Abstract
Development of metastasis in peripheral tissues is a major problem in the fight to cure breast cancer. Although it is becoming evident that chronic inflammation can contribute to tumor progression and metastasis, the effect of acute inflammation in primary tumor is less known. Using mouse models for breast cancer here we show that biopsy of mammary tumors increases the frequency of lung metastases. This effect is associated with the recruitment of inflammatory cells to the lung and elevated levels of certain cytokines such as IL-6 in the lung airways. Antiinflammatory treatment prior to and after the biopsy reduces the development of metastases triggered by the biopsy. In addition, while lack of IL-6 does not affect primary tumor development, it protects from increasing number of metastases upon biopsy. Thus, our studies show that in addition to chronic inflammation, acute immune response caused by invasive procedures in the primary tumor may cause an increased risk on peripheral metastases, but the risk could be decreased by anti-inflammatory treatments.
INTRODUCTION
Breast cancer is the second leading cause of cancer death among women. The areas of diagnosis and therapy have significantly improved relative to other types of cancer. However, despite the number of advances in screening, prevention and treatment, a portion of patients still present with or develop late stage disease resulting in increased mortality. The rate of breast cancer recurrence and death remains high with over 25,000 deaths in 2011 in the USA alone. Metastases were once believed to follow a sequential pattern from primary tumor to regional lymph nodes and then to the distant sites [1]. However, recent evidence suggests that tumor metastases are a far more complex system that does not necessarily follow this ordered pattern. Current models propose that the development of metastasis in distant organs requires additional events than just having circulating cancer cells. Primary tumor cells undergo changes that increase the potential for these cells to seed distant organs. In addition, a hospitable niche (“soil”) in the distant organs is required for disseminated tumor cells which migrate into these organs, to successfully survive and proliferate [2, 3]. This “seed and soil” hypothesis that was initially proposed over two centuries ago [4] can explain why not all breast cancer patients with circulating cancer cells develop metastasis, and why distant metastases may develop long after the primary tumor was diagnosed and treated.
A factor that can clearly affect the environment of the tissues is the inflammatory response. The association between chronic inflammation and cancer has been most evident in diseases such as ulcerative colitis, which carries a significantly elevated risk of colon cancer [5, 6]. Recent studies have shown that chronic arthritis in mice leads to an enhanced frequency of mammary tumors and metastasis [7, 8]. It is becoming evident through animal studies that selective types of immune responses and immune cells can promote tumor progression more than others. The mechanisms are variable and in some cases unclear. Among innate immune cells, tumor resident macrophages (M2 type) have been shown to contribute to angiogenesis by secreting VEGF and EGF [9]. A number of studies both in mouse and human subjects indicate that neutrophils, a key component of the innate immune system, contribute to tumor progression and metastasis in part through the secretion of elastase [10–12]. Within the adaptive immune responses, cytotoxic CD8 T cells and CD4 Th1 cells can mediate antitumor activities, while CD4 Th2 cells are known to promote tumor progression in breast cancer through secretion of cytokines such as IL-13 [13, 14]. It is well known that the antibody response mediated by B cells is not highly effective as an antitumor immune response, but recent studies indicate that B cells can favor progression of some tumors (e.g. skin, prostate) through secretion of cytokines such as lymphotoxin β and other cytokines [15–17]. Since the recruitment of some of the immune cells, such as neutrophils, is a cardinal feature of acute inflammatory responses, acute inflammation in the tumor may also provide an environment that favors tumor growth or metastasis.
Surgery is an important component of the modern, multimodality approach to breast cancer management, and is potentially curative especially in earlier stages of disease. With the advent of widespread breast cancer screening, a greater number of patients present with stage I and II breast cancer amenable to surgical resection. As any other surgical procedure, excision of tumors triggers an acute inflammatory immune response that contributes to the natural wound healing process. This acute inflammatory response has normally minimal consequences in tumor progression since the tumor cells have already been removed. Biopsies are another common surgical procedures used in the diagnosis of breast cancer, and provide highly valuable information for prognosis and treatment. The wound generated by the biopsy, primarily core biopsies, also triggers an acute inflammatory response within the tumor. It remains unclear whether this inflammatory response could have an impact in tumor progression and/or development of metastasis in breast cancer, and whether this may be an independent risk factor in clinical settings.
To address whether biopsies and surgery may influence the risk of metastasis, using a mouse model of mammary cancer we have investigated the effect of biopsy in the frequency of metastases and whether this effect is mediated by the inflammatory response triggered within the tumor or organs that are invaded by tumor cells. We show here that the biopsy of mammary tumors increases the development of micrometastases in the lung in association with the recruitment of neutrophils. Anti-inflammatory agents significantly reduced the frequency of metastasis induced by biopsies. Thus, any potential risk of acute inflammatory induced metastasis may be able to be mitigated through the use of specific anti-inflammatory treatment.
MATERIAL AND METHODS
Mice
The MMTV-PyMT transgenic mice [18] and null IL-6 knockout (KO) mice [19] have been previously described. All mice were housed under pathogen-free conditions at the animal care facility at the University of Vermont. The procedures were approved by the University of Vermont Institutional Animal Care and Use Committee. Survival curves were determined by the time mice need to be euthanized due to overall tumor mass and institutional approved requirements.
Mammary Tumor Biopsies
For “Biopsy mice”, MMTV-PyMT mice were anesthetized with isoflurane and a biopsy of the tumor was performed using a surgical punch biopsy needle (2mm diameter). The small wound in the skin produced by the biopsy was sutured using a single interrupted stitch. For “Control mice”, MMTV-PyMT mice were anesthetized with isoflurane and skin on the tumor was sutured using a single interrupted stitch. Prior to the biopsy, the size of the tumor for the majority of the mice was between 0.15–0.25 cm3. In a few mice, the tumor size was slightly larger but not more than 0.5 cm3. Control and Biopsy mice were always paired according to tumor size at the time of the biopsy. For the ibuprofen treatment, MMTV-PyMT mice received an i.p. administration of ibuprofren (50 mg/Kg) or vehicle in 100 μl four hours before the biopsy, and one daily dose for the next three consecutive days.
Tumor histology
Three or eight days after the biopsy, the mice were euthanized, and tumors containing the biopsy wound were harvested, fixed in formalin and paraffin embedded. Tissue sections were used for staining for hematoxylin and eosin (H & E) or for immunohistochemistry.
Bronchoalveolar lavage (BAL) collection, cell differential and lung histology
For BAL, cold PBS (1 ml) was instilled into the lungs as previously described [20, 21]. For cell differential, 100 μl of BAL were cytospun and stained with Hema-3 (Biochemical Sciences). A hundred cells per high power field were counted and classified as macrophages or neutrophils, by cell morphology and staining. For BAL fluid (BALF), cells were spun, and supernatant was recovered for cytokine determination. After recovering BAL, lungs were inflated with formalin, fixed and embedded in paraffin. Sections were stained for H & E according to routine procedures, or for immunohistochemistry.
Tumor growth and metastases
Tumor volume was measured every other day starting the day after the biopsy, through a caliber and following the formula (width × length × height)/2). For quantification of lung metastases, tissue sections of the paraffin embedded blocks containing all lobes of the lung were H & E stained, and the number of micrometastases per slide was counted. The number of metastases in three sequential sections of the blocks was highly consistent.
Immunohistochemistry
Formalin-inflated lungs and primary tumors were fixed in formalin and embedded in paraffin. Sections cut from paraffin embedded tissues underwent antigen retrieval (DAKO) and stained with antibodies against CD45 (BD Pharmingen), cytokeratin 8 (CK8) (Novus Biologicals), or Ki67 (Thermo Fisher) followed by a secondary antibody conjugated to peroxidase. 3,3′-diaminobenzidine (DAKO) was used as a substrate to develop the staining as we previously described [22]. Histological images were taking at a Olympus BX50 light microscope with an Optronics Magnafire digital camera.
ELISA
Detection of IL-6 in BALF was performed using the mouse IL-6 Duoset according to manufacturer’s instructions (R&D Systems) as we previously described [20].
Statistical Analysis
Time courses of tumor growth were analyzed using a random coefficients model with time included as a quadratic term. Survival curve was analyzed using the log-rank test. All other analysis was performed using the student’s t test. p< 0.05 was considered significant. Error bars represent SEM
RESULTS
A sustained inflammatory response in the tumor triggered by a biopsy is associated with a localized increased proliferative capacity of cancer cells
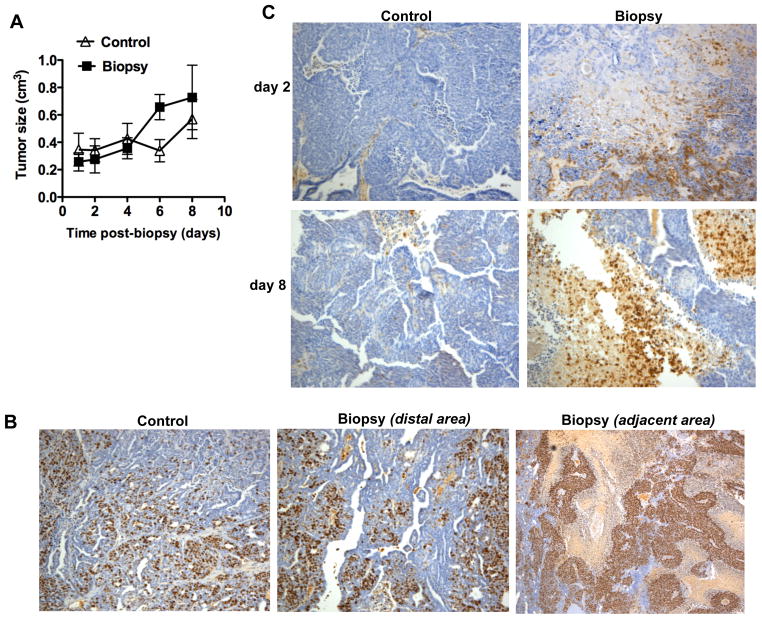
A breast biopsy, a common procedure used in breast cancer diagnosis, can induce the recruitment of inflammatory cells to the tumor as part of the natural wound-healing response. To address whether tumor biopsy may have an effect on tumor progression, we used MMTV-PyMT transgenic mice [18] as a mammary tumor mouse model for breast cancer. In MMTV-PyMT mice, the middle T (MT) antigen of the polyomavirus is expressed under the control of the MMTV (Mouse Mammary Tumor Virus) promoter/enhancer, which drives expression specifically in mammary epithelial cells [18]. We performed a live surgical punch biopsy followed by a surgical stitch on the skin, under anesthesia. Control mice underwent an identical procedure except there was no needle penetration of the tumor, but they also had the surgical stitch on the skin. In order to assess the impact of a biopsy on tumor growth, the size of the targeted tumor was measured over time after the biopsy for a total of eight days. There was only a marginal, but statistically significant, increase in the rate of growth of the tumor in mice that underwent biopsy compared with control mice (Fig. 1A).
Figure 1. Effect of mammary tumor biopsy in tumor progression.
(A) Size of the tumors that underwent biopsy (Biopsy) and control tumors (Control) over time after biopsy (n=6 mice). Tumor growth rate was slightly increased in mice undergoing biopsy (p < 0.05), as determined by random coefficient analysis with time included as a quadratic term. (B) Immunohistochemistry staining for Ki67 in tumors isolated from “Control mice” (control) and “Biopsy mice” (Biopsy) 8 days after biopsy. For “Biopsy mice”, an area distal to the biopsy and an area adjacent to the biopsy site within the same tumor are shown. 100x magnification. (C) Immunohistochemistry staining for CD45 in sections of tumors isolated from Control mice and Biopsy mice that had biopsy 2 days or 8 days prior to tissue harvesting. 100 × magnification.
To address whether the biopsy could have an effect on the proliferative capacity of the tumor cells, we performed immunohistochemical staining for Ki67, a standard clinical parameter of proliferation in cancer, in section of tumors harvested eight days after the biopsy. The relative frequency of Ki67 positive cells in the biopsy-tumors at a region distal from the biopsy wound was comparable to the frequency in tumors from control mice (Fig. 1B). However, an increased ratio of proliferating cells was found in the tumor area adjacent to the biopsy (Fig. 1B), suggesting that either the presence of inflammatory cells, physical disruption of the tumor or increased blood supply caused by the biopsy could promote the growth of the tumor cells.
We therefore examined the presence of inflammation in the tumor after the biopsy as well as in tumors from control mice by immunohistochemical staining with CD45, a marker present in all leukocytes. No large foci of CD45 cells were found in the control tumors but, as expected, there was an accumulation of infiltrating CD45 cells within the area of the tumor where the biopsy was taken, two days following the biopsy (Fig. 1C). Interestingly, eight days after the biopsy procedure infiltrating leukocytes were still present in the tumors (Fig. 1C). Since the external biopsy wound on the skin was fully repaired within two to three days after the biopsy these data indicate that inflammation last longer than the typical acute response. Cytokines and other factors secreted by the inflammatory cells in the proximity of the biopsy could provide a proliferative signal for the tumor cells. Inflammatory cells in the proximity of the biopsy wound are also found in human breast cancer and they are used as a clinical parameter to identify the biopsy location in the tumor after surgery.
Mammary tumor biopsies increase the frequency of lung metastases
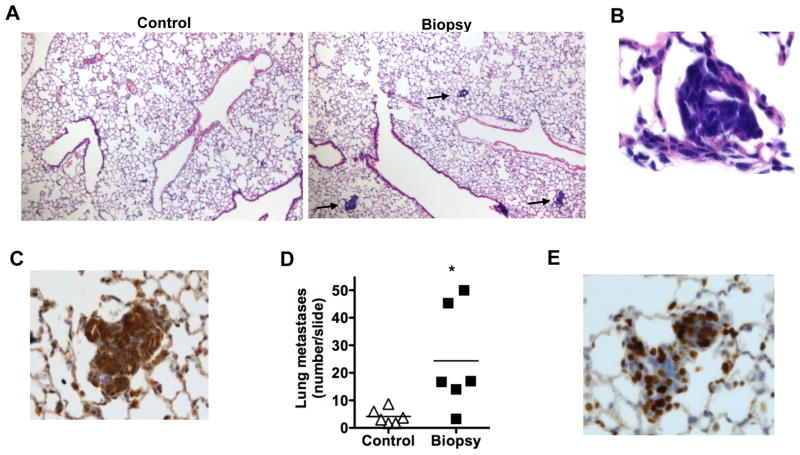
In human breast cancer, the most negative and devastating outcome of a primary tumor is metastasis in specific tissues (e.g. lung, brain, bone). It remains unclear what factors determine the risk of metastasis, although soluble factors such VEGF and inflammatory cytokines have been shown to contribute. We determined whether a biopsy could also have an effect in the development of metastasis. MMTV-PyMT mice are known to develop a low, but detectable, number of metastases in the lung [18]. Eight days after biopsy, lungs were inflated, fixed and stained for H&E. Histological analysis of lung sections from biopsy mice revealed an abundant presence of tumor micrometastases, visualized as a small, dense and intensely stained blue area in the lung (Fig. 2A). A higher magnification further displayed the compact cellularity of the micrometastases (Fig. 2B). Immunohistological staining of lung sections for cytokeratin 8 (CK8), a marker for mammary epithelial tumor cells [23], confirmed the presence of tumor cells in these metastases (Fig. 2C). Interestingly, quantification of the number of metastases per whole lung section showed a prominent increase (3–5 fold) of lung metastases in the mice that underwent biopsy compared with control mice (Fig. 2D). The most abundant micrometastases present in the lungs of biopsy-mice were smaller than the few preexisting metastases found in the control mice, suggesting that the former were newly generated metastases. To address the proliferative capacity of tumor cells in these metastases, lung sections from the mice with biopsy were immunohistochemically stained for Ki67. High frequency of proliferating cells was present in the micrometastases (Fig. 2E). Together, these results indicate that surgical biopsy in mammary tumors can largely increase the frequency of lung metastases.
Figure 2. Increased number of metastases in the lung upon biopsy of the mammary tumor.
(A) H & E sections of lungs from Control mice and Biopsy mice 8 days after the biopsy. Micrometastases are displayed as highly dense nucleated (strong blue) spots within the parenchyma of the lung. The arrow points to representative micrometastases. 100x magnification. (B) A higher magnification (400x) of a micrometasis in the lung of Biopsy mice 8 days after biopsy, H & E staining. (C) Immunohistochemistry staining for CK8 on a metastasis in the lung of Biopsy mice 8 days after biopsy (200x magnification). (D) The number of tumor metastases in a whole lung histological section (H & E staining) from Control and Biopsy mice (n=6) 8 days after biopsy. *, denotes p <0.05, as determined by student’s t test analysis. (E) Immunohistochemistry staining for Ki67 on a metastasis in the lung of Biopsy mice 8 days after biopsy (200x magnification).
Biopsy of mammary tumors triggers a rapid recruitment of inflammatory cells to the lung
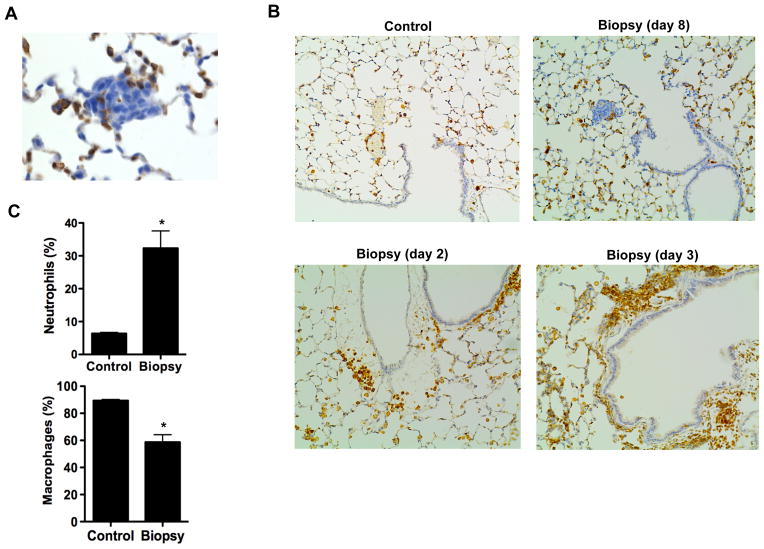
The development of cancer metastasis in peripheral tissues requires the appropriate environment in the targeted tissue that facilitates the seeding, survival and proliferation of the circulating tumor cells. Considering the inflammatory response in the tumor even eight days after biopsy, we examined the presence of inflammatory cells in the proximity of the micrometastases in the lung. Immunostaining for CD45 of lung sections from mice eight days after biopsy showed inflammatory cells present within the micrometastases (Fig. 3A), suggesting that immune cells might have also been recruited to the lung in addition to the tumor cells. No prominent inflammation in the lungs of mice eight days after the biopsy was detected as determined by H&E stained sections (Fig. 1B), or by immunostaining analysis for CD45 (Fig. 3B). However, immunostaining analysis for CD45 in lungs from mice two and three days after biopsy revealed a clear accumulation of inflammatory cells, not present in lungs from control mice (Fig. 3B).
Figure 3. Biopsy of mammary tumors leads to a rapid inflammatory response in the lung.
(A) Immunohistochemistry analysis for CD45 in sections of the lung of Biopsy mice 8 days after biopsy showing the presence of CD45 cells within a micrometastasis. 400x magnification. (B) Immunohistochemistry analysis for CD45 in lung sections of Control mice and Biopsy mice 2, 3 or 8 days after biopsy. 200x magnification. (C) Percentage of macrophages and neutrophils in BAL of Control mice and Biopsy mice (n=3) 3 days after biopsy. *, denotes p < 0.05, as determined by student’s t test analysis.
To further demonstrate an acute recruitment of inflammatory cells into the lung triggered by the biopsy, we examined the type of inflammatory cells in bronchoalveolar lavage (BAL) obtained from mice three days after biopsy or from control mice (undergoing the same procedure). It is well established that alveolar macrophages are the most abundant immune cell type in BAL (lung resident macrophages) in physiological conditions. Accordingly, in the control mice that did not have biopsy, macrophages were the predominant cell population, with a low percentage of neutrophils (Fig. 3C). The presence of macrophages in the airways was not increased, but slightly diminished in mice that had biopsy (Fig. 3C). However, the percentage of neutrophils in the lung airways drastically increased upon biopsy (3–4 fold) (Fig. 3C). Since neutrophils have a short half-life, the high frequency of neutrophils in the lungs of biopsy-mice is indicative of an ongoing inflammatory response in the lung of these mice as a result of the biopsy.
Administration of anti-inflammatory drugs diminishes the increased risk of metastasis caused by the tumor biopsy
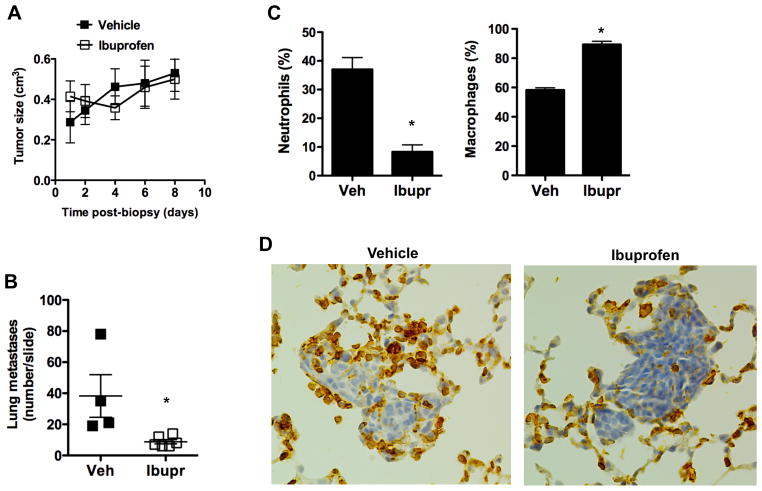
The presence of inflammation both in the tumor and lungs in response to the tumor biopsy suggested that inflammatory response could contribute to tumor growth and/or increased lung metastases caused by the biopsy. We therefore investigate whether the administration of anti-inflammatory drugs could prevent the pro-metastatic effect of the biopsy. Mice that underwent biopsy were divided in two groups that received either ibuprofen or vehicle for three consecutive days after the biopsy. The size of the tumors that had the biopsy was measured over time. There was only a slight delay in the growth of the biopsy-tumors in mice that received ibuprofen and the mice that received vehicle (Fig. 4A).
Figure 4. Anti-inflammatory treatment prevents the increase in lung metastases upon biopsy.
(A) Tumor size at different times after biopsy in mice that received vehicle or ibuprofen (n= 4). There was a slight but statistical significant (p <0.05) decreased in tumor growth with ibuprofen as determined by random coefficient analysis with time included as a quadratic term. (B) The number of tumor metastases in a whole lung histological section (H & E staining) from Biopsy mice (8 days after biopsy) that received ibuprofen (n=5) or vehicle (n=4). *, denotes p <0.05, as determined by student’s t test. (C) Percentage of macrophages and neutrophils in BAL of Biopsy mice (3 days after biopsy) that receive ibuprofen or vehicle (n= 3). *, denotes p < 0.05, as determined by student’s t test analysis. (D) Immunohistochemistry analysis for CD45 in lung sections of Biopsy mice (3 days after biopsy) that received ibuprofen or vehicle. 200x magnification.
Tissue harvesting was conducted 8 days post-biopsy and the number of lung metastases was measured using whole lung histological sections as described above. The number of lung metastases found in mice that underwent biopsy and received vehicle only (Fig. 4B) was comparable to the number of metastases in mice that underwent biopsy with no other administration (Fig. 2B). Interestingly, the number of lung metastases in mice that underwent biopsy and received ibuprofen was markedly lower than in mice administered with vehicle (Fig. 4B), indicating that the increased development of metastasis promoted by biopsy could be minimized by administration of anti-inflammatory medication.
To determine whether the decreased frequency of metastases in the lung obtained by ibuprofen administration was associated with reduced inflammation in the lung, mice underwent biopsy and administered either with vehicle or ibuprofen. After three days, BAL was harvested and the presence of neutrophils was determined. Administration of ibuprofen reduced the presence of neutrophils in the lung (Fig. 4C). We also examined the effect of ibuprofen on the presence of CD45 positive cells in the proximity of lung metastases eight days after biopsy. Fewer inflammatory cells appeared to localize at the remaining metastases in mice that received ibuprofen (Fig. 4D). Together, these results show that inflammation triggered by the biopsy of tumors contributes to increased risk of lung metastasis likely by promoting the recruitment of inflammatory cells such as neutrophils to the lung.
IL-6 contributes to the increased frequency of lung metastases caused by the biopsy of mammary tumors
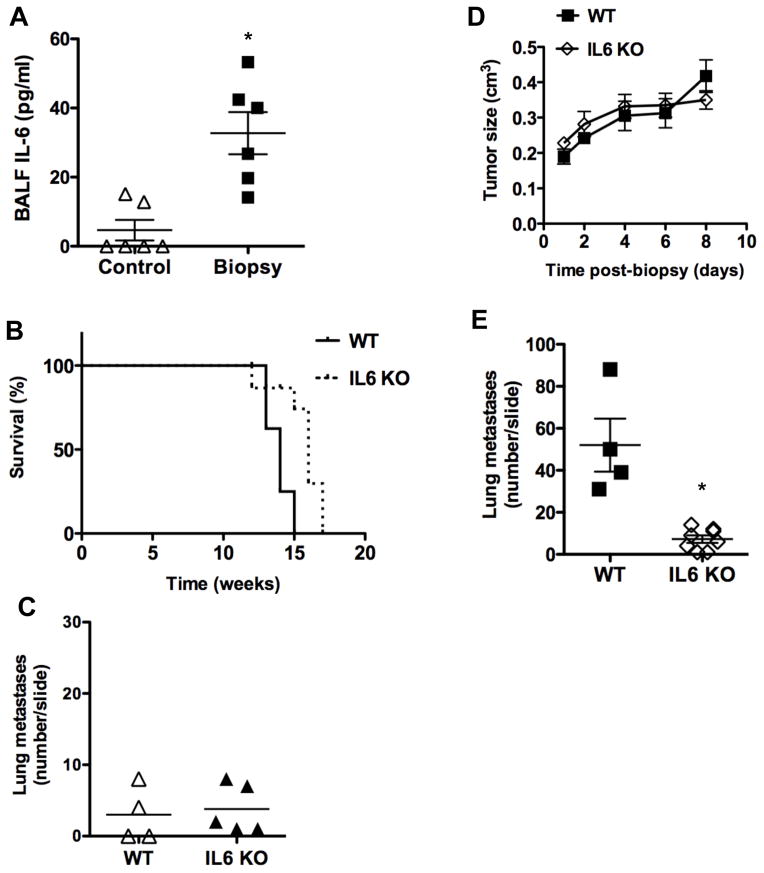
Since some inflammatory cytokines are known to provide survival signals to breast cancer tumor cells, we examined the levels of predominant inflammatory cytokines in BAL fluid (BALF) in control mice or mice undergoing tumor biopsy. No IL-1 or TNFα could be detected in BALF (data not shown). In contrast, we found elevated levels of IL-6 in BALF from mice that underwent biopsy relative to control mice (Fig. 5A).
Figure 5. IL-6 contributes to the increased frequency of lung metastases caused by the biopsy of mammary tumors.
(A) IL-6 production in BALF of Control mice and Biopsy mice (n=6) 3 days after biopsy. *, denotes p < 0.05, as determined by student’s t test analysis. (B) Kaplan-Meier survival curve of untreated MMTV-PyMT (WT) and IL-6 KO x MMTV-PyMT (IL6 KO) mice (n=8). p <0.05 as determined by log-rank test. (C) Number of spontaneous lung metastases in untreated MMTV-PyMT (WT) mice (n=4) and IL-6 KO x MMTV-PyMT (IL6 KO) mice (n=5). No statistically significant difference between WT and IL-6 KO mice was found (p= 0.75) as determined by student’s t test. (D) Tumor size at different times after biopsy in WT (n=4) and IL-6 KO mice (n=7). There was no statistical significant difference in tumor growth, as determined by random coefficient analysis. (E) The number of tumor metastases in a whole lung histological section (H & E staining) from WT mice (n=4) or IL-6 KO mice (n=7) 8 days after biopsy. *, denotes p <0.05, as determined by student’s t test.
IL-6 is now emerging as a cytokine that contributes to tumor progression in some types of cancer such as colon cancer and liver cancer, and there is evidence suggesting that IL-6 can also contribute to metastasis [24–26]. To address a potential role of IL-6 in the effect of biopsy on tumor growth and metastases, we crossed MMTV-PyMT mice with null IL-6 deficient mice. We first examined whether the absence of IL-6 affect mammary tumor development in these mice by examining survival of the wildtype MMTV-PyMT mice and IL6 KO MMTV-PyMT mice. There was just a slight increase (about 1–2 weeks) in the survival curve in IL-6 KO MMTV-PyMT mice compared with the survival in wildtype MMTV-PyMT mice (Fig. 5B). Analysis of the number of spontaneous metastases in the lung at base line prior to any procedure was no different between IL-6 KO and wildtype MMTV-PyMT (Fig. 5C), indicating that the development of metastasis in the absence of an acute inflammation was not dependent on IL-6. We performed tumor biopsy in wildtype and IL-6 KO mice and examined both tumor growth and lung metastases. There was no detectable difference in tumor growth over time between wildtype and IL-6 KO mice after the biopsy (Fig. 5D). However, the number of micrometastases in the lung eight days after the biopsy was markedly reduced in IL-6 KO mice compared with wildtype mice (Fig. 5E). Thus, IL-6 contributes to the increased risk in the development of mammary tumor cell metastasis after biopsy.
DISCUSSION
Using mouse models of mammary cancer, our study demonstrated that performing a biopsy of mammary tumors increases the development of lung metastasis. Biopsy of the primary mammary tumor had only a minor impact on primary tumor growth itself, but it severely increased the development of micrometastases in the lung in association with the recruitment of neutrophils. Anti-inflammatory drugs as well as IL-6 deficiency significantly reduced the risk of metastasis caused by biopsies.
Surgery is a standard modality in the treatment of breast cancer, including total mastectomy and more frequently lumpectomy. The surgical procedure clearly causes a local immune response at the tumor site that attracts inflammatory cells capable of producing different cytokines. Thus, if residual tumor remains at the site of surgery, the inflammation caused by the surgical procedure could increase the risk of proliferation leading to local or distant recurrence. Some data suggest that positive margins following initial breast cancer surgery may be associated with increased risk of recurrence, despite subsequent surgery for margin control. Although speculative, it is possible that proliferation of residual tumor cells in the lumpectomy bed in response to wound healing cytokine following initial surgery may play a role in this phenomenon. Likewise, breast biopsies, which are a standard method of diagnosis in breast cancer, may have a similar impact on tumor proliferation and metastasis in certain cases, especially if there is a long delay between the time of biopsy and definitive surgery. It remains unclear whether such a risk may exist.
Importantly, however, our study also demonstrated that the increased risk in metastasis as a consequence of a wound healing in the primary tumor can be substantially diminished by treatment with standard anti-inflammatory drugs. The fact that ibuprofen was provided only for the first three days after biopsy and reduced the number of metastases indicates that it is the early inflammatory response that contributes to the establishment of the metastases. Thus, not only chronic, but also acute inflammation within the tumor could affect the risk of breast cancer metastases. This model could contrast with previous hypotheses proposing that surgery can have a suppressive effect on immunosurveillance and therefore tumor cells have an opportunity to escape from immune response [27]. However, our data support the concept of inflammation being a factor that favors tumor progression and metastasis. A number clinical studies and mouse models have shown an association of chronic inflammatory diseases and risk of cancer and/or cancer progression [28]. In addition, more recent studies in mouse models have demonstrated that breast cancer-associated metastasis is significantly increased in models of autoimmune arthritis [7, 8].
In addition to tumor cells being released from the primary tumor into circulation, an appropriate microenvironment in the targeted tissue is needed for tumor cells to “seed” peripheral tissues and establish micrometastases [2, 3]. Interestingly, our studies show for the first time that wound healing in mammary tumors causes an inflammatory response in the lung, as determined by the presence of an acute accumulation of neutrophils as well as by the presence of IL-6 in the airways. Lung inflammation is associated with the development of micrometastasis in the parenchyma of the lung and infiltrated immune cells are in a tight contact with tumor cells in the micrometastasis. It is therefore possible that it is the inflammatory state of the lung that facilitates the establishment of metastases. Neutrophils have been associated with poor clinical outcome and decreased survival in some cancers including breast cancer [10–12]. The presence of neutrophil elastase in breast cancer also correlates with poor outcome [29, 30]. It has been suggested that neutrophils may be recruited in part by tumor cells. In exchange, neutrophils could contribute to cancer progression through the production of elastase, MMPs, cytokines and reactive oxygen species, among others [31]. A close interaction in the lung of neutrophils with recent cancer cell emigrants from the primary tumor after the biopsy could provide a protective environment that facilitates the development of the metastases. Future studies should address the potential effect of acute inflammation (e.g. triggered by infection or injury) may have on the development of metastasis.
IL-6 was first identified as a proinflammatory marker [32]. Although for more than two decades there have been a number of reports describing increased levels of IL-6 in serum and tumors of different cancer patients, until recently this cytokine was not considered to be a player in cancer. A number of recent studies in animal models and humans have revealed IL-6 as a regulator of cancer progression primarily in colon and liver cancer [24, 33, 34]. Using our mouse model of mammary tumor, IL-6 alone did not seem to be a major contributor of tumor progression or spontaneous metastasis in the lung. A previous study has reported that IL-6 deficiency impairs mammary tumorigenesis [35], however a different mouse mammary tumor model (Neu-MMTV transgenic mice) and different IL-6 KO mice (not null mice) were used. Nevertheless, we show here that IL-6 is a key factor for the dramatic increase in lung metastasis in response to the biopsy of the tumor. In addition, we also found increased levels of IL-6 in the lung airways (BAL), correlating with the presence of inflammation in the lung. Thus, these results suggest that IL-6 may not be an oncogenic factor itself, but in the context of inflammation it could be a key factor that facilitates the seeding of breast cancer cells in peripheral tissues and the formation of metastasis. While IL-6R expression is primarily restricted to leukocytes and hepatocytes, the presence of soluble IL-6R (sIL-6R) in association with IL-6 can trigger the IL-6 trans-signaling pathway in almost any cells including tumor cells [36]. One of the major sources of sIL-6R is neutrophils through the presence of metalloproteases that mediate the cleavage [36]. Thus, it is possible that the major effect of IL-6 in breast cancer cells is provided by sIL-6R secreted by inflammatory cells. This pathway could facilitate the formation of metastasis in the lung where neutrophils accumulate after the biopsy.
In summary, our study shows that in a mammary mouse model acute inflammation triggered by a biopsy, as part of the natural wound repair response, can enhance the risk of developing peripheral metastasis. The increase in metastases is likely due to the combine effect of inflammation in the primary tumors as well as in targeted organs, favoring the seeding of released tumor cells. Nevertheless, our results also indicate that anti-inflammatory treatment for a few days after biopsy may reduce the risk of metastasis. Further investigating the potential role wound healing has on tumor progression and identifying strategies that could mitigate this process could have a major impact on breast cancer management and survival. Developing anti-inflammatory drugs that have minimal effect on hemostasis might be a potential adjunctive therapy to combine with surgical procedures.
Acknowledgments
This work was funded by Cabot-Wellington Foundation (M.R.), Lake Champlain Cancer Research Organization (M.R.), and P30 RR031158 (M.R.). We would like to thank the Microscopy Imaging Facility (University of Vermont, Burlington, VT) for the advise on immunohistochemistry analysis, Dr. Charles Irvin (University of Vermont) for the use of microscope for images and helpful discussion, Dr. Daniel Weis (University of Vermont) for the use of the Ki67 antibody, and Dr. Marta Cañamero (CNIO, Madrid, Spain) for helping with the pathology.
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest
References
- 1.Grange JM, Stanford JL, Stanford CA. Campbelol De Morgan’s “observations on cancer”, and their relevance today. J R Soc Med. 2002;95:296–299. doi: 10.1258/jrsm.95.6.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Comen EA. Tracking the seed and tending the soil: evolving concepts in metastatic breast cancer. Discov Med. 2012;14:97–104. [PubMed] [Google Scholar]
- 3.Psaila B, Kaplan RN, Port ER, Lyden D. Priming the ‘soil’ for breast cancer metastasis: the pre-metastatic niche. Breast Dis. 2006;26:65–74. doi: 10.3233/bd-2007-26106. [DOI] [PubMed] [Google Scholar]
- 4.Paget S. The distribution of secndary growths in cancer of the breast. Cancer Metastasis Rev. 1889;8:98–101. [PubMed] [Google Scholar]
- 5.Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–2114. e2105. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 6.Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology. 2011;140:1807–1816. doi: 10.1053/j.gastro.2011.01.057. [DOI] [PubMed] [Google Scholar]
- 7.Roy LD, Ghosh S, Pathangey LB, Tinder TL, Gruber HE, Mukherjee P. Collagen induced arthritis increases secondary metastasis in MMTV-PyV MT mouse model of mammary cancer. BMC Cancer. 2011;11:365. doi: 10.1186/1471-2407-11-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das Roy L, Pathangey LB, Tinder TL, Schettini JL, Gruber HE, Mukherjee P. Breast-cancer-associated metastasis is significantly increased in a model of autoimmune arthritis. Breast Cancer Res. 2009;11:R56. doi: 10.1186/bcr2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruffell B, Affara NI, Coussens LM. Differential macrophage programming in the tumor microenvironment. Trends Immunol. 2012;33:119–126. doi: 10.1016/j.it.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jensen HK, Donskov F, Marcussen N, Nordsmark M, Lundbeck F, von der Maase H. Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma. J Clin Oncol. 2009;27:4709–4717. doi: 10.1200/JCO.2008.18.9498. [DOI] [PubMed] [Google Scholar]
- 11.Bellocq A, Antoine M, Flahault A, Philippe C, Crestani B, Bernaudin JF, Mayaud C, Milleron B, Baud L, Cadranel J. Neutrophil alveolitis in bronchioloalveolar carcinoma: induction by tumor-derived interleukin-8 and relation to clinical outcome. Am J Pathol. 1998;152:83–92. [PMC free article] [PubMed] [Google Scholar]
- 12.Azab B, Bhatt VR, Phookan J, Murukutla S, Kohn N, Terjanian T, Widmann WD. Usefulness of the neutrophil-to-lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients. Ann Surg Oncol. 2012;19:217–224. doi: 10.1245/s10434-011-1814-0. [DOI] [PubMed] [Google Scholar]
- 13.Aspord C, Pedroza-Gonzalez A, Gallegos M, Tindle S, Burton EC, Su D, Marches F, Banchereau J, Palucka AK. Breast cancer instructs dendritic cells to prime interleukin 13-secreting CD4+ T cells that facilitate tumor development. J Exp Med. 2007;204:1037–1047. doi: 10.1084/jem.20061120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pedroza-Gonzalez A, Xu K, Wu TC, Aspord C, Tindle S, Marches F, Gallegos M, Burton EC, Savino D, Hori T, et al. Thymic stromal lymphopoietin fosters human breast tumor growth by promoting type 2 inflammation. J Exp Med. 2011;208:479–490. doi: 10.1084/jem.20102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ammirante M, Luo JL, Grivennikov S, Nedospasov S, Karin M. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature. 2010;464:302–305. doi: 10.1038/nature08782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7:411–423. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 17.Schioppa T, Moore R, Thompson RG, Rosser EC, Kulbe H, Nedospasov S, Mauri C, Coussens LM, Balkwill FR. B regulatory cells and the tumor-promoting actions of TNF-alpha during squamous carcinogenesis. Proc Natl Acad Sci U S A. 2011;108:10662–10667. doi: 10.1073/pnas.1100994108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol. 1992;12:954–961. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poli V, Balena R, Fattori E, Markatos A, Yamamoto M, Tanaka H, Ciliberto G, Rodan GA, Constantini F. Interleukin-6 deficient mice are protected from bone loss caused by estrogen depletion. EMBO J. 1994;13:1189–1196. doi: 10.1002/j.1460-2075.1994.tb06368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neveu W, Allard JB, Dienz O, Wargo MJ, Ciliberto G, Whittaker LA, Rincon M. IL-6 is required for airway mucus production induced by inhaled fungal allergens. J Immunol. 2009;183:1732–1738. doi: 10.4049/jimmunol.0802923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allard JB, Poynter ME, Marr KA, Cohn L, Rincon M, Whittaker LA. Aspergillus fumigatus generates an enhanced Th2-biased immune response in mice with defective cystic fibrosis transmembrane conductance regulator. J Immunol. 2006;177:5186–5194. doi: 10.4049/jimmunol.177.8.5186. [DOI] [PubMed] [Google Scholar]
- 22.Rincon M, Broadwater G, Harris L, Crocker A, Weaver D, Dressler H, Berry D, Sutton L, Michaelson R, Messino M, et al. Interleukin-6 and multidrug resistance protein 1 (PGP-1) expression and response to paclitaxel in women with metastatic breast cancer: results of Cancer And Leukemia Group B trial 159806. Breast Cancer Res Treat. 2006;100:301–308. doi: 10.1007/s10549-006-9251-7. [DOI] [PubMed] [Google Scholar]
- 23.Baribault H, Wilson-Heiner M, Muller W, Penner J, Bakhiet N. Functional analysis of mouse keratin 8 in polyoma middle T-induced mammary gland tumours. Transgenic Res. 1997;6:359–367. doi: 10.1023/a:1018427215923. [DOI] [PubMed] [Google Scholar]
- 24.Waldner MJ, Foersch S, Neurath MF. Interleukin-6--a key regulator of colorectal cancer development. Int J Biol Sci. 2012;8:1248–1253. doi: 10.7150/ijbs.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He G, Karin M. NF-kappaB and STAT3 - key players in liver inflammation and cancer. Cell Res. 2011;21:159–168. doi: 10.1038/cr.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tawara K, Oxford JT, Jorcyk CL. Clinical significance of interleukin (IL)-6 in cancer metastasis to bone: potential of anti-IL-6 therapies. Cancer Manag Res. 2011;3:177–189. doi: 10.2147/CMR.S18101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shakhar G, Ben-Eliyahu S. Potential prophylactic measures against postoperative immunosuppression: could they reduce recurrence rates in oncological patients? Ann Surg Oncol. 2003;10:972–992. doi: 10.1245/aso.2003.02.007. [DOI] [PubMed] [Google Scholar]
- 28.Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339:286–291. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foekens JA, Ries C, Look MP, Gippner-Steppert C, Klijn JG, Jochum M. The prognostic value of polymorphonuclear leukocyte elastase in patients with primary breast cancer. Cancer Res. 2003;63:337–341. [PubMed] [Google Scholar]
- 30.Foekens JA, Ries C, Look MP, Gippner-Steppert C, Klijn JG, Jochum M. Elevated expression of polymorphonuclear leukocyte elastase in breast cancer tissue is associated with tamoxifen failure in patients with advanced disease. Br J Cancer. 2003;88:1084–1090. doi: 10.1038/sj.bjc.6600813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Houghton AM. The paradox of tumor-associated neutrophils: fueling tumor growth with cytotoxic substances. Cell Cycle. 2010;9:1732–1737. doi: 10.4161/cc.9.9.11297. [DOI] [PubMed] [Google Scholar]
- 32.Rincon M. Interleukin-6: from an inflammatory marker to a target for inflammatory diseases. Trends Immunol. 2012;33:571–577. doi: 10.1016/j.it.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Grivennikov SI, Karin M. Inflammatory cytokines in cancer: tumour necrosis factor and interleukin 6 take the stage. Ann Rheum Dis. 2011;70(Suppl 1):i104–108. doi: 10.1136/ard.2010.140145. [DOI] [PubMed] [Google Scholar]
- 34.Sansone P, Bromberg J. Targeting the interleukin-6/Jak/stat pathway in human malignancies. J Clin Oncol. 2012;30:1005–1014. doi: 10.1200/JCO.2010.31.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rokavec M, Wu W, Luo JL. IL6-mediated suppression of miR-200c directs constitutive activation of inflammatory signaling circuit driving transformation and tumorigenesis. Mol Cell. 2012;45:777–789. doi: 10.1016/j.molcel.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rose-John S. IL-6 trans-signaling via the soluble IL-6 receptor: importance for the pro-inflammatory activities of IL-6. Int J Biol Sci. 2012;8:1237–1247. doi: 10.7150/ijbs.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]