Abstract
We evaluated the hypothesis that CYP3A5 expression can affect intrarenal tacrolimus accumulation. An oral dose of tacrolimus was administered to 24 healthy volunteers who were selected based on their CYP3A5 genotype. Compared to CYP3A5 nonexpressors, expressors had a 1.6-fold higher oral tacrolimus clearance and 2.0- to 2.7-fold higher metabolite/parent AUC ratios for 31-DMT, 12-HT and 13-DMT. In addition, the apparent urinary tacrolimus clearance was 36% lower in CYP3A5 expressors, compared to nonexpressors. To explore the mechanism behind this observation, we developed a semi-physiological model of renal tacrolimus disposition and predicted that tacrolimus exposure in the renal epithelium of CYP3A5 expressors is 53% of that for CYP3A5 nonexpressors, when normalized to blood AUC. These data suggest that at steady state, intrarenal accumulation of tacrolimus, and its primary metabolites, will depend on the CYP3A5 genotype of the liver and kidneys. This may contribute to inter-patient differences in the risk of tacrolimus-induced nephrotoxicity.
Introduction
The use of tacrolimus, a calcineurin inhibitor, to prevent solid organ transplant rejection is routinely guided by therapeutic blood level monitoring (TDM) (1). Despite close monitoring and individualization of dosing, TDM has not prevented some transplant recipients from developing chronic calcineurin inhibitor nephrotoxicity (CNIT) (2, 3).
The pathogenesis of CNIT is complex and largely unpredictable (4). However, there is evidence that the drug concentration in kidney tissue is more predictive of CNIT than systemic blood concentration (4–6). In addition, some studies suggest that a higher systemic exposure to tacrolimus metabolites or intrarenal production of potentially toxic metabolites may play a contributory role in the development of CNIT (4, 7–9). There is also evidence to suggest that inherited variation in an individual’s genome contributes to the risk of nephrotoxicity (5, 8). This includes variation in CYP3A genes that influence the bioavailability and metabolic clearance of tacrolimus (10). CYP3A4 is expressed in the liver and small intestine of nearly all individuals, but with substantial inter-individual variability that is largely unexplained to date by genetic factors (11–15). In contrast, polymorphic expression of CYP3A5 in the liver, small intestine, kidney and other organs is determined primarily by single-nucleotide variations that distinguish the “active” CYP3A5*1 allele (inferred CYP3A5 expressor phenotype) from the “inactive” CYP3A5*3, *6 or *7 alleles (inferred CYP3A5 nonexpressor phenotype). Individuals carrying two loss-of-function CYP3A5 alleles have a markedly reduced level of CYP3A5 tissue expression and catalytic activity (11–15). There are data suggesting a significant impact of the CYP3A5 polymorphism on the metabolism of tacrolimus. Metabolism of tacrolimus is more efficient with recombinant CYP3A5 than recombinant CYP3A4. In addition, a higher metabolic clearance of tacrolimus has been observed in liver tissue obtained from CYP3A5-expressing organ donors than from nonexpressors (16, 17). In contrast, in the kidneys, only CYP3A5 (and not CYP3A4) is found at levels thought sufficient to affect intra-tissue drug clearance (14, 18). Microsomes from CYP3A5*1/*3 genotyped kidney tissues exhibited at least an 8-fold higher CYP3A5 content and 18-fold higher midazolam or tacrolimus hydroxylation activity than microsomes from CYP3A5*3/*3 genotyped tissues (15, 16); an observation consistent with CYP3A5 being the predominant CYP3A isoform expressed in renal tissue (4, 14).
Pharmacokinetic studies in heart, lung, liver and kidney transplant patients have shown a significant difference in dose-normalized blood tacrolimus level between the CYP3A5 expressors versus nonexpressors; the CYP3A5 expressors typically required a larger tacrolimus dose to achieve the same, therapeutic trough blood concentration (19–22). Thus, inheritance of the CYP3A5*1 allele could affect systemic and intrarenal concentrations of tacrolimus and its metabolites during drug therapy (7, 16), and in turn the risk of CNIT.
To test this hypothesis, we examined the apparent urinary tacrolimus clearance in CYP3A5 expressors compared to CYP3A5 nonexpressors. The relationship between blood concentration and urinary excretion rate (i.e., apparent urinary clearance) should depend upon whether or not CYP3A5-dependent intrarenal tacrolimus metabolism is present; specifically, apparent urinary clearance of tacrolimus should be lower in CYP3A5 expressors compared to nonexpressors, reflecting intrarenal loss due to metabolism. In addition, we evaluated whether a CYP3A5-dependent difference in systemic tacrolimus clearance was associated with a difference in the accumulation of the known primary tacrolimus metabolites in blood that may also contribute to the risk of nephrotoxicity.
Results
Demographic characteristics of healthy volunteers
Twenty-four healthy subjects, comprised of 12 CYP3A5 expressors (CYP3A5*1-allele carriers) and 12 nonexpressors (subjects who carry two copies of a loss-of-function CYP3A5 allele: CYP3A5*3, CYP3A5*6 or CYP3A5*7), were enrolled. There were no significant differences between the two CYP3A5 phenotype groups with respect to distribution of gender, body weight, serum creatinine, creatinine clearance, and estimated GFR (eGFR) (Supplemental Table 1 online). However, the mean age of CYP3A5 expressors was older than that of nonexpressors (30.8 ± 9.9 vs. 23.5 ± 3.5 yrs, P = 0.03). The frequency of ABCB1 3435C>T, 1236C>T, 2677G>T/A SNPs and the 1236T-2677T-3435T (T-T-T) variant haplotype did not differ significantly between the CYP3A5 phenotype groups.
Systemic disposition of tacrolimus and its primary metabolites
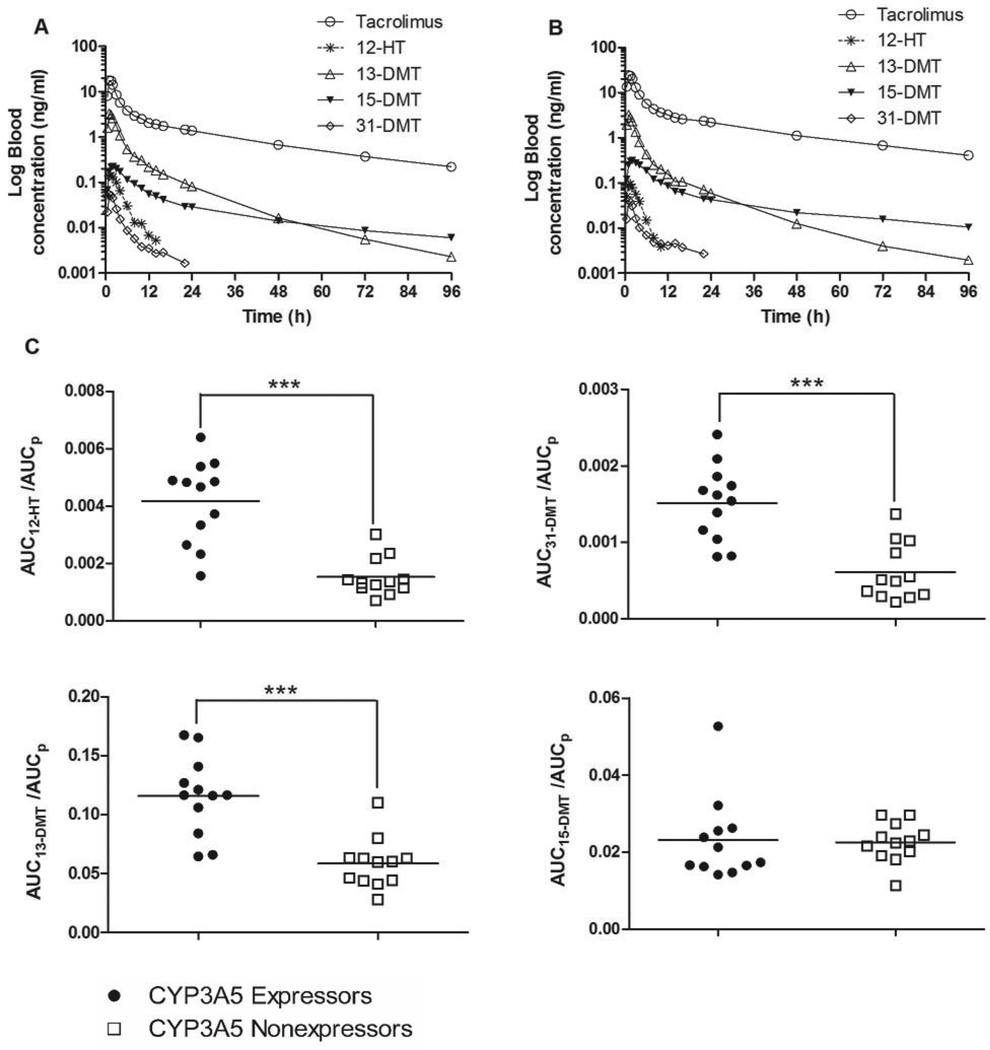
The pharmacokinetics of tacrolimus and its metabolites differed between CYP3A5 expressors and nonexpressors. Blood or plasma tacrolimus concentrations were, on average, lower in CYP3A5 expressors compared to nonexpressors, as reflected in a 1.6-fold higher mean oral tacrolimus clearance (CL/F; which is dependent on both intestinal and hepatic CYP3A activity) for CYP3A5 expressors (Table 1). The peak blood concentration (Cmax) and time to peak concentration (tmax) of tacrolimus were not significantly different; however, the blood concentration at 96 hours after drug administration (Clast) was 1.9-fold higher in CYP3A5 nonexpressors compared to expressors (P = 0.0003). The mean blood concentration–time profiles of tacrolimus and its four primary metabolites after oral tacrolimus administration in CYP3A5 expressors and CYP3A5 nonexpressors are shown in Figures 1A and 1B, respectively. The concentration of all metabolites was less than that of parent drug across all time points. As seen in Figure 1C, the mean AUCmetabolite/AUCparent ratio for 31-DMT, 12-HT and 13-DMT, an indirect measure of the respective metabolite formation clearances, was 2.5-, 2.7- and 2.0-fold higher in CYP3A5 expressors than the ratios for CYP3A5 nonexpressors (p < 0.001 for all). In contrast, the mean ratio for 15-DMT did not differ between the two CYP3A5 phenotype groups.
Table 1.
Tacrolimus pharmacokinetic parameters for study participants stratified by predicted CYP3A5 phenotype.
| Nonexpressors (N=12) | Expressors (N=12) | Difference (%) | P value | |
|---|---|---|---|---|
| AUC0–96 (h•ng/ml) | 209.2 ± 45.0 | 135.6 ± 60.4 | −35.2% | 0.003 |
| AUC0–inf (h•ng/ml) | 228.1 ± 50.0 | 144.8 ± 61.6 | −36.5% | 0.001 |
| t1/2 (h) | 31.7 ± 4.3 | 29.9 ± 4.8 | NS | |
| tmax (h) | 1.3 ± 0.4 | 1.4 ± 0.6 | NS | |
| Cmax (ng/ml) | 27.9 ± 7.7 | 20.8 ± 11.2 | NS | |
| Clast (ng/ml) | 0.4 ± 0.1 | 0.2 ± 0.1 | −46.2% | 0.0003 |
| CL/F (ml/h/kg) | 355.4 ± 88.0 | 550.2 ± 127.7 | 54.8% | 0.0003 |
| CLurinary(ml/h) (based on AUCblood) | 3.15 ± 1.69 | 2.01 ± 0.57 | −36.2% | 0.04 |
| CLurinary/eGFR (%) (based on AUCblood) | 0.048 ± 0.032 | 0.028 ± 0.012 | −41.7% | 0.05 |
| CLurinary (ml/h) (based on AUCplasma) | 97.71 ± 59.89 | 50.15 ± 13.67 | −48.7% | 0.01 |
| CLurinary/eGFR (%) (based on AUCplasma) | 1.48 ± 1.10 | 0.58 ± 0.28 | −53.9% | 0.02 |
AUC, area under the concentration–time curve; tmax, time to reach the maximum blood concentration; Cmax, maximum blood concentration; Clast, blood concentration at 96 hour after tacrolimus administration; CL/F, oral clearance; CLurinary, tacrolimus urinary clearance for the 0 to 96-hour collection interval; eGFR, estimated glomerular filtration rate calculated using the Cockroft-Gault equation (37); NS, not significant.
Figure 1.
Mean log concentration–time profiles of tacrolimus and its four primary metabolites after oral tacrolimus administration in (A) CYP3A5 expressors (n = 12) and (B) CYP3A5 nonexpressors (n = 12). Also shown are AUCmetabolite to AUCparent ratios for the four primary metabolites of tacrolimus (C): AUC12-HT /AUCp, AUC31-DMT/AUCp, AUC13-DMT/AUCp and AUC15-DMT/AUCp. The solid line represents the mean ratios; *** P < 0.0001.
Renal excretion of tacrolimus and its primary metabolites
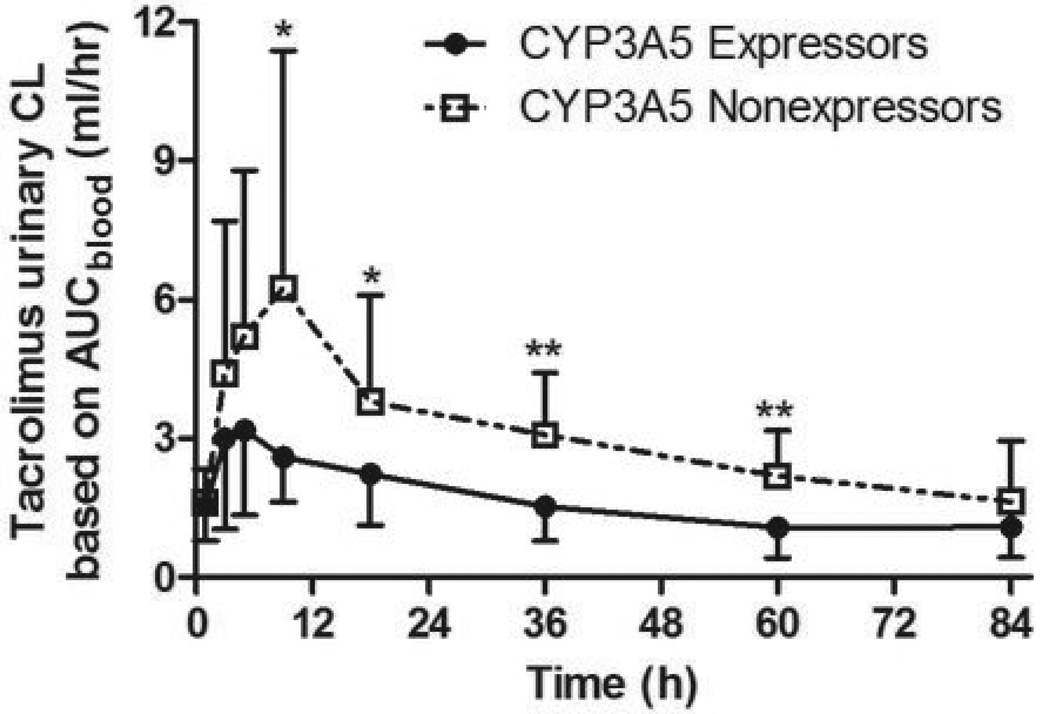
The total amount of tacrolimus excreted in urine as unchanged drug over 96 hours after oral administration was 271.6 ± 147.4 (ng) and 642.6 ± 349.6 (ng) for CYP3A5 expressors and nonexpressors, respectively (P = 0.003); i.e., 58% lower in CYP3A5 expressors compared to nonexpressors. The mean apparent urinary tacrolimus clearance was 36%, based on blood AUC, or 49% based on plasma AUC, lower in CYP3A5 expressors compared to CYP3A5 nonexpressors (Table 1). Similarly, the apparent urinary tacrolimus clearance (based on blood AUC) normalized by eGFR was 42% lower in CYP3A5 expressors compared to CYP3A5 nonexpressors. The between-group difference in urinary tacrolimus clearance was evident over the successive urine collection time intervals (Figure 2).
Figure 2.
Serial calculations of the apparent urinary tacrolimus clearance based on blood concentrations; * P < 0.05; ** P < 0.005.
Semi-physiological model of renal tacrolimus disposition
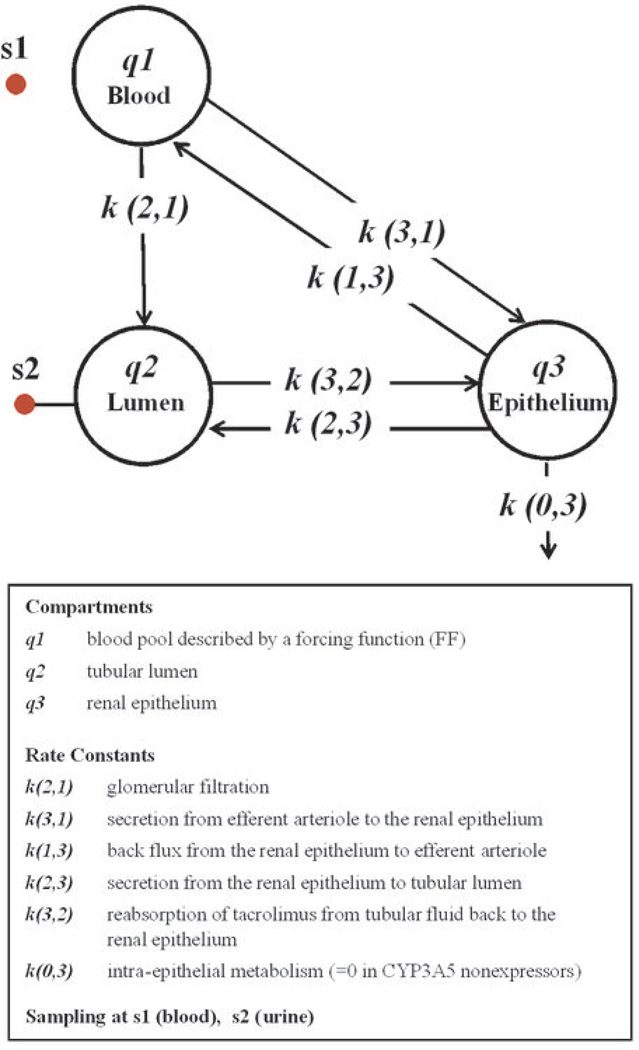
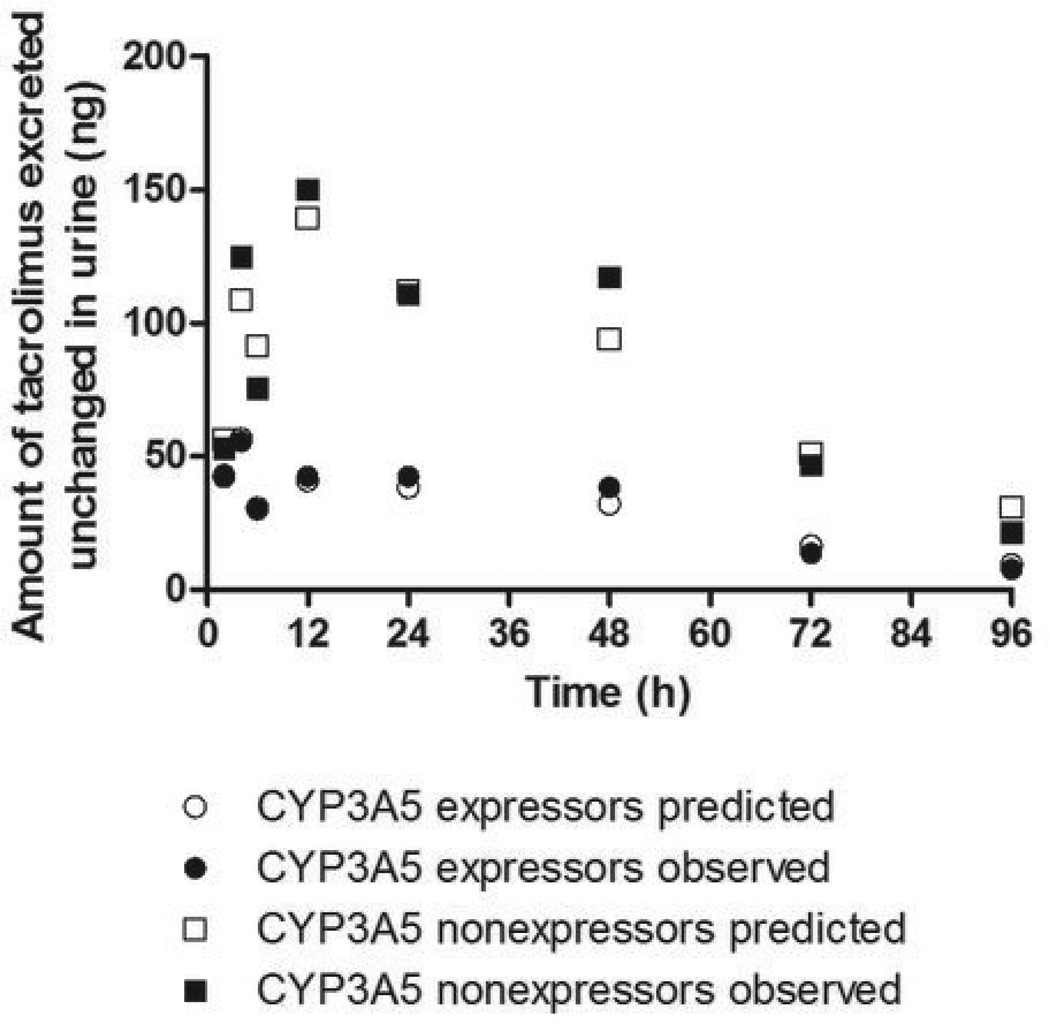
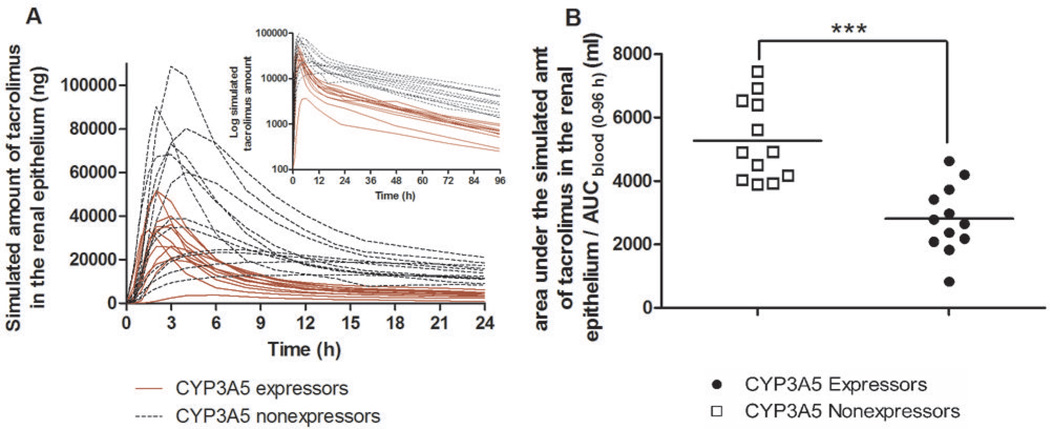
A semi-physiological model of renal tacrolimus disposition was developed in an effort to evaluate how the presence or absence of intrarenal CYP3A5-mediated tacrolimus metabolism might impact epithelial exposure to tacrolimus. A three-compartment model (Figure 3) representing the blood pool, tubular lumen, and tubular epithelium and featuring glomerular filtration, epithelial transport, and metabolism within the epithelium is capable of explaining the relationship between the time course of blood concentration and urinary excretion, as illustrated by the goodness of fit between the observed amount of tacrolimus excreted in the successive urine collection periods and model prediction based on simultaneous fitting of the model to the mean data for the CYP3A5 expressor and nonexpressor groups (Figure 4). The model was fit to individual tacrolimus urine excretion data according to a staged strategy as described in the Methods. Using the parameter estimates from individual model fitting (Table 2), a profile of the amount of tacrolimus in the epithelial compartment over time was simulated (Figure 5A). The simulated tacrolimus exposure in the renal epithelial compartment of CYP3A5 expressors, when normalized to the blood AUC (0–96 h), was on average 53% of that for CYP3A5 nonexpressors (Figure 5B). Large between-subjects variability in the maximum amount (29-fold) and normalized area under the amount-time curve (9-fold) in the epithelial compartment was observed, particularly when data from the two phenotype groups were combined.
Figure 3.
Compartmental model scheme for renal disposition. Rate constant, compartment labels and parameters are defined in the figure.
Figure 4.
Model fit to the mean tacrolimus urine excretion data in CYP3A5 expressors and nonexpressors, simultaneously, using blood concentration as a forcing function.
Table 2.
Parameter estimates (mean ± SD) from model fitting to the individual tacrolimus urine excretion data in CYP3A5 expressors and nonexpressors using blood concentration as a forcing function.
| CYP3A5 expressors | CYP3A5 nonexpressors | ||
|---|---|---|---|
| Parameters fixed to known physiological values | GFR (ml/h) | 7761 ± 1748 | 7124 ± 1585 |
| Qeffart (ml/h) | 48490 ± 11461 | 45731 ± 11158 | |
| Vepi (ml) | 250 ± 76 | 232 ± 52 | |
| Parameters from empirical Bayesian estimation | Kp,kidney | 12.1 ± 0.3*** | 23.4 ± 0.04 |
| Parameters from ordinary least-squares estimation | fub (%) | 0.014 ± 0.015 | 0.011 ± 0.006 |
| k(0,3)(h−1) | 0.55 ± 0.52 | ||
| k(1,3) (h−1) | 0.34 ± 0.05 | 0.36 ± 0.39 | |
| k(2,3) (h−1) | 0.0009 ± 0.0005 | 0.0012 ± 0.0008 | |
| k(3,2) (h−1) | 0.091 ± 0.063 | 0.106 ± 0.057 | |
| ER (%) | 5.1 ± 3.0 | 4.3 ± 4.7 | |
| Parameters derived from the above estimation | k(2,1) (ml/h) | 0.94 ± 0.76 | 0.77 ± 0.51 |
| k(3,1) (ml/h) | 2368 ± 1404 | 1800 ± 1734 |
GFR: values are from estimated glomerular filtration rate calculated using the Cockroft-Gault equation (37)
Qeffart : efferent arteriolar blood flow rate
Vepi : the volume of renal epithelium
Kp,kidney: apparent tissue-to-blood partitioning coefficient of tacrolimus
fub: exchangeable fraction of tacrolimus in blood during glomerular filtration
k(0,3): rate constant representing CYP3A5-mediated intra-epithelial metabolism (=0 in CYP3A5 nonexpressors and assumes a characteristic value for CYP3A5 expressors.)
k(1,3): transfer rate constant representing back flux from the renal epithelium to efferent arteriole
k(2,3): transfer rate constant representing secretion from the renal epithelium to tubular lumen
k(3,2): transfer rate constant representing reabsorption of tacrolimus from tubular fluid back to the renal epithelium
ER: extraction ratio of tacrolimus from the efferent arteriole
k(2,1): transfer rate constant representing the glomerular filtration of tacrolimus
k(3,1): transfer rate constant representing secretion from efferent arteriole to the renal epithelium
P < 0.0001
Figure 5.
The simulated tacrolimus exposure in the renal epithelium. (A) Simulated tacrolimus amount in the renal epithelium (logarithmic amount shown in the upper insert) and (B) Area under the simulated amountrenal epithelium - time curve normalized to blood AUC(0–96h); *** P < 0.0001.
There were no statistical differences in the model parameter estimates (Table 2) except for Kp,kidney, the apparent tissue-to-blood partitioning coefficient of tacrolimus. Kp,kidney in CYP3A5 expressors was estimated to be about one-half of that in CYP3A5 nonexpressors.
The unbound fraction of tacrolimus in whole blood (fub), derived from in vitro measurements of unbound fraction in plasma (fup, 2.1 ± 0.8%) and whole blood-to-plasma distribution ratio (23), was 0.078 ± 0.026% (24), which is significantly higher than the in vivo of 0.012 ± 0.011% estimated from modeling. It should be noted that the exchangeable fraction in vivo, which reflects the interplay of drug binding and dynamic events at the glomerulus, does not necessarily equal the in vitro equilibrium unbound fraction (i.e., ).
The other noteworthy observation with the model parameter estimates in Table 2 is the comparison between the extraction ratio of tacrolimus from the efferent arteriolar flow (ER) and the estimated exchangeable fraction (). The fact that ER was much greater than (by ~400-fold) suggests an exceptionally efficient uptake of tacrolimus at the basolateral aspect of the renal tubular epithelium that goes well beyond the exchangeable fraction operating at the glomerulus.
Discussion
The risk of nephrotoxicity remains a major challenge to the long-term survival of organ transplant patients receiving chronic calcineurin inhibitor therapy despite the fact that tacrolimus dosage is titrated to achieve an accepted therapeutic range of trough blood concentrations. Currently, there is no effective way to identify those that will develop chronic nephrotoxicity from those that will not (7). Plausible susceptibility factors have been proposed, including individual differences in renal metabolism of the drug. In the present study, we evaluated how CYP3A5 genetic variation and the corresponding enzyme phenotype affect systemic and intrarenal tacrolimus metabolism and exposure of kidney tubular epithelium to tacrolimus. A single dose study was conducted in healthy volunteers in order to avoid the confounding effects of changing renal function or drug-drug interactions from concomitant therapies in organ transplant patients (5).
Our results confirm that the mean oral tacrolimus clearance is higher in CYP3A5 expressors than in CYP3A5 nonexpressors, which explains the larger tacrolimus dose that CYP3A5 expressors require in order to maintain the same trough blood concentration as nonexpressors (19–22). The data also indicate that the CYP3A5*1 genotype, and inferred high renal expression phenotype, is associated with a greater extent of renal tacrolimus metabolism and a lower apparent urinary tacrolimus clearance compared to those subjects lacking the active reference allele. Such a relationship between renal metabolism and apparent urinary clearance of unchanged drug was first reported by Sirianni et al., who showed, in the isolated perfused rat kidney, that the urinary clearance of enalapril increased following inhibition of enalapril hydrolysis to enalaprilate by paraoxon (24). In our study, the mean apparent urinary tacrolimus clearance (based on blood AUC) was 36% lower in CYP3A5 expressors compared to CYP3A5 nonexpressors, which is highly indicative of intrarenal CYP3A5-dependent tacrolimus metabolism. Tacrolimus is a substrate of P-glycoprotein (P-gp), encoded by the polymorphic ABCB1 gene and multiple studies, though with conflicting results, have related ABCB1 SNPs to tacrolimus nephrotoxicity (25), suggesting P-gp may affect the renal secretion of tacrolimus. Our analysis of ABCB1 genotype/ haplotype showed that they did not constitute a significant covariate in determining the urinary clearance of tacrolimus, although the study was not powered to test for such a contribution. It follows then that there should be a corresponding difference in the exposure to tacrolimus in the metabolically competent cell types within the kidneys, viz., the tubular epithelia, resulting from the difference in renal CYP3A5 expression. Results from our simulations using a semi-physiological model of renal tacrolimus disposition support this hypothesis. Importantly, the estimated value for the steady-state tissue-to-blood partitioning ratio, Kp,kidney in CYP3A5 expressors was nearly 50% that calculated for CYP3A5 nonexpressors, which is a model prediction that can be evaluated by measuring tacrolimus concentrations in kidney biopsy and blood samples in genotyped patients receiving the immuosuppressant following kidney transplantation.
One predicted consequence of increased CYP3A5-dependent intrarenal tacrolimus metabolism is a reduced risk of tacrolimus-induced nephrotoxicity following solid organ transplantation, if renal tacrolimus concentration is a major driver of toxicity. To date, only a few studies in kidney recipients have considered donor CYP3A5 genotype. Our prediction is supported by results from some of these studies (6, 26), but not by all (27, 28). Such discordant pharmacogenetic findings may reflect the additional complication that tacrolimus metabolites could exert independent effects on renal function. Notably, the significantly increased circulating concentrations of metabolites in CYP3A5 expressors (recipient’s genotype in renal transplantation, for example) may counteract the protective role of CYP3A5 expression in the kidney (donor’s genotype in renal transplantation). In addition, any effects of tacrolimus metabolites on renal function will depend on the efficiency by which they are cleared, as well as the efficiency of their formation systemically and intrarenally. It has been suggested previously that systemic production of primary and related downstream metabolites of tacrolimus might contribute to CNI nephrotoxicity (4, 7–9). A recent study showed that CYP3A5 expressing renal transplant patients with high early tacrolimus dose requirements, had a higher risk of developing CNIT compared with nonexpressors (8). CYP3A5 expressors are also, reportedly, at greater risk of developing de novo arteriolar hyalinization, a histologic sign of CNIT based on a study of 304 de novo renal graft recipients (7).
The blood metabolite AUCs and AUCmetabolite/AUCparent ratios we observed after a single tacrolimus dose allow us to predict abundance of the CYP3A5-catalyzed metabolites in the blood circulation at steady-state. Based on that analysis, there should be greater absolute accumulations of three of the four primary tacrolimus metabolites (13-DMT, 31-DMT, and 12-HT) in CYP3A5 expressors at steady-state when the tacrolimus dose is titrated to achieve the same therapeutic tacrolimus concentration, a prediction consistent with in vitro results showing that the average formation rates of these metabolites were at least 1.7-fold higher in human liver microsomes with a CYP3A5*1/*3 genotype compared to microsomes with a homozygous CYP3A5*3/*3 genotype (16). Interestingly, we found that the 15-DMT/parent AUC ratio and renal excretion of 15-DMT in the two genotyped groups were similar, which is also consistent with in vitro metabolic data (16).
Although the nephrotoxic potential of tacrolimus metabolites has not been studied (5), the metabolites of cyclosporine, another calcineurin inhibitor, has been examined. In a study by Sigal et al. (29) with 61 cyclosporine analogs, it was shown that the ability to induce nephrotoxicity in vivo correlates with the immunosuppression activity of these agents. Another study by Kung et al. (30) showed an association between the degree of calcineurin inhibition in tissue homogenate (greatest in kidney) and susceptibility of that organ to cyclosporine toxicity. With regard to metabolites, in mouse mixed lymphocyte reaction studies, Iwasaki et al. (31) found that 31-DMT had the same immunosuppressive activity as tacrolimus, whereas the IC50 values for other metabolites were at least 10-fold higher. Thus, a higher systemic steady-state level of 31-DMT in CYP3A5 expressors compared to nonexpressors is likely to influence renal exposure to this active metabolite with its entry to the renal tubular cells via either uptake from the efferent arteriole or reabsorption from the tubular lumen following glomerular filtration, and possibly contribute to the nephrotoxicity risk. However, one caveat to this interpretation is the observation that the metabolic clearance of 31-DMT by CYP3A5 was comparable to that of tacrolimus (Supplemental Figure 1) and thus effective elimination of the potential toxic metabolite in CYP3A5 expressors may have a protective effect on renal function.
In summary, our findings demonstrate that CYP3A5 genotype has a significant impact on the metabolism and clearance of tacrolimus in the human kidney, as well as in previously studied and metabolically competent organs, the liver and small intestine. The extent of intrarenal metabolism in CYP3A5 expressing organ transplant patients is such that we predict a much lower accumulation of tacrolimus in the tubular epithelium compared to that in CYP3A5 nonexpressing patients. This difference may contribute to interindividual differences in tacrolimus-induced nephrotoxicity, although greater intrarenal exposure to primary and secondary tacrolimus metabolites in CYP3A5 expressors compared to nonexpressors may also have to be considered. Further prospective studies investigating the impact of CYP3A5 genotype on tacrolimus nephrotoxicity studies would help to clarify this issue by identifying the CYP3A5 genotype(s) of the recipients and their donor kidneys, as well as metabolite concentrations of tacrolimus in blood and renal tissue. A full elucidation of the pharmacogenomics of tacrolimus nephrotoxicity may lead to improved management of tacrolimus pharmacotherapy.
Methods
Clinical Protocol
The human subjects protocol was approved by the University of Washington Institutional Review Board. Written informed consent was obtained from all study participants. A single oral dose of tacrolimus (5 mg) was administered to 24 healthy participants selected based on their CYP3A5 genotype. None of the subjects had a significant medical history or abnormal clinical lab test results, and none had taken a known inhibitor, inducer, or activator of CYP3A4/5 (other than oral contraceptives) for at least 1 month preceding the start of and during the pharmacokinetic investigation, and all abstained from grapefruit and grapefruit juice and alcoholic beverages beginning at one week prior to the start until the end of the study. Sequential blood samples (5 ml) were collected in EDTA glass tubes just before and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 14, 16, 22, 24, 48, 72 and 96 h after oral drug administration beginning at 8 am. Plasma was harvested from an aliquot of the blood samples after incubation at 37°C for 30 min and centrifugation at 37°C. Urine was collected in silanized glass containers over the following post-dose intervals: 0–2 h, 2–4 h, 4–6 h, 6–12 h, 12–24 h, 24–48 h, 48–72h, and 72–96 h. All samples were stored at −80°C until analysis.
Genotyping
Buccal cell DNA was isolated using a DNeasy Blood & Tissue Kit or the Qiagen Gentra Puregene protocol (Qiagen, USA). Single-nucleotide polymorphisms (SNPs) in the CYP3A5 gene (*3, *6 and *7 alleles) were determined from a buccal swab tissue sample, using either previously published methods by Lin et al. (12) or a validated Taqman® allelic discrimination assay from Applied Biosystems (Foster City, CA) (32). The ABCB1 C3435T and C1236T SNPs were genotyped using TaqMan® assays (32). The ABCB1 G2677T/A multivariate SNP was determined by PCR amplification and DNA sequencing using published oligonucleotides (33).
Analytical Methods
Tacrolimus and metabolite concentrations in blood, plasma and urine samples were quantified by improving upon a previously reported LC-MS/MS procedure (34). A full description of the modified assay, along with the procedure for isolation and purification of the tacrolimus metabolites for use as analytical standards, is provided in a recent manuscript submission from our laboratory (Zheng et al., Therapeutic Drug Monitoring).
Pharmacokinetic Analysis
Noncompartmental pharmacokinetic analysis was performed using WinNonlin software version 5.2 (Pharsight, Mountain View, CA). Pharmacokinetic parameters determined for tacrolimus included the maximum concentration in blood (Cmax), the time to reach maximum concentration (Tmax), terminal half-life (t1/2), AUC (0–96 h), AUC (0-infinity), and oral clearance (CL/F normalized to individual body weight in kg). In addition, CLurinary was calculated as the amount of drug or metabolite excreted in urine divided by AUCblood over the collection interval.
Compartmental Model for Renal Metabolism
A semi-physiological model was developed to evaluate the effect of CYP3A5 polymorphism on intrarenal metabolism and tubulo-epithelial exposure to tacrolimus. Blood tacrolimus concentration-time data were entered as a forcing function (FF) for the blood compartment (35), which obviated the need to model the systemic disposition kinetics of tacrolimus. CYP3A5-mediated metabolism is assumed to occur in the epithelial compartment as demonstrated by previous studies (18, 36). The transfer of drug between compartments and the metabolic process is assumed to follow first-order kinetics; the six rate constants are defined in Figure 3. Because blood concentration is used as the driver for the blood compartment, k (2,1) and k(3,1) have the unit of flow (ml/h). All the other first-order rate constants have the typical dimension of reciprocal time (h−1).
To introduce a physiological framework for the model and provide realistic constraint on the parameter estimates, the rate constants for glomerular filtration k(2,1) and uptake from the efferent arteriole k(3,1) were re-parameterized as follows.
where , and GFR = glomerular filtration rate.
where ER = extraction ratio of tacrolimus from the efferent arteriole for either a CYP3A5 expressor (Exp) or CYP3A5 nonexpressor (NEx); and Qeffart = efferent arteriolar blood flow. In turn, ERExp and ERNEx can be expressed as a function of the apparent tissue-to-blood partitioning coefficient of tacrolimus (Kp,Exp or Kp,NEx), epithelial volume (Vepi), fub, GFR, Qeffart, and the intercompartmental transfer rate constants.
Kp,kid is experimentally defined as
where is the steady-state amount of tacrolimus in each of the renal tissue compartments, and Vi is the corresponding compartment volume. Given that CYP3A5 expressors have a metabolic sink in the epithelial compartment (q3), their apparent steady-state Kp,kid is expected to be lower than that in CYP3A5 nonexpressors.
The above model was implemented using SAAM II (The Epsilon Group, Charlottesville, VA). The model was fitted to the amount of tacrolimus excreted in the successive urine collection periods over the 96 hours.
Individual estimated eGFR was calculated using the Cockroft-Gault equation (37), which incorporated the variables of sex, age, weight, serum creatinine concentration and entered into model-fitting as fixed values.
Estimates for Qeffart and Vepi were taken from the literature, scaled to the individual body weight, and entered into model-fitting as fixed values. About 80% of total kidney volume (Vkidney,total) is composed of tubular epithelial cells and cells within the interstitial space (38). We assumed that CYP3A5 is expressed in all tubular epithelial cells, and assigned Vepi a value that equals 80% of Vkidney,total. The mean Vkidney,total was obtained from Cheong et al. (39), 202 ± 36 ml per kidney for men (weight = 90 ± 16 kg) and 154 ± 33 ml per kidney for women (weight = 73 ± 18 kg). Qeffart was estimated as Qaffart minus GFR (40). Further recognition of the following relationship of Qaffart to GFR, filtration fraction (FF), and arterial hematocrit (44, 45) yields the estimating equation for Qeffart.
The afferent arterial hematocrit is approximated by the hematocrit measured from individual venous blood. A mean value of 23.1 ± 0.7% was used as filtration fraction in healthy adults (41). Individual eGFR was used as the input as GFR in the above equations.
The exchangeable fraction of tacrolimus in blood available for glomerular filtration () was estimated from the nonlinear regression fit. While human renal tacrolimus tissue distribution data are not available, a Kp,kid of 12.2 was reported for male rats receiving tacrolimus orally for four days (42). Tacrolimus is a substrate for rat CYP3A isoforms (43) and considering their expression and activity in rat kidneys (44, 45), the rat Kp,kid was entered as a mean for the prior distribution probability in CYP3A5 expressors, along with an assumed standard deviation (SD) of 15% that reflect the usual magnitude of analytical errors. The SD for the Bayesian prior was not sensitive. All inter-compartmental transfer rate constants, viz. k(1,3), k(2,3), k(3,2) and k(0,3), were estimated from the nonlinear least-squares regression fit. A relative weighting scheme (FSD 0.1) was used, assuming a constant coefficient of variation of 10%.
A preliminary model fit was conducted with mean urinary excretion data for the CYP3A5 phenotype groups. The use of mean excretion data offered the benefit of assessing the general plausibility of the three-compartment model in describing the urinary excretion data. It allowed a simultaneous fit of data from both CYP3A5 phenotype groups to evaluate the impact of a metabolic sink in the epithelial compartment on Kp,kid, about which we lack prior information.
Modeling of the individual data sets was then conducted as a two-stage process. In the first stage, data from the CYP3A5 nonexpressors were fitted to the three-compartment model with k(0,3) set to zero. The mean and SD of each of the fitted parameters served as Bayesian priors for the second stage analysis with data from the CYP3A5 expressors; in so doing, k(0,3) became the only parameter that was left to float. The Bayesian prior estimate for Kp,kid values in the CYP3A5 expressors was set to 12.2 ± 2.0, and the prior for Kp,kid in CYP3A5 nonexpressors (23.4 ± 4.0) was set to be about twice of the expressor value based on the preceding simultaneous fit of mean data. The amount of tacrolimus in the epithelial compartment versus time was simulated for each subject using the final parameter estimates.
Statistical Analysis
Descriptive statistics are presented as mean ± standard deviation. Statistical comparisons were conducted using an unpaired two-sided Student’s t-test (GraphPad Prism 5, La Jolla, CA). A P value less than 0.05 was considered significant.
Supplementary Material
Study Highlights.
What is the current knowledge on the topic?
Accumulating studies suggested that local tacrolimus and/or its metabolites concentration in kidney tissue may play a role in the development of chronic calcineurin inhibitor nephrotoxicity. In vitro experiments showed that CYP3A5 contributes significantly to the metabolic clearance of tacrolimus in the liver and kidney.
What question this study addressed?
How CYP3A5 affects intrarenal tacrolimus accumulation and its systemic metabolite exposure in vivo.
What this study adds to our knowledge?
At steady state, intrarenal accumulation of tacrolimus, and its primary metabolites, will depend on the CYP3A5 genotype of the liver and kidney. We predicted lower tacrolimus exposure in the renal epithelium of CYP3A5 expressors, but greater intrarenal accumulation of tacrolimus metabolites in CYP3A5 expressing patients with tacrolimus dose adjustment.
How this might change clinical pharmacology and therapeutics?
The current study highlighted the need to conduct prospective studies investigating the impact of CYP3A5 genotype on tacrolimus nephrotoxicity by identifying the CYP3A5 genotype of recipients and donors, as well as drug concentrations in blood and renal tissue. A full elucidation of the pharmacogenomics of tacrolimus nephrotoxicity may lead to improved management of tacrolimus pharmacotherapy.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health: R01 GM068871, U01 GM092676, P30 ES07033 and UL1 RR025014. The authors would like to thank Ms. Christine Hoffer for her outstanding assistance in the conduct of the clinical study. The authors would also like to thank Taurence Senn for assisting with the verification of tacrolimus metabolite identities by Q-TOF and Dr. Edward Kelly for his assistance with CYP3A5 and ABCB1 genotyping. The authors also appreciate the generosity of Dr. Uwe Christians in providing tacrolimus’s primary metabolites for verification purposes.
Nonstandard abbreviations
- LC-MS/MS
liquid chromatography-mass spectrometry/mass spectrometry
- fub
unbound fraction in whole blood
- fup
unbound fraction in plasma
- CP
plasma concentration
- CWB
whole blood concentration
- Cmax
maximum blood concentration
- Clast
blood concentration at 96 hour after tacrolimus administration
- F
systemic bioavailability
- CL/F
oral clearance
- CLurinary
urinary clearance
- CrCL
renal creatinine clearance
- GFR
glomerular filtration rate
- eGFR
estimated glomerular filtration rate
- CYP3A4
cytochrome P450 3A4
- CYP3A5
cytochrome P450 3A5
- P-gp
P-glycoprotein
- 13-DMT
13-O-desmethyl tacrolimus
- 15-DMT
15-O-desmethyl tacrolimus
- 31-DMT
31-O-desmethyl tacrolimus
- 12-HT
12-hydroxy tacrolimus
- CNIT
chronic calcineurin inhibitor nephrotoxicity
References
- 1.Scott LJ, McKeage K, Keam SJ, Plosker GL. Tacrolimus: a further update of its use in the management of organ transplantation. Drugs. 2003;63:1247–1297. doi: 10.2165/00003495-200363120-00006. [DOI] [PubMed] [Google Scholar]
- 2.Ojo AO, et al. Chronic renal failure after transplantation of a nonrenal organ. The New England journal of medicine. 2003;349:931–940. doi: 10.1056/NEJMoa021744. [DOI] [PubMed] [Google Scholar]
- 3.Ekberg H, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. The New England journal of medicine. 2007;357:2562–2575. doi: 10.1056/NEJMoa067411. [DOI] [PubMed] [Google Scholar]
- 4.Metalidis C, Lerut E, Naesens M, Kuypers DR. Expression of CYP3A5 and P-glycoprotein in renal allografts with histological signs of calcineurin inhibitor nephrotoxicity. Transplantation. 2011;91:1098–1102. doi: 10.1097/TP.0b013e3182177502. [DOI] [PubMed] [Google Scholar]
- 5.Naesens M, Kuypers DR, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. 2009;4:481–508. doi: 10.2215/CJN.04800908. [DOI] [PubMed] [Google Scholar]
- 6.Fukudo M, et al. Impact of MDR1 and CYP3A5 on the oral clearance of tacrolimus and tacrolimus-related renal dysfunction in adult living-donor liver transplant patients. Pharmacogenetics and genomics. 2008;18:413–423. doi: 10.1097/FPC.0b013e3282f9ac01. [DOI] [PubMed] [Google Scholar]
- 7.Kuypers DR, Naesens M, de Jonge H, Lerut E, Verbeke K, Vanrenterghem Y. Tacrolimus dose requirements and CYP3A5 genotype and the development of calcineurin inhibitor-associated nephrotoxicity in renal allograft recipients. Ther Drug Monit. 2010;32:394–404. doi: 10.1097/FTD.0b013e3181e06818. [DOI] [PubMed] [Google Scholar]
- 8.Kuypers DR, de Jonge H, Naesens M, Lerut E, Verbeke K, Vanrenterghem Y. CYP3A5 and CYP3A4 but not MDR1 single-nucleotide polymorphisms determine long-term tacrolimus disposition and drug-related nephrotoxicity in renal recipients. Clinical pharmacology and therapeutics. 2007;82:711–725. doi: 10.1038/sj.clpt.6100216. [DOI] [PubMed] [Google Scholar]
- 9.Min SI, et al. CYP3A5 *1 allele: impacts on early acute rejection and graft function in tacrolimus-based renal transplant recipients. Transplantation. 2010;90:1394–1400. doi: 10.1097/TP.0b013e3181fa93a4. [DOI] [PubMed] [Google Scholar]
- 10.Sattler M, Guengerich FP, Yun CH, Christians U, Sewing KF. Cytochrome P-450 3A enzymes are responsible for biotransformation of FK506 and rapamycin in man and rat. Drug metabolism and disposition: the biological fate of chemicals. 1992;20:753–761. [PubMed] [Google Scholar]
- 11.Lampen A, et al. Metabolism of the immunosuppressant tacrolimus in the small intestine: cytochrome P450, drug interactions, and interindividual variability. Drug metabolism and disposition: the biological fate of chemicals. 1995;23:1315–1324. [PubMed] [Google Scholar]
- 12.Lin YS, et al. Co-regulation of CYP3A4 and CYP3A5 and contribution to hepatic and intestinal midazolam metabolism. Molecular pharmacology. 2002;62:162–172. doi: 10.1124/mol.62.1.162. [DOI] [PubMed] [Google Scholar]
- 13.Kuehl P, et al. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet. 2001;27:383–391. doi: 10.1038/86882. [DOI] [PubMed] [Google Scholar]
- 14.Haehner BD, et al. Bimodal distribution of renal cytochrome P450 3A activity in humans. Molecular pharmacology. 1996;50:52–59. [PubMed] [Google Scholar]
- 15.Givens RC, et al. CYP3A5 genotype predicts renal CYP3A activity and blood pressure in healthy adults. J Appl Physiol. 2003;95:1297–1300. doi: 10.1152/japplphysiol.00322.2003. [DOI] [PubMed] [Google Scholar]
- 16.Dai Y, et al. Effect of CYP3A5 polymorphism on tacrolimus metabolic clearance in vitro. Drug metabolism and disposition: the biological fate of chemicals. 2006;34:836–847. doi: 10.1124/dmd.105.008680. [DOI] [PubMed] [Google Scholar]
- 17.Kamdem LK, et al. Contribution of CYP3A5 to the in vitro hepatic clearance of tacrolimus. Clinical chemistry. 2005;51:1374–1381. doi: 10.1373/clinchem.2005.050047. [DOI] [PubMed] [Google Scholar]
- 18.Bolbrinker J, et al. CYP3A5 Genotype - phenotype Analysis in the Human Kidney Reveals a Strong Site-specific Expression of CYP3A5 in the Proximal Tubule in Carriers of the CYP3A5*1 allele. Drug metabolism and disposition: the biological fate of chemicals. 2012 doi: 10.1124/dmd.111.042648. [DOI] [PubMed] [Google Scholar]
- 19.Hesselink DA, et al. Genetic polymorphisms of the CYP3A4, CYP3A5, and MDR-1 genes and pharmacokinetics of the calcineurin inhibitors cyclosporine and tacrolimus. Clinical pharmacology and therapeutics. 2003;74:245–254. doi: 10.1016/S0009-9236(03)00168-1. [DOI] [PubMed] [Google Scholar]
- 20.Haufroid V, et al. The effect of CYP3A5 and MDR1 (ABCB1) polymorphisms on cyclosporine and tacrolimus dose requirements and trough blood levels in stable renal transplant patients. Pharmacogenetics. 2004;14:147–154. doi: 10.1097/00008571-200403000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Thervet E, et al. Impact of cytochrome p450 3A5 genetic polymorphism on tacrolimus doses and concentration-to-dose ratio in renal transplant recipients. Transplantation. 2003;76:1233–1235. doi: 10.1097/01.TP.0000090753.99170.89. [DOI] [PubMed] [Google Scholar]
- 22.Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clinical pharmacokinetics. 2004;43:623–653. doi: 10.2165/00003088-200443100-00001. [DOI] [PubMed] [Google Scholar]
- 23.Minematsu T, et al. Effect of hematocrit on pharmacokinetics of tacrolimus in adult living donor liver transplant recipients. Transplantation proceedings. 2004;36:1506–1511. doi: 10.1016/j.transproceed.2004.04.097. [DOI] [PubMed] [Google Scholar]
- 24.Sirianni GL, Pang KS. Inhibition of esterolysis of enalapril by paraoxon increases the urinary clearance in isolated perfused rat kidney. Drug metabolism and disposition: the biological fate of chemicals. 1999;27:931–936. [PubMed] [Google Scholar]
- 25.Staatz CE, Goodman LK, Tett SE. Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: Part II. Clinical pharmacokinetics. 2010;49:207–221. doi: 10.2165/11317550-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 26.de Denus S, et al. Association between renal function and CYP3A5 genotype in heart transplant recipients treated with calcineurin inhibitors. J Heart Lung Transplant. 2011;30:326–331. doi: 10.1016/j.healun.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 27.Klauke B, et al. No association between single nucleotide polymorphisms and the development of nephrotoxicity after orthotopic heart transplantation. J Heart Lung Transplant. 2008;27:741–745. doi: 10.1016/j.healun.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Glowacki F, et al. CYP3A5 and ABCB1 polymorphisms in donor and recipient: impact on Tacrolimus dose requirements and clinical outcome after renal transplantation. Nephrol Dial Transplant. 2011 doi: 10.1093/ndt/gfr253. [DOI] [PubMed] [Google Scholar]
- 29.Sigal NH, et al. Is cyclophilin involved in the immunosuppressive and nephrotoxic mechanism of action of cyclosporin A? The Journal of experimental medicine. 1991;173:619–628. doi: 10.1084/jem.173.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kung L, Batiuk TD, Palomo-Pinon S, Noujaim J, Helms LM, Halloran PF. Tissue distribution of calcineurin and its sensitivity to inhibition by cyclosporine. Am J Transplant. 2001;1:325–333. doi: 10.1034/j.1600-6143.2001.10407.x. [DOI] [PubMed] [Google Scholar]
- 31.Iwasaki K, et al. Further metabolism of FK506 (tacrolimus). Identification and biological activities of the metabolites oxidized at multiple sites of FK506. Drug metabolism and disposition: the biological fate of chemicals. 1995;23:28–34. [PubMed] [Google Scholar]
- 32.Hebert MF, et al. Effects of pregnancy on CYP3A and P-glycoprotein activities as measured by disposition of midazolam and digoxin: a University of Washington specialized center of research study. Clinical pharmacology and therapeutics. 2008;84:248–253. doi: 10.1038/clpt.2008.1. [DOI] [PubMed] [Google Scholar]
- 33.Asano T, et al. ABCB1 C3435T and G2677T/A polymorphism decreased the risk for steroid-induced osteonecrosis of the femoral head after kidney transplantation. Pharmacogenetics. 2003;13:675–682. doi: 10.1097/00008571-200311000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Chen YL, Hirabayashi H, Akhtar S, Pelzer M, Kobayashi M. Simultaneous determination of three isomeric metabolites of tacrolimus (FK506) in human whole blood and plasma using high performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;830:330–341. doi: 10.1016/j.jchromb.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 35.Barrett PH, et al. SAAM II: Simulation, Analysis, and Modeling Software for tracer and pharmacokinetic studies. Metabolism. 1998;47:484–492. doi: 10.1016/s0026-0495(98)90064-6. [DOI] [PubMed] [Google Scholar]
- 36.Lohr JW, Willsky GR, Acara MA. Renal drug metabolism. Pharmacol Rev. 1998;50:107–141. [PubMed] [Google Scholar]
- 37.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 38.Klahr S, Morrissey J. Obstructive nephropathy and renal fibrosis. American journal of physiology Renal physiology. 2002;283:F861–F875. doi: 10.1152/ajprenal.00362.2001. [DOI] [PubMed] [Google Scholar]
- 39.Cheong B, Muthupillai R, Rubin MF, Flamm SD. Normal values for renal length and volume as measured by magnetic resonance imaging. Clin J Am Soc Nephrol. 2007;2:38–45. doi: 10.2215/CJN.00930306. [DOI] [PubMed] [Google Scholar]
- 40.Myers BD, Deen WM, Brenner BM. Effects of norepinephrine and angiotensin II on the determinants of glomerular ultrafiltration and proximal tubule fluid reabsorption in the rat. Circulation research. 1975;37:101–110. doi: 10.1161/01.res.37.1.101. [DOI] [PubMed] [Google Scholar]
- 41.Anastasio P, Cirillo M, Spitali L, Frangiosa A, Pollastro RM, De Santo NG. Level of hydration and renal function in healthy humans. Kidney international. 2001;60:748–756. doi: 10.1046/j.1523-1755.2001.060002748.x. [DOI] [PubMed] [Google Scholar]
- 42.Qin XL, et al. Study of the effect of Wuzhi tablet (Schisandra sphenanthera extract) on tacrolimus tissue distribution in rat by liquid chromatography tandem mass spectrometry method. Biomedical chromatography : BMC. 2010;24:399–405. doi: 10.1002/bmc.1305. [DOI] [PubMed] [Google Scholar]
- 43.Wu CY, Benet LZ. Disposition of tacrolimus in isolated perfused rat liver: influence of troleandomycin, cyclosporine, and gg918. Drug metabolism and disposition: the biological fate of chemicals. 2003;31:1292–1295. doi: 10.1124/dmd.31.11.1292. [DOI] [PubMed] [Google Scholar]
- 44.Ronis MJJ, Huang J, Longo V, Tindberg N, IngelmanSundberg M, Badger TM. Expression and distribution of cytochrome P450 enzymes in male rat kidney: Effects of ethanol, acetone and dietary conditions. Biochemical pharmacology. 1998;55:123–129. doi: 10.1016/s0006-2952(97)00381-x. [DOI] [PubMed] [Google Scholar]
- 45.Ghosh SS, et al. Renal and Hepatic Family 3a Cytochromes P450 (Cyp3a) in Spontaneously Hypertensive Rats. Biochemical pharmacology. 1995;50:49–54. doi: 10.1016/0006-2952(95)00110-l. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.