Abstract
We report the use of a novel dual-channel hybrid near infrared optical spectrometer for the measurement of changes in oxidized cytochrome c oxidase (oxCCO) concentration and optical scattering in 11 healthy volunteers during functional activation induced by an anagram-solving task. A haemodynamic response consistent with functional activation was seen in 9 out of 11 subjects and in 13 of the total 22 channels measured; only these channels were retained for further analysis. In each of these 13 channels the haemodynamic response was accompanied by a statistically significant change in [oxCCO], although the direction of this change was heterogenous with a significant increase seen in eight channels and a significant decrease seen in five channels. There was no significant change in the optical scattering coefficent measured at four wavelengths, and the use of differential pathlength factor (DPF) calculated in real time using measured absorption and scattering showed a high level of agreement with a conventional algorithm. The possible reasons for the heterogeneity of the [oxCCO] response are discussed.
1. Introduction
Near infrared spectroscopy (NIRS) is finding increasing utility in the investigation of functional activation, but in most cases its use is limited to the measurement of oxyhaemoglobin (HbO2) and deoxyhaemoglobin (HHb) concentration. However, since the advent of NIRS, there has been great interest in the measurement of oxidized cytochrome c oxidase (oxCCO). CCO is the terminal electron acceptor in the mitochondrial electron transport chain and change in its redox state has been suggested as a potential marker of cellular energy status. The NIRS measurement of [oxCCO] in the adult human brain, however, has posed a number of technical challenges [1], including concerns about the effect on the signal from possible changes in optical scattering. Nevertheless, a number of studies have reported the measurement of [oxCCO] within a number of contexts including during manipulation of cerebral oxygen delivery [2, 3] and in functional activation [4].
Anagram solving has been shown by NIRS to evoke a bilateral frontal haemodynamic response consistent with functional activation, although these changes are associated with potentially confounding changes in systemic physiology [5, 6]. Whilst prior studies have shown an increase in [oxCCO] in response to visual activation [4], theoretical models suggest that either an increase or decrease in [oxCCO] could potentially occur [7].
The aims of this study are to use a hybrid optical spectrometer (pHOS) comprising a novel combination of broadband and frequency domain NIR systems [8] to investigate: (i) the direction and magnitude of [oxCCO] change; (ii) the change in optical scattering in the presence of a haemodynamic response consistent with functional activation of the frontal cortex during anagram-solving and (iii) the effects of the use of a novel algorithm utilising differential pathlength factor (DPF) measured in real-time to scale chromophore concentration changes.
2. Methods
Eleven subjects (7 male, 4 female; age range 21–34 years) were studied. This study was approved by the UCL Ethics Committee and informed written consent was obtained from each subject. Optodes from the pHOS were affixed over the FP1 point bilaterally prior to the initiation of the anagram protocol, which was identical to that previously reported by our group [5, 6]. The protocol consisted of a two-minute baseline recording, followed by the presentation of six alternating 60-second blocks of 4-letter and 7-letter anagrams, each with a single correct solution (e.g. DISEASE = SEASIDE), which the subjects were asked to solve. This was followed by a further two-minute baseline recording.
The pHOS has been described in detail elsewhere [8]. Briefly, it comprises two identical broadband spectrometers and a two-channel frequency domain spectrometer capable of absolute measurements of optical absorption and scattering at 690, 750, 790 and 850nm. [HHb], [HbO2] and [oxCCO] were calculated using the UCLn algorithm [9] by fitting to the changes in NIR attenuation from 740 to 860nm using a 35mm source-detector separation. The quantification of measured chromophore concentration changes was performed (i) using a constant DPF derived from measured optical absorption and scattering during the baseline period of recording and (ii) using a variable DPF updated in real-time (using the DPF derived from optical absorption and scattering measured at 790nm from the frequency domain component of the pHOS and applying additional correction factors for the wavelength dependence of pathlength). Other measurements included continuous blood pressure and heart rate (Portapres®, Finapres Medical Systems) and laser Doppler monitoring of frontal scalp blood flow (FloLab, Moor Instruments).
Two channels were recorded per subject. The resulting NIRS-derived data were post-processed by resampling to a sample period of 3s, application of a linear detrending function and a 5th order Butterworth filter with a 0.08Hz cut-off (MatLab, Mathworks USA).
Individual channels were tested for the presence of a haemodynamic response consistent with functional activation – i.e. an increase in Δ[HbO2] and no increase in Δ[HHb] between baseline and activation windows. The baseline window was defined as the 60 seconds immediately prior to the onset of the anagram exercise; 60 second activation windows were then defined separately for Δ[HbO2] and Δ[HHb] for each channel independently. This was achieved through the use of an automated algorithm scanned the Δ[HbO2] signal following the beginning of the anagram exercise to identify the 60 second window of maximal increase and then scanning for the Δ[HHb] window, starting 57 seconds before and finishing 57 seconds after the Δ[HbO2] activation window, to identify the maximal change in Δ[HHb]. The mean change in Δ[HbO2] and Δ[HHb] were between baseline and activation windows were then compared using an unpaired t-test; channels that thus met the haemodynamic criteria detailed above – i.e. p<0.05 for Δ[HbO2] > 0 and p>0.05 for Δ[HHb] > 0 – were retained for further analysis.
The Δ[oxCCO] change was identified using an algorithm that identified the 60 second window of maximal change in a fashion analogous to that used for Δ[HHb]. Using this time period for each channel, averages were calculated for optical scattering and systemic variables. Significance was then inferred using an unpaired t-test comparing baseline and activation windows, with a significance threshold of p≤0.05.
3. Results
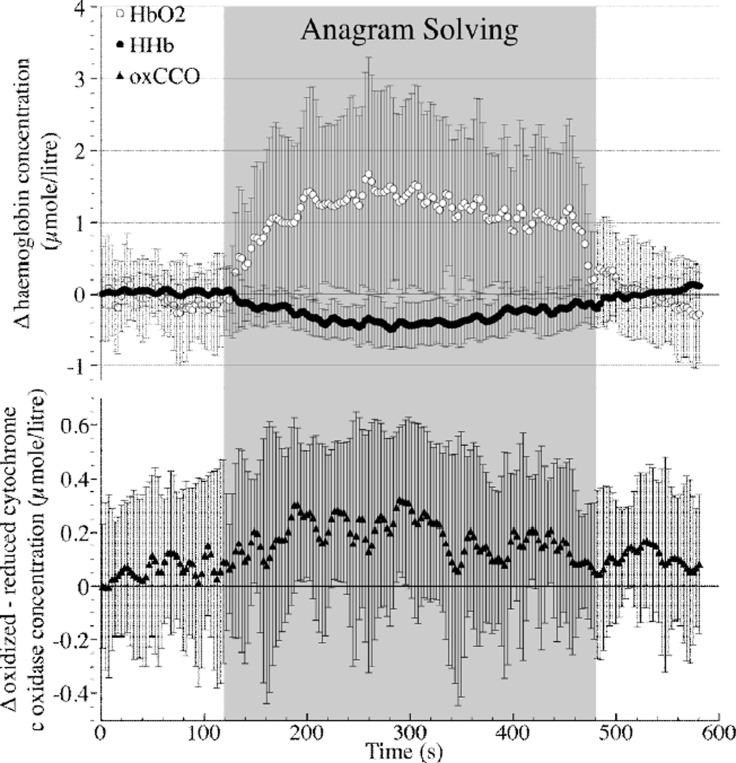
A haemodynamic response consistent with functional activation was seen in nine out of eleven subjects (13/22 channels); mean chromophore concentration changes for these channels are shown in Figure 1 and Table 1. In this group of channels there was no significant change in [oxCCO], although every individual subject did show a statistically significant change in [oxCCO] between baseline and activation. Table 3 shows the magnitude of [oxCCO] change in two groups defined posthoc by its direction. There was no significant change in optical scattering or mean arterial blood pressure (MAP) between baseline and activation, although there was a significant increase in both heart rate and laser Doppler (LD) flux.
Figure 1. Grouped data showing chromophore concentration (mean ± SD) using fixed DPF algorithm).
Table 1. Changes in chromophore concentration, optical scattering and physiological parameters between baseline and activation windows.
| Baseline (mean ± SD) |
Activation (mean ± SD) |
p* | |
|---|---|---|---|
| Δ [HbO2] (μmole/litre) | 0 ± 0.501 | 1.728 ± 1.292 | 0.001 |
| Δ [HHb] (μmole/litre) | 0 ± 0.178 | −0.394 ± 0.268 | 0.00001 |
| Δ [oxCCO] (μmole/litre) | 0 ± 0.281 | 0.142 ± 0.383 | 0.3369 |
|
| |||
| μs 690nm(cm−1) | 10.7 ± 2.31 | 11.1 ± 3.26 | 0.42 |
| μs 750nm(cm−1) | 9.45 ± 2.46 | 9.81 ± 2.87 | 0.49 |
| μs 790nm(cm−1) | 9.43 ± 1.76 | 9.54 ± 1.82 | 0.15 |
| μs 850nm(cm−1) | 8.53 ± 1.91 | 8.28 ± 2.78 | 0.51 |
|
| |||
| MAP (mm Hg) | 72.6 ± 35.5 | 73.5 ± 36.3 | 0.42 |
| HR (bpm) | 81.9 ± 38.2 | 88.5 ± 41.3 | 0.0042 |
| Δ LD flux (%age change) | 0 ± 20.9 | 41.94 ± 26.5 | 0.002 |
paired Students' t-test.
Table 3. Changes in [oxCCO] divided by direction of change during functional activation.
| # subjects |
# channels |
Baseline [oxCCO] (μmole/litre; mean ± SD) |
Activation [oxCCO] (μmole/litre; mean ± SD) |
|
|---|---|---|---|---|
| Increase in [oxCCO] | 7 | 8 | 0 ± 0.279 | 0.485 ± 0.317 |
| Decrease in [oxCCO] | 4 | 5 | 0 ± 0.136 | −0.406 ± 0.145 |
Bland-Altman comparison between fixed DPF- and variable DPF-based algorithms revealed a high level of agreement in the chromophore concentrations derived using the two methods, as shown in Table 2.
Table 2. Bland Altman analysis showing agreement between chromophore concentrations derived using constant DPF and variable DPF algorithms.
| Chromophore | Mean difference | (95%limit of agreement) |
|---|---|---|
| Δ HbO2 | 0.0007551 | (−0.0719 – 0.0734) μmol/litre |
| Δ HHb | −0.0005352 | (−0.0238 – 0.0227) μmol/litre |
| Δ oxCCO | 0.0009688 | (−0.0252 – 0.0272) μmol/litre |
4. Discussion
We have used a novel hybrid NIRS system to simultaneously measure haemodynamic, metabolic and optical scattering changes during functional activation of the frontal cortex. Using a data analysis methodology designed to investigate [oxCCO] changes in the presence of a haemodynamic response consistent with functional activation, we have demonstrated no significant change in the group data in either [oxCCO] or optical scattering, although significant changes in [oxCCO] were seen in each individual channel. Furthermore, we have demonstrated a high level of agreement between the conventional UCLn algorithm for broadband spectroscopy using a fixed DPF and a novel algorithm incorporating real-time measurements DPF derived from real-time measurements of optical scattering, in accordance with previous findings using the pHOS during a Valsalva manoeuvre [10]. In agreement with previous studies [6], we have shown significant increases in heart rate and scalp blood flow during activation, but conflictingly not in MAP; this may be a consequence of our treatment of multiple channels from the same individual as independent variables.
Functional activation may cause an increase or decrease in [oxCCO] depending on the particular haemodynamic and metabolic circumstances present during activation [7]. However, mathematical modeling of brain metabolism suggests that an increase is the most likely outcome [11] and such an increase in [oxCCO] has been shown during passive visual stimulation [4]. Whilst our group data (as shown in Figure 1) are suggestive of an increase in [oxCCO], it is of importance to note that there was a great deal of heterogeneity in our observed [oxCCO] response, with all subjects showing a statistically significant change – that is, increase or decrease – in [oxCCO] between baseline and activation windows, with changes in both directions occurring in the presence of similar haemodynamic changes. There are many potential explanations for this heterogeneity some of which maybe related to the optical measurement of [oxCCO], variations in underlying functional anatomy and differences in individuals’ metabolic responses to the activation task. Further analysis is underway to investigate the possible confounds in the optical measurement of [oxCCO] during functional activation, e.g the choice of source detector separation, which may affect depth sensitivity. In addition to the development of further algorithms utilising the data from the pHOS we are also using a mathematical model of cerebral physiology [11] to aid the interpretation of the observed responses in each individual. This may improve our understanding of the confounds involved in the optical measurement of the metabolic consequences of functional activation.
Acknowledgements
This work was undertaken at University College London Hospitals and partially funded by the Department of Health's National Institute for Health Research Centres funding scheme. This research was supported by MRC programme grant G0701458 and EPSRC grant EP/D0609821/1. IT is supported by Wellcome Trust grant 088429/Z/09/Z
References
- 1.Cooper CE, Cope M, Quaresima V, et al. Measurement of cytochrome oxidase redox state by near infrared spectroscopy. Adv Exp Med Biol. 1997;413:63–73. doi: 10.1007/978-1-4899-0056-2_7. [DOI] [PubMed] [Google Scholar]
- 2.Tisdall MM, Tachtsidis I, Leung TS, et al. Near-infrared spectroscopic quantification of changes in the concentration of oxidized cytochrome c oxidase in the healthy human brain during hypoxemia. J Biomed Opt. 2007;12:24002. doi: 10.1117/1.2718541. [DOI] [PubMed] [Google Scholar]
- 3.Tachtsidis I, Tisdall MM, Leung TS, et al. Relationship between brain tissue haemodynamics, oxygenation and metabolism in the healthy human adult brain during hyperoxia and hypercapnea. Adv Exp Med Biol. 2009;645:315–320. doi: 10.1007/978-0-387-85998-9_47. [DOI] [PubMed] [Google Scholar]
- 4.Heekeren HR, Kohl M, Obrig H, et al. Noninvasive assessment of changes in cyto-chrome-c oxidase oxidation in human subjects during visual stimulation. J Cereb Blood Flow Metab. 1999;19:592–603. doi: 10.1097/00004647-199906000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Tachtsidis I, Leung T, Devoto L, et al. Measurement of frontal lobe functional activation and related systemic effects: a near-infrared spectroscopy investigation. Adv Exp Med Biol. 2008;614:397–403. doi: 10.1007/978-0-387-74911-2_44. [DOI] [PubMed] [Google Scholar]
- 6.Tachtsidis I, Leung TS, Tisdall MM, et al. Investigation of frontal cortex, motor cortex and systemic haemodynamic changes during anagram solving. Adv Exp Med Biol. 2008;614:21–28. doi: 10.1007/978-0-387-74911-2_3. [DOI] [PubMed] [Google Scholar]
- 7.Banaji M. A generic model of electron transport in mitochondria. J Theor Biol. 2006;243:501–516. doi: 10.1016/j.jtbi.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Tachtsidis I, Leung TS, Elwell CE, et al. Multi-Wavelength, Depth Resolved, Scattering and Pathlength Corrected in vivo Near-Infrared Spectroscopy of Brain Tissue. Optical Society of America Biomedical Optics (BIOMED) BTuB. 2010 [Google Scholar]
- 9.Matcher SJ, Elwell CE, Cooper CE, et al. Performance comparison of several published tissue near-infrared spectroscopy algorithms. Anal Biochem. 1995;227:54–68. doi: 10.1006/abio.1995.1252. [DOI] [PubMed] [Google Scholar]
- 10.Gao L, Elwell C, Kohl-Bareis M, et al. Effects of assuming constant optical scattering on haemoglobin concentration measurements using NIRS during a Valsalva Manoeuvre. Adv Exp Med Biol. 2010 doi: 10.1007/978-1-4419-7756-4_3. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banaji M, Mallet A, Elwell C, et al. A model of brain circulation and metabolism: NIRS signal changes during physiological challenges. PLoS Comput. Biol. 2008;4:e1000212. doi: 10.1371/journal.pcbi.1000212. [DOI] [PMC free article] [PubMed] [Google Scholar]