Abstract
17β-estradiol (E2), at high circulating levels, enhances learning and memory in many women, making it a clinical treatment for hormone-related cognitive decline in aging. However, the mechanisms stimulated by E2, which are responsible for its cognitive enhancing effects, remain incompletely defined. Using an ovariectomized rat model, we previously reported that increasing plasma E2 enhances the magnitude of long-term potentiation (LTP) at hippocampal CA3-CA1 synapses, which is caused by a selective increase in current mediated by NR2B-containing NMDARs causing an increase in the NMDAR/AMPAR ratio. Whether the increase in NR2B current is causally related to the ability of E2 to enhance hippocampal dependent learning and memory has yet to be tested. Here, we find that E2 enhances performance in the novel object recognition (NOR) task with the same time course we previously showed E2 enhances the LTP magnitude, temporally linking the increase in LTP to enhanced learning and memory. Furthermore, using the selective NR2B subunit antagonist Ro25-6981, we find that the E2-enhanced NOR, like the enhanced LTP, requires hippocampal NR2B-containing NMDARs, specifically in area CA1. Finally, using whole-cell recordings and the phosphatase inhibitor orthovanadate, we investigated whether the E2-induced increase in NMDAR current is caused by an increase in the density of synaptic NMDARs and/or an increase in NMDAR subunit phosphorylation. We find that both mechanisms are responsible for the enhanced NMDAR current in E2-treated rats. Our results show that the E2-enhanced NOR requires a functional increase in NR2B-containing NMDARs, a requirement shared with the E2-enhanced LTP magnitude at CA3-CA1 synapses, supporting the hypothesis that the increase in LTP likely contributes to the enhanced learning and memory following an increase in plasma E2 levels.
Keywords: Estrogen, LTP, Learning, Memory, Plasticity
Introduction
Elevated plasma levels of the ovarian hormone 17β-estradiol (E2) are associated with heightened hippocampal dependent learning and memory in women and in animal models (Frye et al., 2007; Frye, 1999; Rhodes and Frye, 2004; Rosenberg and Park, 2002; Warren and Juraska, 1997). At the synaptic level, elevated plasma E2 increases dendritic spine density on hippocampal CA1 pyramidal cells in female rats, transmission mediated by NR2B-containing NMDARs which increases the NMDAR/AMPAR ratio (Smith and McMahon, 2005; Smith and McMahon, 2006; Snyder et al., 2010; Woolley et al., 1997), and the magnitude of LTP at CA3-CA1 synapses (Bi et al., 2001; Smith and McMahon, 2005; Warren et al., 1995), a cellular correlate of learning and memory (Malenka and Bear, 2004). However, because a mechanistic link has yet to be demonstrated, it remains unknown whether the E2-induced increase in LTP is in any way associated with the E2-induced enhanced learning and memory. It is assumed this is the case, but an enhanced LTP magnitude can occur independent of an increase in learning and memory, and vice versa (Dutar et al., 2002; Migaud et al., 1998).
Using ovariectomized (OVX) rats, we previously reported that elevated plasma E2 increases the LTP magnitude precisely when transmission mediated by NR2B-containing NMDARs and the NMDAR/AMPAR ratio are also increased (Smith and McMahon, 2005). In fact, the increase in NR2B-mediated transmission is causal to the increase in LTP (Smith and McMahon, 2006; Snyder et al., 2010). It remains unclear how E2 increases current mediated by NR2B-containing NMDARs. E2 may increase NR2B subunit phosphorylation or density of NMDARs containing NR2B subunits. However, western blot analysis has failed to detect a change in either phosphorylation or density (Snyder et al., 2010) and electron microscopy studies also do not detect an increase in NR2B subunits in OVX rats treated with E2 (Adams et al., 2004). Thus, the mechanisms underlying the increase in function of NR2B-containing NMDARs reported using electrophysiology (Smith and McMahon, 2005, 2006, Snyder et al., 2010) requires further investigation. Importantly, because over-expression of NR2B subunits enhance both LTP and learning and memory in transgenic mice (Tang, 1999), the increase in NR2B function stimulated by E2 may be responsible for the E2-induced increase in hippocampal learning and memory.
Here, we used novel object recognition, a hippocampal dependent behavioral task that is enhanced by increased E2 levels (Fernandez et al., 2008; Frye et al., 2007; Luine et al., 2003; Michael C. Lewis, 2008), to test the hypothesis that E2 increases learning and memory only at time points when E2 increases NR2B transmission and LTP, and that like LTP, E2-enhanced learning and memory also requires NR2B-containing NMDARs. We also determined whether E2 increases receptor density, phosphorylation of NMDAR subunits, or both, to functionally increase NR2B transmission.
Methods
All experiments were performed with an approved protocol from the IACUC of the University of Alabama at Birmingham, in accordance with the guidelines from the National Institutes of Health.
Animals
Sprague-Dawley female rats (Charles River Laboratories), on a 12hr light/dark cycle with food and water ad libitum, were 8–12 weeks of age for all experiments except cannula experiments where animals were 16–20 weeks old.
Surgery
All surgeries were performed under 2.5% isoflurane anesthesia in 100% oxygen using aseptic conditions. Animals were given either Tylenol in drinking water or a single carprofen (10mg) subcutaneous injection for analgesia. Ovariectomy was performed at 7–9 weeks of age as described previously (Smith and McMahon 2005). Intra-hippocampal Cannula Placement: After at least 7 days post-OVX, 26 gauge bilateral hippocampal guide cannulae (Plastics One) were inserted into area CA1 using the following coordinates (from Bregma, AP −4.2mm, ML −2.5mm,+2.5mm, DV −2.3mm). Cannulae were secured using dental cement.
Drugs
Hormone treatment
Two daily injections of 10µg 17β-estradiol (E2, Sigma) in 0.1cc cottonseed oil or cottonseed oil vehicle (V) were given subcutaneously (Smith and McMahon, 2005; Woolley and McEwen, 1993). Uterine weights were measured to verify hormone treatment and successful ovary removal. Animals were tested in behavior and sacrificed either 24(E24), 48(E48) or 72(E72) hours after E2 treatment.
Ro25-6981(RO, Sigma)
Systemic:RO was dissolved in saline (2mg/ml) and injected intraperitoneally at a dose of 5mg/kg (Figure 1A). As a concentration of 10mg/kg of RO injected systemically has been shown to block hippocampus dependent learning in rats (Valenzuela-Harrington et al., 2007), we chose 5mg/kg to avoid blocking NOR in V-treated rats. IntraCA1 Infusion: RO (1µl) was infused at a rate of 0.3µL/min. Cannulae were left in place for one minute to allow for diffusion of drug. Correct cannula placement was verified with cresyl violet. Only animals with cannula tips in area CA1 were included for analysis (Figure 2C).
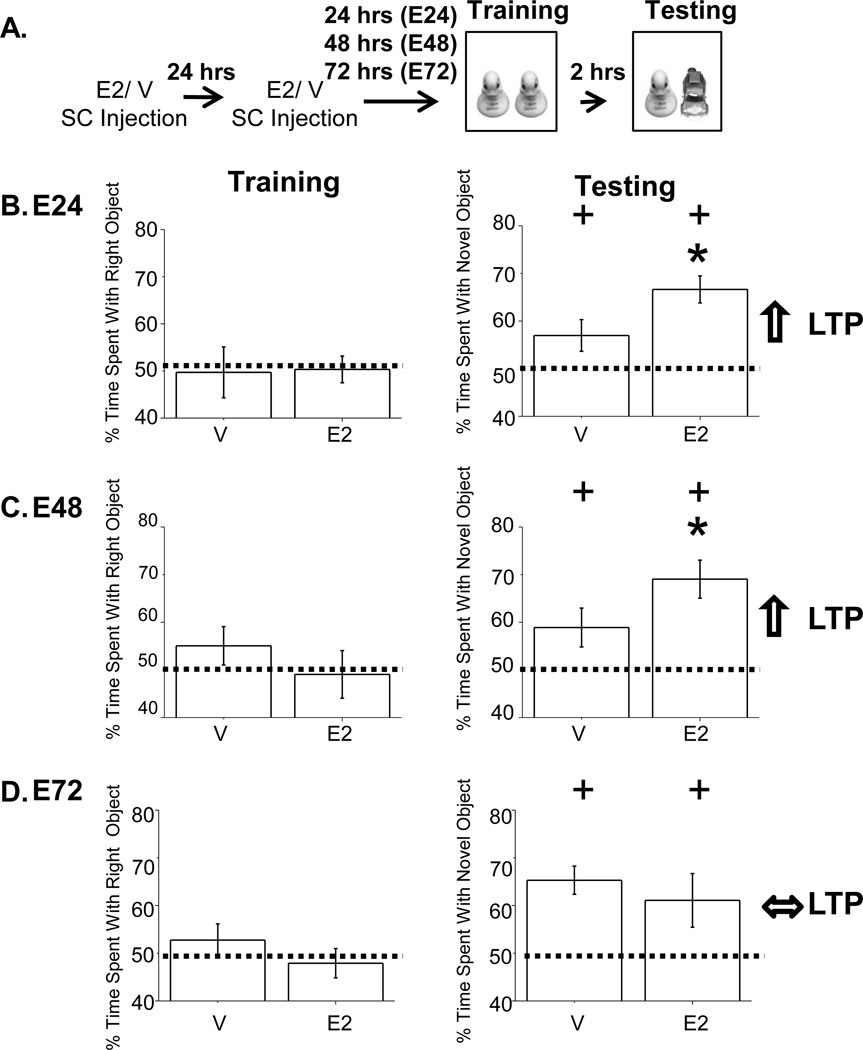
Figure 1. E2-enhanced NOR occurs at 24 and 48, but not at 72 hours post E2 treatment.
A. Experimental timeline. B–D, Left. The average percent time spent investigating the right object during training demonstrates no object preference in E2 and V-treated rats at E24, E48 and E72. B–C, Right. Percent time spent with the novel object at E24 and E48 was significantly greater in E2-treated compared to V-treated rats. D, Right. Percent time spent with novel object at E72 was not different between E2 and V-treated rats. Far right arrows represent LTP at CA3-CA1 synapses for respective time points as reported in Smith and McMahon, 2005. + indicates significance from chance at p<0.05. * indicates significance of hormone treatment at p<0.05.
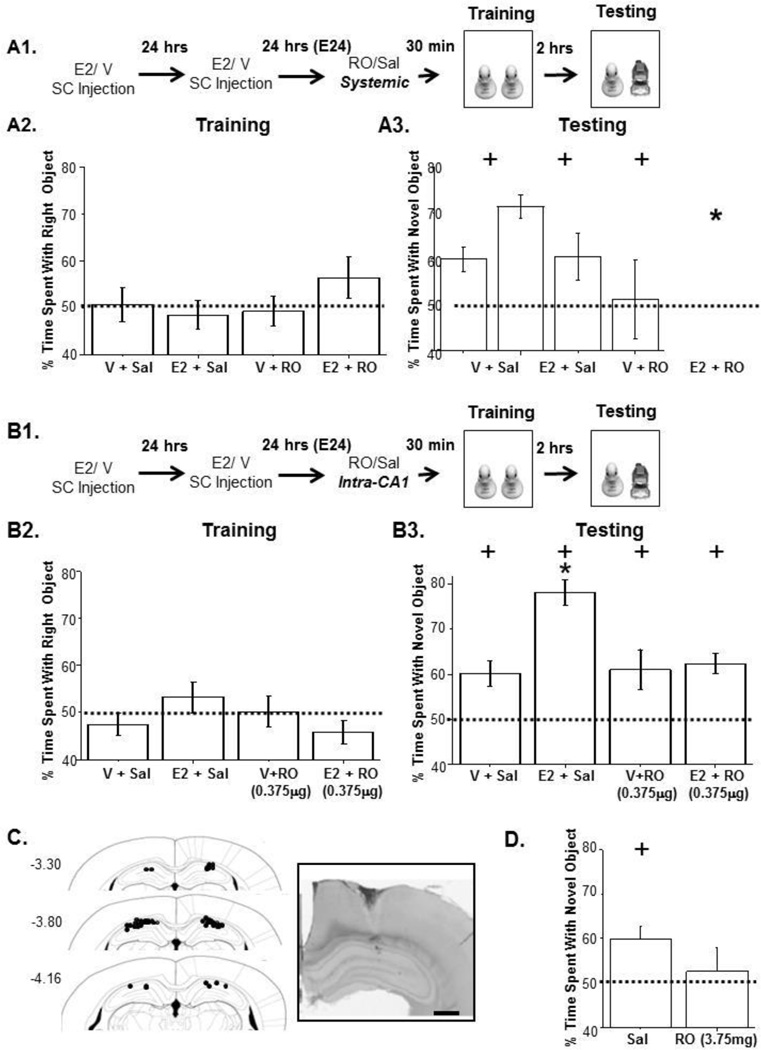
Figure 2. E2-enhanced NOR is prevented by blocking NR2B-containing NMDARs.
A1. Experimental timeline. A2. No object preference during training. A3. Systemic RO (5mg/kg) blocks learning and memory in E2-treated animals only. B1. Experimental timeline for intraCA1 infusion of RO. B2. No object preference during training. B3. IntraCA1 RO (0.375µg/1µL) infusion blocks E2-enhanced learning and memory. Importantly, V-treated animals still spend significantly more time on the novel object compared to chance. C. Cannula tip locations for each animal included in behavioral experiments. Inset: Cresyl violet stained hippocampal brain slice showing representative cannula tip placement. Scale bar represents 100µM. D. Infusion of 3.75µg/1µL RO blocks NOR in V-treated animals. + indicates percent time spent on novel object is significant from chance. *p<0.05 comparing E2 Sal and E2 RO.
Novel Object Recognition (NOR)
Rats were handled for 1 minute and habituated to the empty NOR box (40cm × 40cm × 60cm, black plexi-glass) for 10 minutes. The next day, rats were placed in the NOR box and trained with 2 identical objects for 3 minutes, returned to their home cage for 2 hours and returned to the NOR Box for 3 minutes where one of the training objects (familiar) was replaced by a novel object. All sessions were videotaped and analyzed by blind observers. Investigative behaviors were defined as the rat’s nose coming in direct contact or within 1cm of the object. Training objects and novel object locations during testing were counterbalanced to avoid an effect of object or place preferences. Animals were excluded if they did not have at least 5 total seconds of investigative behavior or spend at least 1 second investigating both objects during the testing session.
Electrophysiology
Whole-cell patch clamp recordings were obtained from CA1 pyramidal cells as done previously (Smith and McMahon, 2006). The non-selective phosphatase inhibitor orthovanadate (100 µM) was added to the pipet solution to saturate phosphorylation by preventing de-phosphorylation. A stimulating electrode was placed in stratum radiatum of area CA1 to evoke glutamate transmission at CA3-CA1 synapses. Picrotoxin (100µM) and DNQX (10µM) were bath applied to isolate NMDAR currents and cells were held at −20mV to relieve the voltage-dependent Mg2+ block. From each animal, recordings were performed with and without orthovandate to allow for within animal comparison, and V and E2-treated animals were interleaved.
Statistics
NOR: Within a treatment group, a one–sample t-test was used to determine whether there was a significant increase compared to chance (50%) in the percent time spent on the novel object out of total time spent investigating during the testing session indicating successful NOR performance and a memory of the familiar object. Percent time spent on the right object during training was compared to chance to rule out side preference. Differences between groups were compared using a student T-test or One-way ANOVA with Tukey post-hoc analysis. Electrophysiology: Maximum NMDAR currents between groups were statistically compared using Two-way ANOVA and Tukey’s test for post-hoc comparison. Statistical significance was declared if p<0.05.
Results
E2-induced enhancement in novel object recognition is time dependent
If the E2-induced increase in LTP is related to the mnemonic enhancing effects of E2, then the increase in learning and memory should occur with an identical time course as the enhanced LTP we published previously (Smith and McMahon, 2005). To test this idea, OVX female rats were treated with E2 or vehicle (V) and working memory was assessed using novel object recognition (NOR) at 24(E24), 48(E48), or 72(E72) hours after E2 treatment. For all treatment groups, the percent time spent with the novel object was significant compared to chance, demonstrating learning (Figures 1B–D, Right, E24; V, t(8)=1.87, p<0.05, E2, t(8)=5.86, p<0.001, E48; V, t(7)=2.18, p<0.05, E2, t(8) = 4.78, p<0.001, E72; V, t(18) = 5.19, p<0.001, E2, t(18) = 3.98, p<0.005). Importantly, we find that E2 induces an increase in NOR beyond that measured in V-treated animals at E24 (Figure 1B, Right, t(14)=1.97, p<0.05) and E48 (Figure 1C, Right, t(15)=1.78, p<0.05), time points when E2 also enhances LTP (Smith and McMahon 2005). However, at E72, performance in NOR is no longer enhanced (Figure 1D, Right, t(36)=1.04, p=0.84, and neither is LTP (Smith and McMahon 2005). The E2-enhanced NOR cannot be explained by an increase in investigative behavior, as the total time spent investigating during the training session with identical objects was not significantly different between V and E2-treated rats (Figures 1B–D, Left, E24; t(14)=0.292, p=0.77, E48; t(15)=1.73, p=0.11, E72; t(36)=0.155, p=0.56). This alleviates the concern that E2 is increasing investigation or motivation, which could non-mnemonically increase percent time spent with the novel object.
E2-enhanced novel object recognition requires NR2B-containing NMDARs
If the E2-induced increase in LTP and NOR are mechanistically linked as suggested by shared temporal relationship, then E2-enhanced NOR should require NR2B-containing NMDARs similarly to the E2-enhanced LTP (Smith and McMahon 2006). To test this, we first blocked NR2B-containing NMDARs systemically using the selective NR2B subunit antagonist Ro25-6981 (RO;5mg/kg,ip) injected 30 minutes prior to NOR training at E24 (Figure 2A1). There was no effect of RO on investigative behavior between groups during the training session (Figure 2A2, F(3,52)=0.33, p=0.80). All groups except E2+RO showed significant NOR (Figure 2A3, V+Sal, t(16)=3.37, p<0.001, V+RO, t(11)=2.07,p<0.05, E2+Sal, t(17)=6.28, p<5.0×10−6, E2+RO, t(8)=0.14, p=0.45). The increase in variance in E2+RO-treated rats indicates that NOR was disrupted, as these animals prefer objects at random, and did not spend significantly more time than chance on the novel object (Figure 2A3). This effect was not seen in V+RO-treated rats, suggesting that NOR is more heavily dependent on NR2B-containing NMDARs in E2-treated rats.
To further define a role for hippocampal NR2B-containing NMDARs, we next asked if blocking these receptors only in hippocampus prevents the E2-induced increase in NOR. RO (0.375µg/1µL) or saline was infused directly into area CA1 of V or E2-treated rats 15 min prior to training (Figure 2B1–2B3). In all animal groups, significantly more time was spent with the novel object compared to chance, demonstrating NOR (Figure 2B3, V+Sal;t(8)=3.47, p<0.005, E2+Sal;t(9)=9.74, p<0.000005, V+RO;t(6)=2.51, p<0.05, E2;+RO;t(9)=5.47, p<0.0005). As predicted given their role in the increased LTP magnitude (Smith and McMahon, 2006), the targeted block of NR2B-containing NMDARs eliminated the enhanced NOR observed in E2-treated rats (Figure 2B3, F(3, 32,)=8.39, P<0.0005), decreasing it to a level not different from that measured in V-treated animals. As before, investigative behavior did not differ between treatment groups during training (Figure 2B2, F(3,33)=2.16, p=0.11). It is important to note that while infusion of RO (0.375µg) into CA1 (see Figure 2C for cannula placement) had no effect on NOR in V-treated control rats, a 10-fold higher concentration of RO (3.75µg) disrupts NOR as denoted by the increase in variance (Figure 2D, V+RO, t(11)=0.50, p=0.63), demonstrating that NR2B containing receptors participate in normal learning and memory, but their role is heightened in E2-treated rats when NOR is increased.
E2-enhanced current through NR2B-containing NMDARs is due to both increased receptor number and phosphorylation
Although elevated E2 selectively increases NMDAR current mediated by NR2B-containing receptors measured in electrophysiological recordings (Smith and McMahon, 2006; Snyder et al., 2010), biochemical assays have been unable to determine whether this increase results from increased NMDAR receptor density, subunit phosphorylation, or both (MacDonald et al., 1989; Snyder et al., 2010). Because of the shared mechanistic requirement in the enhanced LTP and NOR, we were prompted to investigate the mechanism(s) by which E2 increases NMDAR current. In an attempt to distinguish between a possible increase in receptor density and subunit phosphorylation, we included the phosphatase inhibitor orthovanadate in the pipet solution during whole-cell voltage clamp recordings of CA1 pyramidal cells to saturate NMDAR subunit phosphorylation. This strategy has been used previously to demonstrate that phosphorylation increases NMDAR current amplitude (Wang and Salter, 1994). We reasoned that if the E2-induced increase in NMDAR current is due only to an increase in subunit phosphorylation, then orthovanadate will have no effect on the maximum NMDAR current amplitude in cells from E2-treated rats, while in V-treated rats, orthovanadate will significantly increase the maximum current amplitude to the same maximum as in E2-treated animals. Alternatively, if E2 primarily increases receptor density, enhancing phosphorylation with orthovanadate should increase the maximum NMDAR current in cells from both E2- and V-treated groups in parallel, and the NMDAR current amplitude in V-treated rats will not reach the same maximum as in E2-treated rats. Lastly, if E2 increases receptor density and saturates subunit phosphorylation, then the maximum current amplitude in E2-treated rats will be unaffected by orthovanadate as suggested in the first paradigm, but the maximum NMDAR current amplitude in cells from V-treated rats recorded with orthovanadate will be significantly smaller than the maximum NMDAR current in cells from E2-treated rats.
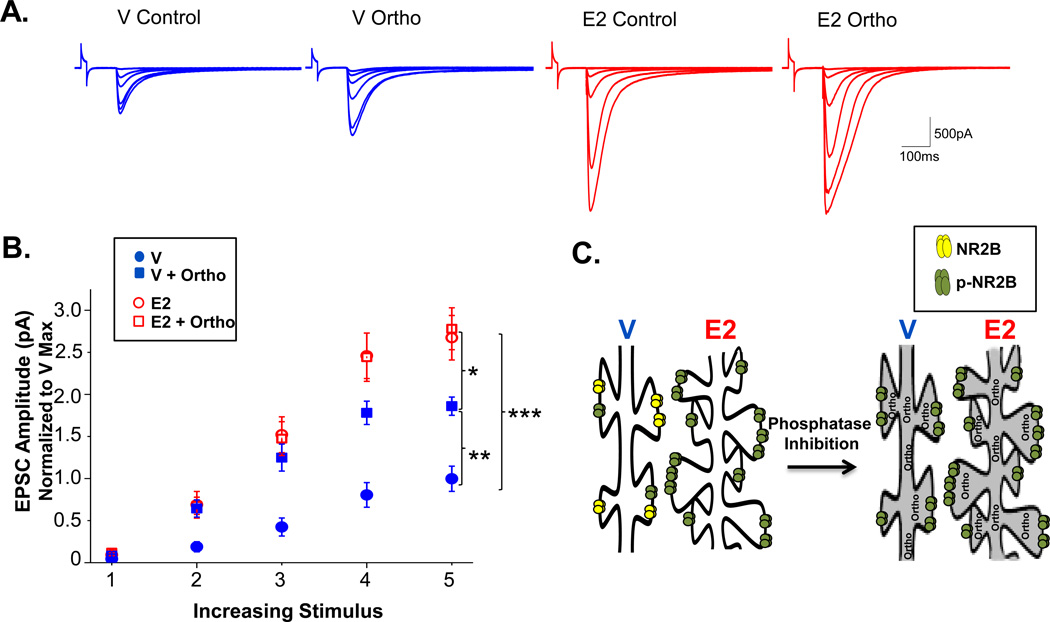
We investigated which of these possible scenarios explains the E2-induced increase in NMDAR current amplitude. In interleaved whole-cell recordings with and without orthovanadate from V and E2-treated rats, we find a significant interaction between drug (control versus orthovanadate) and hormone (V versus E2), F(1,43) = 5.16, p<0.05. We also find main effects of both hormone treatment, F(1,43) = 51.561, p<0.00000001 and drug, F(1,43) = 6.82065, p<0.05. Using Tukey for post-hoc comparison, we find that the maximum NMDAR current amplitude in cells from V-treated rats was significantly greater when cells were recorded with orthovanadate in the pipet solution, demonstrating that NMDAR subunit phosphorylation is not saturated under control conditions (p<0.005, Figure 3A,B). In contrast, the maximum NMDAR current amplitude was not significantly different with or without orthovanadate in cells from E2-treated rats, suggesting that phosphorylation is saturated by E2 (Figure 3A,B). Importantly, the maximum NMDAR current with orthovanadate remains significantly less in cells from V-treated rats compared to the maximum current in cells from E2-treated rats (p<0.05). As mentioned in the scenarios above, these findings are consistent with the interpretation that E2 increases both the density and phosphorylation of NMDARs, which together are responsible for the increase in current measured in CA1 pyramidal cells recorded in E2 versus V-treated rats (Figure 3C).
Figure 3. E2 enhances both phosphorylation of NR2B-containing NMDARs and total NR2B-containing NMDARs in the synapse.
A. Representative traces for each group with over-layed responses for each stimulus intensity. B. Normalized whole-cell recordings of evoked NMDAR EPSCs at increasing stimulus intensities from V-treated animals with (closed squares) or without (closed circles) orthovanadate in the pipet solution and E2-treated animals with (open squares) or without (open circles) orthovanadate. The maximum evoked NMDAR current measured in cells from E2-treated animals is significantly increased above that measured in cells from V-treated rats. Orthovanadate has no effect in cells from E2-treated animals, but significantly enhances the maximum current elicited in cells from V-treated animals. ***p<0.0005, ** p<0.005, *p<0.05. C. Cartoon model illustrating differences in NMDAR receptor density and subunit phosphorylation in CA1 pyramidal cells from E2-treated versus control rats, and the lack of effect of orthovanadate in E2 versus control cells due to E2-induced saturation of subunit phosphorylation.
Discussion
These results support a mechanistic link between the E2-enhanced hippocampal dependent learning and memory and the E2-enhanced LTP at CA3-CA1 synapses reported previously (Smith and McMahon, 2005). The shared requirement for NR2B-containing NMDARs, along with a similar time course of enhanced NOR after E2 treatment suggests that the E2-enhanced LTP may be associated with the E2-enhanced learning and memory.
NOR exploits the natural tendency of rodents to explore a novel object (Ennaceur and Delacour, 1988). This task is sensitive to hormonal fluctuations and is enhanced in cycling female rats at proestrus as well as in OVX rats treated with exogenous E2 (Fernandez et al., 2008; Harburger et al., 2009; Luine et al., 2003; Michael C. Lewis, 2008; Walf et al., 2006). While many brain regions contribute to this task, (Buckley, 2005; Dere et al., 2007; Ennaceur, 2010), the E2-enhanced performance in NOR is specifically due to hippocampal NMDARs (Michael C. Lewis, 2008). We have extended this observation by showing that the E2-enhanced NOR is prevented by selective blockade of NR2B-containing NMDARs in area CA1 of hippocampus (Figure 2B1–3). Interestingly, in contrast to the prevention of NOR in E2-treated rats when RO was administered systemically (Figure 2A1–3), intraCA1 RO infusion only prevented the E2-enhanced learning and memory, rendering NOR in E2+RO treated animals not different from V-treated rats with or without RO (Figure 2B3). This blockade of only the enhanced learning and memory by intraCA1 RO is reminiscent of our previous LTP results showing that bath application of RO to acutely prepared hippocampal slices only prevents the E2-induced increase in LTP magnitude, with the resulting LTP magnitude not different from that measured in slices from V-treated rats with or without RO (Smith and McMahon, 2006). The differential effect of systemic versus intraCA1 RO administration on NOR in E2-treated rats is likely explained by the systemic treatment causing a more complete blockade of NR2B in hippocampus, as well as other brain regions. Importantly, the loss of NOR in E2-treated rats, while V-treated rats are unaffected by systemic RO administration indicates that NOR is more highly dependent upon NR2B-containing NMDARs following elevated E2. This idea is further supported by the 10-fold higher RO concentration (although still selective for NR2B subunits) needed to prevent NOR in V-treated rats, demonstrating that normal learning and memory is dependent upon NR2B-containing NMDARs, but to a lesser degree than in E2-treated animals (Figure 2D).
Biochemical and anatomical studies have thus far have been unable to determine the mechanism causing the increase in NR2B current measured using electrophysiology (Adams et al., 2004; Snyder et al., 2010). Because orthovanadate significantly increases the maximum current amplitude in V-treated rats but is unable to drive the increase to the same maximum as in E2-treated rats, our results are consistent with the interpretation that E2-treatment increases both the number and phosphorylation of synaptic NMDARs, as shown in the model in Figure 3C. Although the E2-induced increase in NMDAR current is entirely due to an increase in NR2B-containing receptors (Smith and McMahon, 2006), in these experiments total NMDAR current is measured (i.e. current mediated by both NR2B-containing and non-NR2B-containing receptors). Therefore, the increase in NMDAR current in cells recorded with orthovanadate in V-treated rats could be due to increased phosphorylation of non-NR2B containing NMDARs, and yet the maximum NMDAR current remains significantly less than in E2-treated rats. This finding further supports the interpretation that E2 increases the density of NMDARs at synapses, which we know contain NR2B subunits (Smith and McMahon, 2006; Snyder et al., 2010). Unfortunately, it is still unclear whether these NR2B-containing receptors are exocytosed into the postsynaptic density or translocated into the synapse from extrasynaptic locations. It also is not known whether these NR2B-containing NMDARs are located within silent synapses (Smith et al., 2009). However, because NR2B-containing receptors are most abundant in early development when the silent synapse density is also highest (Law et al., 2003; Petralia et al., 1999; Sans et al., 2000), the simultaneous increase in dendritic spine density and NR2B-mediated NMDAR transmission (Smith and McMahon, 2005; Smith and McMahon, 2006) with E2-treatment strongly supports the concept that E2 increases the density of silent synapses. Accordingly, the conversion of these newly developed silent synapses into active synapses could underlie the heightened LTP magnitude, which we show here is associated with enhanced NOR.
Estrogen could use a traditional genomic estrogen receptor (ERα or ERβ) and initiate gene transcription which requires hours to days, or E2 could engage a membrane-bound ER, resulting in the rapid activation of signaling cascades to enhance hippocampal LTP, NR2B-mediated current, spine density, and learning and memory. We have shown previously that LTP at CA3-CA1 synapses is not enhanced 24 hours after a single E2 injection (10µg) (Smith and McMahon, 2005), suggesting a genomic mechanism is required for the synaptic changes we observe. However, E2 can enhance NMDAR-mediated responses within minutes of bath application of 1nM E2 in recordings from acute hippocampal slices (Foy et al., 1999). Also, intrahippocampal or intracerebroventricular E2 infusion within 30 min after exposure to objects in the NOR task enhances consolidation, due to activation of a membrane bound estrogen receptor which stimulates PKA and ERK activation (Fernandez et al., 2008; Michael C. Lewis, 2008). Recent data further supports the theory that the ERα agonist PPT can increase dendritic spine density and learning and memory within 40 minutes of a systemic injection (Phan et al., 2011). It is most likely that the effects we observe here are caused by a combination of rapid and genomic effects of E2 that occur in parallel, using separate mechanisms to enhance hippocampal function.
E2 may also act indirectly through cholinergic input into hippocampus to enhance potentiation at CA3-CA1 synapses and learning and memory. E2-enhanced cholinergic function in hippocampus (Gibbs, 2000; Luine, 1985) is critical for E2-enhanced learning and memory (Daniel et al., 2005; Gibbs, 2002; Gibbs, 2007) and increased NMDARs in binding assays (Daniel and Dohanich, 2001). New studies are required to determine a direct linkage between cholinergic innervation into hippocampus and E2-enhanced magnitude of LTP and to further determine whether these effects are causal to the ability of E2 to enhance learning and memory.
Our findings that E2 requires NR2B-containing NMDARs to enhance hippocampal dependent learning and memory are integral for the development of targeted treatments for hormone-related cognitive decline during menopause. We have previously reported that the E2-enhanced magnitude of LTP at CA3-CA1 synapses is lost after long periods of hormone deprivation following ovariectomy in young adulthood (Smith et al., 2010). This loss in E2-enhanced LTP is prevented when rats are aged with their ovaries intact, suggesting that the loss in enhanced plasticity is due primarily to the duration of hormone deprivation, rather than to chronological age. These findings support the theory that a window of opportunity exists after hormone loss during which time E2 can enhance hippocampal LTP. Future studies are required to determine whether E2-enhanced cognition is maintained in aging and through periods of long-term ovarian hormone loss and whether NR2B-containing NMDARS are also critical for these enhancements, should they occur.
Acknowledgements
We would like to thank Dr. Thomas Van Groen, Director of the University of Alabama at Birmingham Behavioral Assessment Core for his assistance with our behavioral assays, Cristin Gavin for her helpful suggestions in performing intraCA1 cannula surgeries and Dr. Karen Gamble for her assistance with our statistical analysis.
Grant sponsor: NIMH; Grant number: MH082304.
Grant sponsor: NINDS; Grant number P30 NS047466
Grant Sponsor: NINDS; Grant number P30 NS057098
Grant Sponsor: The Evelyn F. McKnight Brain Research Foundation
Contributor Information
Lindsey C. Vedder, University of Alabama at Birmingham, Department of Cell, Developmental, and Integrative Biology, 1918 University Boulevard, MCLM964, Birmingham, Al 35294, 205-934-3523.
Caroline C. Smith, Department of Psychiatry, UT Southwestern Medical Center, Dallas, TX, 214-648-5520.
Alaina E. Flannigan, University of Alabama at Birmingham, Department of Cell, Developmental, and Integrative Biology, 1918 University Boulevard, MCLM964, Birmingham, Al 35294, 205-934-3524
Lori L. McMahon, University of Alabama at Birmingham, Department of Cell, Developmental, and Integrative Biology, 1918 University Boulevard, MCLM964, Birmingham, Al 35294, 205-934-3523, McMahon@uab.edu.
References
- Adams MM, Fink SE, Janssen WG, Shah RA, Morrison JH. Estrogen modulates synaptic N-methyl-D-aspartate receptor subunit distribution in the aged hippocampus. J Comp Neurol. 2004;474(3):419–426. doi: 10.1002/cne.20148. [DOI] [PubMed] [Google Scholar]
- Bi R, Foy MR, Vouimba RM, Thompson RF, Baudry M. Cyclic changes in estradiol regulate synaptic plasticity through the MAP kinase pathway. PNAS. 2001;98(23):13391–13395. doi: 10.1073/pnas.241507698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley MJ. The role of the perirhinal cortex and hippocampus in learning, memory, and perception. The Quarterly journal of experimental psychology. B, Comparative and physiological psychology. 2005;58(3–4):246–268. doi: 10.1080/02724990444000186. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Dohanich GP. Acetylcholine mediates the estrogen-induced increase in nmda receptor binding in ca1 of the hippocampus and the associated improvement in working memory. J Neurosci. 2001;21(17):6949–6956. doi: 10.1523/JNEUROSCI.21-17-06949.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Lee CD. Role of hippocampal M2 muscarinic receptors in the estrogen-induced enhancement of working memory. Neuroscience. 2005;132(1):57–64. doi: 10.1016/j.neuroscience.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Dere E, Huston JP, De Souza Silva MA. The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neuroscience & Biobehavioral Reviews. 2007;31(5):673–704. doi: 10.1016/j.neubiorev.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Dutar P, Vaillend C, Viollet C, Billard JM, Potier B, Carlo AS, Ungerer A, Epelbaum J. Spatial learning and synaptic hippocampal plasticity in type 2 somatostatin receptor knock-out mice. Neuroscience. 2002;112(2):455–466. doi: 10.1016/s0306-4522(02)00074-x. [DOI] [PubMed] [Google Scholar]
- Ennaceur A. One-trial object recognition in rats and mice: Methodological and theoretical issues. Behavioural Brain Research. 2010;215(2):244–254. doi: 10.1016/j.bbr.2009.12.036. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behavioural Brain Research. 1988;31(1):47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM. Estradiol-Induced Enhancement of Object Memory Consolidation Involves Hippocampal Extracellular Signal-Regulated Kinase Activation and Membrane-Bound Estrogen Receptors. J. Neurosci. 2008;28(35):8660–8667. doi: 10.1523/JNEUROSCI.1968-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy MR, Xu J, Xie X, Brinton RD, Thompson RF, Berger TW. 17beta-estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. J Neurophysiol. 1999;81(2):925–929. doi: 10.1152/jn.1999.81.2.925. [DOI] [PubMed] [Google Scholar]
- Frye CA, Duffy CK, Walf AA. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiology of Learning and Memory. 2007;88(2):208–216. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye JMVaCA. Progesterone in Conjunction With Estradiol Has Neuroprotective Effects in an Animal Model of Neurodegeneration. Pharmacology Biochemistry and Behavior. 1999;64(4):777–785. doi: 10.1016/s0091-3057(99)00140-9. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Effects of gonadal hormone replacement on measures of basal forebrain cholinergic function. Neuroscience. 2000;101(4):931–938. doi: 10.1016/s0306-4522(00)00433-4. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Basal forebrain cholinergic neurons are necessary for estrogen to enhance acquisition of a delayed matching-to-position T-maze task. Horm Behav. 2002;42(3):245–257. doi: 10.1006/hbeh.2002.1825. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Estradiol enhances DMP acquisition via a mechanism not mediated by turning strategy but which requires intact basal forebrain cholinergic projections. Hormones and Behavior. 2007;52(3):352–359. doi: 10.1016/j.yhbeh.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Harburger LL, Saadi A, Frick KM. Dose-dependent effects of post-training estradiol plus progesterone treatment on object memory consolidation and hippocampal extracellular signal-regulated kinase activation in young ovariectomized mice. Neuroscience. 2009;160(1):6–12. doi: 10.1016/j.neuroscience.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law AJ, Weickert CS, Webster MJ, Herman MM, Kleinman JE, Harrison PJ. Expression of NMDA receptor NR1, NR2A and NR2B subunit mRNAs during development of the human hippocampal formation. Eur J Neurosci. 2003;18(5):1197–1205. doi: 10.1046/j.1460-9568.2003.02850.x. [DOI] [PubMed] [Google Scholar]
- Luine VN. Estradiol increases choline acetyltransferase activity in specific basal forebrain nuclei and projection areas of female rats. Experimental Neurology. 1985;89:484–490. doi: 10.1016/0014-4886(85)90108-6. [DOI] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, MacLusky NJ. Rapid Enhancement of Visual and Place Memory by Estrogens in Rats. Endocrinology. 2003;144(7):2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- MacDonald JF, Mody I, Salter MW. Regulation of N-methyl-D-aspartate receptors revealed by intracellular dialysis of murine neurones in culture. J Physiol. 1989;414:17–34. doi: 10.1113/jphysiol.1989.sp017674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44(1):5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Michael C. Lewis KMK, Orr Patrick T, Frick Karyn M. Estradiol-Induced Enhancement of Object Memory Consolidation Involves NMDA Receptors and Protein Kinase A in the Dorsal Hippocampus of Female C57BL/6 Mice. Behavioral Neuroscience. 2008;122(3):716–721. doi: 10.1037/0735-7044.122.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migaud M, Charlesworth P, Dempster M, Webster LC, Watabe AM, Makhinson M, He Y, Ramsay MF, Morris RGM, Morrison JH, et al. Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature. 1998;396(6710):433–439. doi: 10.1038/24790. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Esteban JA, Wang YX, Partridge JG, Zhao HM, Wenthold RJ, Malinow R. Selective acquisition of AMPA receptors over postnatal development suggests a molecular basis for silent synapses. Nat Neurosci. 1999;2(1):31–36. doi: 10.1038/4532. [DOI] [PubMed] [Google Scholar]
- Phan A, Lancaster KE, Armstrong JN, MacLusky NJ, Choleris E. Rapid Effects of Estrogen Receptor OE± and OE≤ Selective Agonists on Learning and Dendritic Spines in Female Mice. Endocrinology. 2011;152(4):1492–1502. doi: 10.1210/en.2010-1273. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA. Estrogen has mnemonic-enhancing effects in the inhibitory avoidance task. Pharmacology Biochemistry and Behavior. 2004;78(3):551–558. doi: 10.1016/j.pbb.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Rosenberg L, Park S. Verbal and spatial functions across the menstrual cycle in healthy young women. Psychoneuroendocrinology. 2002;27(7):835–841. doi: 10.1016/s0306-4530(01)00083-x. [DOI] [PubMed] [Google Scholar]
- Sans N, Petralia RS, Wang YX, Blahos J, 2nd, Hell JW, Wenthold RJ. A developmental change in NMDA receptor-associated proteins at hippocampal synapses. J Neurosci. 2000;20(3):1260–1271. doi: 10.1523/JNEUROSCI.20-03-01260.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CC, McMahon LL. Estrogen-induced increase in the magnitude of long-term potentiation occurs only when the ratio of NMDA transmission to AMPA transmission is increased. J Neurosci. 2005;25(34):7780–7791. doi: 10.1523/JNEUROSCI.0762-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CC, McMahon LL. Estradiol-induced increase in the magnitude of long-term potentiation is prevented by blocking NR2B-containing receptors. J Neurosci. 2006;26(33):8517–8522. doi: 10.1523/JNEUROSCI.5279-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CC, Vedder LC, McMahon LL. Estradiol and the relationship between dendritic spines, NR2B containing NMDA receptors, and the magnitude of long-term potentiation at hippocampal CA3-CA1 synapses. Psychoneuroendocrinology. 2009;34(Supplement 1):S130–S142. doi: 10.1016/j.psyneuen.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CC, Vedder LC, Nelson AR, Bredemann TM, McMahon LL. Duration of estrogen deprivation, not chronological age, prevents estrogen's ability to enhance hippocampal synaptic physiology. Proceedings of the National Academy of Sciences. 2010;107(45):19543–19548. doi: 10.1073/pnas.1009307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder MA, Cooke BM, Woolley CS. Estradiol potentiation of NR2B-dependent EPSCs is not due to changes in NR2B protein expression or phosphorylation. Hippocampus. 2010;9999(9999) doi: 10.1002/hipo.20756. NA. [DOI] [PubMed] [Google Scholar]
- Tang Y-P. Gentic enhancement of learning and memory in mice. Letters to Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- Valenzuela-Harrington M, Gruart A, Delgado-Garcia JM. Contribution of NMDA receptor NR2B subunit to synaptic plasticity during associative learning in behaving rats. Eur J Neurosci. 2007;25(3):830–836. doi: 10.1111/j.1460-9568.2007.05325.x. [DOI] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiology of Learning and Memory. 2006;86(1):35–46. doi: 10.1016/j.nlm.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YT, Salter MW. Regulation of NMDA receptors by tyrosine kinases and phosphatases. Nature. 1994;369(6477):233–235. doi: 10.1038/369233a0. [DOI] [PubMed] [Google Scholar]
- Warren SG, Humphreys AG, Juraska JM, Greenough WT. LTP varies across the estrous cycle: enhanced synaptic plasticity in proestrus rats. Brain Res. 1995;703(1–2):26–30. doi: 10.1016/0006-8993(95)01059-9. [DOI] [PubMed] [Google Scholar]
- Warren SG, Juraska JM. Spatial and nonspatial learning across the rat estrous cycle. Behav Neurosci. 1997;111(2):259–266. doi: 10.1037//0735-7044.111.2.259. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336(2):293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Weiland NG, McEwen BS, Schwartzkroin PA. Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: correlation with dendritic spine density. J Neurosci. 1997;17(5):1848–1859. doi: 10.1523/JNEUROSCI.17-05-01848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]