekou-Lefaki I, Delli F, Teknetzis A, Euthimiadou R, Karakatsanis G. Low-dose schema of isotretinoin in acne vulgaris. Int J Clin Pharmacol Res. 2003;23:41–6. [PubMed] [Google Scholar]
- 11.Peck GL, Olsen TG, Yoder FW, Strauss JS, Downing DT, Pandya M, et al. Prolonged remissions of cystic and conglobate acne with 13-cis-retinoic acid. N Engl J Med. 1979;300:329–33. doi: 10.1056/NEJM197902153000701. [DOI] [PubMed] [Google Scholar]
- 12.Bellosta M, Vignini M, Miori L, Rabbiosi G. Low-dose isotretinoin in severe acne. Int J Tissue React. 1987;9:443–6. [PubMed] [Google Scholar]
- 13.Sardana K, Sehgal VN. Retinoids: Fascinating up-and-coming scenario. J Dermatol. 2003;30:355–80. doi: 10.1111/j.1346-8138.2003.tb00402.x. [DOI] [PubMed] [Google Scholar]
- 14.Kaymak Y, Ilter N. The effectiveness of intermittent isotretinoin treatment in mild or moderate acne. J Eur Acad Dermatol Venereol. 2006;20:1256–60. doi: 10.1111/j.1468-3083.2006.01784.x. [DOI] [PubMed] [Google Scholar]
- 15.Goulden V, Clark SM, McGeown C, Cunliffe WJ. Treatment of acne with intermittent isotretinoin. Br J Dermatol. 1997;137:106–8. [PubMed] [Google Scholar]
- 16.Zane LT, Leyden WA, Marqueling AL, Manos MM. A population-based analysis of laboratory abnormalities during isotretinoin therapy for acne vulgaris. Arch Dermatol. 2006;142:1016–22. doi: 10.1001/archderm.142.8.1016. [DOI] [PubMed] [Google Scholar]
- 17.Wysowski DK, Pitts M, Beitz J. An analysis of reports of depression and suicide in patients treated with isotretinoin. J Am Acad Dermatol. 2001;45:515–9. doi: 10.1067/mjd.2001.117730. [DOI] [PubMed] [Google Scholar]
- 18.Agarwal US, Besarwal RK, Bhola K. Oral isotretinoin in different dose regimens for acne vulgaris: A randomized comparative trial. Indian J Dermatol Venereol Leprol. 2011;77:688–94. doi: 10.4103/0378-6323.86482. [DOI] [PubMed] [Google Scholar]
- 19.De D, Kanwar AJ. Combination of low-dose isotretinoin and pulsed oral azithromycin in the management of moderate to severe acne: A preliminary open-label, prospective, non-comparative, single-centre study. Clin Drug Investig. 2011;31:599–604. doi: 10.2165/11539570-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 20.Lee JW, Yoo KH, Park KY, Han TY, Li K, Seo SJ, et al. Effectiveness of conventional, low-dose and intermittent oral isotretinoin in the treatment of acne: A randomized, controlled comparative study. Br J Dermatol. 2011;164:1369–75. doi: 10.1111/j.1365-2133.2010.10152.x. [DOI] [PubMed] [Google Scholar]


