Summary
Second primary malignancies have long been associated with chronic lymphocytic leukaemia (CLL). We assessed secondary tumour samples from CLL and control patients for the presence of human papilloma virus (HPV). 132 CLL patients with 44 second malignancies were compared to a matched randomly-identified control population of 264 non-CLL patients with 54 solid malignancies. Polymerase chain reaction was performed with the highly conserved MY09/MY11 HPV primer. None of control samples were HPV-positive, while 53% of samples from the CLL group were positive. This report describes preliminary evidence for the presence of HPV in secondary malignancies, in patients with CLL.
Keywords: human papilloma virus, second primary malignancy, chronic lymphocytic leukaemia
Chronic lymphocytic leukaemia (CLL) is the most common adult leukaemia in the United States (Jemal et al, 2008). Many co-morbid conditions arise as a direct result of CLL, the majority of which may be attributed to the underlying dysfunctional immune system. Second primary malignancies (SPM) have long been recognized as a complication of CLL, with a wide range of published rates (Santoro et al, 1980; Kyasa et al, 2004; Schollkopf et al, 2007) Of the solid tumours described to date, carcinomas of the lung, kidney, prostate, colon and skin have all been shown to occur at a higher frequency in CLL than in age-matched populations, and to exhibit more clinically aggressive behaviour. Unfortunately, the precise contributing mechanism(s) for developing SPM has not been adequately investigated. The commonly suggested aetiology relates to the immune deficiency of CLL, given the increased incidence of secondary solid tumour formation in other immunocompromised populations (Dasanu & Alexandrescu, 2007). However, these same mechanisms have not been elucidated in CLL.
CLL patients exhibit an immune deficiency similar to solid organ transplant patients treated with immunosuppressive agents. Additionally, these patients have a similar predisposition to the development of SPM (Kasiske et al, 2004; Stratta et al, 2008). These tumour types that occur following solid organ transplantation mirror those described in increased numbers in CLL patients. This is particularly true for squamous cell carcinoma of the skin, which has a highly elevated frequency in both populations. More notably, human papilloma virus (HPV) is more frequently associated with skin cancers in solid organ transplants than in otherwise healthy patients diagnosed with squamous cell carcinoma of the skin. We hypothesized that CLL patients might have similar involvement of HPV, and therefore sought to determine the frequency of HPV in carcinomas from CLL patients as compared with a control population without CLL. Our data suggest that HPV may be more commonly associated with SPM in CLL patients, and in particular with squamous cell carcinoma of the skin.
Materials and methods
Patients
Our institutional database identified 132 consecutive patients with CLL, consisting of 100 males (81 white, 17 African-American, 2 Asian) and 32 females (27 white, 5 African-American). Of these CLL patients, 44 were found to have developed second malignancies since the diagnosis of CLL. Fifteen of these cases had paraffin-embedded tissue samples available for analysis. An age-, gender- and ethnicity-matched population was randomly identified as a control population. Data was obtained from 264 patients, of whom 200 were male (162 white, 34 African-American, 4 Asian) and 64 were female (54 white, 10 African-American). Fifty-four malignancies were identified in this control population, of which 16 had paraffin-embedded tissue available.
Tissue sample/polymerase chain reaction
Available archived tumours collected at diagnosis or during therapeutic intervention were eligible for inclusion in this study. Study of these samples and matching to clinical data were approved by the Walter Reed Army Medical Center Institutional Review Board. Archival paraffin-embedded tissue from the CLL group included 8 skin, 3 prostate, 2 head and neck, 1 colon, and 1 breast cancer. The paraffin-embedded tissue from the control group included 13 skin and 3 colon cancers and were randomly chosen from an age-, gender- and race-matched population. CLL and control group samples were extracted simultaneously, and the operator was blinded to the tissue/group association. DNA was extracted using published methods (Qu et al, 1997). Polymerase chain reaction (PCR) was performed using commercially available consensus primers MY09/MY11 (3′ GCCCAGGGAC ATAACAATGG 5′; 5′ CGTCCAAGGGGATACTGAT 3′). These primers recognize a 450 bp fragment of the L1 region that is highly conserved in HPV (Daniel et al, 2000). Primers were utilized per manufacturer’s instructions to amplify HPV DNA. Commercially available positive (HPV) and negative (beta-globin) controls were tested in parallel. Products were typed utilizing a type-specific hybridization according to Qu et al (1997).
Results and discussion
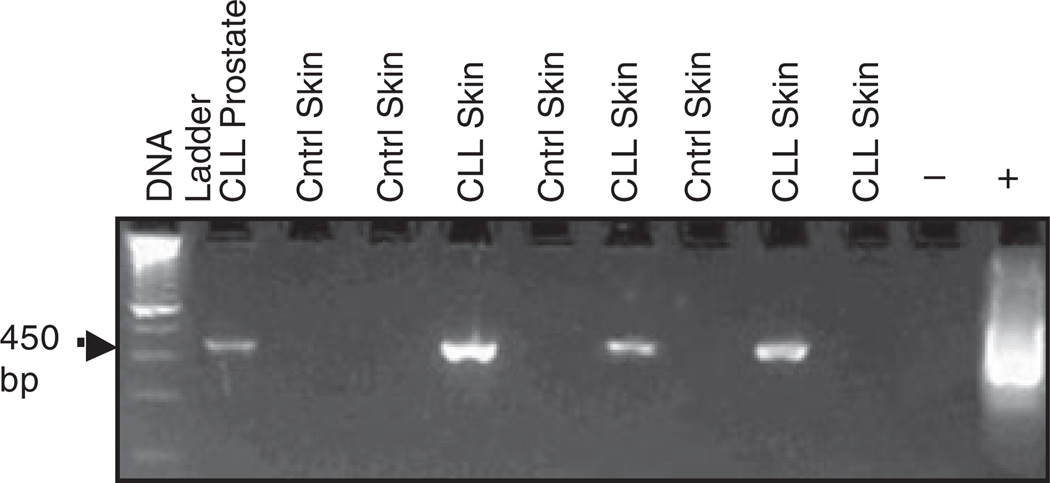
A total of 132 patients with CLL were examined, of whom 44 (33%) had evidence of secondary cancers. This is consistent with reported ranges of second malignancies in CLL, which range from four to greater than 40% (Santoro et al, 1980; Kyasa et al, 2004; Schollkopf et al, 2007). The 264 patient control population had 54 primary cancers (20%). The majority of secondary cancers in each group were localized squamous cell carcinomas of the skin, which did not differ in frequency between the two groups. Specifically, 9 cases (7%) of localized skin cancer were identified in the CLL patients whereas 14 (5%) were identified in the control population, although one biopsy sample from each group was not available for study. Of interest was that 5 of 8 (62·5%) skin biopsies from CLL patients had evidence of HPV by PCR, whereas none of the 13 control patient skin biopsies demonstrated evidence of HPV by PCR (Fig 1). The difference between CLL and normal control patients for HPV association with squamous cell carcinoma of the skin was significant (P = 0·003). Blocks from other tumour types were available in only a minority of patients, preventing comparison of potential HPV association with additional secondary cancers in CLL. Nonetheless, 1 colon cancer and 2 prostate cancer tumours from CLL patients were noted to be positive for HPV DNA by PCR.
Fig 1.
Example PCR. Comparison of DNA from paraffin-embedded CLL patient secondary tumour samples versus tumour samples from non-CLL control patients (cntrl). PCR was performed using consensus primers MY09/MY11 to a highly conserved HPV fragment, producing a 450 bp-amplified product. Products were separated by agarose gel electrophoresis and stained with ethidium bromide.
There are well over 120 different types of HPV found in benign and malignant lesions in humans and the propensity of HPV to promote benign or malignant lesions, such as condyloma acuminatum, anogenital cancer, laryngeal papillomas, head and neck cancers, warts and carcinoma of the uterine cervix, has been well described [International Agency for Research on Cancer (IARC) 2007]. The oncogenic role of HPV is best described in skin cancer, where a high prevalence of HPV DNA has been identified in skin biopsies from immunosuppressed patients (Harwood et al, 2000). Using a comprehensive degenerate PCR technique to compare the HPV status of 148 non-melanoma skin cancers from immunosuppressed and immunocompetent individuals, Harwood et al (2000) detected HPV DNA in 37/44 (84%) squamous cell carcinomas, 18/24 (75%) basal cell carcinomas and 15/17 (88%) premalignant skin lesions from the immunosuppressed population. In contrast, in reviewing the association of HPV in renal allograft patients, Stark et al (1994) demonstrated that up to 79% of viral warts, 42% of premalignant keratoses, and 43% of invasive squamous cell carcinoma contained DNA from various HPV subtypes. This has been confirmed by others (Tieben et al, 1994; Euvrard et al, 1995; Turek & Smith, 1996).
This study identified the presence of HPV DNA in secondary tumours from patients with CLL. The predominant tumour type in archived samples was squamous carcinoma, in which HPV association was significantly higher compared to non-CLL controls. A surprising finding was also the presence of HPV DNA in prostate cancer samples of two of three CLL patients. This study is limited by the small sample size and lack of immunological and molecular characterization of the patient population or HPV subtypes, as a consequence of the retrospective manner that these samples were collected. These observations need to be confirmed in a larger cohort of patients with a clear delineation of variables that may clarify the risk of secondary malignancy. These limitations can be mitigated by expanding the number of tumours evaluated in future prospective studies of well-characterized patients. An important question remains regarding the specific HPV subtypes present in our CLL SPM population, and future hybridization of the PCR products will help to answer this. The results of this study offer interesting prospects for the potential role that HPV may play in carcinogenesis in CLL. With recent advances in the prevention of HPV infection with vaccines and the novel immunomodulating agents under clinical testing in CLL, we believe this observation has potential to lead to new strategies to prevent secondary cancer in CLL.
Acknowledgements
Supported in part by the D. Warren Brown Foundation and Leukaemia and Lymphoma Society.
References
- Daniel RW, Ahdieh L, Hayden D, Cu-Uvin S, Shah KV. Intra-laboratory reproducibility of human papillomavirus identification in cervical specimens by a polymerase chain reaction-based assay. Journal of Clinical Virology. 2000;19:187–193. doi: 10.1016/s1386-6532(00)00142-6. [DOI] [PubMed] [Google Scholar]
- Dasanu CA, Alexandrescu DT. Risk for second nonlymphoid neoplasms in chronic lymphocytic leukemia. Medscape General Medicine. 2007;9:35. [PMC free article] [PubMed] [Google Scholar]
- Euvrard S, Kanitakis J, Pouteil-Noble C, Dureau G, Touraine JL, Faure M, Claudy A, Thivolet J. Comparative epidemiologic study of premalignant and malignant epithelial cutaneous lesions developing after kidney and heart transplantation. Journal of the American Academy of Dermatology. 1995;33:222–229. doi: 10.1016/0190-9622(95)90239-2. [DOI] [PubMed] [Google Scholar]
- Harwood CA, Surentheran T, McGregor JM, Spink PJ, Leigh IM, Breuer J, Proby CM. Human papillomavirus infection and non-melanoma skin cancer in immunosuppressed and immunocompetent individuals. Journal of Medical Virology. 2000;61:289–297. doi: 10.1002/1096-9071(200007)61:3<289::aid-jmv2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 90. Human Papillomaviruses. Lyon, France: IARC; 2007. [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA: A Cancer Journal for Clinicians. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Kasiske BL, Snyder JJ, Gilbertson DT, Wang C. Cancer after kidney transplantation in the United States. American Journal of Transplantation. 2004;4:905–913. doi: 10.1111/j.1600-6143.2004.00450.x. [DOI] [PubMed] [Google Scholar]
- Kyasa MJ, Hazlett L, Parrish RS, Schichman SA, Zent CS. Veterans with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) have a markedly increased rate of second malignancy, which is the most common cause of death. Leukaemia and Lymphoma. 2004;45:507–513. doi: 10.1080/10428190310001612939. [DOI] [PubMed] [Google Scholar]
- Qu W, Jiang G, Cruz Y, Chang CJ, Ho GY, Klein RS, Burk RD. PCR detection of human papillomavirus: comparison between MY09/MY11 and GP5+/GP6+ primer systems. Journal of Clinical Microbiology. 1997;35:1304–1310. doi: 10.1128/jcm.35.6.1304-1310.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro A, Rilke F, Franchi F, Monfardini S. Primary malignant neoplasms associated with chronic lymphocytic leukemia. Tumori. 1980;66:431–437. doi: 10.1177/030089168006600404. [DOI] [PubMed] [Google Scholar]
- Schollkopf C, Rosendahl D, Rostgaard K, Pipper C, Hjalgrim H. Risk of second cancer after chronic lymphocytic leukemia. International Journal of Cancer. 2007;121:151–156. doi: 10.1002/ijc.22672. [DOI] [PubMed] [Google Scholar]
- Stark LA, Arends MJ, McLaren KM, Benton EC, Shahidullah H, Hunter JA, Bird CC. Prevalence of human papillomavirus DNA in cutaneous neoplasms from renal allograft recipients supports a possible viral role in tumour promotion. British Journal of Cancer. 1994;69:222–229. doi: 10.1038/bjc.1994.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratta P, Morellini V, Musetti C, Turello E, Palmieri D, Lazzarich E, Cena T, Magnani C. Malignancy after kidney transplantation: results of 400 patients from a single center. Clinical Transplantation. 2008;22:424–427. doi: 10.1111/j.1399-0012.2008.00802.x. [DOI] [PubMed] [Google Scholar]
- Tieben LM, Berkhout RJ, Smits HL, Bouwes Bavinck JN, Vermeer BJ, Bruijn JA, Van der Woude FJ, Ter Schegget J. Detection of epidermodysplasia verruciformis-like human papillomavirus types in malignant and premalignant skin lesions of renal transplant recipients. British Journal of Dermatology. 1994;131:226–230. doi: 10.1111/j.1365-2133.1994.tb08496.x. [DOI] [PubMed] [Google Scholar]
- Turek LP, Smith EM. The genetic program of genital human papillomaviruses in infection and cancer. Obstetrics and Gynecology Clinics of North America. 1996;23:735–758. doi: 10.1016/s0889-8545(05)70275-8. [DOI] [PubMed] [Google Scholar]