Abstract
Background
The epidermis is an important protective barrier that is essential for maintenance of life. Maintaining this barrier requires continuous cell proliferation and differentiation. Moreover, these processes must be balanced to produce a normal epidermis. The stem cells of the epidermis reside in specific locations in the basal epidermis, hair follicle and sebaceous glands and these cells are responsible for replenishment of this tissue.
Scope of review
A great deal of effort has gone into identifying protein epitopes that mark stem cells, in identifying stem cell niche locations, and in understanding how stem cell populations are related. We discuss these studies as they apply to understanding normal epidermal homeostasis and skin cancer.
Major conclusions
An assortment of stem cell markers have been identified that permit assignment of stem cells to specific regions of the epidermis, and progress has been made in understanding the role of these cells in normal epidermal homeostasis and in conditions of tissue stress. A key finding is the multiple stem cell populations exist in epidermis that give rise to different structures, and that multiple stem cell types may contribute to repair in damaged epidermis.
General significance
Understanding epidermal stem cell biology is likely to lead to important therapies for treating skin diseases and cancer, and will also contribute to our understanding of stem cells in other systems. This article is part of a Special Issue entitled Biochemistry of Stem Cells.
Keywords: Stem cell Hair follicle, Interfollicular stem cell, Epidermis, Keratinocyte
1. Introduction — the epidermis
The epidermis provides an important barrier that protects against environmental stress and loss of body fluids. As such it generates a number of structures that provide key homeostatic functions including the epidermis, hair follicles, and sebaceous and sweat glands. This dynamic structure renews continually to maintain the epidermal barrier and to respond to damage. Construction of an appropriate and functional barrier requires a balance between keratinocyte proliferation and differentiation [37], in addition to apoptosis when the tissue is stressed [49,50].
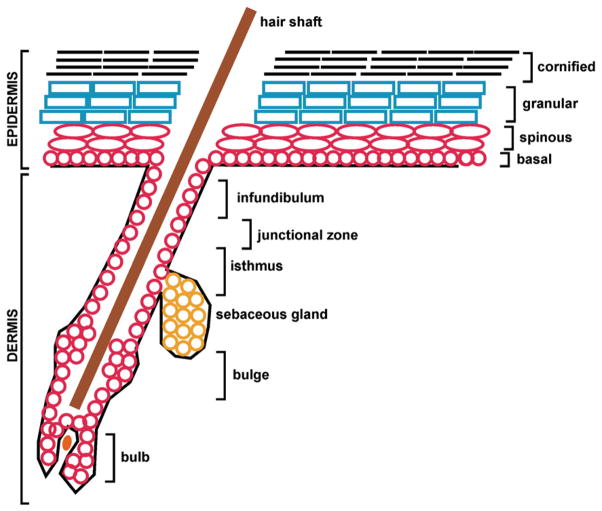
During mouse development, the embryonic epidermis begins as a single layer of cells until embryonic day 12.5 when keratinocytes initiate stratification and multiple layers are established. As a multilayered tissue the epidermis displays a number of morphologically distinct zones, including the basal, spinous, granular and cornified layers (Fig. 1) [37]. The basal layer contains the undifferentiated cells that possess proliferative capacity. These cells are anchored to the basement membrane, a barrier that separates the epidermis from the underlying dermis. As such, cells of the basal layer are polarized with their basal surface anchored by integrins to the basement membrane extracellular matrix components (including laminin and collagen IV secreted by keratinocytes). The integrins include a variety of α- and β-integrin subunits, such as α6 integrin and β4 integrin, putative epidermal stem cell markers, which act as heterodimeric transmembrane receptors [82,125]. In particular, integrins have an important role in organizing hemidesmosomes which anchor the basal keratinocytes to the extracellular matrix [120].
Fig. 1.
Structure of the hair follicle and the epidermis. The schematic shows hair follicle structures including the infundibulum, junctional zone, isthmus, sebaceous gland, bulge, bulb and hair shaft. The epidermal layers (basal, spinous, granulate and cornified) are also shown. The interfollicular epidermis is the stratified epidermis located between the hair follicles. The text describes various regions of these structures where specific stem cell populations, identified by specific markers, are located.
At intervals, some of these basal cells lose the ability to proliferate and initiate a program of terminal differentiation which involves maturation as the cells are pushed upwards towards the cell surface. Ultimately, the role of these cells is to form corneocytes, which are dead cells, that consist of a covalently crosslinked (cornified) envelope surrounding stabilized keratin bundles [37,94]. In the course of assembly of this terminal structure, the nucleus and all other organelles are destroyed [57]. Ultimately, these cells are shed from the skin surface as the terminal event in differentiation. This tissue includes three major functional zones, the proliferative cells of the basal layer (which include the stem cells), partially differentiated cells of the spinous and granular layers which are viable but non-proliferating, and the terminal differentiated dead cells of the cornified layer. Regulation of cell survival is important in each of these zones (Fig. 1). The molecular processes that control differentiation are complex and involve many gene products [36,38].
Keratinocyte stem cells (KSC) are proliferation units that display limited and slow division, can self-renew and are responsible for generating the various lineages present in the mature tissue [17,18,21,56,127–129]. These were initially identified as label-retaining cells [16,19,20]. Current studies identify three populations of KSCs in epidermis. These include the interfollicular (IF) epidermal stem cells in the epidermal basal layer, the hair follicle (HF) stem cells of the bulge and the sebaceous gland (SG) stem cells located immediately above the hair bulge. The IF epidermis is the skin surface located between hair follicles. Under normal conditions these stem cell populations appear to function independently to produce the IF epidermis, hair follicle and sebaceous gland. However, recent studies suggest that each of these cell populations can function to replicate any skin structure [46,73], which may be particularly important when the epidermis is stressed.
Specific differences are evident in the microanatomy and composition of the stem cells between mice and humans. These differences emphasize the importance in distinguishing among animal models when studying epidermal stem cells. For instance, the hair bulge is a very distinct structure in the follicle and is a primary source of epidermal stem cells in the mouse, while in humans the hair bulge is less distinct and IF stem cells appear to be the more abundant stem cell. However, studies of label-retaining cells indicated that human hair follicle stem cells like those in the mouse can also be found in the bulge-like structure [79,98].
Stem cells, located in the IF epidermis in the basal layer, are an important population of cells involved in maintaining the IF epidermis. Barrandon and Green characterized differences in proliferative potential of keratinocyte cultures prepared from the human epidermis [11]. Three populations of cells were identified and called holo-, para- and metaclones. The holoclones give rise to the largest colonies in clonal growth assays and are rich in markers of the epidermal basal layer, including β1-integrin [69]. Subsequent studies reveal that these cells are rich in α6-integrin and express low levels of CD71 (transferrin receptor) [128,129]. α6-integrinbri/CD71dim cells display many stem cell features, including quiescence and long-term growth capacity (Table 1) [74,128,129]. These cells are generally detected at the downward tip of the rete ridges. However, another population of cells, that are β1-integrin+/MCSP (melanoma chondroitin sulfate proteoglycan)+/Lrg1+, are present in the upper segment of the rete ridge and these share stem cell properties [65,66,71]. Additional markers of these cells have also been identified, including survivin [80]. The existing information suggests that IF epidermal stem cells are randomly distributed in the basal layer and represent 1% to 7% of the basal cell population. The 1% level was determined by labeling experiments using human epidermal organotypic skin cultures [91] and the higher values were determined based on detection of α6-integrinbri/CD71dim human cells [129]. It is interesting that neonatal foreskin includes more stem cells (7%) than skin derived from adults (5%) [128,129]. The localization of the murine IF epidermal stem cells is controversial. Evidence suggests that Lgr6+ cells, present in the HF, near the infundibulum, serve as a stem cell reservoir for the murine IF epidermis [133]. CD133 is a putative marker of the murine dermal papilla [141].
Table 1.
Distribution of stem cell markers in human and murine epidermis.
| Marker | Location | References | Species |
|---|---|---|---|
| α6bri/CD71dim | IF epidermis | [74,128,129] | Human |
| β1+/MCSP+/Lrg1+ | IF epidermis | [65,66,71] | Human |
| Survivin+ | IF epidermis | [80] | Human |
| Lgr6+ | IF epidermis | [133] | Mouse |
| CD133 (prominin) | IF epidermis | [141] | Human |
| CD200+ | HF (bulge) | [98,99] | Human |
| K15+ | HF (bulge) | [88] | Mouse |
| CD200+/CD34−/K15bri | HF (bulge) | [64] | Human |
| CD200+/CD34−/K15dim | HF (bulge) | [64] | Human |
| CD34+ | HF (bulge) | [22] | Mouse |
| K15 Promoter | HF (bulge) | [88,118] | Mouse |
| K19 | HF (bulge) | [85] | Mouse |
| α6dim/MTS24+/Lrig1+ | HF (isthmus) | [96] | Mouse |
| Sca-1+ | HF (infundibulum) | [67] | Mouse |
| Lrig1+ | HF (isthmus) | [65] | Mouse |
| Lgr6+ | SG (isthmus) | [112] | Mouse |
| Blimp1+ | SG | [63] | Mouse |
| NGFRp75, nestin, Oct-4 | MSC (dermis) | [76] | Mouse |
| Neural crest (bulge area) | [140] | Mouse | |
| CD133+ | Cancer stem | [100] | Human |
| α6bri/β1dim/CD34bri α6bri/β1dim/CD34dim |
Cancer stem | [106] | Mouse |
Various other proteins have also been identified that help to distinguish epidermal stem cells. For instance, connexin43 (Cx43) a gap junction protein is absent in 10% of human basal keratinocytes which also exhibited clonal ability, small size and low granularity. Cx43dim cells also helped distinguish p63+/ABCG2+/β1-integrin+ label-retaining cells in the human limbal epithelium [31]. Desmoglein3 (Dsg3) is another intercellular junction protein that may be a useful marker for distinguishing epithelial stem cells. High β1-integrin expressing human keratinocytes have been shown to express low levels of Dsg3 and exhibit greater long-term proliferative capacity in vitro than integrin β1hi/Dsg3hi cells. The β1hi/Dsg3lo cells isolated from the adult human palm show comparable clonogenic ability to α6hi/CD71lo cells [121,122]. Some evidence suggests that CD146, melanoma cell adhesion molecule (MCAM), may also distinguish stem cells. For example, CD146lo selection, in conjunction with selection for other markers, including CD200+, CD24lo, CD34lo, CD71lo, isolates human hair follicle cells with high colony-forming efficiency [98]. Other markers that have been studied include human EGFRlo (epidermal growth factor receptor) cells which undergo long-term expansion and produce a stratified epidermis in models of skin reconstruction [45]. Low major histocompatibility complex, MHC Class I-HLA expression is observed in pluripotent stem cells and also in a subpopulation of basal human keratinocytes [83].
2. Two models of epidermal stem cell amplification
In addition to holoclones, Barrandon and Green identified other dividing cells called paraclones, which give rise to abortive colonies that differentiate after only limited proliferation and meroclones which are intermediate in morphology and proliferative capacity [11]. Based on these and other findings, it has been theorized that the IF epidermis includes a mixture of proliferating cells consisting of holoclones and paraclones [11]. The holoclones are thought to correspond to the label-retaining stem cell population and the paraclones to the transient amplifying cells. These cells are distinguished based on differences in label-retention [28], cell surface marker expression, proliferation frequency, and ability to grow as clones in culture [11,11,28,64,96,98,117]. In murine epidermis cell populations have been distinguished as epidermal stem cells which are label-retaining and occasionally give rise to an identical daughter stem cell (symmetrical division) and a transient amplifying cell (asymmetrical division). Unlike the epidermal stem cell, the transit amplifying cell divides rapidly and, after several rounds of cell division, undergoes terminal differentiation.
However, fate mapping experiments question the existence of transient-amplifying cells [34,68]. These studies used inducible genetic labeling to track progenitor cells in murine tail epidermis for one year. Results showed that the average number of basal layer cells per clone increases in a linear fashion with time and does not follow an “epidermal proliferation unit” pattern which would be expected if transient amplifying cells were present. Since the clones remained cohesive and expand in size over time, this suggests that only one type of proliferative stem cell exists that undergoes an unlimited number of symmetrical divisions. If these results can be replicated in areas outside the tail region, it would suggest a new model for stem cell renewal in the epidermis.
3. Stem cells of the hair follicle
The hair follicle is a structure that varies from the interfollicular epidermis in several important ways. First, it projects down into the dermis where the cells are exposed to a different environment and, second, the hair follicle undergoes intermittent cycles of growth, regression and quiescence [92]. In each growth cycle, the follicle is generated from a specific set of stem cells that are resident in the hair bulge. In mouse, the hair follicle bulge is a highly recognizable structure. In human hair, the bulge is not as readily recognized. The mouse and human systems also differ in other ways. The human hair follicle cycle takes nearly a decade while the murine hair cycle is on the order of weeks [23].
CD200 (cluster of differentiation 200), a type I membrane glyco-protein which contains two immunoglobulin domains, is a reliable marker of human bulge cells (Table 1), but not of murine bulge cells [98,99]. In contrast, keratin 15 (K15), is a reliable marker in mouse bulge cells [88], but is not as specific for human hair bulge stem cells [97]. Distinct bulge-localized K15-positive stem cell subpopulations have been identified in human epidermis which are CD200+/CD34−/K15bri (basal) and CD200+/CD34−/K15dim (suprabasal) [64]. The CD200+/CD34−/K15bri cells form larger clonal growth colonies [64].
CD34 (cluster of differentiation 34), a single-pass transmembrane sialomucin protein, marks bulge cells in murine follicles [22], but is absent from human bulge tissue [23,61]. In murine hair follicle bulge cells, the CD34+ cells are label-retaining and readily form clonal colonies [118,119]. Keratin 19 (K19) also stains label-retaining cells in mice [85]. An extremely useful finding is that the K15 promoter also marks these cells [88,118].
The region between the bulge and the IF epidermis in murine epidermis also contains putative stem cells. This region, the isthmus (Fig. 1), contains a population of MTS24+/Lrig1+/α6dim cells that are highly clonogenic [96]. These cells are also marked by an absence of CD34, K15 and Sca-1. In contrast, Sca-1+ cells are present in the infundibulum [67] and these cells can repopulate the IF epidermis but not the HF. Lrig1+ cells have also been identified in the junctional zone (Fig. 1) [65]. Lrig1 probably acts in these cells to maintain quiescence and in reconstitution assays these cells give rise to all epidermal cell lineages [65].
4. Sebaceous gland stem cells
The SG is an important structure in the epidermis. In mouse, stem cells that produce this gland are located in a region of the HF above the CD34+/K15+ bulge region. This is evidenced by the presence of a population of Lgr6+ cells at this location that can produce sebaceous cells [112]. This population is also multipotent and can replace HF and IF epidermis and SG structures in mouse [112]. However, these cells are not label-retaining. The Lrg6+ cells are particularly interesting in that they can renew the sebaceous gland and sebaceous gland stem cells which are Blimp-1 positive [63]. Lineage-tracking experiments indicate that these cells give rise to the entire gland [63]. Although its role is not well understood, Blimp-1 is an interesting protein that must have a negative control role on gland growth, as suppression of Blimp-1 expression results in SG hyperplasia [63]. The bulge area also contains melanocyte stem cells and stem cells with neural crest properties [76,140].
5. Skin cancer stem cells
Non-melanoma skin cancer is the most common cancer in human populations. Basal cell carcinoma (BCC) is frequent and can be highly destructive. The second type of skin cancer, squamous cell carcinoma (SCC), is extremely frequent. SCC precancerous stages are well characterized with respect to clinical, histological and molecular features and metastasis [9,14]. Immunosuppressed patients are two-hundred times more likely to get aggressive and metastatic SCC. Skin cancer progression is linked to ultraviolet (UV) exposure. Early-age UV exposure contributes to BCC development, and chronic UV exposure is thought to be responsible for SCC development and progression [9]. Moreover, due to the presence of environmental irritants and exposure to UV irradiation, skin cancer incidence is increasing [60]. Thus, skin cancer is an important health concern.
The cancer stem cell theory states that tumor stem cells are self-renewing, slow cycling, quiescent cells that are not impacted by anti-cancer agents that kill rapidly growing tumor cells [3]. Moreover, it is thought that a limited number of cancer stem cells give rise to highly proliferative non-stem cells that comprise the bulk of the tumor. Since cancer stem cells give rise to other tumor cells, eliminating tumor stem cells is thought to be necessary to halt tumor formation [3]. Well-developed mouse models are available to study the role of epidermal skin cancer stem cells [14,21,51,58,59,109,127].
It is thought that mutations in rarely dividing long-lived stem cells lead to accumulation of genetic changes that overcome cell control and lead to cancer growth. Squamous cell carcinoma progression involves mutational inactivation of p53. Ultraviolet light is a common skin mutagen and DNA changes characteristic of UV exposure are often observed in skin cancer cells and tumors [25]. As mutated p53 suppresses wild-type p53 activity, the cells are rendered more resistant to apoptosis [143]. It is clear that these slowly-dividing stem cells accumulate mutations in the 7,12-dimethyl-1,2-benz[a]anthracene (DMBA)/tetradecanoylphorbol-13-acetate (TPA) two-stage cancer protocol and that these cells then expand during tumor expansion [70,86,87,111]. Thus, stem cells appear to be an important carcinogen target.
Human SCC is thought to arise from IF stem cells that give rise to squamous tumors. SCC generally occurs at sun-exposed sites and manifests UV-associated p53 mutation [26]. This is consistent with the fact that basal IF epidermis is accessible to UV irradiation. Actinic keratosis is the apparent precursor lesion to SCC [5,9]. However, a strict origin of SCC in the IF epidermis may not hold under specific experimental conditions. For example, tumors arising in the two stage carcinogenesis model, following sequential treatment of DMBA and TPA, are associated with DMBA-dependent H-ras mutation. Most of the tumors arise from stem cells located in the HF bulge [89]. Moreover, SCC still develops when the IF epidermis is removed and mice are UV-irradiated [43]. This is presumably due to an impact on HF bulge stem cells. This suggests that various cell populations can give rise to tumors following UV exposure. Moreover, when SCC-related mutations (Ras mutation/p53 deletion) are targeted to the bulge stem cells, these cells give rise to SCC [131]. Taken together, these studies suggest that stem cells are the likely target of environmental damage, and that depending upon the circumstance, SCC can arise from various epidermal stem cell populations. Moreover, it is interesting that the targeted mutation of transient amplifying cells does not produce tumors [131], further supporting the idea that stem cells are the target. Identifying tumor stem cells in human tumor samples has recently progressed, as Vogel and coworkers showed that CD133+ cells are enriched for tumor-initiating cells [100]. A distinct spatial distribution of stem cell markers is also observed in murine SCC, as the cancer stem cells are located along the dermal/tumor interface and are integrin-positive and CD34bri or CD34dim [106]. Other protein markers also identify putative cancer stem cells. These markers have been identified in other epithelia, such as mammary cancer. For instance, aldehyde dehydrogenase (ALDH) is expressed in human epidermis [32] and also in normal mammary stem cells [53]. Likewise, CD44 (hyaluronic acid receptor) has also been identified as a marker of mammary stem cells [4] and head and neck squamous cell cancer stem cells [101]. CD24 is a glycoprotein and CD24neg expression is present in more primitive, or less differentiated mammary stem cells, and is a marker of post-mitotic human keratinocytes [15].
Basal cell carcinoma is restricted to areas of the epidermis with hair, and the marker protein profile observed in BCC is consistent with a hair follicle-origin of the tumors [109]. Moreover, mutation in Ptch1, the Sonic hedgehog receptor, which is involved in hair follicle regeneration, is observed [8,75], and the level of Lgr5, a HF stem cell marker, is increased in most BCCs [116]. These findings strongly suggest that HF stem cells give rise to BCC, although recent results suggest that this model may be more complicated than previously appreciated [55,123,139].
6. Other stem cells in the epidermis
Non-epithelial stem cells are also found in the epidermis. These include bone marrow-derived stem cells (BMSCs) which are found in the epidermis during epidermal regeneration. Several studies suggest that BMSCs have the ability to derive both dermal and epidermal cells during regeneration [10,24,29,35,42]. An alternative explanation for such results is the demonstrated ability of BMSCs to fuse with other cells in vitro. However, while ex vivo studies have demonstrated the ability of BMSCs to fuse with keratinocytes [12], several studies have shown that in vivo fusion does not occur [24,62,134] and that BMSCs differentiate into epidermal cells [135].
7. Signaling proteins in epidermal stem cells
A requirement for stem cell survival is that the quiescent state be tightly controlled. This involves a number of signaling cascades, some of which are discussed here. The hair follicle utilizes sonic hedgehog (Shh) and Wnt signaling to control cell function. Wnt/β-catenin signaling regulates cell fate. Wnt is a secreted glycoprotein that binds to Frizzled receptors to trigger a cascade that results in displacement of GSK-3β from the APC/Axin/GSK-3β complex. In the absence of Wnt signaling, β-catenin is phosphorylated and targeted for degradation by the APC/Axin/GSK-3β complex. In the presence of Wnt, Dishevelled is phosphorylated and activated and recruits GSK-3β from the complex. This leads to β-catenin stabilization and Rac1 nuclear translocation and recruitment of LEF/TCF DNA binding factors to activate transcription [102]. Wnt signaling, via its impact on TCF3 transcription factor, is essential for maintenance of HF stem cells [48,84,144], and blocking Wnt action causes cells to differentiate into keratinocytes and sebocytes [78,95]. These findings indicate that Wnt signaling controls commitment of HF bulge cells in mouse to maintain the stem state or permit entry into a differentiation pathway.
Sonic hedgehog (Shh) is a ligand that binds to its membrane-spanning receptor, Patched (Ptc). Under resting conditions, Ptc binds to and inhibits Smoothened (Smo), a transmembrane protein. The hedgehog signaling complex is downstream of Smo. When Shh binds to Ptc, the inhibitor impact on Smo is relieved and Smo becomes phosphorylated. Another protein called Ci is then released and enters the nucleus to drive expression of hedgehog target genes. Sonic hedgehog (Shh) is a second important controller of HF bulge stem cells. Shh is a key gene that is activated in order to organize dermal cells to form the dermal papilla [104]. Inhibition of the Shh pathway during embryonic development arrests the follicle at the hair bud stage after placode formation and initial ingrowth [114]. Furthermore, inhibition of Shh in adult skin prevents anagen hair growth [124].
Notch and EGFR signaling are also key pathways in the IF epidermis [47]. Notch signaling is pronounced in the basal layer of the epidermis with high activity in areas featuring stem cells [77]. Notch signaling also drives keratinocyte differentiation as the cells depart the stem cell niche [77]. In mouse epidermis differentiation and maintenance of IF epidermis are balanced by the opposing actions of Notch and another key signaling molecule, p63 [47,126]. The mitogen-activated protein kinases (MAPK) and epidermal growth factor receptor (EGFR) are also important. Numerous studies indicate that EGFR and MAPK signaling are required for normal epidermal differentiation, particularly of the IF keratinocyte [2,38–41,52].
8. MicroRNA regulation of epidermal stem cells
MicroRNAs (miRNAs) are small (~19–24 nucleotides) noncoding RNAs that regulate gene expression. One way that miRNAs modulate gene expression is by interacting with the complimentary target messenger RNA (mRNA) to alter RNA stability or translation. It has been estimated that miRNAs regulate more than one-third of the protein-coding genome. As a consequence, it is expected that many biological processes are affected. Several groups have demonstrated that mice with epidermal specific deletion of Dicer, an essential component of the miRNA regulatory machinery, are born normal but eventually lose weight and die neonatally without developing a hair coat [6,137]. The lack of hair is manifested by underdeveloped and misaligned hair follicles, which exhibited increased apoptosis, and failure to invaginate into the dermis [6,137]. This is due to the loss of the follicular stem cell pool, as evidenced by the lack of bulge stem cell markers K15 and CD34 [6]. In contrast, the interfollicular epidermis of these mice was unaffected [6,137]. Epidermal-specific deletion of co-factor Dgcr8 reveals a similar phenotype, including neonatal lethality, dehydrated skin, a disordered dermis, and the presence of apoptotic cells in the HFs [137].
Specific miRNAs are expressed in developing mouse skin and HFs [6,137]. Several miRNAs appear to exert specific functions [1,136]. For instance, two independent studies found that miR-203 is enriched in IF epidermis compared to HFs [6,137]. Another study emphasizes the importance of miRNA regulation in development. For instance, miR-203 is barely detectable in mouse E13.5 skin when epithelium is still one layer, but after E15.5 and stratification begins miR-203 is among the most abundant miRNAs. These data suggest that its expression is induced during differentiation and stratification [138]. Studies using in situ hybridization of mature skin revealed that miR-203 is expressed at high levels only in differentiated cells such as those in the suprabasal epidermis or the inner root sheath of the HF, but not in progenitor/stem cells such as those in basal epidermis, in the HF bulge, or in the HF matrix [138]. Similar results were also demonstrated in zebrafish [132] and human skin [130], indicating a conserved function across species. Furthermore, the expression of miR-203 is rapidly upregulated when keratinocytes are induced to differentiate in vitro by calcium [72,138], by phorbol ester [113] or by vitamin D treatment. Functional analysis of miR-203 with transgenic mice engineered to over-express miR-203 in the epidermal basal layer showed that animals frequently died shortly after birth, and histological evaluation revealed a thinner epidermis and depletion of K5+ basal cells [138].
In these studies, increased miR-203 expression reduced colony formation capacity and caused cell cycle exit in vitro [72,93]; yet demonstrated limited ability to promote terminal keratinocyte differentiation. This suggests that the primary role of miR-203 expression may be in the differentiation of the transient amplifying cells or to limit their proliferative lifespan. Interestingly, miR-203 has been shown to target p63 another putative stem cell marker of epidermal keratinocytes. The transcription factor p63 has been shown, in several species, to be required to maintain the stem cell pool in epidermis. In mice, its deletion produces a complete loss of stratified epithelia [110]. Studies have shown that the expression of p63 and miR-203 is mutually exclusive, and that miR-203 directly represses p63 expression as demonstrated by its ability to facilitate cell cycle arrest during the transition in cells from the basal layer to suprabasal layer [72,138]. Specifically, one mechanism by which p63 regulates this cell cycle progression in keratinocytes is through repression of miR-34 family members [7], while in cancer, p63 regulates tumor and metastasis suppression through the transcriptional regulation of Dicer and miR-130b [115].
HF cycling involves stages of expansion, regression and quiescence, and involves dramatic changes in the epidermal and HF architecture [105]. Key regulators of this process are miRNA targets. For example, miR-31 was shown to increase during follicular growth and decrease during regression and quiescence [81]. MiR-31 negatively regulates Fgf10, the components of the Wnt and BMP signaling pathways, sclerostin and BAMBI, and Dlx3 transcription factor, as well as keratin genes. Luciferase reporter assays show that it directly targets K16, K17, Dlx3 and Fgf10 [81]. More recently, it is reported that miR-125b represses skin stem cell differentiation in mouse. In this study, miR-125b expression was high in mouse stem cells and decreased in progeny cells. Moreover, knocking down miR-125b in transgenic mice results in hyper-thickened epidermis, enlarged sebaceous glands, and failure to generate a hair coat [142], indications that miR-125b alters stem cell differentiation. Potential targets of miR-125b included Blimp1 and vitamin D receptor (VDR) [142].
9. MicroRNA in melanoma
Several miRNAs have been discovered that regulate the metastatic or proliferative behavior of melanoma cells and, in some cases, their target or mode of action has been identified. For instance, miR-196a suppression increases tumor cell migration via an impact on HOX-B7 and HOC-C8 [27,90]. MiR-137 downregulation of microphthalmia-associated transcription factor (MITF) in melanoma cell lines is associated with melanoma metastasis [13]. MiR-182 increases migration of melanoma cell lines and their metastatic potential by repressing MITF and FOXO3 expression [108]. MiR-15b upregulation is associated with increased cell proliferation and decreased apoptosis, though its targets are unclear [103]. Let-7a repression increases the invasive behavior of melanocytes by impacting β3-integrin [33], while miR-221/222 overexpression increases cell proliferation, migration and invasion via p27 and c-Kit receptor [44]. miR-125b is reduced in primary melanomas that exhibit early metastases but its target genes are currently unknown [54]. In contrast to these miRs which facilitate melanoma malignancy, other miRNAs are repressors. For instance, miR-193b overexpression represses cell proliferation by increasing cyclin D1 in melanoma cell lines [30], while Let-7b inhibits cell cycle progression and anchorage-independent growth when overexpressed [107].
10. Conclusions
These studies indicate that much progress has been made in understanding the processes that control stem cell quiescence and differentiation in epidermis. Particular progress has been made using mouse models where targeted changes in cell populations can be produced using genetic methods. Studies are less advanced in studies of human epidermal stem cells, but progress is accelerating due to the ability to grow human keratinocytes as organotypic cultures with appropriate fibroblast support. It appears reasonable to assume that tumors arising in epidermis are derived from stem cells, localized either in the HF or IF epidermis. Moreover, the stem cell niche and location of the stem cells relative to the mutagenic stimulus profoundly influences cancer development. For example, UV irradiation is not likely to impact stem cells deep in the HF as compared to the IF epidermal stem cells. This progress bodes well for the application of new knowledge to the understanding of stem cell biology and treatment of stem cell-related conditions, including cancer and other skin diseases.
Acknowledgments
This work was supported by grants to R. Eckert (NIH R01-AR053851, NIH R01-CA131074) and a grant from the State of Maryland Stem Cell Fund.
Abbreviations
- HF
hair follicle
- IF
interfollicular
- SG
sebaceous gland
- miRNA
microRNA
- SCC
squamous cell carcinoma
- KSC
keratinocyte stem cell
Footnotes
This article is part of a Special Issue entitled Biochemistry of Stem Cells.
References
- 1.Aberdam D, Candi E, Knight RA, Melino G. miRNAs, ‘stemness’ and skin. Trends Biochem Sci. 2008;33:583–591. doi: 10.1016/j.tibs.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Adhikary G, Chew YC, Reece EA, Eckert RL. PKC-delta and -eta, MEKK-1, MEK-6, MEK-3, and p38-delta are essential mediators of the response of normal human epidermal keratinocytes to differentiating agents. J Invest Dermatol. 2010;130:2017–2030. doi: 10.1038/jid.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 3.Al-Hajj M, Clarke MF. Self-renewal and solid tumor stem cells. Oncogene. 2004;23:7274–7282. doi: 10.1038/sj.onc.1207947. [DOI] [PubMed] [Google Scholar]
- 4.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ananthaswamy HN, Loughlin SM, Ullrich SE, Kripke ML. Inhibition of UV-induced p53 mutations by sunscreens: implications for skin cancer prevention. J Investig Dermatol Symp Proc. 1998;3:52–56. [PubMed] [Google Scholar]
- 6.Andl T, Murchison EP, Liu F, Zhang Y, Yunta-Gonzalez M, Tobias JW, Andl CD, Seykora JT, Hannon GJ, Millar SE. The miRNA-processing enzyme dicer is essential for the morphogenesis and maintenance of hair follicles. Curr Biol. 2006;16:1041–1049. doi: 10.1016/j.cub.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antonini D, Russo MT, De RL, Gorrese M, Del VL, Missero C. Transcriptional repression of miR-34 family contributes to p63-mediated cell cycle progression in epidermal cells. J Invest Dermatol. 2010;130:1249–1257. doi: 10.1038/jid.2009.438. [DOI] [PubMed] [Google Scholar]
- 8.Athar M, Li C, Tang X, Chi S, Zhang X, Kim AL, Tyring SK, Kopelovich L, Hebert J, Epstein EH, Jr, Bickers DR, Xie J. Inhibition of smoothened signaling prevents ultraviolet B-induced basal cell carcinomas through regulation of Fas expression and apoptosis. Cancer Res. 2004;64:7545–7552. doi: 10.1158/0008-5472.CAN-04-1393. [DOI] [PubMed] [Google Scholar]
- 9.Backvall H, Asplund A, Gustafsson A, Sivertsson A, Lundeberg J, Ponten F. Genetic tumor archeology: microdissection and genetic heterogeneity in squamous and basal cell carcinoma. Mutat Res. 2005;571:65–79. doi: 10.1016/j.mrfmmm.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Badiavas EV, Abedi M, Butmarc J, Falanga V, Quesenberry P. Participation of bone marrow derived cells in cutaneous wound healing. J Cell Physiol. 2003;196:245–250. doi: 10.1002/jcp.10260. [DOI] [PubMed] [Google Scholar]
- 11.Barrandon Y, Green H. Three clonal types of keratinocyte with different capacities for multiplication. Proc Natl Acad Sci U S A. 1987;84:2302–2306. doi: 10.1073/pnas.84.8.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baxter MA, Wynn RF, Jowitt SN, Wraith JE, Fairbairn LJ, Bellantuono I. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells. 2004;22:675–682. doi: 10.1634/stemcells.22-5-675. [DOI] [PubMed] [Google Scholar]
- 13.Bemis LT, Chen R, Amato CM, Classen EH, Robinson SE, Coffey DG, Erickson PF, Shellman YG, Robinson WA. MicroRNA-137 targets microphthalmia-associated transcription factor in melanoma cell lines. Cancer Res. 2008;68:1362–1368. doi: 10.1158/0008-5472.CAN-07-2912. [DOI] [PubMed] [Google Scholar]
- 14.Benitah SA. Epidermal stem cells in skin homeostasis and cutaneous carcinomas. Clin Transl Oncol. 2007;9:760–766. doi: 10.1007/s12094-007-0137-x. [DOI] [PubMed] [Google Scholar]
- 15.Bergoglio V, Larcher F, Chevallier-Lagente O, Bernheim A, Danos O, Sarasin A, Rio MD, Magnaldo T. Safe selection of genetically manipulated human primary keratinocytes with very high growth potential using CD24. Mol Ther. 2007;15:2186–2193. doi: 10.1038/sj.mt.6300292. [DOI] [PubMed] [Google Scholar]
- 16.Bickenbach JR. Identification and behavior of label-retaining cells in oral mucosa and skin. J Dent Res. 1981;60(Spec No C):1611–1620. doi: 10.1177/002203458106000311011. [DOI] [PubMed] [Google Scholar]
- 17.Bickenbach JR. Isolation, characterization, and culture of epithelial stem cells. Methods Mol Biol. 2005;289:97–102. doi: 10.1385/1-59259-830-7:097. [DOI] [PubMed] [Google Scholar]
- 18.Bickenbach JR, Grinnell KL. Epidermal stem cells: interactions in developmental environments. Differentiation. 2004;72:371–380. doi: 10.1111/j.1432-0436.2004.07208003.x. [DOI] [PubMed] [Google Scholar]
- 19.Bickenbach JR, Holbrook KA. Label-retaining cells in human embryonic and fetal epidermis. J Invest Dermatol. 1987;88:42–46. doi: 10.1111/1523-1747.ep12464857. [DOI] [PubMed] [Google Scholar]
- 20.Bickenbach JR, McCutecheon J, Mackenzie IC. Rate of loss of tritiated thymidine label in basal cells in mouse epithelial tissues. Cell Tissue Kinet. 1986;19:325–333. doi: 10.1111/j.1365-2184.1986.tb00684.x. [DOI] [PubMed] [Google Scholar]
- 21.Bickenbach JR, Stern MM. Plasticity of epidermal stem cells: survival in various environments. Stem Cell Rev. 2005;1:71–77. doi: 10.1385/SCR:1:1:071. [DOI] [PubMed] [Google Scholar]
- 22.Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 23.Boehnke K, Falkowska-Hansen B, Stark HJ, Boukamp P. Stem cells of the human epidermis and their niche: composition and function in epidermal regeneration and carcinogenesis. Carcinogenesis. 2012 doi: 10.1093/carcin/bgs136. (Electronic publication ahead of print) [DOI] [PubMed] [Google Scholar]
- 24.Borue X, Lee S, Grove J, Herzog EL, Harris R, Diflo T, Glusac E, Hyman K, Theise ND, Krause DS. Bone marrow-derived cells contribute to epithelial engraftment during wound healing. Am J Pathol. 2004;165:1767–1772. doi: 10.1016/S0002-9440(10)63431-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boukamp P. Non-melanoma skin cancer: what drives tumor development and progression? Carcinogenesis. 2005;26:1657–1667. doi: 10.1093/carcin/bgi123. [DOI] [PubMed] [Google Scholar]
- 26.Boukamp P. UV-induced skin cancer: similarities–variations. J Dtsch Dermatol Ges. 2005;3:493–503. doi: 10.1111/j.1610-0387.2005.05037.x. [DOI] [PubMed] [Google Scholar]
- 27.Braig S, Mueller DW, Rothhammer T, Bosserhoff AK. MicroRNA miR-196a is a central regulator of HOX-B7 and BMP4 expression in malignant melanoma. Cell Mol Life Sci. 2010;67:3535–3548. doi: 10.1007/s00018-010-0394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Braun KM, Watt FM. Epidermal label-retaining cells: background and recent applications. J Investig Dermatol Symp Proc. 2004;9:196–201. doi: 10.1111/j.1087-0024.2004.09313.x. [DOI] [PubMed] [Google Scholar]
- 29.Brittan M, Braun KM, Reynolds LE, Conti FJ, Reynolds AR, Poulsom R, Alison MR, Wright NA, Hodivala-Dilke KM. Bone marrow cells engraft within the epidermis and proliferate in vivo with no evidence of cell fusion. J Pathol. 2005;205:1–13. doi: 10.1002/path.1682. [DOI] [PubMed] [Google Scholar]
- 30.Chen J, Feilotter HE, Pare GC, Zhang X, Pemberton JG, Garady C, Lai D, Yang X, Tron VA. MicroRNA-193b represses cell proliferation and regulates cyclin D1 in melanoma. Am J Pathol. 2010;176:2520–2529. doi: 10.2353/ajpath.2010.091061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Z, Evans WH, Pflugfelder SC, Li DQ. Gap junction protein connexin 43 serves as a negative marker for a stem cell-containing population of human limbal epithelial cells. Stem Cells. 2006;24:1265–1273. doi: 10.1634/stemcells.2005-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheung C, Smith CK, Hoog JO, Hotchkiss SA. Expression and localization of human alcohol and aldehyde dehydrogenase enzymes in skin. Biochem Biophys Res Commun. 1999;261:100–107. doi: 10.1006/bbrc.1999.0943. [DOI] [PubMed] [Google Scholar]
- 33.Christoph T, Muller-Rover S, Audring H, Tobin DJ, Hermes B, Cotsarelis G, Ruckert R, Paus R. The human hair follicle immune system: cellular composition and immune privilege. Br J Dermatol. 2000;142:862–873. doi: 10.1046/j.1365-2133.2000.03464.x. [DOI] [PubMed] [Google Scholar]
- 34.Clayton E, Doupe DP, Klein AM, Winton DJ, Simons BD, Jones PH. A single type of progenitor cell maintains normal epidermis. Nature. 2007;446:185–189. doi: 10.1038/nature05574. [DOI] [PubMed] [Google Scholar]
- 35.Deng W, Han Q, Liao L, Li C, Ge W, Zhao Z, You S, Deng H, Murad F, Zhao RC. Engrafted bone marrow-derived flk-(1+) mesenchymal stem cells regenerate skin tissue. Tissue Eng. 2005;11:110–119. doi: 10.1089/ten.2005.11.110. [DOI] [PubMed] [Google Scholar]
- 36.Eckert RL, Crish JF, Efimova T, Dashti SR, Deucher A, Bone F, Adhikary G, Huang G, Gopalakrishnan R, Balasubramanian S. Regulation of involucrin gene expression. J Invest Dermatol. 2004;123:13–22. doi: 10.1111/j.0022-202X.2004.22723.x. [DOI] [PubMed] [Google Scholar]
- 37.Eckert RL, Crish JF, Robinson NA. The epidermal keratinocyte as a model for the study of gene regulation and cell differentiation. Physiol Rev. 1997;77:397–424. doi: 10.1152/physrev.1997.77.2.397. [DOI] [PubMed] [Google Scholar]
- 38.Eckert RL, Efimova T, Balasubramanian S, Crish JF, Bone F, Dashti S. p38 mitogen-activated protein kinases on the body surface — a function for p38delta. J Invest Dermatol. 2003;120:823–828. doi: 10.1046/j.1523-1747.2003.12120.x. [DOI] [PubMed] [Google Scholar]
- 39.Efimova T, Broome AM, Eckert RL. A regulatory role for p38 delta MAPK in keratinocyte differentiation. Evidence for p38 delta-ERK1/2 complex formation. J Biol Chem. 2003;278:34277–34285. doi: 10.1074/jbc.M302759200. [DOI] [PubMed] [Google Scholar]
- 40.Efimova T, Deucher A, Kuroki T, Ohba M, Eckert RL. Novel protein kinase C isoforms regulate human keratinocyte differentiation by activating a p38 delta mitogen-activated protein kinase cascade that targets CCAAT/enhancer-binding protein alpha. J Biol Chem. 2002;277:31753–31760. doi: 10.1074/jbc.M205098200. [DOI] [PubMed] [Google Scholar]
- 41.Efimova T, LaCelle P, Welter JF, Eckert RL. Regulation of human involucrin promoter activity by a protein kinase C, Ras, MEKK1, MEK3, p38/RK, AP1 signal transduction pathway. J Biol Chem. 1998;273:24387–24395. doi: 10.1074/jbc.273.38.24387. [DOI] [PubMed] [Google Scholar]
- 42.Fathke C, Wilson L, Hutter J, Kapoor V, Smith A, Hocking A, Isik F. Contribution of bone marrow-derived cells to skin: collagen deposition and wound repair. Stem Cells. 2004;22:812–822. doi: 10.1634/stemcells.22-5-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Faurschou A, Gniadecki R. TNF-alpha stimulates Akt by a distinct aPKC-dependent pathway in premalignant keratinocytes. Exp Dermatol. 2008;17:992–997. doi: 10.1111/j.1600-0625.2008.00740.x. [DOI] [PubMed] [Google Scholar]
- 44.Felicetti F, Errico MC, Bottero L, Segnalini P, Stoppacciaro A, Biffoni M, Felli N, Mattia G, Petrini M, Colombo MP, Peschle C, Care A. The promyelocytic leukemia zinc finger-microRNA-221/-222 pathway controls melanoma progression through multiple oncogenic mechanisms. Cancer Res. 2008;68:2745–2754. doi: 10.1158/0008-5472.CAN-07-2538. [DOI] [PubMed] [Google Scholar]
- 45.Fortunel NO, Hatzfeld JA, Rosemary PA, Ferraris C, Monier MN, Haydont V, Longuet J, Brethon B, Lim B, Castiel I, Schmidt R, Hatzfeld A. Long-term expansion of human functional epidermal precursor cells: promotion of extensive amplification by low TGF-beta1 concentrations. J Cell Sci. 2003;116:4043–4052. doi: 10.1242/jcs.00702. [DOI] [PubMed] [Google Scholar]
- 46.Fuchs E, Horsley V. More than one way to skin. Genes Dev. 2008;22:976–985. doi: 10.1101/gad.1645908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fuchs E, Nowak JA. Building epithelial tissues from skin stem cells. Cold Spring Harb Symp Quant Biol. 2008;73:333–350. doi: 10.1101/sqb.2008.73.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gambardella L, Barrandon Y. The multifaceted adult epidermal stem cell. Curr Opin Cell Biol. 2003;15:771–777. doi: 10.1016/j.ceb.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 49.Gandarillas A. Epidermal differentiation, apoptosis, and senescence: common pathways? Exp Gerontol. 2000;35:53–62. doi: 10.1016/s0531-5565(99)00088-1. [DOI] [PubMed] [Google Scholar]
- 50.Gandarillas A, Goldsmith LA, Gschmeissner S, Leigh IM, Watt FM. Evidence that apoptosis and terminal differentiation of epidermal keratinocytes are distinct processes. Exp Dermatol. 1999;8:71–79. doi: 10.1111/j.1600-0625.1999.tb00350.x. [DOI] [PubMed] [Google Scholar]
- 51.Gerdes MJ, Yuspa SH. The contribution of epidermal stem cells to skin cancer. Stem Cell Rev. 2005;1:225–231. doi: 10.1385/SCR:1:3:225. [DOI] [PubMed] [Google Scholar]
- 52.Gilfix BM, Eckert RL. Coordinate control by vitamin A of keratin gene expression in human keratinocytes. J Biol Chem. 1985;260:14026–14029. [PubMed] [Google Scholar]
- 53.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, Schott A, Hayes D, Birnbaum D, Wicha MS, Dontu G. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Glud M, Rossing M, Hother C, Holst L, Hastrup N, Nielsen FC, Gniadecki R, Drzewiecki KT. Downregulation of miR-125b in metastatic cutaneous malignant melanoma. Melanoma Res. 2010;20:479–484. doi: 10.1097/CMR.0b013e32833e32a1. [DOI] [PubMed] [Google Scholar]
- 55.Grachtchouk M, Pero J, Yang SH, Ermilov AN, Michael LE, Wang A, Wilbert D, Patel RM, Ferris J, Diener J, Allen M, Lim S, Syu LJ, Verhaegen M, Dlugosz AA. Basal cell carcinomas in mice arise from hair follicle stem cells and multiple epithelial progenitor populations. J Clin Invest. 2011;121:1768–1781. doi: 10.1172/JCI46307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Greco V, Chen T, Rendl M, Schober M, Pasolli HA, Stokes N, Dela Cruz-Racelis J, Fuchs E. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell. 2009;4:155–169. doi: 10.1016/j.stem.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Green H. The keratinocyte as differentiated cell type. Harvey Lect. 1980;74:101–139. [PubMed] [Google Scholar]
- 58.Grinnell KL, Bickenbach JR. Skin keratinocytes pre-treated with embryonic stem cell-conditioned medium or BMP4 can be directed to an alternative cell lineage. Cell Prolif. 2007;40:685–705. doi: 10.1111/j.1365-2184.2007.00464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grinnell KL, Yang B, Eckert RL, Bickenbach JR. De-differentiation of mouse interfollicular keratinocytes by the embryonic transcription factor Oct-4. J Invest Dermatol. 2006;40:685–705. doi: 10.1038/sj.jid.5700531. [DOI] [PubMed] [Google Scholar]
- 60.Gupta S, Mukhtar H. Chemoprevention of skin cancer: current status and future prospects. Cancer Metastasis Rev. 2002;21:363–380. doi: 10.1023/a:1021275330385. [DOI] [PubMed] [Google Scholar]
- 61.Gutierrez-Rivera A, Pavon-Rodriguez A, Jimenez-Acosta F, Poblet E, Braun KM, Cormenzana P, Ciria JP, Larretxea R, Cardenas JM, Izeta A. Functional characterization of highly adherent CD34+ keratinocytes isolated from human skin. Exp Dermatol. 2010;19:685–688. doi: 10.1111/j.1600-0625.2010.01075.x. [DOI] [PubMed] [Google Scholar]
- 62.Harris RG, Herzog EL, Bruscia EM, Grove JE, Van Arnam JS, Krause DS. Lack of a fusion requirement for development of bone marrow-derived epithelia. Science. 2004;305:90–93. doi: 10.1126/science.1098925. [DOI] [PubMed] [Google Scholar]
- 63.Horsley V, O’Carroll D, Tooze R, Ohinata Y, Saitou M, Obukhanych T, Nussenzweig M, Tarakhovsky A, Fuchs E. Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell. 2006;126:597–609. doi: 10.1016/j.cell.2006.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Inoue K, Aoi N, Sato T, Yamauchi Y, Suga H, Eto H, Kato H, Araki J, Yoshimura K. Differential expression of stem-cell-associated markers in human hair follicle epithelial cells. Lab Invest. 2009;89:844–856. doi: 10.1038/labinvest.2009.48. [DOI] [PubMed] [Google Scholar]
- 65.Jensen KB, Collins CA, Nascimento E, Tan DW, Frye M, Itami S, Watt FM. Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell Stem Cell. 2009;4:427–439. doi: 10.1016/j.stem.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jensen KB, Watt FM. Single-cell expression profiling of human epidermal stem and transit-amplifying cells: Lrig1 is a regulator of stem cell quiescence. Proc Natl Acad Sci U S A. 2006;103:11958–11963. doi: 10.1073/pnas.0601886103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jensen UB, Yan X, Triel C, Woo SH, Christensen R, Owens DM. A distinct population of clonogenic and multipotent murine follicular keratinocytes residing in the upper isthmus. J Cell Sci. 2008;121:609–617. doi: 10.1242/jcs.025502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jones PH, Simons BD, Watt FM. Sic transit gloria: farewell to the epidermal transit amplifying cell? Cell Stem Cell. 2007;1:371–381. doi: 10.1016/j.stem.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 69.Jones PH, Watt FM. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell. 1993;73:713–724. doi: 10.1016/0092-8674(93)90251-k. [DOI] [PubMed] [Google Scholar]
- 70.Kangsamaksin T, Park HJ, Trempus CS, Morris RJ. A perspective on murine keratinocyte stem cells as targets of chemically induced skin cancer. Mol Carcinog. 2007;46:579–584. doi: 10.1002/mc.20355. [DOI] [PubMed] [Google Scholar]
- 71.Legg J, Jensen UB, Broad S, Leigh I, Watt FM. Role of melanoma chondroitin sulphate proteoglycan in patterning stem cells in human interfollicular epidermis. Development. 2003;130:6049–6063. doi: 10.1242/dev.00837. [DOI] [PubMed] [Google Scholar]
- 72.Lena AM, Shalom-Feuerstein R, Rivetti di Val Cervo P, Aberdam D, Knight RA, Melino G, Candi E. miR-203 represses ‘stemness’ by repressing DeltaNp63. Cell Death Differ. 2008;15:1187–1195. doi: 10.1038/cdd.2008.69. [DOI] [PubMed] [Google Scholar]
- 73.Levy V, Lindon C, Zheng Y, Harfe BD, Morgan BA. Epidermal stem cells arise from the hair follicle after wounding. FASEB J. 2007;21:1358–1366. doi: 10.1096/fj.06-6926com. [DOI] [PubMed] [Google Scholar]
- 74.Li A, Simmons PJ, Kaur P. Identification and isolation of candidate human keratinocyte stem cells based on cell surface phenotype. Proc Natl Acad Sci U S A. 1998;95:3902–3907. doi: 10.1073/pnas.95.7.3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li C, Chi S, Xie J. Hedgehog signaling in skin cancers. Cell Signal. 2011;23:1235–1243. doi: 10.1016/j.cellsig.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li L, Fukunaga-Kalabis M, Yu H, Xu X, Kong J, Lee JT, Herlyn M. Human dermal stem cells differentiate into functional epidermal melanocytes. J Cell Sci. 2010;123:853–860. doi: 10.1242/jcs.061598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lowell S, Jones P, Le Roux I, Dunne J, Watt FM. Stimulation of human epidermal differentiation by delta-notch signalling at the boundaries of stem-cell clusters. Curr Biol. 2000;10:491–500. doi: 10.1016/s0960-9822(00)00451-6. [DOI] [PubMed] [Google Scholar]
- 78.Lowry WE, Blanpain C, Nowak JA, Guasch G, Lewis L, Fuchs E. Defining the impact of beta-catenin/Tcf transactivation on epithelial stem cells. Genes Dev. 2005;19:1596–1611. doi: 10.1101/gad.1324905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lyle S, Christofidou-Solomidou M, Liu Y, Elder DE, Albelda S, Cotsarelis G. The C8/144B monoclonal antibody recognizes cytokeratin 15 and defines the location of human hair follicle stem cells. J Cell Sci. 1998;111(Pt 21):3179–3188. doi: 10.1242/jcs.111.21.3179. [DOI] [PubMed] [Google Scholar]
- 80.Marconi A, Dallaglio K, Lotti R, Vaschieri C, Truzzi F, Fantini F, Pincelli C. Survivin identifies keratinocyte stem cells and is downregulated by anti-beta1 integrin during anoikis. Stem Cells. 2007;25:149–155. doi: 10.1634/stemcells.2006-0165. [DOI] [PubMed] [Google Scholar]
- 81.Mardaryev AN, Ahmed MI, Vlahov NV, Fessing MY, Gill JH, Sharov AA, Botchkareva NV. Micro-RNA-31 controls hair cycle-associated changes in gene expression programs of the skin and hair follicle. FASEB J. 2010;24:3869–3881. doi: 10.1096/fj.10-160663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Margadant C, Charafeddine RA, Sonnenberg A. Unique and redundant functions of integrins in the epidermis. FASEB J. 2010;24:4133–4152. doi: 10.1096/fj.09-151449. [DOI] [PubMed] [Google Scholar]
- 83.Matic M. A subpopulation of human basal keratinocytes has a low/negative MHC class I expression. Hum Immunol. 2005;66:962–968. doi: 10.1016/j.humimm.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 84.Merrill BJ, Gat U, DasGupta R, Fuchs E. Tcf3 and Lef1 regulate lineage differentiation of multipotent stem cells in skin. Genes Dev. 2001;15:1688–1705. doi: 10.1101/gad.891401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Michel M, Torok N, Godbout MJ, Lussier M, Gaudreau P, Royal A, Germain L. Keratin 19 as a biochemical marker of skin stem cells in vivo and in vitro: keratin 19 expressing cells are differentially localized in function of anatomic sites, and their number varies with donor age and culture stage. J Cell Sci. 1996;109(Pt 5):1017–1028. doi: 10.1242/jcs.109.5.1017. [DOI] [PubMed] [Google Scholar]
- 86.Morris RJ, Fischer SM, Slaga TJ. Evidence that the centrally and peripherally located cells in the murine epidermal proliferative unit are two distinct cell populations. J Invest Dermatol. 1985;84:277–281. doi: 10.1111/1523-1747.ep12265358. [DOI] [PubMed] [Google Scholar]
- 87.Morris RJ, Fischer SM, Slaga TJ. Evidence that a slowly cycling subpopulation of adult murine epidermal cells retains carcinogen. Cancer Res. 1986;46:3061–3066. [PubMed] [Google Scholar]
- 88.Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- 89.Morris RJ, Tryson KA, Wu KQ. Evidence that the epidermal targets of carcinogen action are found in the interfollicular epidermis of infundibulum as well as in the hair follicles. Cancer Res. 2000;60:226–229. [PubMed] [Google Scholar]
- 90.Mueller DW, Bosserhoff AK. MicroRNA miR-196a controls melanoma-associated genes by regulating HOX-C8 expression. Int J Cancer. 2011;129:1064–1074. doi: 10.1002/ijc.25768. [DOI] [PubMed] [Google Scholar]
- 91.Muffler S, Stark HJ, Amoros M, Falkowska-Hansen B, Boehnke K, Buhring HJ, Marme A, Bickenbach JR, Boukamp P. A stable niche supports long-term maintenance of human epidermal stem cells in organotypic cultures. Stem Cells. 2008;26:2506–2515. doi: 10.1634/stemcells.2007-0991. [DOI] [PubMed] [Google Scholar]
- 92.Myung P, Ito M. Dissecting the bulge in hair regeneration. J Clin Invest. 2012;122:448–454. doi: 10.1172/JCI57414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Myzak MC, Dashwood RH. Histone deacetylases as targets for dietary cancer preventive agents: lessons learned with butyrate, diallyl disulfide, and sulforaphane. Curr Drug Targets. 2006;7:443–452. doi: 10.2174/138945006776359467. [DOI] [PubMed] [Google Scholar]
- 94.Nemes Z, Steinert PM. Bricks and mortar of the epidermal barrier. Exp Mol Med. 1999;31:5–19. doi: 10.1038/emm.1999.2. [DOI] [PubMed] [Google Scholar]
- 95.Niemann C, Owens DM, Hulsken J, Birchmeier W, Watt FM. Expression of DeltaNLef1 in mouse epidermis results in differentiation of hair follicles into squamous epidermal cysts and formation of skin tumours. Development. 2002;129:95–109. doi: 10.1242/dev.129.1.95. [DOI] [PubMed] [Google Scholar]
- 96.Nijhof JG, Braun KM, Giangreco A, van Pelt C, Kawamoto H, Boyd RL, Willemze R, Mullenders LH, Watt FM, de Gruijl FR, van Ewijk W. The cell-surface marker MTS24 identifies a novel population of follicular keratinocytes with characteristics of progenitor cells. Development. 2006;133:3027–3037. doi: 10.1242/dev.02443. [DOI] [PubMed] [Google Scholar]
- 97.Ohyama M. Hair follicle bulge: a fascinating reservoir of epithelial stem cells. J Dermatol Sci. 2007;46:81–89. doi: 10.1016/j.jdermsci.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 98.Ohyama M, Terunuma A, Tock CL, Radonovich MF, Pise-Masison CA, Hopping SB, Brady JN, Udey MC, Vogel JC. Characterization and isolation of stem cell-enriched human hair follicle bulge cells. J Clin Invest. 2006;116:249–260. doi: 10.1172/JCI26043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ohyama M, Vogel JC, Amagai M. Gene ontology analysis of human hair follicle bulge molecular signature. J Dermatol Sci. 2007;45:147–150. doi: 10.1016/j.jdermsci.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 100.Patel GK, Yee CL, Terunuma A, Telford WG, Voong N, Yuspa SH, Vogel JC. Identification and characterization of tumor-initiating cells in human primary cutaneous squamous cell carcinoma. J Invest Dermatol. 2012;132:401–409. doi: 10.1038/jid.2011.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 2007;104:973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 103.Satzger I, Mattern A, Kuettler U, Weinspach D, Voelker B, Kapp A, Gutzmer R. MicroRNA-15b represents an independent prognostic parameter and is correlated with tumor cell proliferation and apoptosis in malignant melanoma. Int J Cancer. 2010;126:2553–2562. doi: 10.1002/ijc.24960. [DOI] [PubMed] [Google Scholar]
- 104.Schmidt-Ullrich R, Paus R. Molecular principles of hair follicle induction and morphogenesis. Bioessays. 2005;27:247–261. doi: 10.1002/bies.20184. [DOI] [PubMed] [Google Scholar]
- 105.Schneider MR, Schmidt-Ullrich R, Paus R. The hair follicle as a dynamic miniorgan. Curr Biol. 2009;19:R132–R142. doi: 10.1016/j.cub.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 106.Schober M, Fuchs E. Tumor-initiating stem cells of squamous cell carcinomas and their control by TGF-beta and integrin/focal adhesion kinase (FAK) signaling. Proc Natl Acad Sci U S A. 2011;108:10544–10549. doi: 10.1073/pnas.1107807108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schultz J, Lorenz P, Gross G, Ibrahim S, Kunz M. MicroRNA let-7b targets important cell cycle molecules in malignant melanoma cells and interferes with anchorage-independent growth. Cell Res. 2008;18:549–557. doi: 10.1038/cr.2008.45. [DOI] [PubMed] [Google Scholar]
- 108.Segura MF, Hanniford D, Menendez S, Reavie L, Zou X, Alvarez-Diaz S, Zakrzewski J, Blochin E, Rose A, Bogunovic D, Polsky D, Wei J, Lee P, Belitskaya-Levy I, Bhardwaj N, Osman I, Hernando E. Aberrant miR-182 expression promotes melanoma metastasis by repressing FOXO3 and microphthalmia-associated transcription factor. Proc Natl Acad Sci U S A. 2009;106:1814–1819. doi: 10.1073/pnas.0808263106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sellheyer K. Basal cell carcinoma: cell of origin, cancer stem cell hypothesis and stem cell markers. Br J Dermatol. 2011;164:696–711. doi: 10.1111/j.1365-2133.2010.10158.x. [DOI] [PubMed] [Google Scholar]
- 110.Senoo M, Pinto F, Crum CP, McKeon F. p63 is essential for the proliferative potential of stem cells in stratified epithelia. Cell. 2007;129:523–536. doi: 10.1016/j.cell.2007.02.045. [DOI] [PubMed] [Google Scholar]
- 111.Singh A, Park H, Kangsamaksin T, Singh A, Readio N, Morris RJ. Keratinocyte stem cells and the targets for nonmelanoma skin cancer. Photochem Photobiol. 2012 doi: 10.1111/j.1751-1097.2012.01079.x. (Electronic publication ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Snippert HJ, Haegebarth A, Kasper M, Jaks V, van Es JH, Barker N, van de Wetering M, van den Born M, Begthel H, Vries RG, Stange DE, Toftgard R, Clevers H. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science. 2010;327:1385–1389. doi: 10.1126/science.1184733. [DOI] [PubMed] [Google Scholar]
- 113.Sonkoly E, Wei T, Pavez LE, Suzuki H, Kato M, Torma H, Stahle M, Pivarcsi A. Protein kinase C-dependent upregulation of miR-203 induces the differentiation of human keratinocytes. J Invest Dermatol. 2010;130:124–134. doi: 10.1038/jid.2009.294. [DOI] [PubMed] [Google Scholar]
- 114.St-Jacques B, Dassule HR, Karavanova I, Botchkarev VA, Li J, Danielian PS, McMahon JA, Lewis PM, Paus R, McMahon AP. Sonic hedgehog signaling is essential for hair development. Curr Biol. 1998;8:1058–1068. doi: 10.1016/s0960-9822(98)70443-9. [DOI] [PubMed] [Google Scholar]
- 115.Su X, Chakravarti D, Cho MS, Liu L, Gi YJ, Lin YL, Leung ML, El-Naggar A, Creighton CJ, Suraokar MB, Wistuba I, Flores ER. TAp63 suppresses metastasis through coordinate regulation of Dicer and miRNAs. Nature. 2010;467:986–990. doi: 10.1038/nature09459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tanese K, Fukuma M, Yamada T, Mori T, Yoshikawa T, Watanabe W, Ishiko A, Amagai M, Nishikawa T, Sakamoto M. G-protein-coupled receptor GPR49 is up-regulated in basal cell carcinoma and promotes cell proliferation and tumor formation. Am J Pathol. 2008;173:835–843. doi: 10.2353/ajpath.2008.071091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tani H, Morris RJ, Kaur P. Enrichment for murine keratinocyte stem cells based on cell surface phenotype. Proc Natl Acad Sci U S A. 2000;97:10960–10965. doi: 10.1073/pnas.97.20.10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Trempus CS, Dang H, Humble MM, Wei SJ, Gerdes MJ, Morris RJ, Bortner CD, Cotsarelis G, Tennant RW. Comprehensive microarray transcriptome profiling of CD34-enriched mouse keratinocyte stem cells. J Invest Dermatol. 2007;127:2904–2907. doi: 10.1038/sj.jid.5700917. [DOI] [PubMed] [Google Scholar]
- 119.Trempus CS, Morris RJ, Bortner CD, Cotsarelis G, Faircloth RS, Reece JM, Tennant RW. Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. J Invest Dermatol. 2003;120:501–511. doi: 10.1046/j.1523-1747.2003.12088.x. [DOI] [PubMed] [Google Scholar]
- 120.Tsuruta D, Hashimoto T, Hamill KJ, Jones JC. Hemidesmosomes and focal contact proteins: functions and cross-talk in keratinocytes, bullous diseases and wound healing. J Dermatol Sci. 2011;62:1–7. doi: 10.1016/j.jdermsci.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wan H, Stone MG, Simpson C, Reynolds LE, Marshall JF, Hart IR, Hodivala-Dilke KM, Eady RA. Desmosomal proteins, including desmoglein 3, serve as novel negative markers for epidermal stem cell-containing population of keratinocytes. J Cell Sci. 2003;116:4239–4248. doi: 10.1242/jcs.00701. [DOI] [PubMed] [Google Scholar]
- 122.Wan H, Yuan M, Simpson C, Allen K, Gavins FN, Ikram MS, Basu S, Baksh N, O’Toole EA, Hart IR. Stem/progenitor cell-like properties of desmoglein 3dim cells in primary and immortalized keratinocyte lines. Stem Cells. 2007;25:1286–1297. doi: 10.1634/stemcells.2006-0304. [DOI] [PubMed] [Google Scholar]
- 123.Wang GY, Wang J, Mancianti ML, Epstein EH., Jr Basal cell carcinomas arise from hair follicle stem cells in Ptch1(+/−) mice. Cancer Cell. 2011;19:114–124. doi: 10.1016/j.ccr.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang LC, Liu ZY, Gambardella L, Delacour A, Shapiro R, Yang J, Sizing I, Rayhorn P, Garber EA, Benjamin CD, Williams KP, Taylor FR, Barrandon Y, Ling L, Burkly LC. Regular articles: conditional disruption of hedgehog signaling pathway defines its critical role in hair development and regeneration. J Invest Dermatol. 2000;114:901–908. doi: 10.1046/j.1523-1747.2000.00951.x. [DOI] [PubMed] [Google Scholar]
- 125.Watt FM. The stem cell compartment in human interfollicular epidermis. J Dermatol Sci. 2002;28:173–180. doi: 10.1016/s0923-1811(02)00003-8. [DOI] [PubMed] [Google Scholar]
- 126.Watt FM, Estrach S, Ambler CA. Epidermal Notch signalling: differentiation, cancer and adhesion. Curr Opin Cell Biol. 2008;20:171–179. doi: 10.1016/j.ceb.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Watt FM, Jensen KB. Epidermal stem cell diversity and quiescence. EMBO Mol Med. 2009;1:260–267. doi: 10.1002/emmm.200900033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Webb A, Kaur P. Epidermal stem cells. Front Biosci. 2006;11:1031–1041. doi: 10.2741/1861. [DOI] [PubMed] [Google Scholar]
- 129.Webb A, Li A, Kaur P. Location and phenotype of human adult keratinocyte stem cells of the skin. Differentiation. 2004;72:387–395. doi: 10.1111/j.1432-0436.2004.07208005.x. [DOI] [PubMed] [Google Scholar]
- 130.Wei T, Orfanidis K, Xu N, Janson P, Stahle M, Pivarcsi A, Sonkoly E. The expression of microRNA-203 during human skin morphogenesis. Exp Dermatol. 2010;19:854–856. doi: 10.1111/j.1600-0625.2010.01118.x. [DOI] [PubMed] [Google Scholar]
- 131.White AC, Tran K, Khuu J, Dang C, Cui Y, Binder SW, Lowry WE. Defining the origins of Ras/p53-mediated squamous cell carcinoma. Proc Natl Acad Sci U S A. 2011;108:7425–7430. doi: 10.1073/pnas.1012670108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wienholds E, Kloosterman WP, Miska E, Alvarez-Saavedra E, Berezikov E, De Bruijn E, Horvitz HR, Kauppinen S, Plasterk RH. MicroRNA expression in zebrafish embryonic development. Science. 2005;309:310–311. doi: 10.1126/science.1114519. [DOI] [PubMed] [Google Scholar]
- 133.Woo WM, Oro AE. SnapShot: hair follicle stem cells. Cell. 2011;146:334. doi: 10.1016/j.cell.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648–2659. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 135.Wu Y, Zhao RC, Tredget EE. Concise review: bone marrow-derived stem/progenitor cells in cutaneous repair and regeneration. Stem Cells. 2010;28:905–915. doi: 10.1002/stem.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yi R, Fuchs E. MicroRNA-mediated control in the skin. Cell Death Differ. 2010;17:229–235. doi: 10.1038/cdd.2009.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yi R, Pasolli HA, Landthaler M, Hafner M, Ojo T, Sheridan R, Sander C, O’Carroll D, Stoffel M, Tuschl T, Fuchs E. DGCR8-dependent microRNA biogenesis is essential for skin development. Proc Natl Acad Sci U S A. 2009;106:498–502. doi: 10.1073/pnas.0810766105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yi R, Poy MN, Stoffel M, Fuchs E. A skin microRNA promotes differentiation by repressing ‘stemness’. Nature. 2008;452:225–229. doi: 10.1038/nature06642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Youssef KK, Van KA, Lapouge G, Beck B, Michaux C, Achouri Y, Sotiropoulou PA, Blanpain C. Identification of the cell lineage at the origin of basal cell carcinoma. Nat Cell Biol. 2010;12:299–305. doi: 10.1038/ncb2031. [DOI] [PubMed] [Google Scholar]
- 140.Yu H, Kumar SM, Kossenkov AV, Showe L, Xu X. Stem cells with neural crest characteristics derived from the bulge region of cultured human hair follicles. J Invest Dermatol. 2010;130:1227–1236. doi: 10.1038/jid.2009.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yu Y, Flint A, Dvorin EL, Bischoff J. AC133-2, a novel isoform of human AC133 stem cell antigen. J Biol Chem. 2002;277:20711–20716. doi: 10.1074/jbc.M202349200. [DOI] [PubMed] [Google Scholar]
- 142.Zhang L, Stokes N, Polak L, Fuchs E. Specific microRNAs are preferentially expressed by skin stem cells to balance self-renewal and early lineage commitment. Cell Stem Cell. 2011;8:294–308. doi: 10.1016/j.stem.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang W, Remenyik E, Zelterman D, Brash DE, Wikonkal NM. Escaping the stem cell compartment: sustained UVB exposure allows p53-mutant keratinocytes to colonize adjacent epidermal proliferating units without incurring additional mutations. Proc Natl Acad Sci U S A. 2001;98:13948–13953. doi: 10.1073/pnas.241353198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhou P, Byrne C, Jacobs J, Fuchs E. Lymphoid enhancer factor 1 directs hair follicle patterning and epithelial cell fate. Genes Dev. 1995;9:700–713. doi: 10.1101/gad.9.6.700. [DOI] [PubMed] [Google Scholar]