Abstract
Background
The role of maternal avoidance diets in the prevention of food allergies is currently under debate. Little is known regarding the effects of such diets on human milk (HM) composition or induction of infant humoral responses.
Objective
To assess the association of maternal cow’s milk (CM) avoidance during breastfeeding with specific IgA levels in HM and development of cow’s milk allergy (CMA) in infants.
Methods
We utilized HM and infant serum samples from a prospective birth cohort of 145 dyads. Maternal serum and HM samples were assessed for casein and beta-lactoglobulin (BLG)-specific IgA and IgG by ELISA; 21 mothers prophylactically initiated a strict maternal CM avoidance diet due to a sibling’s history of food allergy and 16 due to atopic eczema or regurgitation/vomiting seen in their infants within the first 3 months of life. Infants’ sera were assessed for casein and BLG-specific IgG, IgA and IgE; CMA was confirmed by an oral food challenge. The impact of HM on BLG uptake was assessed in transcytosis assays utilizing Caco-2 intestinal epithelial cell line.
Results
Mothers avoiding CM had lower casein- and BLG-specific IgA in HM than mothers with no CM restriction (p=0.019 and p=0.047). Their infants had lower serum casein- and BLG-specific IgG1 (p=0.025 and p<0.001) and BLG-specific IgG4 levels (p=0.037) and their casein- and BLG-specific IgA levels were less often detectable than those with no CM elimination diet (p=0.003 and p=0.007). Lower CM-specific IgG4 and IgA levels in turn were associated with infant CMA. Transcytosis of BLG was impaired by HM with high, but not low levels of specific IgA.
Conclusions
Maternal CM avoidance was associated with lower levels of mucosal specific IgA levels and development of CMA in infants.
Clinical relevance
HM IgA may play a role in preventing excessive, uncontrolled food antigen uptake in the gut lumen and thereby in the prevention of CMA.
Keywords: Breast feeding, breast milk, human milk, cow’s milk, avoidance, restriction diet, infants, cow’s milk allergy, IgA, secretory IgA, epithelium
INTRODUCTION
Cow’s milk allergy (CMA) is typically the first phenomenon of atopic symptomatology and the “allergic march” because cow’s milk proteins are typically the first foreign proteins consumed in large quantities by an infant. CMA results from a defect in the development or breakdown of oral tolerance i.e. immunological hyporesponsiveness to ingested innocuous antigen. Mucosal tissue homeostasis is the result of the perinatal establishment of mucosally induced immune tolerance,[1] and immunomodulatory factors in human milk are thought to influence the development and maturation of the mucosal immune system of the infants.[2] By reinforcing the epithelial barrier, secretory IgA (SIgA) inhibits inappropriate immune activation by microorganisms and antigens in the lumen of the intestinal and respiratory tracts. Although B cells are present in gut tissue during early development, plasma cells producing dimeric IgA are only generated after birth to provide SIgA to the lumen. Maternal SIgA is provided by breast milk during the early postnatal period.[1]
Breast milk is a rich source of SIgA with lesser amounts of IgG and IgM.[3] IgA in human milk is synthesized by resident B-cells in the mammary gland that have migrated from the mother’s intestine (“enteromammary link”) [4] and thereby the antibody specificity of breast milk reflects the antigenic stimulation encountered by the maternal gut.[5,6] Although studies have reported no consistent association between total and food-specific IgA levels in breast milk and development of allergic disease in older children,[7–9] we and others have shown that lower levels of total and CM-specific IgA are present in colostrum and breast milk of mothers with offspring developing CMA.[10,11] The etiology of low breast milk IgA is unknown but unrelated to maternal atopy.[7,10]
In the present study, we sought to investigate whether regulation of breast milk specific IgA could be related to maternal elimination of CM. This was done by utilizing human milk samples from a birth cohort of infants and their mothers on CM elimination diets. Furthermore, we assessed the effect of maternal CM avoidance during lactation on offspring’s risk of development of CM-specific IgG, IgA and IgE antibodies and clinical food allergy by utilizing paired infant serum samples and clinical data from the same human birth cohort. Lastly, we investigated the role of breast milk antibodies in food antigen uptake utilizing a human intestinal epithelial cell line.
MATERIAL AND METHODS
Subjects
We utilized stored human milk and paired maternal and infant serum samples from a prospective birth cohort, designed to assess the association between immunologic factors in human milk and development of food allergies in breastfed infants. The results for total and CM-specific IgA in HM on a subpopulation of this cohort have been previously published.[10] In brief, mothers who volunteered for the study were recruited at birth, as described before.[10] Mothers and infants were followed prospectively at 0–2 weeks, 1, 3, 6, 12 and 18 months to assess for any signs or symptoms suggestive of food allergies. Infants from two groups of differing risks for atopy were recruited: those with an increased risk of food allergy defined either as presence of an older sibling with food allergy and those with low risk as defined by having only non-atopic first degree relatives. All infants were born full-term and had no other chronic diseases. They had diets appropriate for their age. A total of 145 mother-infant pairs were included in the analyses. Among them, we utilized breast milk and/or serum samples from a total of 286 visits with an average of 2 (range, 1 to 4) visits per each mother-infant pair.
Maternal and infant diets were assessed during the visits by the investigating physician and the dietician, as well as by dietary record. A total of 37 mothers started a strict maternal CM avoidance diet within the first 3 months postpartum: 16 of them prophylactically at birth and another 5 within the first month due to sibling’s history of food allergy; and another 16 mothers restricted milk due to mild atopic eczema or gastrointestinal symptoms such as diarrhea or recurrent vomiting/regurgitation seen in their infant between 1 and 2.5 months of lactation. Another 49 mothers started a CM elimination diet between 3 and 7 months of lactation due to the above-mentioned symptoms seen in their infants. All together, breast milk samples were available for 23 such mothers who started a CM elimination diet and 56 mothers who continued CM in their diet without restrictions.
All together 75 infants developed challenge-proven CMA. Their oral food challenges (OFC) were performed at median 5.8 months of age (IQR, 3.9–7.3 months). Of this group, 43 infants had an immediate-type CMA evidenced by hives (n=26), immediate maculopapular rash (n=11) or multi-system involvement (n=6), and 32 had delayed-type CMA, presenting with exacerbation of eczema (n=9), delayed GI symptoms such as diarrhea and regurgitation (n=9), or a combination of eczema and GI symptoms (n=13). Cow’s milk-specific IgE (Phadia/Thermofisher, Uppsala, Sweden) was detected in 13 infants with a median level of 5.8 kUA/L (range 0.1 to >100 kUA/L), skin prick test to milk [10] were positive in 24. Median age of symptom onset was 2 months (IQR, 0.75–3 months). Of the 37 mothers who started milk elimination within the first 3 months of lactation, 5 infants had no CMA, 16 had immediate-type CMA and 16 had delayed-type CMA. Of those 49 mothers who started a milk elimination diet beyond 3 months of age, 7 had no CMA, 27 had immediate-type CMA and 15 had delayed-type CMA. 59 mothers never went on a CM-restricted diet and none of them had infants with CMA. Clinical characteristics of the study infants are presented in Table 1. Atopic diseases were more common in infants whose mothers started milk elimination diet during the first 3 months, and food allergy was more common in their siblings. Accordingly, use of hypoallergenic infant formula was more common and the onset of CM elimination diet was earlier in the infants with maternal elimination diets during first three months of lactation. Gastrointestinal symptoms, asthma, and diagnosis of CMA were frequent in both groups although somewhat more common in those with maternal elimination diet. Otherwise, groups were comparable in terms of length of breastfeeding, use of cow’s milk-based formula in the nursery, and the age of CM-based formula introduction. (Table 1)
Table 1.
Clinical characteristics of study infants whose mothers were on cow’s milk (CM) elimination diets and those whose mothers were on unrestricted diets during the first 3 months of lactation.
| Maternal CM elimination N=37 |
No maternal CM elimination N= 108 |
p-value | |
|---|---|---|---|
| Maternal atopy | 30 (83%) | 52 (49%) | <0.0001 |
| Asthma | 7 (19%) | 6 (6%) | 0.04 |
| Allergic rhinitis | 24 (65%) | 41 (38%) | 0.007 |
| Atopic dermatitis | 12 (32%) | 11 (10%) | 0.003 |
| Food allergy | 8 (22%) | 8 (7%) | 0.03 |
| Paternal atopy | 19 (73%) | 39 (41%) | 0.007 |
| Asthma | 2 (5%) | 5 (5%) | 1.0 |
| Allergic rhinitis | 15 (41%) | 25 (23%) | 0.05 |
| Atopic dermatitis | 5 (14%) | 7 (7%) | 0.18 |
| Food allergy | 5 (14%) | 6 (6%) | 0.15 |
| Sibling’s atopy | 23 (74%) | 40 (42%) | 0.003 |
| Asthma | 7 (19%) | 5 (5%) | 0.127 |
| Allergic rhinitis | 5 (14%) | 9 (8%) | 0.35 |
| Atopic dermatitis | 1 (3%) | 3 (3%) | 1.0 |
| Food allergy | 22 (59%) | 31 (29%) | 0.001 |
| Exclusive breastfeeding (mo) | 2.5 (0.75-4) | 3 (1.5-4) | 0.26 |
| Partial breastfeeding (mo) | 7 (4.9-10) | 7 (4.5-8) | 0.11 |
| CM formula in nursery | 4 (11%) | 9 (8%) | 0.74 |
| Use of hypoallergenic formula | 18 (49%) | 20 (19%) | 0.002 |
| Age CM formula introduction (mo) | 3.25 (1.6-7) | 4 (1.5-6) | 0.81 |
| Age begin maternal CM elimination (mo) | 1 (0-2) | 4 (3.125-5) | <0.0001 |
| Symptom age (mo) | 1 (0.5-2) | 3 (1-4) | 0.06 |
| Symptoms | |||
| Atopic dermatitis | 24 (65%) | 57 (53%) | 0.20 |
| Gastrointestinal* | 23 (62%) | 44 (41%) | 0.024 |
| Asthma | 7 (19%) | 13 (12%) | 0.023 |
| Diagnosis of delayed CMA | 16 (43%) | 15 (14%) | 0.0002 |
| Diagnosis of immediate CMA | 16 (43%) | 27 (25%) | 0.04 |
| IgE-mediated CMA | 8 (22%) | 21 (19%) | 0.77 |
| Multiple food allergies | 15 (41%) | 27 (25%) | 0.09 |
CM, cow’s milk; CMA, cow’s milk allergy
regurgitation, vomiting, diarrhea, abdominal pain/colic
The initial cohort study was approved by the ethics committees of the Skin and Allergy Hospital of the Helsinki University Central Hospital and the City of Helsinki. Written informed consent was obtained from the mothers. Internal Review Boards of the Albany Medical College, Albany, NY (# 3139) and the Icahn School of Medicine at Mount Sinai, New York, NY (HS# 11–01838) approved the use of clinical data and stored and frozen historical samples for the additional antibody assays.
Oral food challenge
The diagnosis of CMA was based on typical symptoms, their amelioration on a CM elimination diet and re-reappearance in an open food challenge performed either as an in-patient or out-patient facility as described previously.[10] Symptoms considered as positive were anaphylaxis, urticaria, eczema, vomiting and diarrhea. Immediate-type CMA was defined as symptoms developing within 2 hours from the last dose and those that occurred thereafter were regarded as delayed-type CMA.
Samples
Human milk and serum samples were collected on each clinical follow-up visit if possible. Milk samples were collected in the morning and processed immediately. Samples were centrifuged (400 g, 15 min), fat was removed, and supernatant collected, frozen and stored at −80°C. The samples from mothers who had mastitis during the preceding 4 weeks were excluded. The serum samples were collected by venipuncture, and frozen, stored at −80°C.
Serum and breast milk CM-specific antibodies
Mothers’ milk samples were assessed for ß-lactoglobulin (BLG)- and casein (Cs)-specific IgA and IgG by ELISA. Mothers’ serum samples were assessed for BLG- and Cs-specific IgA and IgG. Infants’ serum samples were assessed for BLG- and Cs-specific IgA, IgG1, and IgG4 by ELISA. Microtiter plates (Nunc MaxiSorb, ThermoFisher Scientific) were coated either with bovine BLG or casein (Sigma-Aldrich, St Louis, MO, USA) at a concentration of 20 ug/ml in phosphate-buffered saline (PBS) and incubated at 4°C overnight. After washing with PBS-0.05% Tween (PBS-T), plates were blocked with 2% normal sheep serum in PBS-T for 1 hour at room temperature followed by washing with PBS-T. Sera were diluted at 1:10 in 1% sheep serum in PBS-T for adult serum specific IgA, 1:100 for adult serum specific IgG, 1:50 for infant serum specific IgG1 and IgG4 and incubated at 4°C overnight. Human milk was diluted 1:100 for total IgA, 1:4 for specific IgA and 1:2 for specific IgG. Purified human IgA and IgG (Bethyl Laboratories, Montgomery, TX) were used as a standard curve for IgA and IgG. For IgG1 and IgG4, the reference serum comprised a pool of 10 adult sera with high antibody levels, which concentrations was assigned to 1000 AU. After washing, biotinylated mouse anti-human IgA1/IgA2 (BD Pharmingen) at 1:1000, IgG 1:20000, IgG1 1:500, or IgG4 1:10000 was applied to the wells followed by incubation of 1 h at room temperature. After washing, avidin-peroxidase was applied to the wells for 30 min followed by washing. Finally, 100 uL of tetramethyl-benzidine (TMB) liquid substrate (Sigma Aldrich) was applied to the plates, and the reaction was stopped and the optical density was measured at 450 nm.
FITC-labeling of BLG
FITC was added under constant stirring to BLG according to the manufacturer’s instruction (Sigma, St. Louis, MO, USA). Labeled proteins were purified from unconjugated FITC by PD-10 Desalting Columns (GE Healthcare, Little Chalfont, UK) and subsequent overnight dialysis. Aliquots of FITC-labeled BLG were frozen and stored at −80°C.
Transcytosis studies
For transcytosis studies, Caco-2 cells, a human intestinal cell line (American Type Culture Collection, Rockville, MD, USA) that forms a polarized monolayer with tight junctions were grown in Dulbecco’s modified Eagle media (Corning, Corning, NY, U.S.A.) supplemented with 10% (v/v) fetal calf serum, penicillin G and streptomycin at 37 °C, 5% CO2. Cells were fed every 3 or 4 days and subcultured every 7 days using 0.25% trypsin/0.2% EDTA in PBS. For experimentation, cells were seeded at a density of 3×105 cells/cm2 on 12-mm Transwell (Corning, Corning, NY, U.S.A.) permeable polyester filters (0.4 μm pore size). The filters were fed every 2 or 3 days. Transepithelial resistance of the Caco-2 monolayers was measured using an EVOM Epithelial Voltohmmeter (World Precision Instruments, Hamden, CT, U.S.A.) with a pair of chopstick electrodes. Only transwells with a steady transepithelial resistance, minimum 300 ohms, which was typically attained in 10–14 days, were used for further studies. After washing with PBS, 500 ug FITC-labeled BLG was added on the apical side and incubated for 24 hr at 37°C/5% CO2 in the absence or presence of increasing concentrations of pooled breast milk (3, 10 or 30% of total volume applied apically). Pooled breast milk from mothers with high (n=15, mean 259, range 94 to 659 ng/ml) or low BLG-specific IgA levels (n=15, undetectable, <45 ng/ml) was used. Assays were performed in triplicates. Subsequently, aliquots of basal side of filters were collected and measured by anti-FITC ELISA. The paracellular permeability of FITC-conjugated dextran (MW = 3 kDa, Invitrogen, Eugene, OR) in the absence or presence of pooled breast milk was used as a control and measured fluorometrically.
Anti-FITC ELISA
Microtiter plates were coated with serial dilutions of samples and known concentrations of FITC-labeled BLG as a standard curve and incubated at 4°C overnight. After washing with PBS-0.05% Tween (PBS-T), plates were blocked with 10% fetal calf serum in PBS-T for 2 hours at room temperature. After washing, biotinylated anti-FITC rabbit IgG (Invitrogen) at 1:5000 was applied to the wells followed by incubation of 2 h at room temperature. After washing, avidin-peroxidase was applied to the wells for 30 min followed by washing. Finally, 100 uL of tetramethyl-benzidine (TMB) liquid substrate (Sigma Aldrich) was applied to the plates, and the reaction was stopped and the optical density was measured at 450 nm.
Statistical analysis
Statistical comparison of breast milk and infant serum immunoglobulin levels was by a Mann-Whitney nonparametric test for two groups or by a Kruskal-Wallis test followed by Dunn’s method for multiple comparisons for three groups. Nonparametric methods were used because of non-normality with floor and ceiling effects for most of the measurements. Statistical significance for contingency tables was assessed using chi-square tests (or Fisher’s exact test if any cell has less than five counts). Statistica (v 6; StatSoft Inc) and MiniTab (v 14; MiniTab Statistical Software) statistical software was used. Individual data points are plotted along with using box plots on a log scale. On the box plots the median is represented by a horizontal line within the box representing the 25th to 75th percentile, whiskers show the 5th to 95th percentile and the square symbol is the geometric mean.
Transcytosis assays have been analyzed using ANOVA followed by Newman-Keuls method for multiple comparisons by GraphPad Prism 4 software (GraphPad Software, Inc.) Results are shown as mean (SEM).
RESULTS
Specific IgA levels in breast milk are suppressed in mothers avoiding CM
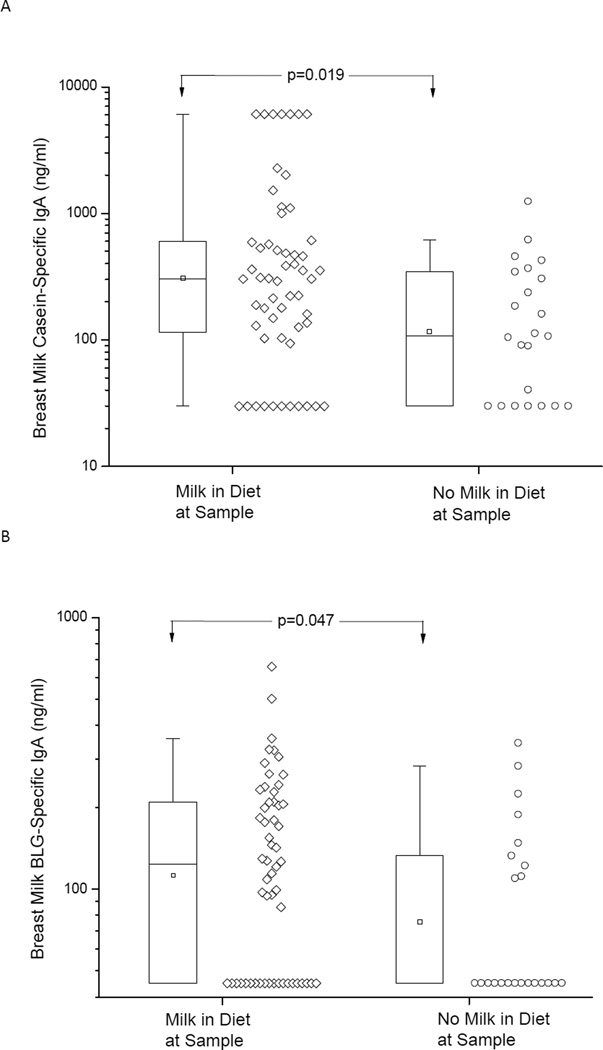
The effect of maternal CM avoidance diet on breast milk specific antibody levels was assessed by utilizing 23 samples from mothers who avoided cow’s milk and 56 samples from mothers who had no dietary restrictions. For this analysis we utilized the earliest milk sample available for each mother, and mothers had eliminated cow’s milk from their diets for a mean of 2.2 (SD= 2.7) months and the duration of breastfeeding at that time was a mean 3.7 (SD 3.4) months as compared to 2.3 (SD 2.4) months in those without elimination (p=0.0459). The levels of casein- and BLG-specific IgA in human milk were lower in those mothers avoiding CM when compared to those with no CM restriction, p=0.019 and 0.047, respectively. (Fig. 1) As a control, total IgA or rice-specific IgA levels in breast milk were comparable between mothers who avoided CM and those who did not, as rice was not restricted in the diet by any of the mothers (data not shown). Maternal atopy was not associated with breast milk specific IgA levels (p=0.78 and 0.9 for casein and BLG, respectively), neither was the duration of lactation. Breast milk specific IgG levels were generally low and comparable between the two groups of mothers (data not shown). Mothers’ serum specific IgA or IgG levels were not affected by the mothers’ diet (data not shown).
Figure 1.
Breast milk IgA levels specific to casein (A) and beta-lactoglobulin (BLG) (B) in mothers who were not eliminating cow’s milk (Milk) in their diet and in those with strict milk elimination diet. On the box plots the median is represented by a horizontal line within the box representing the 25th to 75th percentile, whiskers show the 5th to 95th percentile and the square symbol is the geometric mean.
Maternal CM avoidance was associated with suppressed infant serum specific IgG and IgA levels
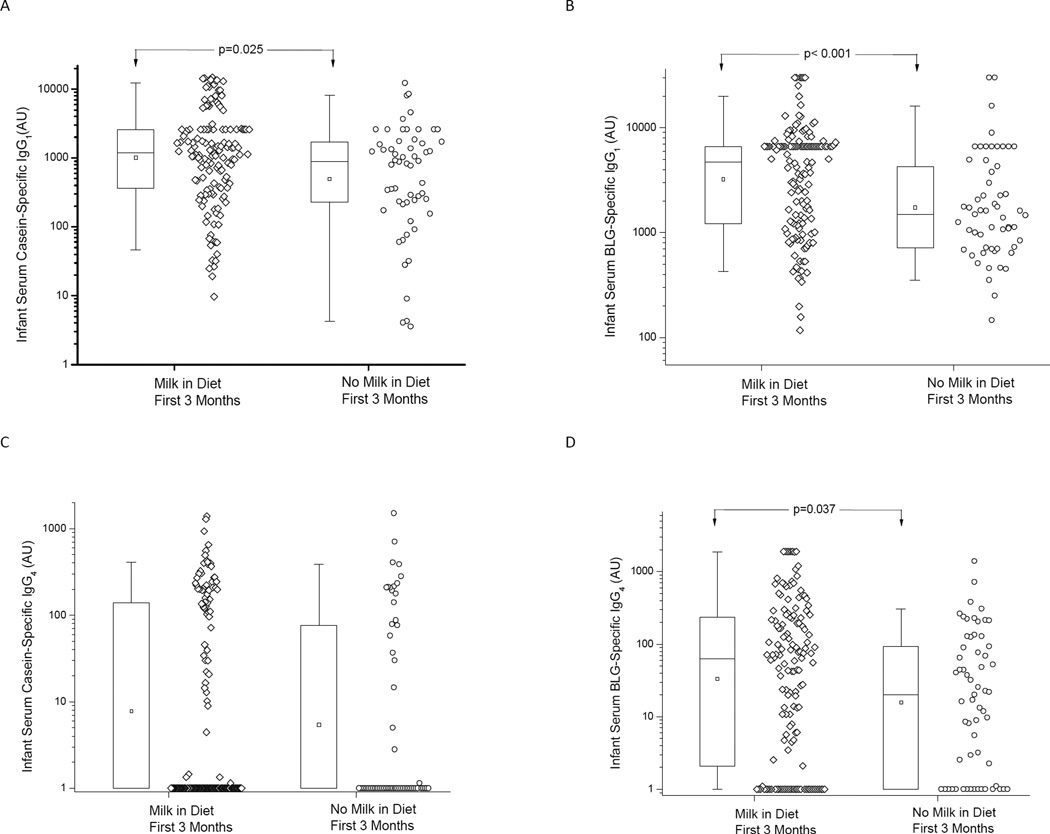
Next we assessed the association of maternal CM avoidance with infants’ specific antibody levels. The infants from those mothers who started milk elimination within the first 3 months of lactation were compared against those who started milk avoidance beyond 3 months of life or did not restrict milk intake in their diet at any time point. The rationale for this dichotomization was to assess the impact of early and in many cases prophylactic maternal CM avoidance during the time period of exclusive breastfeeding in most infants on the development of infant humoral responses. Data was first analyzed using all serum samples available including 0–6 months, which also contain transplacentally acquired IgG from the mother, and then by using only serum samples taken between 6 and 18 months of age to assess for infants’ own antibody production.
When all the infant samples available were used, Cs- and BLG-specific IgG1 levels were higher in those infants who were breastfed by mothers with no CM elimination diet (p=0.025 and p<0.001, respectively), as were the BLG-specific IgG4 levels (p=0.037) when compared to those infants breastfed by mothers on CM elimination diet (Fig. 2). Samples were taken at a mean of 6.2 (SD 5.1) months in those with maternal elimination and a mean of 7.5 (SD 5.3) months with no elimination (p=0.1). Cs-specific IgA levels were detectable (> 50 AU) in 22 of 145 (15%) infants of mothers with no CM avoidance compared to only 1 out of 60 (1.7%) infants of mothers with CM elimination diet (p=0.003). Similarly, BLG-specific IgA levels were detectable (>50 AU) in 45 of 140 (32%) infants of mothers with no CM elimination as compared to 4 out of 38 (11%) infants of mothers with CM elimination diet (p=0.007). When only 1 serum sample per patient taken between 6 and 18 months of age was analyzed, the results held for Cs-specific IgG1 and IgG4 levels (data not shown).
Figure 2.
Serum IgG1 (A, B) and IgG4 (C, D) levels specific to casein and BLG in infants of mothers who were not avoiding cow’s milk (Milk) and in those with cow’s milk avoidance diets. On the box plots the median is represented by a horizontal line within the box representing the 25th to 75th percentile, whiskers show the 5th to 95th percentile and the square symbol is the geometric mean.
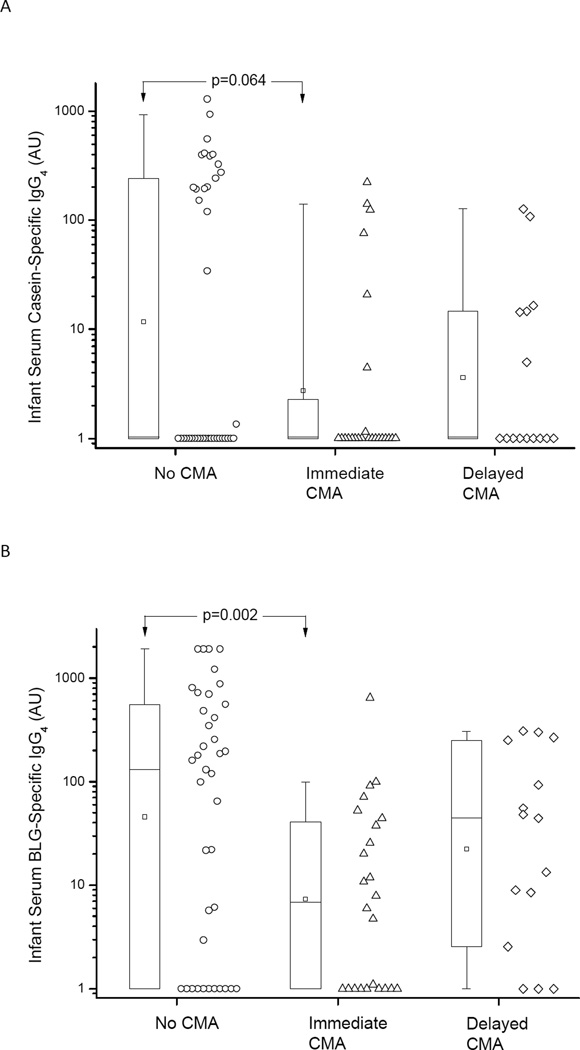
Suppressed serum specific IgG4 and IgA levels were associated with CMA
In the next step, we sought to assess the impact of infant specific IgG and IgA levels on development of clinical CMA. Only one sample per patient taken between 6 and 18 months of age was analyzed, taken at a mean of 9.7 (SD 3.4) months in immediate CMA, a mean of 10.5 (SD 3.3) months in delayed CMA and a mean of 10.5 (SD 4.0) months in healthy infants (p=0.48). Infants with immediate-type CMA had significantly lower BLG-specific IgG4 levels than the healthy infants (p=0.002) (Fig. 3). There was a similar trend for lower Cs-specific IgG4 (P=0.064) when compared to healthy infants (Fig. 3). CM-specific IgG1 levels were comparable between the three groups (data not shown). Detectable levels of infant serum Cs-specific IgA (>50 AU) were present in 18% (7/39) of infants without CMA compared to 4% (1/25) and 0% (0/12) of those with immediate and delayed CMA, respectively (chi-square test; p = 0.049). For infant serum detectable BLG-specific IgA levels were present in 47% (15/32), 17% (3/18) and 10% (1/10) of those without, with immediate and with delayed CMA, respectively (p=0.019).
Figure 3.
Serum IgG4 levels specific to casein (A) and BLG (B) in infants with no cow’s milk allergy (CMA) and in those with either immediate-type or delayed-type CMA. On the box plots the median is represented by a horizontal line within the box representing the 25th to 75th percentile, whiskers show the 5th to 95th percentile and the square symbol is the geometric mean.
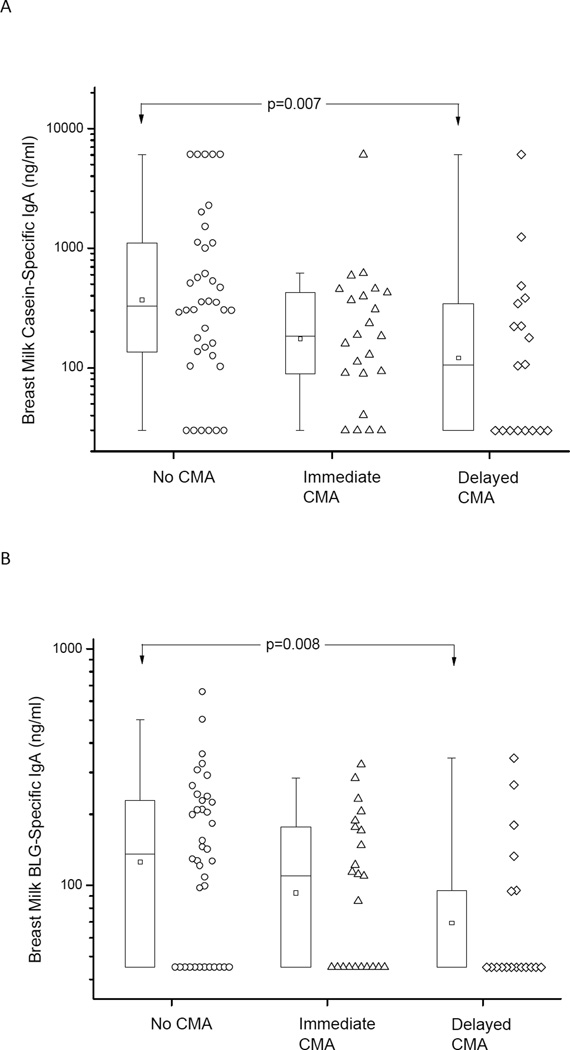
Low breast milk CM-specific IgA levels are associated with development of CMA in the offspring
Lastly, we were interested in investigating the possible direct correlation of CM-specific IgA levels in breast milk with clinical CMA development in infants. Utilizing one sample per mother, breast milk IgA specific to Cs and BLG was lower in those mothers with an infant with delayed-type CMA than in those with healthy infants, p=0.007 and 0.008, respectively.(Fig 4) Multiple logistic regression analysis comparing the effect of diet and that of breast milk CM-specific IgA on development of CMA revealed that these two variables were very closely associated with each other and the effect of presence or absence of CM in maternal diet therefore overwhelmed the effects of CM-specific IgA levels in breast milk on development of CMA (OR 0.03, 95% CI 0.0- 0.23 for milk in diet; OR 0.78, 95% 0.54–1.11 for human milk specific IgA).
Figure 4.
Breast milk IgA levels specific to casein (A) and beta-lactoglobulin (BLG) (B) in infants with no cow’s milk allergy (CMA) and in those with either immediate-type or delayed-type CMA. On the box plots the median is represented by a horizontal line within the box representing the 25th to 75th percentile, whiskers show the 5th to 95th percentile and the square symbol is the geometric mean.
Transcytosis of BLG through enterocytes is impaired by presence of breast milk with high levels of specific IgA
Given the impact of specific IgA in breast milk on development of infant’s humoral immune responses and development of CMA, we assessed the impact of breast milk antibodies on antigen trafficking in vitro. Presence of high levels of BLG-specific IgA in breast milk significantly reduced transcytosis of BLG across Caco-2 monolayers when compared to transcytosis in the absence of breast milk (Fig. 5). Pooled breast milk from mothers with undetectable levels of specific IgA did not have such a blocking effect and with the lowest concentration in fact enhanced antigen uptake. The blocking effect of breast milk was likely due to the presence of specific antibodies as transcytosis of dextran was not affected by the presence of breast milk (Fig. 5).
Figure 5.
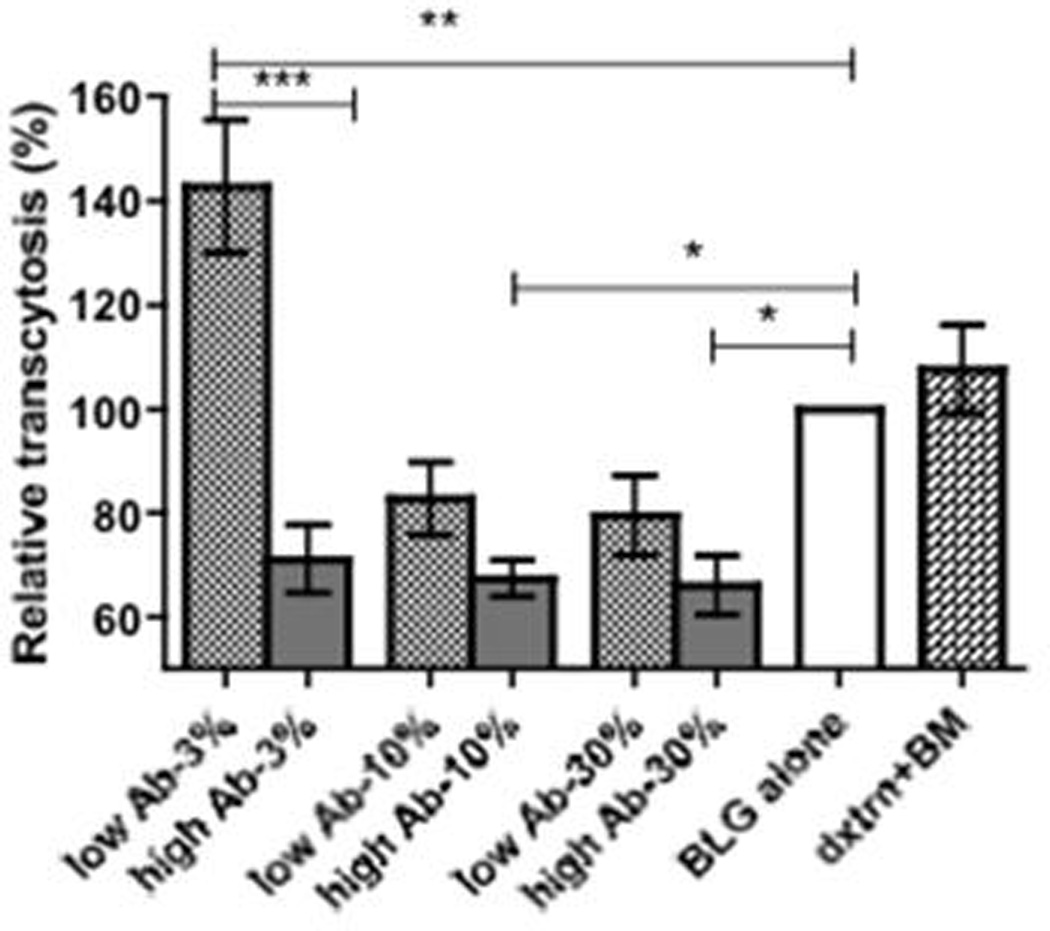
Transcytosis of BLG in vitro. Transport of FITC-labeled BLG across polarized Caco-2 monolayers in the presence or absence of increasing concentrations of pooled breast milk (BM) from mothers with either high or low antibody titers to BLG. Dextran transcytosis in the presence of 30% breast milk is shown as a control. Results are shown as relative transcytosis in the presence of BM; median uptake with BLG (or dextran) alone equals 100%. Results are representative of three independent experiments each performed in triplicates and shown as mean (SEM). * p<0.05, ** p<0.001
DISCUSSION
In the present study we have shown that maternal CM avoidance is associated with lower levels of mucosal CM-specific IgA levels, as measured in mother’s milk, as well as down-regulation of serum specific IgG1, IgG4 and IgA levels in their infants. Low CM-specific IgG4, in turn, was associated with CMA. Low breast milk CM-specific IgA levels were associated with increased risk for development of CMA, and mechanistically were shown to be unable to prevent uptake of cow’s milk antigen as effectively as breast milk with high levels of specific IgA.
In humans, we and others have shown that lower levels of total and milk-specific IgA are present in colostrum and milk of mothers with offspring developing cow’s milk allergy.[10,11] The etiology of low breast milk IgA is unknown but unrelated to maternal atopy.[10] In the present study, we hypothesized that low breast milk specific IgA levels could be secondary to lack of antigenic stimulation during the breastfeeding period, which commonly occurs when foods of high allergenic potential are eliminated in the maternal diet due to suspicion of food allergies in the infant. This is supported by the finding that IgA responses to microbes are constantly modified to reflect the microbiota present in the gut lumen suggesting that continued oral exposure to a particular Ag is required to maintain IgA responses.[12] Our results support also those found in poor Guatemalan mothers with no access to milk when compared to urban privileged mothers with access to milk.[15] Contrary findings were reported in Swedish and African mothers on implemented milk-avoidance diets, with concerns about their compliance to the diet [13,14]. Knowledge on how IgA levels in mother’s milk are regulated is critically important to developing a better understanding of the effect of maternal elimination diets.
Maternal avoidance diets were also associated with lower infant serum cow’s milk-specific IgG levels. Low IgG4 in turn was associated with the development of CMA. Lower specific IgG levels may be due to reduced exposure by the infant’s immune system to cow’s milk protein during the first few months of life, which may represent a window of opportunity for neonatal oral tolerance induction. During this critical time period, introduction of a foreign food protein in the context of immunoregulatory factors in breast milk may have a tolerizing effect. This was shown by Strobel,[16] who analyzed the effects of antigen dose during different time points on induction of neonatal tolerance in mice by maternal antigen transfer from breast milk. Feeding mothers beyond the first 3 days of life demonstrated tolerance induction in their pups, although suckling animals received a small dose of antigen typically associated with sensitization/priming.[17]
The function of breast milk IgA antibodies is not completely understood, although it has been suggested that that they play a role in protection against food allergy.[18,19] By reinforcing the epithelial barrier, SIgA inhibits inappropriate immune activation by microorganisms and antigens in the lumen of the intestinal and respiratory tracts.[1] This immune exclusion could mediate tolerance by providing protection against excessive and uncontrolled antigen influx similar to that seen with microorganisms, [20,21] supported by our transcytosis assays. The data furthermore suggests that antibodies were likely responsible for this effect as a there was a dose response with increasing antibody titers and transcytosis of dextran was not affected by human milk. However, if immune exclusion was the only mechanism providing protection, then complete antigen avoidance, although difficult, should provide a similar effect. Alternatively, breast milk antibodies could favor focused antigen uptake, e.g. via M cells as immune complexes which could target the antigen for presentation favoring tolerance development, suggested by reports showing SIgA targeted to Peyer’s patch dendritic and T cells after transport by intestinal M cells.[22, 23] This possibility is supported by our findings of facilitated peanut uptake to Peyer’s patches in the presence of peanut-specific antibodies in mice (Järvinen et al., unpublished data). Lastly, it is possible that changes in maternal diet and milk IgA are related to her gut microbiota.
The limitations of the study include the fact that a large proportion of mothers who avoided milk early on or even prophylactically were atopic and turned out to have infants with CMA, and therefore it is impossible to dissect out the chicken or egg paradigm. It is possible that the disease process and not maternal diet was driving down-regulation of breast milk IgA and the possibility of reverse causation exists. Therefore randomized controlled clinical trials are needed to address the impact of maternal elimination diets on food allergy risk in offspring. The study cohort was heavily populated with families with an older sibling with food allergies. This limits the applicability of the findings to the general population. We also do not know whether findings can be generalized to other food antigens such as peanut as peanut allergy was uncommon in this cohort. As cow’s milk in the diet is the major stimulant for the development of IgG antibodies, it would have been valuable to compare IgG levels in infants prior to initiation of CM formula or prior to the diagnosis of CMA (accompanied by initiation of milk avoidance); however samples were too few to allow enough power for such comparisons. Furthermore, at such an early age, infant samples would also have contained maternally acquired IgG. The strengths of this study include the prospective setup and availability of paired breast milk, and serum samples from mothers and infants.
At the moment clear dietary recommendations for pregnant and breastfeeding mothers are lacking regarding allergy prevention in allergic and non-allergic families, largely due to the controversy in the literature.[24] We found that maternal elimination diets resulted in lower levels of breast milk specific IgA, which are associated with the development of CMA in infants suggesting that high specific IgA levels in breast milk have a protective effect against food allergy. The protective mechanism may involve immune exclusion although focused antigen uptake to Peyer’s patches could also favor tolerance development. Therefore early initiated and especially prophylactic maternal dietary restrictions may in fact be detrimental in the development of neonatal oral tolerance.
ACKNOWLEDGEMENTS
Funding: The project described was supported by Grant Number K08 AI091655 (K.M. Järvinen) from the National Institute of Allergy and Infectious Diseases. H.A. Sampson is supported in part by grants from the NIH, RR026134, AI44236 and AI066738. Cecilia Berin is supported in part by NIH grant AI093577. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health. ImmunoCAPs were provided by Phadia/ThermoFischer, Uppsala, Sweden.
The authors thank Sarita Wagh, M.Sc. for her technical assistance and Nick Mantis, Ph.D. for his help with the Caco-2 epithelial cell line.
ABBREVIATIONS
- BLG
beta-lactoglobulin
- CM
cow’s milk
- CMA
cow’s milk allergy
- OFC
oral food challenge
Footnotes
The authors have no conflict of interest.
REFERENCES
- 1.Renz H, Brandtzaeg P, Hornef M. The impact of perinatal immune development on mucosal homeostasis and chronic inflammation. Nat Rev Immunol. 2011;12:9–23. doi: 10.1038/nri3112. [DOI] [PubMed] [Google Scholar]
- 2.Verhasselt V, Milcent V, Cazareth J, Kanda A, Fleury S, Dombrowicz D, Glaichenhaus N, Julia V. Breast milk-mediated transfer of an antigen induces tolerance and protection from allergic asthma. Nat Med. 2008;14:170–175. doi: 10.1038/nm1718. [DOI] [PubMed] [Google Scholar]
- 3.Koenig A, de Albuquerque Diniz EM, Barbosa SF, Vaz FA. Immunologic factors in human milk: the effects of gestational age and pasteurization. J Hum Lact. 2005;21:439–443. doi: 10.1177/0890334405280652. [DOI] [PubMed] [Google Scholar]
- 4.Hanson LA, Korotkova M. The role of breastfeeding in prevention of neonatal infection. Semin Neonatol. 2002;7:275–281. doi: 10.1016/s1084-2756(02)90124-7. [DOI] [PubMed] [Google Scholar]
- 5.Lonnerdal B. Nutritional and physiologic significance of human milk proteins. Am J Clin Nutr. 2003;77:1537S–1543S. doi: 10.1093/ajcn/77.6.1537S. [DOI] [PubMed] [Google Scholar]
- 6.Morrow AL, Rangel JM. Human milk protection against infectious diarrhea: implications for prevention and clinical care. Semin Pediatr Infect Dis. 2004;15:221–228. doi: 10.1053/j.spid.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Savilahti E, Siltanen M, Kajosaari M, Vaarala O, Saarinen KM. IgA antibodies, TGF-beta1 and -beta2, and soluble CD14 in the colostrum and development of atopy by age 4. Pediatr Res. 2005;58:1300–1305. doi: 10.1203/01.pdr.0000183784.87452.c6. [DOI] [PubMed] [Google Scholar]
- 8.Pesonen M, Kallio MJ, Siimes MA, Savilahti E, Ranki A. Serum immunoglobulin A concentration in infancy, but not human milk immunoglobulin A, is associated with subsequent atopic manifestations in children and adolescents: a 20-year prospective follow-up study. Clin Exp Allergy. 2011;41:688–696. doi: 10.1111/j.1365-2222.2011.03707.x. [DOI] [PubMed] [Google Scholar]
- 9.Kuitunen M, Kukkonen AK, Savilahti E. Impact of maternal allergy and use of probiotics during pregnancy on breast milk cytokines and food antibodies and development of allergy in children until 5 years. Int Arch Allergy Immunol. 2012;159:162–170. doi: 10.1159/000336157. [DOI] [PubMed] [Google Scholar]
- 10.Jarvinen KM, Laine ST, Jarvenpaa AL, Suomalainen HK. Does low IgA in human milk predispose the infant to development of cow's milk allergy? Pediatr Res. 2000;48:457–462. doi: 10.1203/00006450-200010000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Savilahti E, Tainio VM, Salmenpera L, Arjomaa P, Kallio M, Perheentupa J, Siimes MA. Low colostral IgA associated with cow's milk allergy. Acta Paediatr Scand. 1991;80:1207–1213. doi: 10.1111/j.1651-2227.1991.tb11810.x. [DOI] [PubMed] [Google Scholar]
- 12.Hapfelmeier S, Lawson MA, Slack E, Kirundi JK, Stoel M, Heikenwalder M, Cahenzli J, Velykoredko Y, Balmer ML, Endt K, Geuking MB, Curtiss R, 3rd, McCoy KD, Macpherson AJ. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science. 2010;328:1705–1709. doi: 10.1126/science.1188454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falth-Magnusson K. Breast milk antibodies to foods in relation to maternal diet, maternal atopy and the development of atopic disease in the baby. Int Arch Allergy Appl Immunol. 1989;90:297–300. doi: 10.1159/000235041. [DOI] [PubMed] [Google Scholar]
- 14.Mascart-Lemone F, Donnen P, Paluku B, Brasseur D, Van den Broeck J, Vaerman JP, Hennart P, Duchateau J. Serum and breast milk antibodies to food antigens in African mothers and relation to their diet. Adv Exp Med Biol. 1991;310:201–206. doi: 10.1007/978-1-4615-3838-7_26. [DOI] [PubMed] [Google Scholar]
- 15.Cruz JR, Garcia B, Urrutia JJ, Carlsson B, Hanson LA. Food antibodies in milk from Guatemalan women. J Pediatr. 1981;99:600–602. doi: 10.1016/s0022-3476(81)80269-7. [DOI] [PubMed] [Google Scholar]
- 16.Strobel S. Immunity induced after a feed of antigen during early life: oral tolerance v. sensitisation. Proc Nutr Soc. 2001;60:437–442. doi: 10.1079/pns2001119. [DOI] [PubMed] [Google Scholar]
- 17.Lamont AG, Mowat AM, Parrott DM. Priming of systemic and local delayed-type hypersensitivity responses by feeding low doses of ovalbumin to mice. Immunology. 1989;66:595–599. [PMC free article] [PubMed] [Google Scholar]
- 18.Brandtzaeg P. Update on mucosal immunoglobulin A in gastrointestinal disease. Curr Opin Gastroenterol. 2010;26:554–563. doi: 10.1097/MOG.0b013e32833dccf8. [DOI] [PubMed] [Google Scholar]
- 19.Brandtzaeg P. Food allergy: separating the science from the mythology. Nat Rev Gastroenterol Hepatol. 2010;7:380–400. doi: 10.1038/nrgastro.2010.80. [DOI] [PubMed] [Google Scholar]
- 20.Peterson DA, McNulty NP, Guruge JL, Gordon JI. IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell Host Microbe. 2007;2:328–339. doi: 10.1016/j.chom.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 21.van der Waaij LA, Limburg PC, Mesander G, van der Waaij D. In vivo IgA coating of anaerobic bacteria in human faeces. Gut. 1996;38:348–354. doi: 10.1136/gut.38.3.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rey J, Garin N, Spertini F, Corthésy B. Targeting of secretory IgA to Peyer's patch dendritic and T cells after transport by intestinal M cells. J Immunol. 2004;172:3026–3033. doi: 10.4049/jimmunol.172.5.3026. [DOI] [PubMed] [Google Scholar]
- 23.Mantis NJ, Cheung MC, Chintalacharuvu KR, Rey J, Corthesy B, Neutra MR. Selective adherence of IgA to murine Peyer's patch M cells: evidence for a novel IgA receptor. J Immunol. 2002;169:1844–1851. doi: 10.4049/jimmunol.169.4.1844. [DOI] [PubMed] [Google Scholar]
- 24.Kramer MS, Kakuma R. Maternal dietary antigen avoidance during pregnancy or lactation, or both, for preventing or treating atopic disease in the child. Cochrane Database Syst Rev. 2012 Sep 12;9:CD000133. doi: 10.1002/14651858.CD000133.pub3. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]