Abstract
Background
One-third of all patients with epilepsy have persistent seizures despite medical treatment. If the origin of the seizures can be localized to a particular site in the brain, epilepsy surgery is a treatment option that addresses the cause of the problem.
Methods
The presurgical assessment and surgical treatment of epilepsy are discussed on the basis of a selective literature review and the authors’ clinical experience.
Results
Recent studies give further evidence that surgical treatment is superior to continued medical treatment for patients with seizures of focal origin that persist despite treatment with two antiepileptic drugs. Modern imaging and electrophysiological techniques enable the demonstration of subtle structural and functional changes of the cerebral cortex as a basis for individually tailored surgical resection. 60–80% of surgically treated patients become seizure-free. According to recent reviews, epilepsy surgery is associated with a permanent morbidity of 6% and with a mortality well under 1%; these figures are in the typical range for neurosurgical procedures. In the authors’ series, 2% of patients had permanent complications, and the death rate was less than 0.1%.
Conclusion
Advances in presurgical assessment and the broad range of available surgical techniques have widened the applicability of surgical treatment for children and adults with medically refractory epilepsy. Patients should be referred early in the course of their disease to an epilepsy center for evaluation of the surgical options.
One-third of all patients with epilepsy (in Germany, more than 200 000 people) have persistent seizures despite optimal medical treatment. Even though pharmacotherapy has improved with the introduction of new drugs that have fewer side effects and interactions, the problem of medically refractory epilepsy has not been solved (1– 3). If the patient’s seizures fail to respond to the first two antiepileptic drugs that are tried, the probability of achieving a lasting seizure-free state with further changes in medication is only 5–10 % (4). Epilepsy patients are exposed to multiple risks for as long as their seizures persist: the annual incidence of status epilepticus in such persons is 1% (5), while that of physical injury—most commonly contusions, open wounds, and fractures—is 27% (e1), and 41 of 100 000 patients per year die a sudden, unexpected death during an epileptic seizure (SUDEP) (6, e2). These patients also suffer from restricted mobility, scarce job options, and the emotional stress arising from their condition and the associated stigmatization.
Two prospective randomized trials provide grade I evidence for the superiority of epilepsy surgery to pharmacotherapy for patients with temporal lobe epilepsy not responding to treatment with two antiepileptic drugs (2). On the basis of these trials and numerous case series with similar findings, there is now an international consensus that persons with epilepsy originating in a circumscribed, localizable area of the brain should be referred to an epilepsy center if their seizures have not responded to treatment with two drugs (1, 7; see also the guidelines of the German Neurological Society). The specialists at the epilepsy center confirm medical intractability, rule out the possibility that the patient is being treated inappropriately for seizures that are actually not epileptic, and carry out a comprehensive diagnostic assessment to determine whether the patient might benefit from surgical treatment.
The preoperative evaluation of epilepsy
In preoperative evaluation, the presence of focal epilepsy is confirmed and the site and extent of the focus are determined in a multimodal assessment. Furthermore, the risks of surgery, especially regarding cognitive function, are assessed and weighed against the risks associated with further medical treatment. Finally, the chance of controlling seizures and improving the patient’s quality of life with surgery are discussed with the patient (8).
Identifying suitable candidates for epilepsy surgery
The prerequisite for a resective surgical procedure to treat epilepsy is the presence of seizures that arise in a circumscribed area of the brain, known as the epileptogenic focus. The manifestations (semiology) of the seizures can provide important clues to their localization: there may be an aura, asymmetrical motor signs, or a speech disturbance during or after the seizure. Evidence of focal seizure onset can also be derived from regional EEG slowing or spikes. Further highly suggestive, but not in itself conclusive, evidence may come from the demonstration of a lesion on the patient’s magnetic resonance scan.
Localiza+tion of the epileptogenic area
No single currently available method suffices by itself to identify the epileptogenic area in the brain, for a number of reasons: many patients have focal epilepsy that is “cryptogenic” with respect to imaging studies, seizure semiology that apparently localizes the lesion may actually reflect propagation of epileptogenic activity to sites outside the focus, and spike-generating zones may extend well beyond the epileptogenic zone and even into the opposite hemisphere. Thus, in centers where preoperative evaluation for epilepsy surgery is performed (www.dgfe.org/home/index,id,47,selid,95,type,VAL_MEMO.html), the diagnostic assessment is multimodal, and the brain area to be resected is determined on the basis of concordant findings from different diagnostic modalities. Moreover, for an individualized assessment of the risk of surgery, the spatial relationship of the epileptogenic area to functionally important (“eloquent”) brain areas is investigated (e3).
Recording of epileptic activity and seizure semiology with video-EEG monitoring—To record epileptic activity sensitively and completely, long-term recordings are made during periods of sleep and wakefulness. In general, the doses of antiepileptic drugs that suppress interictal spikes are lowered before recording. Long-term recording enables the analysis of interictal spikes and sharp waves, and high density EEG recordings enable the localization of generators in relation to the patient’s individual brain morphology (Figure 1a, b).
Figure 1.
Preoperative evaluation for epilepsy surgery.
a–b) Precise determination of the source of epileptic activity with multichannel surface EEG recording (256 EEG channels);
c–d) High-resolution magnetic resonance imaging revealing multiple lesions: intrasulcal left fronto-insular lipomas with associated focal cortical dysplasia; “pancake view” revealing migration abnormalities in a side-to-side comparison (in this case: right perisylvian polymicrogyria);
e) Stereo-EEG trajectories, in this case for the invasive evaluation of the epileptogenicity of a temporopolar encephalocele;
f) Subdural electrodes in the interhemispheric fissure and over the cerebral convexity for the precise definition of an epileptogenic area next to the primary motor cortex
The central element of preoperative assessment for epilepsy surgery is the recording of the patient’s typical seizures with simultaneous video and EEG. It can be established in this way that the patient’s seizures are indeed epileptic, and they can then be more precisely classified (9). A detailed analysis of seizure semiology in relation to electroencephalographic seizure patterns enables the individual clinical features of a seizure to be ascribed to specific areas of the brain: for example, complex visual hallucinations can be traced to temporo-occipital association areas, ictal changes of heart rate to the insular cortex, and asymmetrical posturing during the seizure to the supplementary motor cortex.
Structural imaging—Advances in magnetic resonance imaging have improved our ability to identify structural lesions causing focal epilepsy (e4). Some types of epileptogenic lesion are easily seen (e.g., tumors, vascular malformations, infarcts, or post-traumatic defects), while others may be overlooked unless specific, high-resolution imaging techniques are used. For example, thin sections perpendicular to the longitudinal axis of the hippocampus (“temporally angled cuts”) are indispensable for detailed assessment of this structure, and three-dimensional data sets with voxels smaller than 1 mm are needed in order to distinguish subtle abnormalities of brain architecture from partial-volume effects (Table 1; Figure 1c, d). With good imaging, experts can identify epileptogenic lesions in up to 90% of patients (e5) .
Table 1. Minimal requirements for magnetic resonance imaging of the brain in epilepsy of structural origin (DGN).
| Weighting | Plane | Orientation |
|---|---|---|
| T1 | sagittal | standard |
| T1 | coronal | standard* |
| T2-TSE | axial | standard |
| T2-TSE | coronal | temporally angled* |
| FLAIR | axial-coronal | standard* |
*The authors consider temporal angulation to be preferable for these sequences as well.
DGN, German Neurological Society (Deutsche Gesellschaft für Neurologie).
In addition to the demonstration of cortical changes, diffusion tensor imaging (DTI) is increasingly being used to detect white-matter changes. DTI can also be used to identify important fiber tracts that should be preserved in neurosurgical procedures (10).
Neuropsychological testing—Standardized neuro-psychological test batteries are used to evaluate specific types of epilepsy-associated cognitive dysfunction and to assess the functional risks of surgery. Predictors of a good cognitive outcome include preoperatively evident dysfunction of the focus region, lateralization to the non-dominant hemisphere for language, young age at the time of surgery, and high general intelligence (e3).
Functional imaging—Functional imaging techniques are especially useful if structural imaging studies have not revealed any potentially epileptic lesion, or if multiple such lesions are present. Hypometabolic areas can be detected with interictal FDG-PET, and areas of increased perfusion during seizures can be detected with ictal SPECT. Subtraction techniques for ictal versus interictal perfusion and the superposition of functional data with three-dimensional MRI data sets have substantially increased the sensitivity of functional imaging (e6).
Functional MRI can also be used to display the motor cortex and the important cortical areas for language and memory that are to be spared during surgery. It has largely replaced Wada testing for this purpose.
Intracranial EEG recording—The epileptogenic area can usually be defined without difficulty if the seizure semiology, findings of structural imaging studies, and interictal and ictal EEG changes are concordant. Additional intracranial (i.e., invasive) EEG recording is needed under the following circumstances:
high-resolution imaging fails to reveal a lesion;
discrepant findings are obtained because of the spread of epileptic activity through the brain;
multifocality must be excluded (e.g., when non-invasive studies have revealed multiple lesions or foci of epileptic activity);
a tailored resection is indicated in order to spare neighboring eloquent brain areas.
Invasive EEG recording can be carried out with subdural electrodes that are implanted on the surface of the brain, which are also highly suitable for defining the borders of functional brain areas by electrostimulation, or else with stereotactically implanted depth electrodes for the detection of seizure generators in sulci or deeper structures such as the insula, the amygdala, or the hippocampus (Figure 1 e, f). Recent developments in invasive EEG recording allow the analysis of the activity of single neurons (11), the use of high-resolution (i.e., narrowly spaced) electrode grids, and the use of new biomarkers for epileptogenicity, such as high-frequency oscillations (12, 13).
Neurosurgical techniques
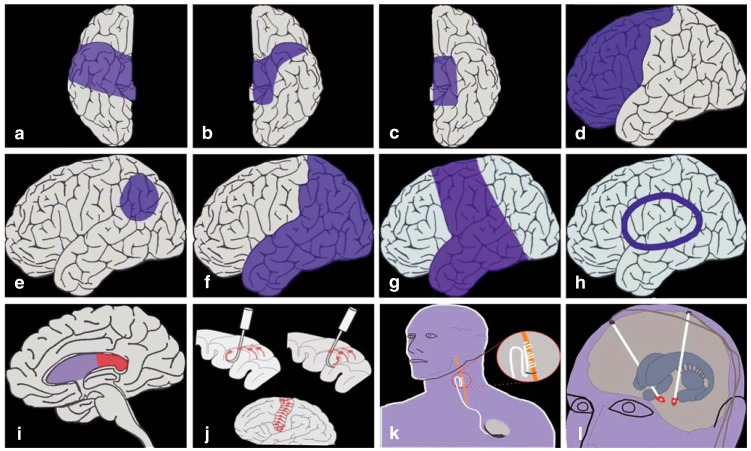
Neurosurgical techniques have widened the spectrum of treatable epilepsy far beyond the cases that can be treated with medications alone. Such techniques can be either resective or functional; in the former, the epileptogenic area of the brain is removed, while in the latter the epileptogenic network is interrupted—with the common purpose of preventing the generation, or at least the spread, of epileptic activity. (Figure 2 contains an overview of current neurosurgical methods.
Figure 2.
Schematic depiction of techniques of epilepsy surgery. Temporal resection: a) two-third resection, b) keyhole approach,
c) selective amygdalohippocampectomy. Extratemporal resection: d) lobectomy, e) topectomy, f) multilobectomy, g) hemispherectomy,
h) hemispherotomy, i) callosotomy, j) multiple subpial transection. Stimulation techniques: k): vagus nerve stimulation, l) deep brain stimulation.
The classic neurosurgical procedure for temporal-lobe seizures is an anterior two-third temporal lobectomy (14, 15). Epilepsy of predominantly mesiotemporal origin can be treated by hippocampectomy through a temporopolar or keyhole approach. In selective amygdalohippocampectomy, a further technical refinement, the epileptogenic mesiotemporal structures can be removed with preservation of the lateral temporal cortex.
In the early era of epilepsy surgery, lobectomy was the preferred treatment of extratemporal seizures. The current goal is a selective, circumscribed resection of a precisely localized epileptogenic area (“topectomy”) within a lobe of the brain. Intracranial recording of interictal and ictal EEG activity is often needed for this purpose. The first step in extraoperative electrocorticography is the implantation of subdural or intracerebral electrodes. These can be used not only for the recording of epileptic activity, but also for the functional mapping of important areas, e.g., the language area or the motor cortex. The epileptogenic area is then resected in a second neurosurgical procedure. On the other hand, a single operative procedure, with intraoperative electrocorticography performed under the same general anesthesia as the resection, is often adequate, provided that the non-invasive EEG and MRI findings unequivocally show where the epileptogenic area lies. Surgery is performed under local anesthesia in some centers so that the language area can be precisely localized. For this, the patient’s cooperation is essential.
Patients with epilepsy due to extensive lesions in a single hemisphere, e.g., porencephalic cyst, hemiatrophy, hemimegalencephaly, Rasmussen’s encephalitis, or a large Sturge-Weber angioma, can be successfully treated with hemispherectomy (16– 18). In Rasmussen’s functional hemispherectomy (now the most common technique), the temporal and parietal lobes are removed, while the frontal and occipital lobes are disconnected but are left anatomically intact. Hemispherotomy is a further technical refinement in which the entire hemisphere is disconnected through a trans-sylvian or peri-insular approach but is left anatomically intact.
If the epileptogenic area is in a functionally important area of the brain, multiple subpial transection (MST) is a treatment option (19). In this technique, which is based on encouraging findings from animal experiments, the cortex is not removed, but rather transected with parallel cuts spaced 5 mm apart. This is intended to interrupt the horizontal fiber pathways through which epilepsy is spread while sparing the functionally important vertical fiber pathways. MST is less effective than a resective procedure.
Callosotomy (20) is intended to prevent the spread of epileptic activity across the corpus callosum to the opposite hemisphere. It is now only rarely used to treat otherwise intractable tonic drop attacks, which are caused by the rapid interhemispheric propagation of epileptic activity.
Vagus nerve stimulation is performed mainly when no circumscribed, surgically accessible epileptogenic area can be identified (21). Deep brain stimulation, a technique now attracting increasing attention, can be performed either directly in the epileptogenic focus or at a distant site, usually the anterior thalamic nucleus (22, 23). The main target for direct electrical stimulation of an epileptogenic focus is the hippocampus (24– 26).
Success rates
70% to 80% of patients become completely free of seizures after temporal procedures, and 10% to 20% have a significant reduction in seizure frequency (27– 33, e8– e11) (Table 2). Hemispherotomy leads to freedom from seizures in about 90% of patients. Extratemporal procedures have somewhat lower success rates, with 60% to 70% of patients becoming seizure-free. Favorable predictors for seizure control include a circumscribed lesion such as a benign tumor or cavernoma, complete resection of the lesion, the absence of bilateral tonic-clonic seizures, and early surgery. Palliative techniques such as callosotomy, multiple subpial transection, and vagus nerve stimulation only rarely lead to a seizure-free state, but about 50% of patients have a significantly reduced seizure frequency.
Table 2. Pertinent clinical studies*.
| Author | Type of study | Follow-up | Type of surgery | Outcome variables |
|---|---|---|---|---|
| Rate of freedom from seizures | ||||
| Edelvik et al., 2013 (e8) |
prospective, population-based | 5/10 years | various types of resection |
postoperatively, 62%, vs. 14% under drug treatment |
| Englot et al., 2013 (e9) |
meta-analysis of extratemporal epilepsy surgery in childhood |
variable | extratemporal operations |
postoperatively, 56% (mean) |
| Engel et al., 2012 (2) |
randomized study of epilepsy surgery vs. drug treatment |
2 years | temporal resection (anterior 2/3 resection) |
postoperatively, 73% , vs. 0% under drug treatment |
| Schmidt & Stavem, 2004 (e10) |
meta-analysis | variable | various types of resection |
postoperatively, 44%, vs. 12 % under drug treatment |
| Wiebe et al., 2001 (e11) |
randomized study of epilepsy surgery vs. drug treatment |
1 year | temporal resection (anterior 2/3 resection) |
postoperatively, 58%, vs. 8% under drug treatment |
| Cognitive function | ||||
| Skirrow et al., 2011 (e12) |
prospective, case-controlled | 6 years | temporal resection |
postoperative IQ improvement in surgically treated patients compared to medically treated ones |
| Psychosocial function | ||||
| Smith et al., 2011 (e13) |
prospective, case-controlled (children) |
2 years | temporal and extratemporal resections |
postoperative seizure control led to improvements on scales of depression, anxiety, and disease-related stress |
| Quality of life | ||||
| Hamid et al., 2014 (e14) |
prospective, multicenter cohort study | 5 years | temporal and extratemporal resections |
postoperative seizure control improves the quality of life |
| Mortality | ||||
| Sperling et al., 2005 (e15) |
prospective cohort study | 5 years | resective and disconnecting procedures at various sites |
postoperative freedom from seizures was associated with normalization of mortality, while the mortality of patients with persistent seizures was 5.7 times that of the normal poulation |
*Class I evidence indicates the superiority of resective surgery to continued medical treatment if treatment with two antiepileptic drugs has not led to seizure control. Moreover, successful surgery is associated with improvements in childhood cognitive and psychosocial development and in quality of life (34) and lowers the risk of death due to epileptic seizures (cf. Ref. e7). Comment: Many other studies that are not listed here document the efficacy of the surgical techniques that are mentioned in the text, but not in this table.
Risks
The frequency and severity of complications depend on the type of procedure (diagnostic or therapeutic) and its localization (temporal or extratemporal), as well as on the age of the patient (child or adult) (35). In a systematic analysis of literature published from 1990 to 2008 (76 articles with data on nearly 5000 patients [e16]), the surgical and neurological complications of epilepsy surgery were enumerated and classified as either temporary (completely resolved within 3 months) or permanent (persistent beyond 3 months). Electrode implantation for diagnostic purposes was associated with a temporary morbidity of 7.7% and a permanent morbidity of 0.6%, mainly consisting of cerebrospinal fluid leaks and extra-axial hematomas. Resective surgery was associated with temporary surgical morbidity of 5.1% and permanent surgical morbidity of 1.5%, consisting of complications such as cerebrospinal fluid leak, meningitis, intracranial hematoma, and hydrocephalus. The neurological complications of resective surgery led to a temporary morbidity of 10.9% and a permanent morbidity of 4.7%; the more common ones were visual field defects, hemiparesis, aphasia, and cranial nerve palsies. All types of neurological complication were more common among children and after extratemporal procedures. Psychiatric complications, in contrast, mainly occurred after temporal-lobe surgery in adults. The perioperative mortality was 0.4% for temporal procedures and 1.2% for extratemporal procedures. The permanent morbidity (of all types) for diagnostic procedures was below 1% (6% for therapeutic procedures), with mortality well below 1%. In the authors’ series, the permanent morbidity of therapeutic procedures (both surgical and neurological) was 2%, and the mortality was less than 0.1%.
Surgery can cause functional impairment, e.g., when a hippocampus is removed that still had some memory function. This is an important consideration for dominant temporal-lobe surgery. On the other hand, the removal of the disruptive influence of ictal and interictal epileptic activity can lead to functional improvement. Preoperative neuropsychological testing and functional MRI are used to evaluate the patient’s baseline cognitive function and the spatial localization of functionally relevant areas. These findings provide a basis for assessing the risks of surgery and the expected benefit to the patient’s quality of life.
Epilepsy surgery in childhood
Epilepsy surgery has proved to be an important treatment option for children with medically refractory epilepsy. The negative effects of epileptic seizures and of anticonvulsant drugs on the immature brain are now well known; thus, freedom from seizures after surgery can be expected to aid the child’s cognitive development (36). The special features of epilepsy surgery in childhood are the broad spectrum of pediatric epilepsy syndromes and their causes and the high variability of clinical and electroencephalographic seizure patterns (37). The most common etiologies of epilepsy in childhood are cortical dysplasia (40–60%) and tumors (20–30%), followed, in order of decreasing frequency, by the phakomatoses (tuberous sclerosis, Sturge-Weber syndrome), hemispheric syndromes (Rasmussen’s encephalitis, hemimegalencephaly), perinatal ischemia/infarction, hypothalamic hamartoma, and mesial temporal sclerosis (38).
In view of the known facts that epilepsy often arises in early childhood, that childhood epilepsy is often severe, and that it is drug-resistant in about 30% of cases, the surgical treatment of epilepsy in infants and young children has become more common in recent years (39). Earlier studies showed that surgical treatment early in the course of epilepsy leads to better seizure control and better postoperative psychomotor development; the benefit to psychomotor development is greatest if a seizure-free state is achieved.
Epilepsy surgery is of comparable effectiveness in infants, children, and adolescents, despite the marked differences in brain maturation across these three groups. Depending on the type of underlying lesion and the site and extent of the epileptogenic area, 60–80% of surgically treated children become free of seizures (40). This high success rate and the prospect of better psychomotor development after successful surgery should encourage treating physicians in all pertinent specialties to consider the option of epilepsy surgery and to refer their young, medically intractable patients for evaluation in an epilepsy center before the persistent seizures lead to secondary cognitive and psychosocial impairment.
Key Messages.
Epilepsy surgery is an important option for the treatment of patients with drug-resistant epilepsy.
In preoperative evaluation for epilepsy surgery, the epileptogenic area is identified by means of clinical observation, neuroimaging, and electrophysiologic studies.
Selective, tailored resection of the epileptogenic area gives 60% to 80% of patients lasting freedom from seizures while preserving physiological brain function.
Early epilepsy surgery in childhood can prevent the adverse cognitive, emotional, and social sequelae of chronic epilepsy.
The broad spectrum of diagnostic and therapeutic techniques now used for epilepsy surgery are only available in specially qualified and equipped epilepsy centers.
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
References
- 1.Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51:1069–1077. doi: 10.1111/j.1528-1167.2009.02397.x. [DOI] [PubMed] [Google Scholar]
- 2.Engel J, McDermott MP, Wiebe S, et al. Early surgical therapy for drug-resistant temporal lobe epilepsy. JAMA. 2012;307:922–930. doi: 10.1001/jama.2012.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schulze-Bonhage A. Epilepsien und ihre medikamentöse Behandlung. Medizinische Monatsschrift Pharmakologie. 2010;33:207–214. [PubMed] [Google Scholar]
- 4.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- 5.Hauser WA. Status epilepticus: epidemiologic considerations. Neurology. 1990;40:9–13. [PubMed] [Google Scholar]
- 6.Schulze-Bonhage A. Der unerwartete Tod bei Epilepsiepatienten. Schweizerische Zeitschrift für Psychiatrie & Neurologie. 2007;3:6–8. [Google Scholar]
- 7.Kwan P, Schachter SC, Brodie MJ. Drug-resistant epilepsy. N Engl J Med. 2011;365:919–926. doi: 10.1056/NEJMra1004418. [DOI] [PubMed] [Google Scholar]
- 8.Schulze-Bonhage A. Epilepsiechirurgie - Welche Patienten profitieren am meisten? Nervenheilkunde. 2007;26:1006–1012. [Google Scholar]
- 9.Oehl B, Altenmüller DM, Schulze-Bonhage A. Zur Notwendigkeit prächirurgischer Video-EEG-Registrierungen bei läsionellen Epilepsiepatienten. Nervenarzt. 2009;80:464–467. doi: 10.1007/s00115-009-2672-x. [DOI] [PubMed] [Google Scholar]
- 10.Anastasopoulos C, Reisert M, Kiselev VG, Nguyen-Thanh TH, Schulze-Bonhage A, Zentner J, Mader I. Local and Global Fiber Tractography in Patients with Epilepsy. AJNR. 2014 doi: 10.3174/ajnr.A3752. im Druck. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hefft S, Brandt A, Zwick S, et al. Safety of hybrid electrodes for single neuron recordings in Humans. Neurosurgery. 2013;73:78–85. doi: 10.1227/01.neu.0000429840.76460.8c. [DOI] [PubMed] [Google Scholar]
- 12.Kerber K, Levan P, Dumpelmann M, et al. High frequency oscillations mirror disease activity in patients with focal cortical dysplasia. Epilepsia. 2013;54:1428–1436. doi: 10.1111/epi.12262. [DOI] [PubMed] [Google Scholar]
- 13.Dümpelmann M, Jacobs J, Kerber K, Schulze-Bonhage A. Automatic 80-250 Hz „ripple“ high frequency oscillation detection in invasive subdural grid and strip recordings in epilepsy by a radial basis function neural network. Clin Neurophysiol. 2012;123:1721–1731. doi: 10.1016/j.clinph.2012.02.072. [DOI] [PubMed] [Google Scholar]
- 14.Spencer DD, Inserni J. Temporal lobectomy. In: Lüders H, editor. Epilepsy Surgery. New York: Raven Press; 1992. pp. 533–546. [Google Scholar]
- 15.Zentner J, Hufnagel A, Wolf HK, et al. Surgical treatment of temporal lobe epilepsy: Clinical, radiological and histopathological findings in 178 patients. J Neurol Neurosurg Psychiatry. 1995;58:666–673. doi: 10.1136/jnnp.58.6.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Villemure JG. Hemispherectomy techniques. In: Lüders HO, editor. Epilepsy Surgery. New York: Raven Press; 1992. pp. 569–578. [Google Scholar]
- 17.Rasmussen T. Hemispherectomy for seizures revisited. Can J Neurol Sci. 1983;10:71–78. doi: 10.1017/s0317167100044668. [DOI] [PubMed] [Google Scholar]
- 18.Schramm J, Behrens E, Entzian W. Hemispherical deafferentation: an alternative to functional hemispherectomy. Neurosurgery. 1995;36:509–516. doi: 10.1227/00006123-199503000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Morrell F, Whisler WW, Bleck TP. Multiple subpial transsection: a new approach to the surgical treatment of focal epilepsy. J Neurosurg. 1989;70:231–239. doi: 10.3171/jns.1989.70.2.0231. [DOI] [PubMed] [Google Scholar]
- 20.Zentner J. Surgical aspects of corpus callosum section. In: Tuxhorn I, Holthausen H, Boenigk H, editors. Paediatric epilepsy syndromes and their surgical treatment. London: John Libbey; 1997. pp. 830–839. [Google Scholar]
- 21.Penry JK, Deen JC. Prevention of intractable partial seizures by intermittent vagal stimulation in humans: preliminary results. Epilepsia. 1990;32:40–43. doi: 10.1111/j.1528-1157.1990.tb05848.x. [DOI] [PubMed] [Google Scholar]
- 22.Sandok E, Neal J, Handforth A, et al. SANTE Study Group: Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51:899–908. doi: 10.1111/j.1528-1167.2010.02536.x. [DOI] [PubMed] [Google Scholar]
- 23.Chabardes S, Kahane P, Minotti L, Koudsie A, Hirsch E, Benabid AL. Deep brain stimulation in epilepsy with particular reference to the subthalamic nucleus. Epileptic Disord. 2002;4:83–93. [PubMed] [Google Scholar]
- 24.Boon P, Vonck K, De Herdt V, et al. Deep brain stimulation in patients with refractory temporal lobe epilepsy. Epilepsia. 2007;48:1551–1560. doi: 10.1111/j.1528-1167.2007.01005.x. [DOI] [PubMed] [Google Scholar]
- 25.Morrell MJ. RNS System in Epilepsy Study Group: Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77:1295–1304. doi: 10.1212/WNL.0b013e3182302056. [DOI] [PubMed] [Google Scholar]
- 26.Schulze-Bonhage A, Coenen V. Epilepsiebehandlung - Periphere und zentrale Stimulationsverfahren. Nervenarzt. 2013;84:517–529. doi: 10.1007/s00115-013-3749-0. [DOI] [PubMed] [Google Scholar]
- 27.Fauser S, Schulze-Bonhage A, Honegger J, et al. Focal cortical dysplasias: surgical outcome in 67 patients in relation to histological subtypes and dual pathology. Brain. 2004;127:2406–2418. doi: 10.1093/brain/awh277. [DOI] [PubMed] [Google Scholar]
- 28.Kim DW, Lee SK, Chu K, et al. Predictors of surgical outcome and pathologic considerations in focal cortical dysplasia. Neurology. 2009;72:211–216. doi: 10.1212/01.wnl.0000327825.48731.c3. [DOI] [PubMed] [Google Scholar]
- 29.Kral T, Clusmann H, Blümcke I, et al. Outcome of epilepsy surgery in focal cortical dysplasia. J Neurol Neurosurg Psychiatry. 2003;74:183–188. doi: 10.1136/jnnp.74.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zentner J, Hufnagel A, Ostertun B, et al. Surgical treatment of extratemporal epilepsy: Clinical, radiological and histopathological findings in 60 patients. Epilepsia. 1996;37:1072–1080. doi: 10.1111/j.1528-1157.1996.tb01027.x. [DOI] [PubMed] [Google Scholar]
- 31.Davies KG, Weeks RD. Cortical resections for intractable epilepsy of extratemporal origin: experience with seventeen cases over eleven years. Br J Neurosurg. 1993;7:343–353. doi: 10.3109/02688699309103488. [DOI] [PubMed] [Google Scholar]
- 32.Fauser S, Bast T, Altenmüller DM, et al. Factors influencing surgical outcome in patients with focal cortical dysplasia. J Neurol Neurosurg Psychiatry. 2008;79:103–105. doi: 10.1136/jnnp.2007.116038. [DOI] [PubMed] [Google Scholar]
- 33.Ramantani G, Cosandier-Rimele D, Schulze-Bonhage A, Maillard L, Zentner J, Dumpelmann M. Source reconstruction based on subdural EEG recordings adds to the presurgical evaluation in refractory frontal lobe epilepsy. Clin Neurophysiol. 2013;124:481–491. doi: 10.1016/j.clinph.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 34.Schulze-Bonhage A, Metternich B, Biethahn S, Zentner J, Wagner K. Lebensqualität nach extratemporaler Epilepsiechirurgie. Nervenarzt. 2009;80:445–451. doi: 10.1007/s00115-009-2668-6. [DOI] [PubMed] [Google Scholar]
- 35.Behrens E, Schramm J, Zentner J, et al. Surgical and neurological complications in a series of 708 epilepsy surgery procedures. Neurosurgery. 1997;41:1–10. doi: 10.1097/00006123-199707000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Ramantani G, Kadish NE, Strobl K, et al. Seizure and cognitive outcomes of epilepsy surgery in infancy and early childhood. Eur J Paediatr Neurol. 2013;17:498–506. doi: 10.1016/j.ejpn.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 37.Cross JH, Jayakar P, Nordli D, et al. International league against epilepsy, subcommission for paediatric epilepsy surgery; Commissions of Neurosurgery and Paediatrics. Proposed criteria of referral and evaluation of children for epilepsy surgery: recommendations of the Subcommission for Pediatric Epilepsy Surgery. Epilepsia. 2006;47:952–959. doi: 10.1111/j.1528-1167.2006.00569.x. [DOI] [PubMed] [Google Scholar]
- 38.Harvey AS, Cross JH, Shinnar S, Mathern BW. ILAE Pediatric Epilepsy Surgery Taskforce: Defining the spectrum of international practice in pediatric epilepsy surgery patients. Epilepsia. 2008;49:146–155. doi: 10.1111/j.1528-1167.2007.01421.x. [DOI] [PubMed] [Google Scholar]
- 39.Wirrell E, Wong-Kisiel L, Mandrekar J, Nickels K. Predictors and course of medically intractable epilepsy in young children presenting before 36 months of age: A retrospective, population-based study. Epilepsia. 2012;53:1563–1569. doi: 10.1111/j.1528-1167.2012.03562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spencer S, Huh L. Outcomes of epilepsy surgery in adults and children. Lancet Neurol. 2008;7:525–537. doi: 10.1016/S1474-4422(08)70109-1. [DOI] [PubMed] [Google Scholar]
- e1.Beghi E, Cornaggia C. Morbidity and accidents in patients with epilepsy: results of a European cohort study. Epilepsia. 2002;43:1076–1083. doi: 10.1046/j.1528-1157.2002.18701.x. [DOI] [PubMed] [Google Scholar]
- e2.Holst AG, Winkel BG, Risgaard B, et al. Epilepsy and risk of death and sudden unexpected death in the young: a nationwide study. Epilepsia. 2013;54:1613–1620. doi: 10.1111/epi.12328. [DOI] [PubMed] [Google Scholar]
- e3.Helmstaedter C. Neuropsychological aspects of epilepsy surgery. Epilepsy Behav. 2004;5:45–55. doi: 10.1016/j.yebeh.2003.11.006. [DOI] [PubMed] [Google Scholar]
- e4.Knake S, Triantafyllou C, Wald LL, et al. 3T phased array MRI improves the presurgical evaluation in focal epilepsies: a prospective study. Neurology. 2005;65:1026–1031. doi: 10.1212/01.wnl.0000179355.04481.3c. [DOI] [PubMed] [Google Scholar]
- e5.Von Oertzen J, Urbach H, Jungbluth S, Kurthen M, Reuber M, Fernández G, Elger CE. Standard magnetic resonance imaging is inadequate for patients with refractory focal epilepsy. J Neurol Neurosurg Psychiatry. 2002;73:643–647. doi: 10.1136/jnnp.73.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e6.Desai A, Bekelis K, Thadani VM, et al. Interictal PET and ictal subtraction SPECT: sensitivity in the detection of seizure foci in patients with medically intractable epilepsy. Epilepsia. 2013;54:341–350. doi: 10.1111/j.1528-1167.2012.03686.x. [DOI] [PubMed] [Google Scholar]
- e7.Perry MS, Duchowny M. Surgical versus medical treatment for refractory epilepsy: Outcomes beyond seizure control. Epilepsia. 2013;54:2060–2070. doi: 10.1111/epi.12427. [DOI] [PubMed] [Google Scholar]
- e8.Edelvik A, Rydenhag B, Olsson I, Flink R, Kumlien E, Källén K, Malmgren K. Longterm outcomes of epilepsy surgery in Sweden: a national prospective and longitudinal study. Neurology. 2013;81:1244–1251. doi: 10.1212/WNL.0b013e3182a6ca7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e9.Englot DJ, Breshears JD, Sun PP, Chang EF, Auguste KI. Seizure outcomes after resective surgery for extra-temporal lobe epilepsy in pediatric patients. J Neurosurg Pediatr. 2013;12:126–133. doi: 10.3171/2013.5.PEDS1336. [DOI] [PubMed] [Google Scholar]
- e10.Schmidt D, Stavem K. Long-term seizure outcome of surgery versus no surgery for drug-resistant partial epilepsy: a review of controlled studies. Epilepsia. 2004;50:1301–1309. doi: 10.1111/j.1528-1167.2008.01997.x. [DOI] [PubMed] [Google Scholar]
- e11.Wiebe S, Blume WT, Girvin JP, Eliasziw M. Effectiveness and efficiency of surgery for temporal lobe epilepsy study group. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311–318. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- e12.Skirrow C, Cross JH, Cormack F, Harkness W, Vargha-Khadem F, Baldewag T. Long-term intellectual outcome after temporal lobe surgery in childhood. Neurology. 2011;76:1330–1337. doi: 10.1212/WNL.0b013e31821527f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e13.Smith ML, Kelly K, Kadis DS, Elliott IM, Olds J, Whiting S, Snyder T. Self-reported symptoms of psychological well-being in young adults who underwent resective epilepsy surgery in childhood. Epilepsia. 2011;52:891–899. doi: 10.1111/j.1528-1167.2011.03026.x. [DOI] [PubMed] [Google Scholar]
- e14.Hamid H, Blackmon K, Cong X, et al. Mood, anxiety, and incomplete seizure control affect quality of life after epilepsy surgery. Neurology. 2014 doi: 10.1212/WNL.0000000000000183. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- e15.Sperling MR, Harris A, Nei M, Liporace JD, O’Connor MJ. Mortality after epilepsy surgery. Epilepsia. 2005;46:49–53. doi: 10.1111/j.1528-1167.2005.00410.x. [DOI] [PubMed] [Google Scholar]
- e16.Hader WJ, Tellez-Zenteno J, Metcalfe A, Hernandez-Ronquillo L, Wiebe S, Kwon CS, Jette N. Complications of epilepsy surgery: a systematic review of focal surgical resections and invasive EEG-monitoring. Epilepsia. 2013;54:840–847. doi: 10.1111/epi.12161. [DOI] [PubMed] [Google Scholar]