Abstract
Efficacy of monoclonal anti-EGFR antibodies (cetuximab, panitumumab) used in combination with chemotherapy or alone has been demonstrated in clinical trials of patients with mCRC. Both drugs block signaling EGFR pathway in malignant cells (blocking ligand binding and EGFR dimerization). Obtaining treatment responses with anti-EGFR agents is possible only in a selected subgroup of patients with mCRC. Successful treatment with cetuximab and panitumab is possible almost exclusively in patients without RAS mutations. Research on predictive value of EGFR gene copy number, PI3KCA gene mutations, P53 and PTEN, and EGFR their ligands concentrations is ongoing. Cetuximab, as IgG1 class antibody, can cause antibody dependent cellular cytotoxicity against neoplasm cells, while panitumumab, as IgG2 class antibody, does not induce such effect. Therefore a potential predictor cetuximab therapy may be the presence of different polymorphic forms of the genes for receptor immunoglobulin Fc fragments: FcγRIIa and FcγRIII subclasses.
Keywords: cetuximab, panitumumab, metastatic colorectal cancer, mCRC, EGFR, ADCC
Introduction
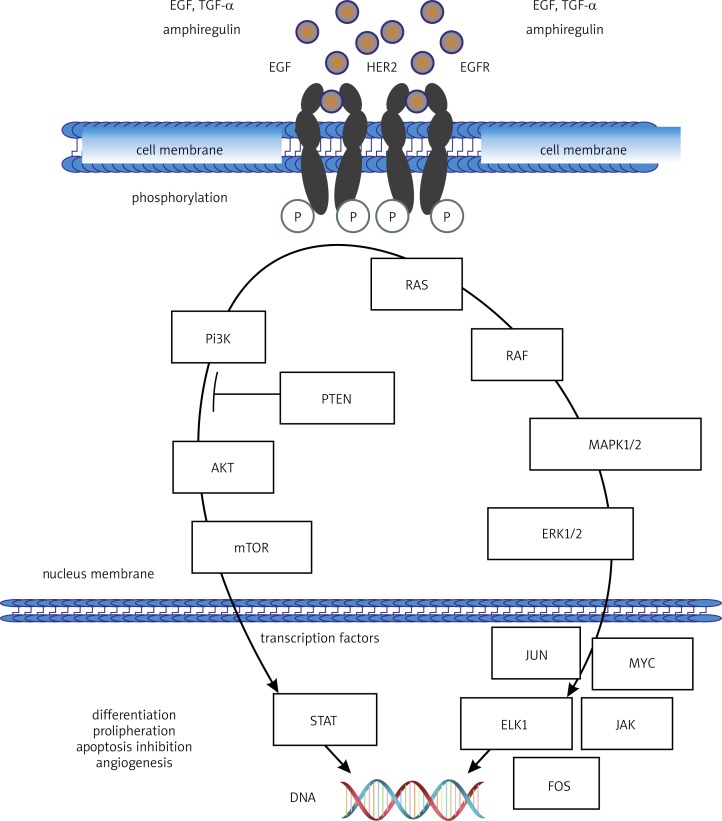
Colorectal cancer (CRC) is the second (after lung cancer) most common cause of death from malignancy in Poland. In 2010, it led to the death of 3,944 men and 3,435 women [1]. Similarly to many other cancer types, high mortality is a consequence of delayed diagnosis. It often happens that by the time the disease is detected, it has already disseminated and developed distant metastases. A major therapeutic modality for colorectal cancer is systemic treatment including chemotherapy and molecularly targeted therapies. Three molecularly targeted drugs: bevacizumab, cetuximab and panitumumab have been used in the therapy of advanced colorectal cancer. Bevacizumab is an anti-VEGF (vascular endothelial growth factor) monoclonal antibody which acts by inhibiting the process of neoangiogenesis and normalizing the formation of blood vessels within the tumour. Cetuximab and panitumumab are anti-EGFR (epidermal growth factor receptor) monoclonal antibodies. Their mechanism of action involves blocking cancer cell proliferation signal through the inhibition of the signalling pathways EGFR/Pi3K/AKT/mTOR or EGFR/Ras/Raf/MAPK/ERK. Signal blocking leads to the inhibition of cell divisions in the G1 phase due to the lack of required transcription factors, followed by cell elimination by apoptosis (Fig. 1).
Fig. 1.
Intracellular signalling pathway originating at EGFR and inducing the activation of proliferation, inhibition of apoptosis, and differentiation of epithelial and cancer cells
Direct inhibition of the binding of a ligand to EGFR through the blocking of the extracellular domain of the receptor by monoclonal antibodies is also accompanied by the process of EGFR homo- or heterodimerization with another member of the HER family, which is a prerequisite for the activation of a signal cascade inside cancer cells. This also leads to the internalization of the EGFR receptor. The therapeutic effect of cetuximab (and, to a limited extent, also panitumumab) also seems to be dependent on the cytotoxic response of the immune system induced against cancer cells coated with EGFR-bound antibodies (antibody-dependent cell-mediated cytotoxicity – ADCC), and the activation of the complement system [2–6].
According to some studies, panitumumab has a higher potential for binding to EGFR, however it is currently believed that both drugs demonstrate similar capacity for receptor binding. Moreover, both drugs achieve comparable therapeutic concentrations in blood plasma. There are, nevertheless, certain differences which may have an impact on the efficacy of therapy and on the potential for adverse reactions of both drugs. The differences result from the molecular structures of both antibodies. Cetuximab belongs to the class of IgG1 antibodies. It is a chimeric molecule containing a murine antigen-binding region. The remaining parts of heavy and light chains are of human origin (allergic reactions occur in 2–4% of treated patients, and corticosteroid and antihistamine premedication is required). Measurable concentrations of human anti-chimeric antibodies (HACA) have been detected in 3.4% of patients treated with cetuximab. The formation of HACA, however, is not associated with the development of hypersensitivity reactions to cetuximab, and no HACA-induced neutralizing effect on cetuximab is observed. Panitumumab is a fully human IgG2 antibody which induces allergic reactions in less than 1% of treated patients. It should be noted, though, that contrary to IgG1 antibodies (cetuximab), IgG2 antibodies have no ability to induce ADCC immune response. Importantly, the blood plasma half-life of cetuximab is up to one week, and for panitumumab it reaches two weeks, which is why cetuximab is administered every seven days and panitumumab – every 14 days. As there are no clinical trials directly comparing the efficacy of both agents, they have been approved for use in Poland and in the EU for similar indications in the treatment of colorectal cancer [4, 5].
Indications for cetuximab or panitumumab, and results of major clinical trials conducted in colorectal cancer patients
Cetuximab is indicated for the treatment of patients with epidermal growth factor receptor (EGFR)-expressing, KRAS wild-type metastatic colorectal cancer, in combination with irinotecan-based chemotherapy, in first-line in combination with FOLFOX, as a single agent in patients who who have failed oxaliplatin- and irinotecan-based therapy and who are intolerant to irinotecan. Cetuximab monotherapy is regulated within the framework of a drug programme. Panitumumab has similar indications to cetuximab, however according to the SPC it is approved for first-line treatment in combination with FOLFOX, for second-line treatment in combination with FOLFIRI in patients who have received first-line fluoropyrimidine-based chemotherapy (excluding irinotecan) and in monotherapy after failure of chemotherapy regimens containing fluoropyrimidine, oxaliplatin and irinotecan [7].
The efficacy of cetuximab used in monotherapy or in combination with chemotherapy has been analyzed, among others, in five large randomized clinical trials which enrolled over 3,700 patients with mCRC. Study EMR 62 202-013 conducted in patients with KRAS wild-type gene demonstrated superiority of first-line FOLFIRI chemotherapy combined with cetuximab over chemotherapy alone in all the analyzed characteristics. Significant increases were observed for median overall survival (OS) from 20 to 23.5 months (p = 0.0093) and median progression-free survival (PFS) from 8.4 to 9.9 months, accompanied by an increase in response rate (RR) from 39.7% to 57.3%. No comparable efficacy was found in patients with KRAS gene mutations [8–10]. Study CA225006 compared treatment with cetuximab plus irinotecan with irinotecan monotherapy in patients with progressive disease (PD) following oxaliplatin- and fluoropyrimidine-based therapy. The study showed a significant increase in median PFS (4 vs. 2.6 months, p < 0.0001) and an increase in objective response rate from 4.2% to 16.4% regardless of the status of the KRAS gene in the group of patients treated with cetuximab combined with irinotecan. No difference was noted in median overall survival (ca. 10 months) between the two study groups [11]. Study CA225025 sought to compare cetuximab used in monotherapy with placebo in patients with progressive disease following treatment with oxaliplatin, irinotecan and fluoropyrimidine. Cetuximab proved to be similarly effective to best supportive care (BSC) exclusively in patients with KRAS wild-type gene (response to treatment was seen exclusively in this group, in 12.8% of subjects). Median OS was found to have increased significantly from 4.8 to 9.5 months, and median PFS – from 1.9 to 3.7 months. In the group of patients with KRAS gene mutations parameters describing the efficacy of cetuximab were almost identical to the BSC-treated group [12].
The efficacy of panitumumab in patients with mCRC was comparable to the efficacy of cetuximab for the same therapeutic regimens. Four major randomized studies involved a total of 3,885 patients. In PRIME study, panitumumab was used in combination with FOLFOX for first-line treatment. In patients with no KRAS gene mutation the addition of panitumumab to chemotherapy induced a statistically significant increase in therapeutic response rate (48% vs. 57%), a prolongation of median PFS (8.6 vs. 10 months) and median OS (19.7 vs. 23.9 months). Panitumumab used in patients with a KRAS gene mutation had no effect on RR (ca. 40%). A significant reduction in median PFS and an insignificant reduction in median OS compared to chemotherapy alone were noted in this group of patients (7.4 and 9.2 months, and 15.5 and 19.2 months, respectively) [13, 14]. The effects of panitumumab plus FOLFIRI vs. FOLFIRI alone as second-line therapy was investigated in study NCT00339183. Among KRAS gene wild-type patients a statistically significant increase in the response rate (10% vs. 36%), an extension of median PFS (6.6 months vs. 7.6 months) and median OS (12.5 months vs. 14.5 months) were achieved. In the group of KRAS mutation positive patients the efficacy of the FOLFIRI regimen was similar regardless of whether it was combined with panitumumab or used alone [15]. Administered in monotherapy, panitumumab – similarly to cetuximab – induced an objective treatment response only among patients with KRAS wild-type gene. Median PFS in this group of patients was 16 weeks, as opposed to 8 weeks in the placebo group. A median PFS of 8 weeks was observed in the group of KRAS mutation positive patients receiving panitumumab or placebo [16].
Role of determining EGFR expression for the eligibility of treatment with cetuximab or panitumumab
Activation of the signal transduction pathway which originates at EGFR in abnormal cells plays a central role in the development of many types of cancer including the two most common, i.e. non-small-cell lung carcinoma (NSCLC) and colorectal cancer. These cancers are usually associated with elevated blood plasma levels of EGFR ligands including EGF, amphiregulin (AREG), epiregulin (EREG) and TGF-α(transforming growth factor α), and high expression of HER family membrane receptors: HER1 (EGFR), HER2, HER3 and HER4 on the surface of cancer cells. EGFR expression on CRC cells is identified in over 80% of patients. Data on correlations existing between the degree of EGFR expression on cancer cells and the degree of clinical advancement of CRC, survival time, and rate and extent of metastatic spread, are controversial. Some studies have demonstrated that EGFR expression is the greatest in the most invasive tumour areas, in locations where cancer infiltrates peri-intestinal tissues, and in lymph node and distant metastases. It appears, then, that high expression of EGFR (and its ligands such as amphiregulin) may be a poor prognostic factor in CRC patients. Several other studies [17–21], however, have found no evidence to support the above hypothesis.
Considering that cetuximab and panitumumab act by blocking the extracellular domain of EGFR, it appeared that the efficacy of both drugs would be conditional on the presence of EGFR on the surface of cancer cells. The requirement for immunohistochemical (IHC) detection of EGFR expression in tumour material preserved in paraffin blocks to determine eligibility of mCRC patients for cetuximab therapy is included in the SPC of the drug [20]. In Poland, the opinion issued by the Consultative Council of the Agency for Health Technology Assessment (AOTM) has been used as a basis for the development of drug programmes under which eligibility for cetuximab or panitumumab treatment is limited to patients with positive EGFR expression and lack of KRAS gene mutations in cancer cells.
Most early clinical trials required an assessment of EGFR expression for determining eligibility for cetuximab or panitumumab therapy. The predictive value of the degree of EGFR expression, however, was not confirmed in two randomized studies (CRYSTAL and OPUS) [8, 9]. In the randomized phase III COIN trial (Continuous Chemotherapy plus Cetuximab or Intermittent Chemotherapy) EGFR expression was no longer listed among inclusion criteria for cetuximab treatment. The study showed no significant benefits of adding cetuximab to chemotherapy mainly due to treatment delays and the necessity to reduce doses of cytostatic agents due to toxicity effects. Cetuximab significantly increased the response rate (57% vs. 64%) and prolonged median PFS in patients receiving cetuximab with a regimen containing oxaliplatin and fluorouracil in combination with folic acid (Modified de Gramont with Oxaliplatin – OxMdG) [10]. Moreover, study results have been published indicating that positive EGFR expression is not a precondition for the efficacy of cetuximab, while response to treatment is possible in patients with negative receptor expression. In one of the first reports Chung et al. revealed a potential for achieving response to cetuximab treatment alone or in combination with irinotecan in 25% of patients with chemotherapy refractory EGFR-negative metastatic CRC [22]. Similar results were obtained by Hebbar et al., who even concluded that response to treatment with cetuximab combined with irinotecan was more frequently observed in those of oxaliplatin- and irinotecan-refractory subjects who were EGFR expression-negative than EGFR expression-positive [23]. Han et al. demonstrated that the finding could be attributed to different monoclonal antibodies used in immunohistochemical diagnostic assays for EGFR expression which gave false negative results of EGFR expression on cancer cells [24]. CE/IVD certified IHC tests which are currently commonly used in CRC patients for the detection of EGFR expression, e.g. EGFR PharmDx (Dako), are expected to detect the presence of receptor on cancer cells in over 95% of patients [20]. In view of the results of studies cited above, it is increasingly claimed that there are no grounds for IHC diagnostic tests determining EGFR expression to assess eligibility of mCRC patients for anti-EGFR antibody treatment. This is especially important in view of large differences in results of EGFR expression assays obtained in different Polish medical centres. In some of them, a considerable number of patients may, in fact, be erroneously excluded from molecularly targeted therapy on the basis of lack of EGFR expression despite the presence of wild-type KRAS gene in tumour cells. The provision included in the therapeutic programme, however, remains unchanged and in order to be considered eligible for therapy with anti-EGFR antibodies, mCRC patients must be EGFR expression-positive.
Studies investigating the predictive value of the assessment of the number of EGFR gene copies for therapy with anti-EGFR antibodies in mCRC cancer patients having a wild-type KRAS gene have failed to yield unambiguous results. With the help of suitable techniques including fluorescence in situ hybridization (FISH), chromogenic in situ hybridization (CISH) or, less commonly, silver in situ hybridization (SISH) it has been shown that an incorrect EGFR gene copy number occurs heterogeneously in different areas of the CRC tumour. The majority of studies have demonstrated correlations between polysomy or amplification of the EGFR gene and the potential for achieving objective response to treatment and prolongation of PFS. In addition, in many studies the OS of patients treated with anti-EGFR antibodies has been similar regardless of having a normal or increased number of copies of the EGFR gene [6, 25]. In the study conducted by Scartozzi et al. among subjects treated with irinotecan-cetuximab the PFS of patients with a high number of EGFR gene copies was found to be significantly longer, whereas in the studies by Laurent-Puig et al. and Personeni et al. conducted in cetuximab-treated patients there was a slight increase of OS in subjects with a high number of EGFR gene copies compared to patients with a low number of copies of the gene [26–28]. The most spectacular results regarding the efficacy of cetuximab or panitumumab in different lines of treatment were obtained by Algars et al. who observed a significant prolongation of PFS and OS in patients without KRAS gene mutations and with more than four copies of the EGFR gene compared to patients with a low number of copies of that gene. According to the authors, clinical benefit of anti-EGFR antibody therapy occurred in 82% of KRAS wild-type gene patients with a high number of copies of the EGFR gene (with remission noted in 36% of patients), whereas in subjects with a low number of EGFR gene copies remission and stable disease were rarely observed (6% and 13%, respectively) [29]. In 2013, Jiang et al. published a metaanalysis of eight studies on the effects of EGFR gene polysomy on the efficacy of cetuximab or panitumumab in different therapeutic regimens used in patients with mCRC. The authors demonstrated that a high number of copies of the EGFR gene causes a significant increase in OS (HR = 0.62) and PFS (HR = 0.65) in patients receiving anti-EGFR antibodies, and is associated with a higher incidence of skin rash during the therapy. On that basis, it can be assumed that an assessment of EGFR gene copy number alterations provides a good predictive factor for the eligibility of mCRC patients for anti-EGFR antibody treatment [30, 31]. It seems that the assessment may prove to be much more valuable for appropriate qualification of patients for this type of treatment than IHC-based assays for EGFR expression.
Equally debatable are the results of studies investigating correlations between various polymorphic forms of the EGFR gene and the efficacy of anti-EGFR antibodies in mCRC patients. Genetic polymorphism refers to the simultaneous occurrence of various forms of the same gene in a population (e.g. single-nucleotide polymorphism), which may lead to differences in the structure and characteristics of the protein encoded by this gene. As opposed to driver mutations (there have only been reports on isolated CRC patients with mutations in exons 20 and 21 of the EGFR gene), genetic polymorphism occurs in at least 1% of members of a given population, and affects not only cancer cells but all body cells. Intron 1 of the EGFR gene can be affected by a polymorphism causing variation in the number of tandem CA repeats. The longer form of intron 1 is associated with a reduced transcriptional capability of the EGFR gene and hence lower expression of the EGFR protein on the surface of epithelial cells. Analyses were also performed for other polymorphisms in the EGFR gene: G216T and G497A, and in the EGF gene: A61G. Graziano et al. demonstrated the presence of the short variant of the EGFR gene intron-1 and the G allele in codon 61 of the EGF gene (higher EGF production) to be a favourable predictive factor for cetuximab-irinotecan therapy. In addition, a smaller number of CA repeats in intron 1 is associated with more frequent adverse reactions accompanying treatment, manifested as skin rash [6, 25, 31].
Impact of mutations in KRAS and BRAF genes, and other rare mutations on the efficacy of cetuximab or panitumumab
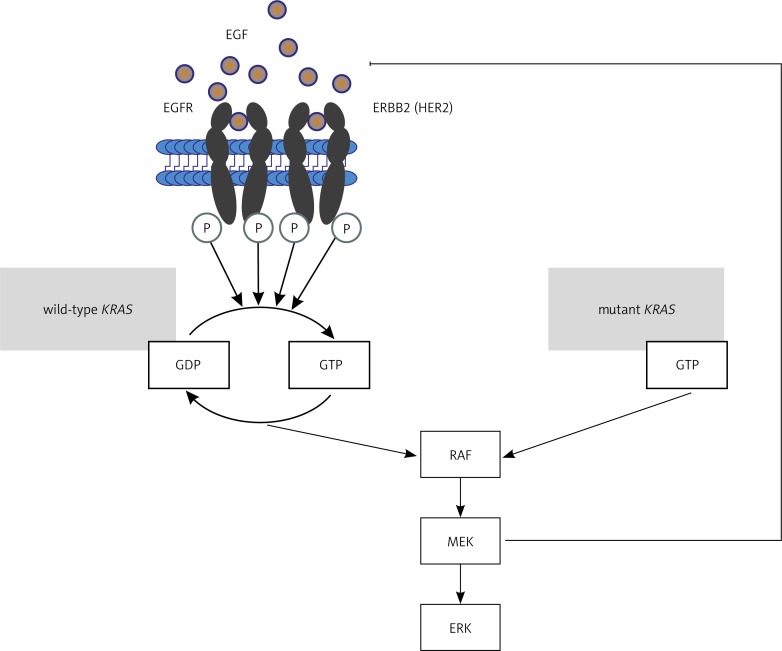
Mutations in the KRAS gene and possibly also in the BRAF gene are the fundamental negative predictive factors in anti-EGFR antibody treatment of mCRC patients. Mutations in the KRAS oncogene are the most common genetic abnormalities identified in CRC cells and in the majority of human cancers in general. They are detected in 20–50% of CRC patients. High discrepancy of results defining the incidence of KRAS gene mutations stems from the diversity of diagnostic methods and types of mutations under study. The most important KRAS gene mutations occur in exons 1 and 2 in codons 12 (most commonly), 13 and 61. The mutations represent single-nucleotide substitutions resulting in the replacement of glycine in codons 12 and 13, and glutamine in codon 61, with another amino acid (G12C, G12V, G12D, G12R, G12A, G12S, G13D, G13C, Q61K, Q61R, Q61L). Since the KRAS protein plays a key role in the intracellular transduction cascade originating at EGFR, KRAS damage and excessive activity generates a signal for cell proliferation or differentiation regardless of EGFR activation or lack of it. What this means is that effectors continuously transmit signal to the cell nucleus, activating appropriate transcription factors (Fig. 2) [32–37].
Fig. 2.
Role of normal and mutated KRAS protein in the regulation of the signalling pathway associated with EGFR activation
Large clinical trials have shown that the majority of chemotherapy-refractory mCRC patients with mutations in the KRAS gene (regardless of mutation type) are also refractory to chemotherapy combined with anti-EGFR antibody treatment. Objective response to this treatment modality is observed in 2–15% of patients with KRAS gene mutations and in ca. 35–40% of patients with wild-type KRAS gene [11, 15]. Assessment of the influence of KRAS gene mutations on the efficacy of chemotherapy and cetuximab in patients who have had no previous chemotherapy is ambiguous. The addition of cetuximab or panitumumab to chemotherapy compared to chemotherapy alone in untreated mCRC patients with wild-type KRAS gene increases the response rate from over 35% to nearly 60%, prolongs PFS to over 9 months and extends OS by a mean of ca. 4 months [8, 9, 13]. The PRIME study even demonstrated that panitumumab added to chemotherapy in the treatment of patients with KRAS-mutated colorectal cancers reduced progression-free survival compared to patients treated by chemotherapy alone [13]. The outcomes of the studies seem to suggest that there are no benefits of adding anti-EGFR antibodies to chemotherapy in patients with a mutated KRAS gene, however on account of the fact that the efficacy of this type of treatment depends on multiple factors (e.g. crossover to alternative treatment after disease progression, and use of subsequent lines of therapy), it is extremely difficult to evaluate the effect of anti-EGFR monoclonal antibodies in this patient group. The role of KRAS gene mutations in the development of refractoriness to cetuximab and panitumumab monotherapy is discussed above [12, 16]. In other studies, Karapetis et al. report objective response to cetuximab monotherapy in just one patient with a KRAS gene mutation (1.8% of mutation-bearing patients) and in 12.8% of mutation-free patients. In the group of cetuximab-treated patients with wild-type KRAS gene the authors report longer PFS (3.7 months) and OS (9.5 months) compared to patients with mutated KRAS gene receiving this antibody (1.8 and 4.5 months, respectively). Moreover, in the latter group of patients there were no differences in median PFS and OS depending on the type of treatment [36]. Amado et al. identified similar differences in response rates in patients treated with panitumumab monotherapy, achieving response in 17% of patients with wild-type KRAS gene and no response in patients with mutated KRAS gene. Panitumumab-treated patients with wild-type KRAS gene had longer PFS and OS than patients with KRAS mutations who received the same antibody therapy [38].
In view of the study results presented above and the indisputable role of KRAS gene mutations as a negative predictive factor both for cetuximab and panitumumab therapy, subsequent clinical studies always incorporated an analysis of KRAS gene mutations to determine patient eligibility for treatment. The first of these was the COIN study mentioned above. Despite multiple divergences from the protocol, COIN still represented a prospective study involving an analysis of mutations in the KRAS gene. Cetuximab combined with the FOLFOX chemotherapy regimen or capecitabine plus oxaliplatin (CAPOX) in patients with wild-type KRAS gene increased median OS to 23 months and PFS to 8.3 months – an outcome that was impossible to achieve in patients with KRAS mutations receiving the same therapy (13.4 and 5.5 months, respectively) [10]. In two other studies, CAIRO-1 (Capecytabine, oxalipaltyn and Bevacizumab with or without Cetuximab in First-Line Advanced Colorectal Cancer) and PACCE (Panitumumab Advanced Colorectal Cancer Evaluation) [39, 40], subjects with wild-type KRAS gene also failed to benefit from anti-EGFR antibody treatment added to chemotherapy, if the efficacy of treatment were to be compared to chemotherapy alone.
In Poland, AOTM's explicit opinion was used as a basis for developing a drug programme under which anti-EGFR antibody treatment can be prescribed to mCRC patients provided that cancer cells are free from KRAS gene mutations (without further specification mutation types).
KRAS gene mutation types have been analyzed retrospectively in the context of assessing their importance for the development of refractoriness to anti-EGFR antibodies. Research conducted over the past two years has established unambiguously that a mutation in codon 12 is a negative predictive factor. On the other hand, it seems possible to achieve response to cetuximab and panitumumab therapy in the presence of mutations in codon 13 of the KRAS gene – regardless of treatment line or modality. In the study by Pentheroudakis et al., patients with a mutation in codon 12 of the KRAS gene who received chemotherapy combined with cetuximab had a median overall survival of 19 months, whereas patients with other KRAS mutations and KRAS-wild type gene subjects treated with the same modality had a considerably longer median survival of nearly 30 months [41–44]. Furthermore, there are many reports on patients with the wild-type KRAS gene who are refractory to anti-EGFR treatment. The cause of refractoriness has been identified in ca. 15% of mCRC patients as mutations in the BRAF gene, primarily V600E substitution. Raf family proteins are downstream of Ras proteins in the signal transduction pathway originating at EGFR. It comes as no surprise, then, that activating mutations in the BRAF gene occurring in cancer cells have a similar clinical effect to KRAS gene mutations, making them refractory both to cetuximab and panitumumab [6, 27, 45–47]. Di Nicolantonio et al. reviewed patients treated with these antibodies, finding objective response in two patients with KRAS gene mutations (6%) and in 22 mutation-free subjects (28%). In the group of patients with wild-type KRAS gene the authors identified a total of 11 patients with BRAF mutations (14%). Among them, there were no objective responses to anti-EGFR treatment, and PFS and OS were reduced. In in vitro cultures the authors successfully overcame refractoriness of BRAF-mutated cancer cells to cetuximab using a combination of cetuximab and the multikinase inhibitor sorafenib which inhibits, among others, Raf kinase [48]. Findings on the impact of BRAF mutations on the efficacy of anti-AGFR antibody treatment (both in monotherapy and in combination with chemotherapy) in mCRC patients have also been corroborated by other authors. Tol et al. have identified mutations in the BRAF gene as an unfavourable prognostic factor [49]. Pentherodaukis et al. showed that the presence of BRAF mutations in patients treated with chemotherapy combined with cetuximab is even a weaker predictive factor for this type of treatment than a mutation in codon 12 of the KRAS gene. As previously mentioned, patients having both genes of the wild-type who are treated with anti-EGFR antibodies had a median survival close to 30 months. By contrast, subjects with a mutation in codon 12 of the KRAS gene had a median survival of 19 months and those with a mutation in the BRAF gene – only 12 months [41].
During the 2013 ASCO Annual Meeting, however, there were reports stating that the presence of mutations in the BRAF gene had no predictive value for anti-EGFR therapy and was a negative prognostic factor only in CRC patients. The studies showed that negative predictive factors in panitumumab therapy included not only common mutations in the KRAS gene but also rare mutations in genes encoding RAS proteins. The authors characterized the effects of additional mutations in codons 59, 117 and 146 of the KRAS gene, and mutations in codons 12, 13, 59, 61, 117 and 146 of the NRAS gene, on the efficacy of panitumumab combined with FOLFOX6 chemotherapy in first-line mCRC treatment (PEAK study), and the efficacy of panitumumab monotherapy (20020408 study). The retrospective analysis revealed that the occurrence of these rare mutations (the incidence of NRAS mutations ranges from 5 to 8.3%, while that of mutations in codons 59, 117 and 146 of the KRAS gene does not exceed 10%) reduced the chance of achieving response to panitumumab therapy and shortened PFS following the introduction of this therapy. Consequently, the SPC of the drug will soon be revised to include the requirement to assess all mutations in KRAS and NRAS genes in the process of determining eligibility for panitumumab treatment. One obstacle which currently hinders compliance with the requirement is the fact that there are no methods certified for the detection of this group of mutations, since the SURVEYOR platform applied for the analysis of tissue material in PRIME and 20020408 studies is not in widespread use [50–52].
Phosphatidylinositol 3-kinase (Pi3K) consists of two subunits: one regulatory and one catalytic (subunit P110α) which is responsible for the phosphorylation of phosphatidylinositol followed by activation of the AKT/mTOR pathway. Pi3K/AKT is a pathway alternative to Ras/Raf/MAPK, transmitting signals from the activated EGFR protein to the cellular nucleus. A regulatory role in the pathway is played by the PTEN protein (phosphatase and tensin homolog) encoded by the suppressor gene PTEN. It can thus be concluded that activating mutations of the PI3KCA oncogene (phosphatidylinositol-4, 5-bisphosphate 3-kinase catalytic subunit α isoform) and reduced expression of the PTEN protein may affect the efficacy of EGFR-inhibiting cancer treatments (Fig. 1) [6, 41, 46, 47, 53–56].
Activating mutations of the PI3KCA gene occur most commonly in exons 9 (E542K, E545K) and 20 (H1047R). The mutations are detected in 6–10% of CRC patients. Typically, they exist independently of mutations affecting the KRAS gene. The value of studies investigating the influence of PI3KCA gene mutations on the efficacy of anti-EGFR antibody treatment is limited due to their retrospective nature and small study groups. Studies by Lievre et al. and Perrone et al. found that carriers of mutations in the PI3KCA gene were non-responders to anti-EGFR treatment [6, 53]. By contrast, studies by Sartore-Bianchi et al. and Peren et al. demonstrated a possibility of achieving response to anti-EGFR therapy in patients with PI3KCA mutations (13% of patients bearing a PI3KCA mutation and 11% of mutation-free patients had an objective response to treatment) [54, 55]. Pentheroudakis et al. identified no correlations between mutations in the PI3KCA gene and the efficacy of chemotherapy combined with cetuximab. An attempt can be made at explaining divergences in results as attributable to differences in the extent of Pi3K activation induced by mutations in exons 9 and 20 of the PI3KCA gene [41]. De Roock et al. argue that the main factor responsible for the excessive activity of phosphatidylinositol kinase is H1047G substitution. There is, as yet, no strong evidence in favour of extending the current genetic test panel to include mutations of the PI3KCA gene as another element of determining eligibility of mCRC patients for anti-EGFR treatment [56].
While it is relatively easy to detect mutations present in KRAS, BRAF and even PI3KCA genes with the aid of dedicated CE/IVD-certified tests based on real-time PCR, an assessment of epigenetic phenomena is extremely difficult and subjective, and may yield contradictory results. This applies to attempts at investigating abnormalities within the PTEN gene and their predictive value for the efficacy of anti-EGFR treatment given to mCRC patients. PTEN is a potential site for a number of mutations – and for the formation of pseudogenes, hypermethylation of the promoter region, amplification of the entire gene, etc. As a result, the majority of authors confine themselves to an assessment of PTEN expression within cancer cells by immunohistochemistry. Laurent-Puig et al. found that the lack of PTEN expression in KRAS mutation-free patients (ca. 20% of CRC patients) was correlated with reduced survival. Loupakis et al. established that a KRAS mutation and absence of PTEN expression were negative predictive factors for response to treatment based on cetuximab in combination with irinotecan. Also, Razis et al. showed that the deletion of a fragment of the PTEN gene detected by FISH – unlike the absence of the PTEN protein expression – was a negative factor for such therapy [27, 46, 47, 53, 57–59].
Assessment of expression of EGFR ligands as predictive factors in cetuximab and panitumumab therapy
Ligands of the HER family receptors include EGF, amphiregulin, epiregulin and TGF-α. High concentrations of these ligands and high expression of their mRNA in the CRC tissue are frequently observed, and are essential for the proliferation of cancer cells. Higher concentrations of EGFR ligands are presumed to be correlated with faster tumour growth and metastatic ability. Moreover, patients with overexpression of EGFR ligands are less commonly identified with mutations in the KRAS gene because the carcinogenesis pathway is, in this case, independent of the mutation. Khambata-Ford et al. noted more frequent disease control and longer PFS for cetuximab monotherapy in patients whose cancers had high levels of EREG or AREG expression than in subjects with low expression levels of these ligands. Jacobs et al. examined KRAS wild-type patients treated with cetuximab and irinotecan, reporting a median survival of 65 weeks in patients with high EREG expression and just 31 weeks in patients with low ligand expression. Also in studies by Pentheroudakis et al. and Ohchi et al. high expression levels of AREG and EREG (high mRNA levels for these ligands) were favourable predictive factors (with a potential for the achievement of response to treatment and prolongation of overall survival) for cetuximab plus chemotherapy in KRAS wild-type mCRC patients [6, 41, 60–64].
Antibody-dependent cell-mediated cytotoxicity and activation of the complement system as a mechanism of action of cetuximab
Antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) belong to the most important processes allowing IgG1 antibodies to destroy microorganisms, parasites and cancer cells. Antibodies alone are unable to destroy the target cell: they can only bind specifically to epitopes of cancer antigens. If the antigen is a receptor, as in anti-EGFR therapy, the intracellular transduction pathway is blocked but also cytotoxic cells become activated (if the antibody, like cetuximab, belongs to the IgG1 class). After coating the cancer cell, antibodies bind to NK cells and other immune cells which have receptors for the antibody Fc fragment on their surface. Some of them (IgM and IgG antibodies, with the exception of IgG4) can also bind to the C1q molecule. C1q-associated proteases then induce enzymatic conversion of C1r and C1s cells, thus initiating the classical complement activation pathway. NK cells bound to the target cell become degranulated releasing perforins, granulysins and granzymes which induce apoptosis of cancer cells. Similarly, the membrane of the target cell can become lysed as a result of activation of components of the complement system [65–68].
Evidence for the impact of ADCC on the efficacy of cetuximab and absence of any influence on the efficacy of panitumumab therapy is found in in vitro studies (cell cultures). Other evidence was to be derived from observations into the relationship between the occurrence of polymorphic forms of genes coding receptors for the antibody Fc region and the effect of cetuximab treatment in mCRC patients. Unfortunately, results of these studies are often contradictory. Two polymorphisms with a major role for the receptor function (i.e. the degree of their affinity to IgG1) have been identified: H131R substitution in the FcγRIIa gene and F158V substitution in the FcγRIIIa gene. The first studies by Zheng et al. and Bibeau et al. showed the H allele in codon 131 in the FcγRIIa gene to be correlated with long time to progression in cetuximab-treated patients. Results of both studies indicate that individuals with HH homozygous genotypes in codon 131 of the FcγRIIa gene benefit significantly from cetuximab treatment. Identical conclusions were reached by Rodriguez et al. The studies, however, provided conflicting results on the association between the presence of the F or V alleles in the FcγRIIIa gene and the efficacy of cetuximab. Bibeau et al. report, however, that the time to progression in cetuximab-treated mCRC patients with wild-type KRAS gene is prolonged to 9.6 months in the subgroup of subjects with HH homozygous genotypes in codon 131 of the FcγRIIa gene or VV homozygous genotypes in codon 158 of the FcγRIIIa gene – compared to 4.6 months in subjects with other genotypes of receptor genes for the Fc fragment of immunoglobulins. Just one year later, however, studies by Pander et al. contradicted the finding that greater benefits of cetuximab therapy are achieved in patients with VV homozygous genotypes in codon 158 of the FcγRIIIa gene. The effect, the authors claimed, is observed rather in carriers of the FF genotype in codon 158 of the FcγRIIIa gene. Lurje et al. studied a larger patient group (n = 130) without detecting any associations between the efficacy of cetuximab and the genotype of genes for receptors for the Fc fragment of the antibodies. Pander et al., investigating a group of 246 patients with CRC to determine polymorphism V176F (818A > C) and the efficacy of cetuximab, found that the C allele was an unfavourable predictive factor for cetuximab treatment. Carriers of the allele had a median PFS of just 8.2 months, whilst AA homozygous individuals survived without signs of progression for 12.8 months. In view of the fact that different authors have obtained divergent study results, the role of ADCC in the mechanism of action of cetuximab continues to be an object of debate. The study by Lopez-Albeitero et al., conducted among 170 patients with head and neck cancer seems to point to the major role of ADCC in cetuximab's mechanism of action and to the modulation of its efficacy by polymorphisms within the FcγRIIIa gene. In addition, as there is no possibility of ADCC induction by panitumumab, the majority of studies have found no evidence for any relationship between the efficacy of the drug and the occurrence of various polymorphic forms of the FcγRIIIa gene [65–76].
Summary
The efficacy of monoclonal anti-EGFR antibodies has been proven in mCRC patients and, for cetuximab, also in individuals with squamous cell carcinoma of the head and neck. Advanced clinical trials (LUCAS and FLEX) with cetuximab have also been performed in patients with non-small-cell lung carcinoma (NSCLC). They were not successful, though, and did not result in the approval of cetuximab for treatment of this cancer type. It has been established clearly that response to anti-EGFR antibody treatment is only possible in selected patient groups with various cancer types. Predictive factors for the efficacy of anti-EGFR therapy have been best elucidated in CRC patients. A factor identified in multiple studies as essential for appropriate assessment of eligibility for cetuximab or panitumumab treatment is the absence of KRAS gene mutations. EGFR expression on the surface of cancer cells does not seem to have a decisive influence on the efficacy of the therapy. There are ongoing studies assessing the predictive value of the number of copies of the EGFR gene, mutations in the NRAS, PI3KCA, P53 and PTEN genes, concentration of EGFR ligands and polymorphisms in the EGF and EGFR, and the FcγRIIa and FcγRIIIa, genes. These factors, however, have not as of yet been examined in large randomized prospective studies and hence should not be used as a basis for mCRC patient eligibility for cetuximab or panitumumab treatment. Merck provided a medical writing grant to support the manuscript development, however, Merck made no contributions to the content of the manuscript.
Authors declare no conflict of interest.
References
- 1.Krajowy Rejestr Nowotworów. http://www.epid.coi.waw.pl/krn/.
- 2.Gullick WJ. The epidermal growth factor system of ligands and receptors in cancer. Eur J Cancer. 2009;45(suppl. 1):205–210. doi: 10.1016/S0959-8049(09)70035-8. [DOI] [PubMed] [Google Scholar]
- 3.Spano JP, Fagard R, Soria JC, Rixe O, Khayat D, Milano G. Epidermal growth factor receptor signaling in colorectal cancer: preclinical data and therapeutic perspectives. Ann Oncol. 2005;16:189–94. doi: 10.1093/annonc/mdi057. [DOI] [PubMed] [Google Scholar]
- 4.Petrelli F, Borgonovo K, Cabiddu M, Ghilardi M, Barni S. Cetuximab and panitumumab in KRAS wild-type colorectal cancer: a meta-analysis. Int J Colorectal Dis. 2011;26:823–33. doi: 10.1007/s00384-011-1149-0. [DOI] [PubMed] [Google Scholar]
- 5.Meriggi F, Di Biasi B, Abeni C, Zaniboni A. Anti-EGFR therapy in colorectal cancer: how to choose the right patient. Curr Drug Targets. 2009;10:1033–40. doi: 10.2174/138945009789577891. [DOI] [PubMed] [Google Scholar]
- 6.Di Fiore F, Sesboüé R, Michel P, Sabourin JC, Frebourg T. Molecular determinants of anti-EGFR sensitivity and resistance in metastatic colorectal cancer. Br J Cancer. 2010;103:1765–72. doi: 10.1038/sj.bjc.6606008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Narodowy Fundusz Zdrowia. http://www.nfz.gov.pl/new.
- 8.Van Cutsem E, Köhne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–17. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 9.Bokemeyer C, Bondarenko I, Makhson A, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27:663–71. doi: 10.1200/JCO.2008.20.8397. [DOI] [PubMed] [Google Scholar]
- 10.Adams RA, Meade AM, Seymour MT, et al. MRC COIN Trial Investigators. Intermittent versus continuous oxaliplatin and fluoropyrimidine combination chemotherapy for first-line treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet Oncol. 2011;12:642–53. doi: 10.1016/S1470-2045(11)70102-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sobrero AF, Maurel J, Fehrenbacher L, et al. EPIC: Phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:2311–9. doi: 10.1200/JCO.2007.13.1193. [DOI] [PubMed] [Google Scholar]
- 12.Jonker DJ, O'Callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal cancer. N Eng J Med. 2007;357:2040–8. doi: 10.1056/NEJMoa071834. [DOI] [PubMed] [Google Scholar]
- 13.Douillard JY, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28:4697–705. doi: 10.1200/JCO.2009.27.4860. [DOI] [PubMed] [Google Scholar]
- 14.Stec R, Bodnar L, Smoter M, Mączewski M, Szczylik C. Optimal chemotherapy treatment for patients with advanced colorectal cancer. Contemp Oncol (Pozn) 2011;15:31–9. [Google Scholar]
- 15.Peeters M, Price TJ, Cervantes A, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol. 2010;28:4706–13. doi: 10.1200/JCO.2009.27.6055. [DOI] [PubMed] [Google Scholar]
- 16.Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658–64. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 17.Spano JP, Lagorce C, Atlan D, et al. Impact of EGFR expression on colorectal cancer patient prognosis and survival. Ann Onn. 2005;16:102–8. doi: 10.1093/annonc/mdi006. [DOI] [PubMed] [Google Scholar]
- 18.McKay JA, Murray LJ, Curran S, Ross VG, Clark C, Murray GI, Cassidy J, McLeod HL. Evaluation of the epidermal growth factor receptor (EGFR) in colorectal tumours and lymph node metastases. Eur J Cancer. 2002;38:2258–64. doi: 10.1016/s0959-8049(02)00234-4. [DOI] [PubMed] [Google Scholar]
- 19.Mayer A, Takimoto M, Fritz E, Schellander G, Kofler K, Ludwig H. The prognostic significance of proliferating cell nuclear antigen, epidermal growth factor receptor, and mdr gene expression in colorectal cancer. Cancer. 1993;71:2454–60. doi: 10.1002/1097-0142(19930415)71:8<2454::aid-cncr2820710805>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 20.Goldstein NS, Armin M. Epidermal growth factor receptor immunohistochemical reactivity in patients with American Joint Committee on Cancer Stage IV colon adenocarcinoma: implications for a standardized scoring system. Cancer. 2001;92:1331–1346. doi: 10.1002/1097-0142(20010901)92:5<1331::aid-cncr1455>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 21.Saltz LB, Meropol NJ, Loehrer PJ, Sr, Needle MN, Kopit J, Mayer RJ. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol. 2004;22:1201–8. doi: 10.1200/JCO.2004.10.182. [DOI] [PubMed] [Google Scholar]
- 22.Chung KY, Shia J, Kemeny NE, et al. Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol. 2005;23:1803–10. doi: 10.1200/JCO.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 23.Hebbar M, Wacrenier A, Desauw C, Romano O, Cattan S, Triboulet JP, Pruvot FR. Lack of usefulness of epidermal growth factor receptor expression determination for cetuximab therapy in patients with colorectal cancer. Anticancer Drugs. 2006;17:855–7. doi: 10.1097/01.cad.0000217425.44584.9f. [DOI] [PubMed] [Google Scholar]
- 24.Han HS, Chang HJ, Hong YS, Kim SY, Lee KS, Jung KH. Epidermal growth factor receptor expression discrepancies in metastatic colorectal cancer patients treated with cetuximab plus irinotecan-based chemotherapy refractory to irinotecan and oxaliplatin. Dis Colon Rectum. 2009;52:1144–51. doi: 10.1007/DCR.0b013e31819edbf9. [DOI] [PubMed] [Google Scholar]
- 25.Graziano F, Ruzzo A, Loupakis F, et al. Pharmacogenetic profiling for cetuximab plus irinotecan therapy in patients with refractory advanced colorectal cancer. J Clin Oncol. 2008;26:1427–34. doi: 10.1200/JCO.2007.12.4602. [DOI] [PubMed] [Google Scholar]
- 26.Scartozzi M, Bearzi I, Mandolesi A, et al. Epidermal growth factor receptor (EGFR) gene copy number (GCN) correlates with clinical activity of irinotecan-cetuximab in K-RAS wild-type colorectal cancer: a fluorescence in situ (FISH) and chromogenic in situ hybridization (CISH) analysis. BMC Cancer. 2009;9:303. doi: 10.1186/1471-2407-9-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laurent-Puig P, Cayre A, Manceau G, et al. Analysis of PTEN, BRAF, and EGFR status in determining benefit from cetuximab therapy in wild-type KRAS metastatic colon cancer. J Clin Oncol. 2009;27:5924–30. doi: 10.1200/JCO.2008.21.6796. [DOI] [PubMed] [Google Scholar]
- 28.Personeni N, Fieuws S, Piessevaux H, et al. Clinical usefulness of EGFR gene copy number as a predictive marker in colorectal cancer patients treated with cetuximab: a fluorescent in situ hybridization study. Clin Cancer Res. 2008;14:5869–76. doi: 10.1158/1078-0432.CCR-08-0449. [DOI] [PubMed] [Google Scholar]
- 29.Algars A, Lintunen M, Carpén O, Ristamäki R, Sundström J. EGFRgene copy number assessment from areas with highest EGFR expression predicts response to anti-EGFR therapy in colorectal cancer. Br J Cancer. 2011;105:255–62. doi: 10.1038/bjc.2011.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang Z, Li C, Li F, Wang X. EGFR gene copy number as a prognostic marker in colorectal cancer patients treated with cetuximab or panitumumab: a systematic review and metaanalysis. PLoS One. 2013;8(2):56205. doi: 10.1371/journal.pone.0056205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Góralczyk A, Sokołowska-Wojdyło M, Kowalczyk A, Szczerkowska-Dobosz A, Barańska-Rybak W. Case reports Effective therapy of epidermal growth factor receptor inhibitor-associated dermatologic side effects in a patient with metastatic colorectal cancer: a case report and review of literature. Postep Derm Alergol. 2012;29:324–9. [Google Scholar]
- 32.Benvenuti S, Sartore-Bianchi A, Di Nicolantonio F, et al. A Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res. 2007;67:2643–8. doi: 10.1158/0008-5472.CAN-06-4158. [DOI] [PubMed] [Google Scholar]
- 33.Di Fiore F, Blanchard F, Charbonnier F, et al. Clinical relevance of KRAS mutation detection in metastatic colorectal cancer treated by cetuximab plus chemotherapy. Br J Cancer. 2007;96:1166–9. doi: 10.1038/sj.bjc.6603685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lièvre A, Bachet JB, Le Corre D, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66:3992–5. doi: 10.1158/0008-5472.CAN-06-0191. [DOI] [PubMed] [Google Scholar]
- 35.Lièvre A, Bachet JB, Boige V, et al. KRAS mutation as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol. 2008;26:374–9. doi: 10.1200/JCO.2007.12.5906. [DOI] [PubMed] [Google Scholar]
- 36.Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–65. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 37.Domagala P, Hybiak J, Sulzyc-Bielicka V, Cybulski C, Ryś J, Domagala W. KRAS mutation testing in colorectal cancer as an example of the pathologist's role in personalized targeted therapy: a practical approach. Pol J Pathol. 2012;3:145–64. doi: 10.5114/pjp.2012.31499. [DOI] [PubMed] [Google Scholar]
- 38.Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–34. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 39.Tol J, Koopman M, Cats A, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;360:563–72. doi: 10.1056/NEJMoa0808268. [DOI] [PubMed] [Google Scholar]
- 40.Hecht JR, Mitchell E, Chidiac T, et al. A randomised phase IIIb trial of chemotherapy, bevacizumab and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol. 2009;27:672–680. doi: 10.1200/JCO.2008.19.8135. [DOI] [PubMed] [Google Scholar]
- 41.Pentheroudakis G, Kotoula V, De Roock W, et al. Biomarkers of benefit from cetuximab-based therapy in metastatic colorectal cancer: interaction of EGFR ligand expression with RAS/RAF, PIK3CA genotypes. BMC Cancer. 2013;13:49. doi: 10.1186/1471-2407-13-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Roock W, Piessevaux H, De Schutter J, et al. KRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectal cancer treated with cetuximab. Ann Oncol. 2008;19:508–15. doi: 10.1093/annonc/mdm496. [DOI] [PubMed] [Google Scholar]
- 43.Loupakis F, Ruzzo A, Cremolini C, et al. KRAS codon 61, 146 and BRAF mutations predict resistance to cetuximab plus irinotecan in KRAS codon 12 and 13 wild-type metastatic colorectal cancer. Br J Cancer. 2009;101:715–21. doi: 10.1038/sj.bjc.6605177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peeters M, Douillard JY, Van Cutsem E, Siena S, Zhang K, Williams R, Wiezorek J. Mutant KRAS codon 12 and 13 alleles in patients with metastatic colorectal cancer: assessment as prognostic and predictive biomarkers of response to panitumumab. J Clin Oncol. 2013;31:759–65. doi: 10.1200/JCO.2012.45.1492. [DOI] [PubMed] [Google Scholar]
- 45.Van Cutsem E, Köhne CH, Láng I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011–9. doi: 10.1200/JCO.2010.33.5091. [DOI] [PubMed] [Google Scholar]
- 46.Saridaki Z, Tzardi M, Papadaki C, et al. Impact of KRAS, BRAF, PIK3CA mutations, PTEN, AREG, EREG expression and skin rash in ( 2 line cetuximab-based therapy of colorectal cancer patients. PLoS One. 2011;6(1):15980. doi: 10.1371/journal.pone.0015980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Roock W, De Vriendt V, Normanno N, Ciardiello F, Tejpar S. KRAS, BRAF, PIK3CA, and PTEN mutations: implications for targeted therapies in metastatic colorectal cancer. Lancet Oncol. 2011;12:594–603. doi: 10.1016/S1470-2045(10)70209-6. [DOI] [PubMed] [Google Scholar]
- 48.Di Nicolantonio F, Martini M, Molinari F, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol. 2008;26:5705–12. doi: 10.1200/JCO.2008.18.0786. [DOI] [PubMed] [Google Scholar]
- 49.Tol J, Dijkstra JR, Klomp M, et al. Markers for EGFR pathway activation as predictor of outcome in metastatic colorectal cancer patients treated with or without cetuximab. Eur J Cancer. 2010;46:1997–2009. doi: 10.1016/j.ejca.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 50.Vaughn CP, Zobell SD, Furtado LV, Baker CL, Samowitz WS. Frequency of KRAS, BRAF, and NRAS mutations in colorectal cancer. Genes Chromosomes Cancer. 2011;50:307–12. doi: 10.1002/gcc.20854. [DOI] [PubMed] [Google Scholar]
- 51.Schwartzber L, Rivera F, Karthau M. Analysis of KRAS/NRAS mutations in PEAK: a randomized phase 2 study of FOLFOX6 + panitumumab or bevacizumab as 1st-line treatment of wild type (wt) KRAS (exon 2) metastatic colorectal cancer (mCRC) J Clin Oncol. 2013;31(suppl) [abstr.3631] [Google Scholar]
- 52.Patterson SD, Peeters M, Siena S. Comprehensive analysis of KRAS and NRAS mutations as a predictive biomarkers for single agent panitumumab response in a randomized, pase 3 metastatic colorectal cancer (mCRC) study (20020408) J Clin Oncol. 2013;31(suppl) [abstr.3617] [Google Scholar]
- 53.Perrone F, Lampis A, Orsenigo M, et al. PI3KCA/PTEN deregulation contributes to impaired responses to cetuximab in metastatic colorectal cancer patients. Ann Oncol. 2009;20:84–90. doi: 10.1093/annonc/mdn541. [DOI] [PubMed] [Google Scholar]
- 54.Sartore-Bianchi A, Martini M, Molinari F, et al. PIK3CA mutations in colorectal cancer are associated with clinical resistance to EGFR-targeted monoclonal antibodies. Cancer Res. 2009;69:1851–7. doi: 10.1158/0008-5472.CAN-08-2466. [DOI] [PubMed] [Google Scholar]
- 55.De Roock W, Claes B, Bernasconi D, et al. PIK3CA mutations are not a major determinant of resistance to the epidermal growth factor receptor inhibitor cetuximab in metastatic colorectal cancer. Clin Cancer Res. 2009;15:3184–8. doi: 10.1158/1078-0432.CCR-08-2961. [DOI] [PubMed] [Google Scholar]
- 56.De Roock W, Claes B, Bernasconi D, et al. Effects of KRAS, BRAF, NRAS and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic clorocetal cancer: a retrospective consortium analysis. Lancet Oncol. 2010;11:753–62. doi: 10.1016/S1470-2045(10)70130-3. [DOI] [PubMed] [Google Scholar]
- 57.Laurent-Puig P, Cayre A, Manceau G, et al. Analysis of PTEN, BRAF, and EGFR status in determining benefit from cetuximab therapy in wild-type KRAS metastatic colon cancer. J Clin Oncol. 2009;27:5924–30. doi: 10.1200/JCO.2008.21.6796. [DOI] [PubMed] [Google Scholar]
- 58.Loupakis F, Pollina L, Stasi I, et al. PTEN expression and KRAS mutations on primary tumors and metastases in the prediction of benefit from cetuximab plus irinotecan for patients with metastatic colorectal cancer. J Clin Oncol. 2009;27:2622–9. doi: 10.1200/JCO.2008.20.2796. [DOI] [PubMed] [Google Scholar]
- 59.Razis E, Briasoulis E, Vrettou E, et al. Potential value of PTEN in predicting cetuximab response in colorectal cancer: an exploratory study. BMC Cancer. 2008;8:234. doi: 10.1186/1471-2407-8-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khambata-Ford S, Garrett CR, Meropol NJ, et al. Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J Clin Oncol. 2007;25:3230–7. doi: 10.1200/JCO.2006.10.5437. [DOI] [PubMed] [Google Scholar]
- 61.Jacobs B, De Roock W, Piessevaux H, et al. Amphiregulin and epiregulin mRNA expression in primary tumors predicts outcome in metastatic colorectal cancer treated with cetuximab. J Clin Oncol. 2009;27:5068–5074. doi: 10.1200/JCO.2008.21.3744. [DOI] [PubMed] [Google Scholar]
- 62.Baker JB, Dutta D, Watson D, et al. Tumour gene expression predicts response to cetuximab in patients with KRAS wild-type metastatic colorectal cancer. Br J Cancer. 2011;104:488–95. doi: 10.1038/sj.bjc.6606054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oliveras-Ferraros C, Massaguer Vall-Llovera A, Carrion Salip D, et al. Evolution of the predictive markers amphiregulin and epiregulin mRNAs during long-term cetuximab treatment of KRAS wild-type tumor cells. Invest New Drugs. 2012;30:846–52. doi: 10.1007/s10637-010-9612-2. [DOI] [PubMed] [Google Scholar]
- 64.Ohchi T, Akagi Y, Kinugasa T, et al. Amphiregulin is a prognostic factor in colorectal cancer. Anticancer Res. 2012;32:2315–21. [PubMed] [Google Scholar]
- 65.Yang X, Zhang X, Mortenson ED, Radkevich-Brown O, Wang Y, Fu YX. Cetuximab-mediated tumor regression depends on innate and adaptive immune responses. Mol Ther. 2013;21:91–100. doi: 10.1038/mt.2012.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang W, Gordon M, Schultheis AM, et al. FCGR2A and FCGR3A polymorphisms associated with clinical outcome of epidermal growth factor receptor expressing metastatic colorectal cancer patients treated with single-agent cetuximab. J Clin Oncol. 2007;25:3712–8. doi: 10.1200/JCO.2006.08.8021. [DOI] [PubMed] [Google Scholar]
- 67.Bibeau F, Lopez-Crapez E, Di Fiore F, et al. Impact of Fc{gamma}RIIa-Fc{gamma}RIIIa polymorphisms and KRAS mutations on the clinical outcome of patients with metastatic colorectal cancer treated with cetuximab plus irinotecan. J Clin Oncol. 2009;27:1122–9. doi: 10.1200/JCO.2008.18.0463. [DOI] [PubMed] [Google Scholar]
- 68.Rodríguez J, Zarate R, Bandres E, et al. Fc gamma receptor polymorphisms as predictive markers of Cetuximab efficacy in epidermal growth factor receptor downstream-mutated metastatic colorectal cancer. Eur J Cancer. 2012;48:1774–80. doi: 10.1016/j.ejca.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 69.Pander J, Gelderblom H, Antonini NF, Tol J, van Krieken JH, van der Straaten T, Punt CJ, Guchelaar HJ. Correlation of FCGR3A and EGFR germline polymorphisms with the efficacy of cetuximab in KRAS wild-type metastatic colorectal cancer. Eur J Cancer. 2010;46:1829–34. doi: 10.1016/j.ejca.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 70.Lurje G, Nagashima F, Zhang W, et al. Polymorphisms in cyclooxygenase-2 and epidermal growth factor receptor are associated with progression-free survival independent of K-ras in metastatic colorectal cancer patients treated with single-agent cetuximab. Clin Cancer Res. 2008;14:7884–95. doi: 10.1158/1078-0432.CCR-07-5165. [DOI] [PubMed] [Google Scholar]
- 71.López-Albaitero A, Lee SC, Morgan S, Grandis JR, Gooding WE, Ferrone S, Ferris RL. Role of polymorphic Fc gamma receptor IIIa and EGFR expression level in cetuximab mediated, NK cell dependent in vitro cytotoxicity of head and neck squamous cell carcinoma cells. Cancer Immunol Immunother. 2009;58:1853–62. doi: 10.1007/s00262-009-0697-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pander J, Gelderblom H, Antonini NF, Tol J, van Krieken JH, van der Straaten T, Punt CJ, Guchelaar HJ. Correlation of FCGR3A and EGFR germline polymorphisms with the efficacy of cetuximab in KRAS wild-type metastatic colorectal cancer. Eur J Cancer. 2010;46:1829–34. doi: 10.1016/j.ejca.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 73.Kang X, Patel D, Ng S, et al. High affinity Fc receptor binding and potent induction of antibody-dependent cellular cytotoxicity (ADCC) in vitro by anti-epidermal growth factor receptor antibody cetuximab. J Clin Oncol. 2007;18:3041. [Google Scholar]
- 74.Barriere J, Fischel J, Formento P, et al. Cetuximab-mediated antibody-dependent cellular cytotoxicity (ADCC) against tumor cell lines characterized for EGFR expression and K-ras mutation. J Clin Oncol. 2009;27:14583. [Google Scholar]
- 75.Patel D, Guo X, Ng S, et al. IgG isotype, glycosylation, and EGFR expression determine the induction of antibody-dependent cellular cytotoxicity in vitro by cetuximab. Hum Antibodies. 2010;19:89–99. doi: 10.3233/HAB-2010-0232. [DOI] [PubMed] [Google Scholar]
- 76.Patel D, Saxena B, Zhou Q, et al. Differential induction of antibody-dependent cellular cytotoxicity (ADCC) against human EGFR-expressing NSCLC cell lines by necitumumab, cetuximab, and panitumumab. J Clin Oncol. 2011;29:21075. [Google Scholar]