Abstract
Cancer micrometastasis relies on the ability of cancer cells to secrete angiogenic modulators, to interact with the vascular endothelium, and to overcome the resistance offered by the endothelial-barrier. Being an essential step prior to metastasis, blockage of micrometastasis can have potential applications in cancer therapy and metastasis prevention. Due to poorly known molecular mechanisms leading to micrometastasis, developing therapeutic strategies to target prostate cancer utilizing drugs that block micrometastasis is far from reality. Here we demonstrate the potential benefits of simvastatin in the inhibition of prostate cancer micrometastasis and reveal the novel molecular mechanisms underlying this process. First, we showed that simvastatin inhibited the ability of human PC3 prostate cancer cells for transendothelial migration in vitro. Second, our data indicated that simvastatin modulates the expression of tumor derived factors such as angiopoietins and VEGF-A at the mRNA and protein levels by the PC3 cells, thus preventing endothelial-barrier disruption. Third, simvastatin directly activated endothelial cells and enhances endothelial-barrier resistance. Apart from this, our study revealed that simvastatin-mediated effect on PC3 micrometastasis was mediated through inhibition of integrin αvβ3 activity and suppression of interaction between prostate cancer cell integrin αvβ3 with endothelial ICAM-1.
Keywords: Prostate cancer, micrometastasis, integrin αvβ3, ICAM-1, simvastatin
Introduction
Although slow growing, prostate cancer is the leading cause of cancer-related death among men. Using the prostate specific antigen (PSA) testing and Gleason score analysis (USPSTF, 2012), although many patients get diagnosed for prostate cancer at an early stage, many of them later develop castration-resistant metastatic prostate cancer. This demands additional research in developing procedures for prevention and/or the treatment of advanced stage prostate cancer.
Statins are lipid-lowering agents inhibiting HMG-CoA reductase enzyme, a key component of the cholesterol synthesis machinery (Alberts et al., 1980). Apart from this, statins elicit pleiotropic effects on multiple cell types in regulating various cellular functions (Liao and Laufs, 2005). Previous studies have shown that while statins are vascular protective via activation of endothelial cells (Li and Losordo, 2007), it inhibits proliferation of malignant cells such as hyper-active smooth muscle cells in the atherosclerotic plaques, leading to plaque stabilization and decreasing coronary artery disease-related mortality (Kwak et al., 2003, Takemoto and Liao, 2001). This clearly indicates the ‘normalizing’ potential of statins by activating cells at rest and inhibiting hyper-active malignant cells. This normalizing ability of statins has gathered specific interests for its potential applications in cancer for prevention and chemotherapy in combination with other drugs, thereby cancer therapy can be more effective and at the same time side-effects of chemotherapy can be minimized. In support of this view, a recent report indicates that long-term use of statins help to reduce cancer-related mortality by 15%, equivalent to the success rate of chemotherapy (Nielsen et al., 2012).
During the last several years, various statins have been tested for their anti-cancer efficacy on different cancer cell types in vitro and animal models in vivo (Jakobisiak and Golab, 2010). Despite several controversies on the beneficial vs. adverse effects of statins on various cancers, investigations validating the use of statins for prostate cancer therapy have been highly promising (Papadopoulos et al., 2011). A recent clinical study has reported 45% reduction in the biochemical recurrence of prostate cancer after radical prostatectomy in patients taking statins (Hamilton et al., 2010). Statins have been reported to be safe for humans even at doses 10–50 times higher than that is prescribed for cardiovascular disease (Holstein et al., 2006, Gauthaman et al., 2009). Previous studies from our group has demonstrated the anti-cancer efficacy of simvastatin, a highly lipophilic statin on androgen-responsive LNCaP cells and androgen-insensitive PC3 prostate cancer cell lines in vitro and tumor xenografts in vivo (Kochuparambil et al., 2011). Simvastatin also induced apoptosis in prostate cancer cells via simultaneous modulation of intrinsic cell survival and extrinsic apoptotic pathways (Goc et al., 2012b). Simvastatin-induced effects on prostate cancer cells were mainly mediated through the inhibition of Akt, a serine-threonine kinase that has been implicated to be essential for prostate cancer progression and metastasis (Hammarsten et al., 2012, Goc et al., 2011, Goc et al., 2012d). Our studies have also demonstrated the pivotal role of Akt in mediating prostate cancer micrometastasis via activation of integrin αvβ3 (Goc et al., 2012d), which have been reported to be elevated in prostate cancer cells (McCabe et al., 2007).
The process of micrometastasis involves intravasation and extravasation of cancer cells into the blood vessels and is a pre-requisite for the metastasis of prostate cancer cells to distant tissues such as bone and lungs (Tantivejkul et al., 2004). Due to this rate-limiting nature of the micrometastasis step in cancer progression, its blockage can be developed into an effective strategy for the prevention of prostate cancer metastasis, thus providing longer window for the surgical removal of the cancer tissue. Since simvastatin inhibits Akt pathway in prostate cancer cells (Kochuparambil et al., 2011) and Akt is important for prostate cancer micrometastasis (Goc et al., 2012d) and vascular maturation (Chen et al., 2005, Somanath et al., 2008), this combined with the vascular protective role of statins lead us to hypothesize that simvastatin can be highly effective in preventing prostate cancer micrometastasis.
In the current study, we explored the effects of simvastatin on prostate cancer micrometastasis. We first demonstrated that simvastatin inhibited expression of VEGF and enhanced expression of angiopoietin-1 at the RNA and protein levels as well as other signaling molecules such as IGF-I, integrins and PDGFβ etc., implicating its effects on stabilizing the endothelial-barrier. Our results provide strong evidence that while simvastatin performs vascular normalization through Akt-mediated activation of endothelial cells, thus protecting the endothelial-barrier; it prevents micrometastasis of prostate cancer cells in vitro via suppression of interactions between prostate cancer cell integrin αvβ3 and endothelial ICAM-1. To our knowledge, we provide the first evidence demonstrating the potential application of statins in the prevention of interactions between prostate cancer and the endothelium and inhibition of prostate cancer micrometastasis.
Materials and Methods
Cell culture
PC3 human prostate cancer cells were grown in DMEM/High glucose media supplemented with 10% FBS and 100 U/mL of penicillin-streptomycin (Fisher Scientific, Pittsburgh, PA). Human Microvascular Endothelial Cells (HMVECs) were grown in EBM-2 Basal Medium supplemented with EGM-2 MV SingleQuot Kit and Blasticidine (12.5 mg/ml) (Lonza, Fisher Scientific, Pittsburgh, PA).
Real-time PCR
Upon reaching 90% confluence, cells were treated with activated Simvastatin 25 µM vs. control for 12 h. Cells were harvested and lysed for mRNA using RNeasy Mini Kit (Qiagen, Valecia, CA), cDNA was then produced from mRNA using RT2 First Strand Kit (SA Biosciences, Valecia, CA). A total of 25 µg of cDNA was applied on each Cancer PathwayFinder PCR Array® (SA Biosciences, Valecia, CA) well, and PCR was run using an Eppendorff realplex2 equipment. Results were plugged into the associated tool available in SA Biosciences website to compare difference in expression using ΔΔC method after normalization to housekeeping genes.
Protein precipitation and immunoblotting
A ratio of 1:4 Trichloroacetic acid and the conditioned media was mixed and kept overnight in 4°C. Precipitated proteins were pelleted at 14000 rpm, washed twice with acetone and dried for 5 minutes at 95°C and then mixed with lamelli sample buffer and β-mercaptoethanol (9:1) followed by boiling for 5 min at 95°C. Equal volumes of samples were loaded in a 12% SDS-PAGE and subjected for Western blotting as done previously (Goc et al., 2012a).
Western blotting
HMVECs were treated with Simvastatin vs. control for 0, 1, 6 and 12 h and lysates were prepared using RIPA buffer as previously described (Kochuparambil et al., 2011). Equal amounts of protein (50 µg) were loaded on SDS-PAGE and subjected for Western analysis using specific antibodies for pS473Akt, pS9,21GSK, VE-Cadherin, β-Catenin (All from Cell Signaling, Danvers, MA), and Actin (Sigma Aldrich, St. Louis, MO).
Immunocytochemistry
PC3 cells were plated on glass cover-slips, grown to confluence, treated with simvastatin vs control. At endpoint, media was removed, cells were washed using PBS and fixed using 4% paraformaldehyde washed thrice with PBS, blocked using 5% goat serum albumin/0.3% Triton X-100 for 30 min, incubated with primary antibodies for VE-Cadherin, or β-Catenin (both from Cell Signaling, Danvers, MA) overnight at 4°C. Cells were washed three times with PBS and then incubated with secondary antibodies labeled with alexa-488 (goat anti-mouse) or alexa-597 (goat anti-rabbit) for 1 h followed by three times washing with PBS. Cover-slips were mounted on to the microscope slides using DAPI-containing vectashield (Vector laboratories, Burlingame, CA). Slides were viewed using Carl-Zeiss Fluorescent microscope (Carl Zeiss, Germany).
Electric Cell Substrate Impedance Sensing (ECIS)
Electric Cell Substrate Impedance Sensing (ECIS) (Applied Biophysics, Troy, NY) was performed according to manufacturer’s recommendations. Briefly, arrays were first washed with 10 mM glycine for 10 minutes, washed with media, stabilized using the device, and HMVECs were plated on the array wells. Upon reaching monolayer, cells were treated with: Simvastatin (5, 10 and 25 µM vs. control), detached PC-3 cells, or media collected from Simvastatin-treated PC-3 cells. For cell detachment, cells were first treated with Simvastatin (5, 10 and 25 µM vs. control), media was washed with PBS, and then a sterile Ethylenediamine Tetraacetic acid (EDTA, 20 mM) containing PBS was added to cells (for 5–7 minutes, 37°C) to detach them from ECM without digesting cell surface receptors. Cells were then collected, pelleted, re-suspended in media and counted. A total of 5×104 cells were added to each ECIS array well. For media collection, cells were treated with Simvastatin for 3 h and then media was removed, washed with serum free medium and incubated further in serum free medium to collect secreted factors over 12 h. Following this, media was collected, centrifuged to remove the cell debris, and a total of 200 µl of the conditioned media was added to each ECIS array well. For AP7.4 (β3-integrin activation antibodies; kindly provided by Thomas Kunicki) experiments, PC-3 cells were grown to a 70% confluence, treated with Simvastatin for 10 h, treated with AP7.4 (5 µg/ml) for another two hours, collected using cell dissociation buffer followed by adding to the ECIS array wells containing endothelial monolayer. Real-Time resistance/impedance was measured and recorded by the equipment automatically.
Cancer cell endothelium adhesion assay
PC3 cells were infected with GFP-expressing empty vector adenovirus particles. Briefly, 10 µl of a 109 PFU of adenovirus particles were added to a 6-well plate. 48 h later, cells were treated with Simvastatin (25 µM) vs. control for 4 h and 16 h, followed by plating of cells on top of a monolayer of HMVECs for adhesion. After 1 h, excess cells that were not attached were washed off, fixed with 2% paraformaldehyde and viewed under fluorescent microscope. Attached cells were identified by the GFP fluorescence and quantified.
Integrin αvβ3-ligand binding assays
PC3 cells were plated in a 12-well plate and cultured until monolayer, then treated with simvastatin vs. control for 12 h. Media was removed and integrin αvβ3 specific ligand Fibrinogen-Alexa-488 (12.5 µg/ml or 100 µg/ml, respectively; Invitrogen, Calrsbad, CA) was added to the wells for 40 min. Excess Fibrinogen-Alexa-488 was washed off and fluorescence was measured at 488/519 using a BioTek multi-plate reader (Biotek, Winooski, VT).
Activation-dependent ligand, WOW-1 Fab (30 µg/ml; kindly gifted by Sanford Shattil, Scripps Research Institute, CA), was used to determine the activation status of integrin αvβ3 in PC-3 cells as previously described (Goc et al., 2012d). A monolayer of PC3 cells was treated with simvastatin for 12 h. WOW-1 Fab (30 µg/ml) was added and incubated for additional 40 min. Wells were washed once with PBS, incubated for 1 h with secondary goat anti-mouse antibody labeled with Alexa-488, washed three times with PBS, fixed with 2% paraformaldehyde and fluorescence was measured at 488/519 using a BioTek multi-plate reader (Biotek, Winooski, VT).
PC3 cell ICAM-1 adhesion assay
Human soluble ICAM-1 (R&D Systems, Minneapolis, MN) at a final concentration of 12.5 µg/ml was plated on to each well of a 12 well plate and PC3 cells, pre-treated with various concentrations of simvastatin (5 and 25 µM) were added on top of the wells pre-coated with ICAM-1 at a concentration of 1 × 104 cells/well. After 40 Min, wells were washed three times with PBS to remove unbound cells and adhered cells were stained with crystal violet and counted.
Assessment of tumor angiogenesis in nude mice tumor xenografts
Tumor xenografts were implanted and collected as done previously (Kochuparambil et al., 2011). Tumor sections were stained for antibodies specific to laminin (Sigma, St. Louis, MO) followed by incubation with Alexa-488 labeled secondary antibodies (Invitrogen, Calrsbad, CA). Slides were then mounted on slides using vectashield with DAPI and imaged using Carl-Zeiss Fluorescent microscope (Carl Zeiss, Germany).
Statistical analysis
All the data are presented as mean ± SD. To determine significant differences between treatment and control values, we used the Student’s two-tailed t test. The significance was set at 0.05 levels (marked with symbols wherever data are statistically significant).
Results
Simvastatin inhibits PC3 cell interactions with the endothelium
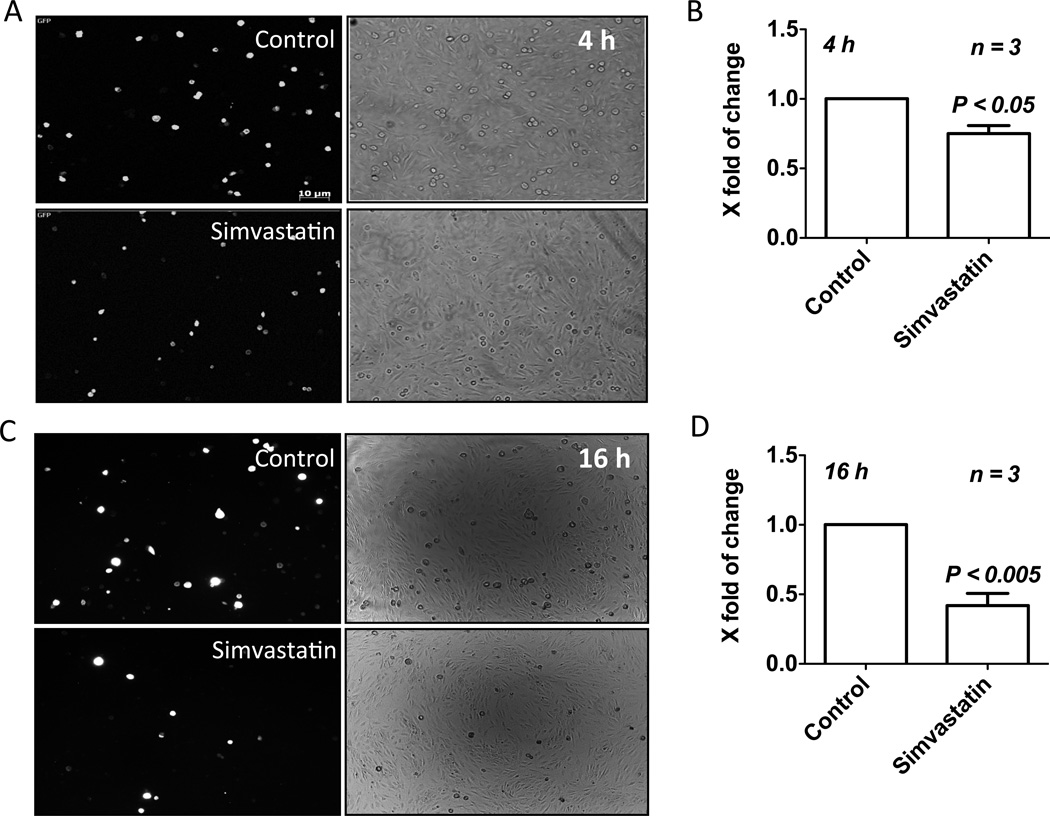
Although the effects of simvastatin-modulated factors on prostate cancer cells have been demonstrated (Goc et al., 2012c, Brown et al., 2012, Vainio et al., 2011) our results suggested that the simvastatin-mediated inhibition of prostate cancer cell micrometastasis is reliant on tumor-host (or EC) cell interactions. The first line of evidence was the modulation of integrin expression in PC3 cells by simvastatin (Table 1). Hence, in the next step, we investigated the effect of simvastatin on interactions between prostate tumor cell and the endothelial cells. To do this, equal number of washed GFP transfected PC3 cells treated with simvastatin for 12 h were plated onto a monolayer of endothelial cells for a 1 hour adhesion assay. Our results indicated that simvastatin significantly inhibited adhesion of PC3 cells to the endothelial monolayer in a time-dependent manner (Figure 1 A–D). While 20% decrease in PC3 cell adhesion to the endothelium was observed in 4 h post simvastatin treatment (Figure 1 A and B), there was >60% inhibition of PC3 cell adhesion to the endothelium after 16 h (Figure 1 C and D).
Table 1.
Key angiogenic genes modulated by simvastatin in prostate cancer (PC3) cells
| Gene Symbol |
Gene description | Change (X-fold) |
|---|---|---|
| AKT1 | V-akt murine thymoma viral oncogene homolog 1 | 4.6↓ |
| ANGPT1 | Angiopoietin 1 | 4.0↑ |
| ANGPT2 | Angiopoietin 2 | 1.8↓ |
| CHEK2 | CHK2 checkpoint homolog | 1.6↓ |
| COL18A1 | Collagen, type XVIII, alpha 1 | 4.7↓ |
| E2F1 | E2F transcription factor 1 | 8.2↓ |
| ERBB2 | V-erb-b2 erythroblastic leukemia viral oncogene homolog 2 | 6.3↓ |
| FGFR2 | Fibroblast growth factor receptor 2 | 1.8↓ |
| FOS | V-fos FBJ murine osteosarcoma viral oncogene homolog | 1.6↓ |
| IFNB1 | Interferon, beta 1, fibroblast | 2.4↓ |
| IGF1 | Insulin-like growth factor 1 (somatomedin C) | 3.2↓ |
| ITGA4 | Integrin, alpha 4 (CD49D) | 3.6↓ |
| ITGB3 | Integrin, beta 3 (glycoprotein IIIa, CD61) | 2.2↓ |
| ITGB5 | Integrin, beta 5 | 5.5↓ |
| JUN | Jun oncogene | 5.1↓ |
| MMP2 | Matrix metallopeptidase 2 | 2.3↓ |
| MTA2 | Metastasis associated 1 family, member 2 | 3.8↓ |
| MTSS1 | Metastasis suppressor 1 | 2.0↓ |
| MYC | V-myc myelocytomatosis viral oncogene homolog | 3.7↓ |
| PDGFB | Platelet-derived growth factor-β | 2.0↓ |
| PLAUR | Plasminogen activator, urokinase receptor | 1.8↓ |
| PNN | Pinin, desmosome associated protein | 1.5↓ |
| SNCG | Synuclein, gamma | 3.1↓ |
| SYK | Spleen tyrosine kinase | 2.0↓ |
| TEK | TEK tyrosine kinase, endothelial | 1.9↓ |
| VEGFA | Vascular endothelial growth factor A | 4.1↓ |
Figure 1.
Simvastatin inhibits binding of PC3 cells to the endothelial monolayer. (A–D) PC3 cells expressing GFP were treated with 25 µM simvastatin for 4 h (A and B) and 12 h (C and D), and introduced into 12-well plates containing a confluent monolayer of HMVECs. One hour later, medium was removed, wells were washed with PBS and the number of GFP-positive PC3 cells were determined.
Simvastatin inhibits expression of prostate tumor cell-derived factors
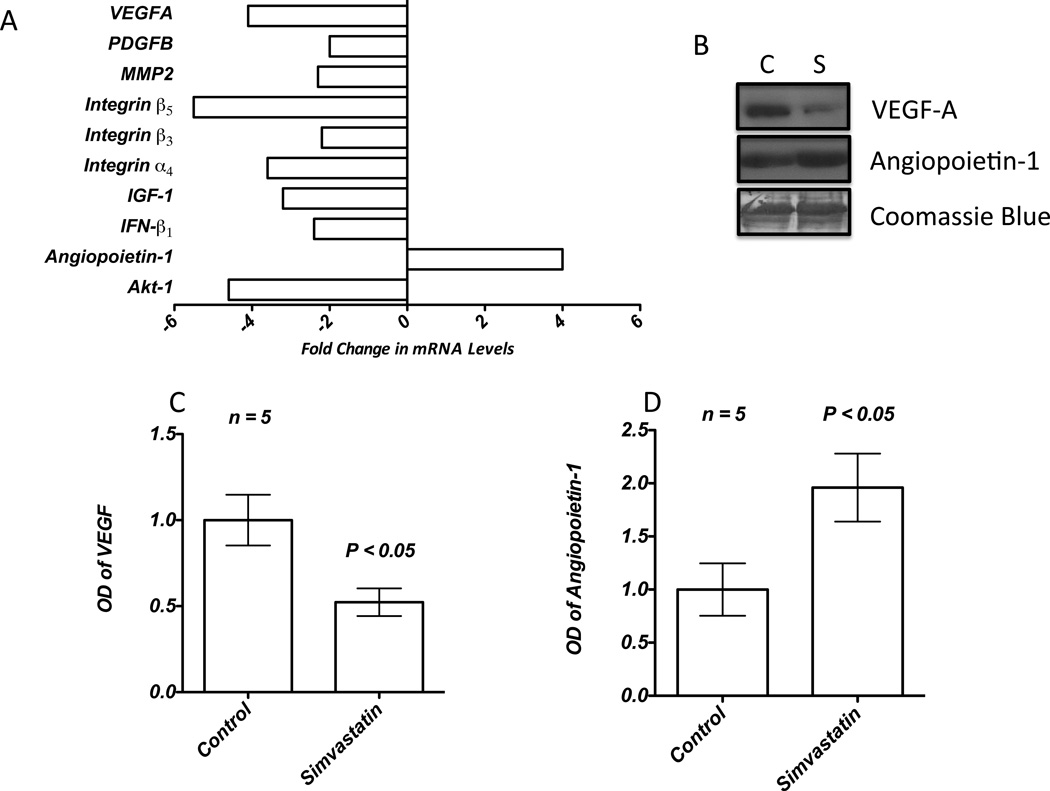
Next, we sought to identify the key growth factors and cytokines regulating simvastatin-mediated inhibition of HMVEC-barrier disruption by PC3 tumor cell-derived factors. First, we performed a qRealTime-PCR array for PC3 cells pre-treated with 25 µM simvastatin for 12 h. Our results indicated that while simvastatin inhibited the mRNA expression of pro-angiogenic and/or endothelial-barrier disrupting growth factors such as VEGF-A, IGF-1 and angiopoietin-2, it resulted in the increased expression of angiopoietin-1, a growth factor known to arrest endothelial-barrier break-down and enhance vascular maturation (Figure 2A and Table 1). These results were corroborated with our Western analysis data, which showed that while simvastatin treatment resulted in significant decrease in the secretion of VEGF-A (2-fold) into the conditioned media by the PC3 cells, secretion of angiopoietin-1 was significantly increased (2-fold) (Figure 2B–D). These results demonstrated the effect of simvastatin on the secretion of various pro- and anti-angiogenic factors such as VEGF-A and angiopoietins in the modulation of endothelial-barrier resistance.
Figure 2.
Simvastatin modulates expression of secreted angiogenic factors by the PC3 cells. (A) Bar graph showing qRT-PCR array data indicating key genes modulated by simvastatin. (B) Western analysis of PC3 cell conditioned medium concentrated by TCA-precipitation showing changes in the expression of VEGF-A and Ang-1. (C and D) Band-densitometry analysis of the Western blots of PC3 cell conditioned medium indicating changes in the expression of VEGF-A and Ang-1 normalized to coomassie staining.
Simvastatin has no significant effect on the rate of tumor angiogenesis
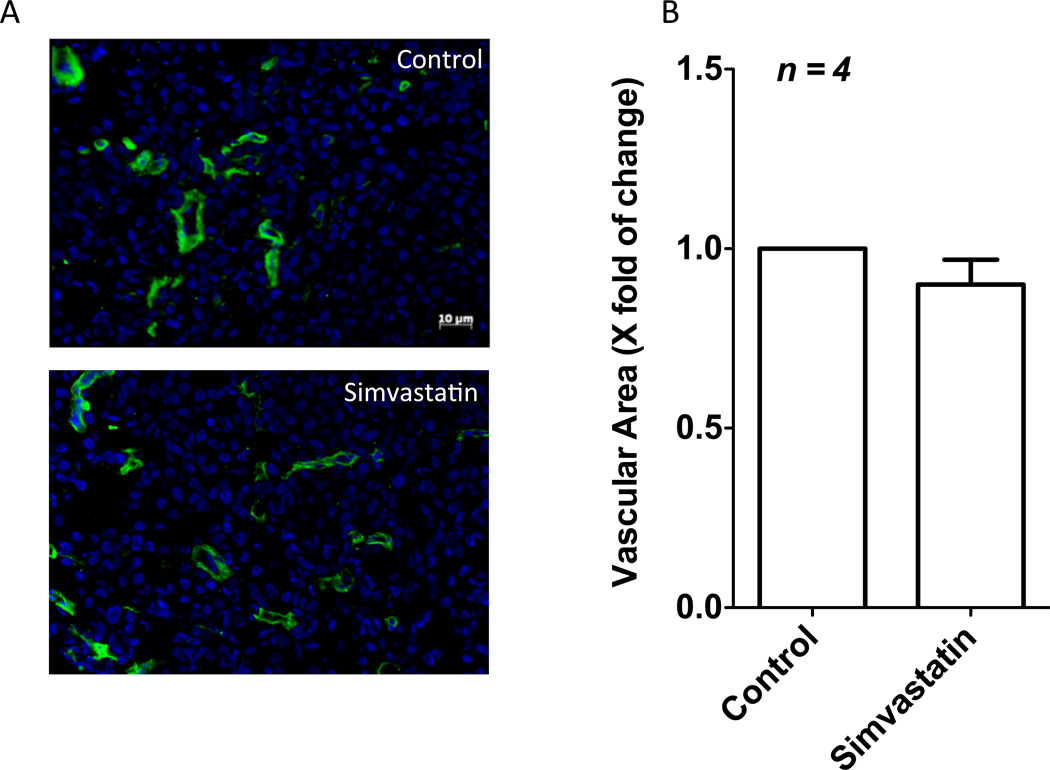
Since expression of pro-angiogenic and vascular permeability modulators VEGF-A and Angiopoietin-1 in PC3 cells were altered by simvastatin treatment, we investigated the effects of simvastatin on tumor angiogenesis in vivo in PC3 tumor xenografts developed in athymic nude mice (Kochuparambil et al., 2011) (Figure 3). Interestingly, simvastatin treatment did not inflict any significant changes in the vascular area in PC3 cell tumor xenografts as evidenced by the laminin statining, demonstrating that simvastatin is more involved in the normalization of tumor vasculature than modulating tumor angiogenesis.
Figure 3.
Simvastatin has no effect on prostate tumor angiogenesis. (A) Immunofluorescence staining of PC3 tumor xenograft sections from nude mice showing laminin staining as a measure of tumor angiogenesis. (B) Bar graph showing no differences in the vascular area in tumor xenografts between c ontrol (saline) and simvastatin (2mg/kg/12 h) treated mice.
Inhibition of prostate cancer cell interactions with the endothelium is mediated through integrin αvβ3
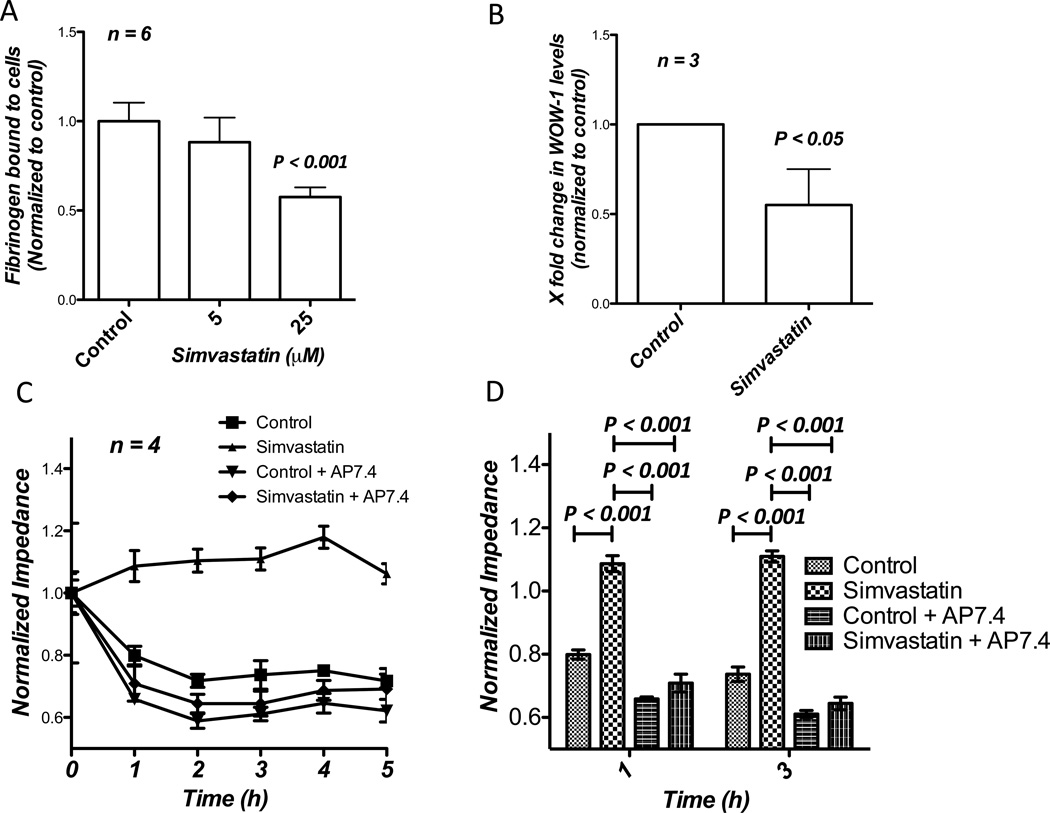
Cell-surface integrin αvβ3 has been implicated in mediating interactions between lymphocytes and vascular endothelial cells in mediating diapedesis (Steffen et al., 1994). Integrin αvβ3 is highly expressed in invasive and metastatic prostate cancer cells and our previous study has demonstrated the role of Akt-integrin αvβ3 cooperation in mediating prostate cancer cell micrometastasis (Goc et al., 2012d). Therefore, we tested if simvastatin-mediated inhibitory effects on prostate cancer cell interactions with the endothelium involved integrin αvβ3. Our study utilizing Alexa 488-labelled fibrinogen, a specific ligand for integrin αvβ3 and WOW-1 Fab that detects only active integrin αvβ3 on the cell surface demonstrated significantly impaired interaction of fibrinogen-Alexa 488 (Figure 4A) and WOW-1 (Figure 4B) with PC3 cells pre-treated with different doses of simvastatin for 12 h, indicating impaired affinity of integrin αvβ3 for its extracellular matrix ligands.
Figure 4.
Simvastatin abolishes PC3 cell interaction with the endothelium via inhibition of PC3 cell surface integrin αvβ3 affinity for its ligands. (A) Bar graph showing the effect of simvastatin on PC3 cell interactions with Alexa486-labelled fibrinogen, an integrin αvβ3 ligand. (B) Bar graph showing impaired binding of WOW-1, a specific ligand for activated integrin αvβ3, by the PC3 cells treated with 25 µM simvastatin compared to control. (C and D) Figure showing significant inhibition of PC3 cell transendothelial migration with 25 µM simvastatin compared to control saline treated cells. Co-treatment with AP7.4, integrin αvβ3 activating antibodies abolished simvastatin-mediated effects.
To confirm this further, we performed a rescue experiment utilizing AP7.4, a specific clone of antibodies that bind to the extracellular domain of the cell-surface integrin αvβ3 and induce activating conformational changes. Treatment of PC3 cells with AP7.4 enhanced their micrometastasis, compared to the control (Figure 4C). While simvastatin impaired the ability of PC3 cells to micrometastasize, our results indicated that pre-treatment of PC3 cells with AP7.4 significantly rescued simvastatin-mediated inhibition of micrometastasis in vitro on HMVECs (Figure 4C and D).
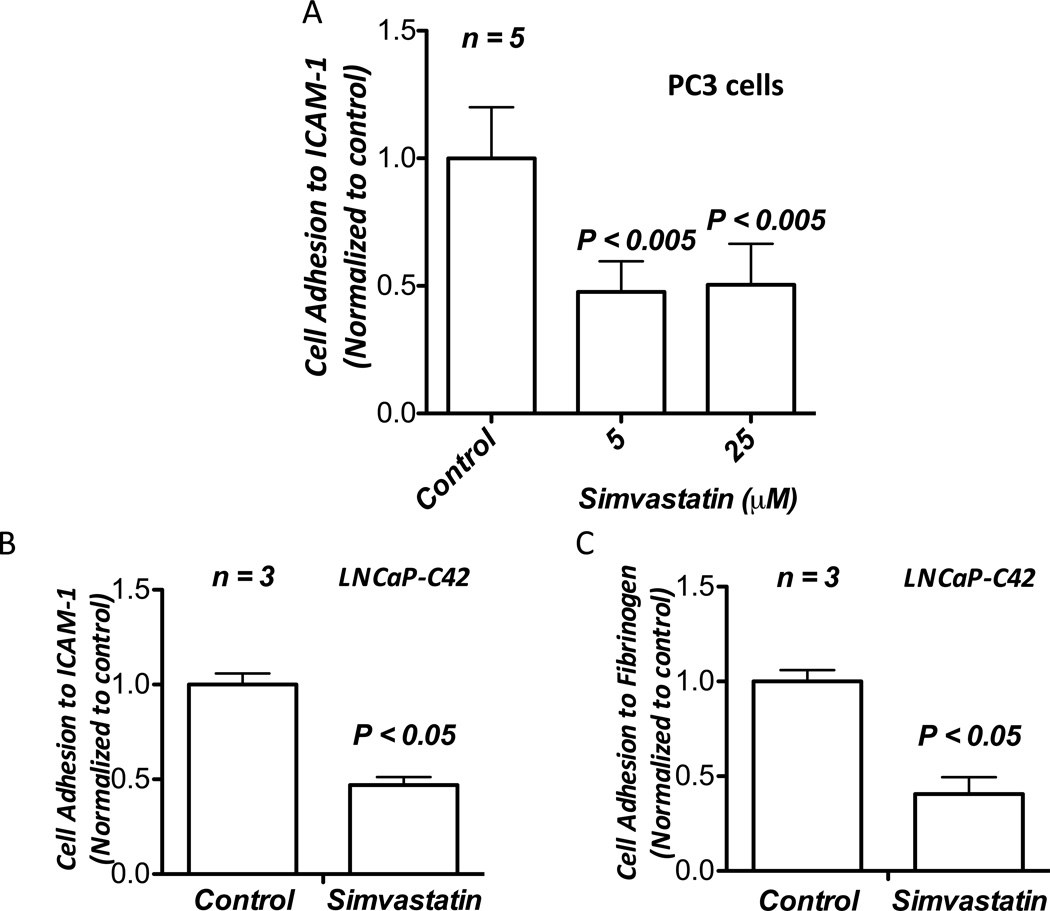
Simvastatin suppresses interactions between PC3 cell integrin αvβ3 and endothelial ICAM-1
One mechanism by which circulating cells adhere to endothelium to mediate diapedesis is by utilizing their surface integrin αvβ3 to bind to specific ligands-cum-adhesion molecules on the endothelium such as VCAM-1 and ICAM-1 (Steiner et al., 2010). Since integrin αvβ3 is also necessary for prostate cancer micrometastasis, we tested whether simvastatin treatment had any effect on interactions between PC3 cell-surface integrin αvβ3 and ICAM-1, adhesion molecules that are abundant on endothelial cell surface. Our data indicated that treatment with various doses of simvastatin significantly inhibited PC3 cell interaction with ICAM-1 (Figure 5A). In order to confirm that observed inhibition of interactions between PC3 cell-surface integrin αvβ3 and ICAM-1 due to simvastatin treatment is not specific to only one cell type, we performed adhesion assay using a second human invasive prostate cancer LNCaP-C42 cell line to study the effect of simvastatin on culture plates pre-coated with fibrinogen and ICAM-1. Treatment with 25 µM simvastatin resulted in significant inhibition of LNCaP-C42 cell adhesion to ICAM-1 and fibrinogen (Figure 5B and C), thus demonstrating that simvastatin suppressed interactions between prostate cancer cell-surface integrin αvβ3 and ICAM-1 that are abundantly expressed by endothelial cells.
Figure 5.
Simvastatin inhibits interaction between prostate cancer cell-surface integrin αvβ3 and endothelial ICAM-1. (A) Bar graph showing significant inhibition of adhesion of PC3 cells on pre-coated soluble human ICAM-1 by treatment with 5 and 25 µM doses of simvastatin. (B) Bar graph showing inhibition of adhesion of LNCaP-C42 cells on pre-coated soluble human ICAM-1 by treatment with 25 µM simvastatin. (C) Bar graph showing inhibition of adhesion of LNCaP-C42 cells on pre-coated fibrinogen by treatment with 25 µM simvastatin.
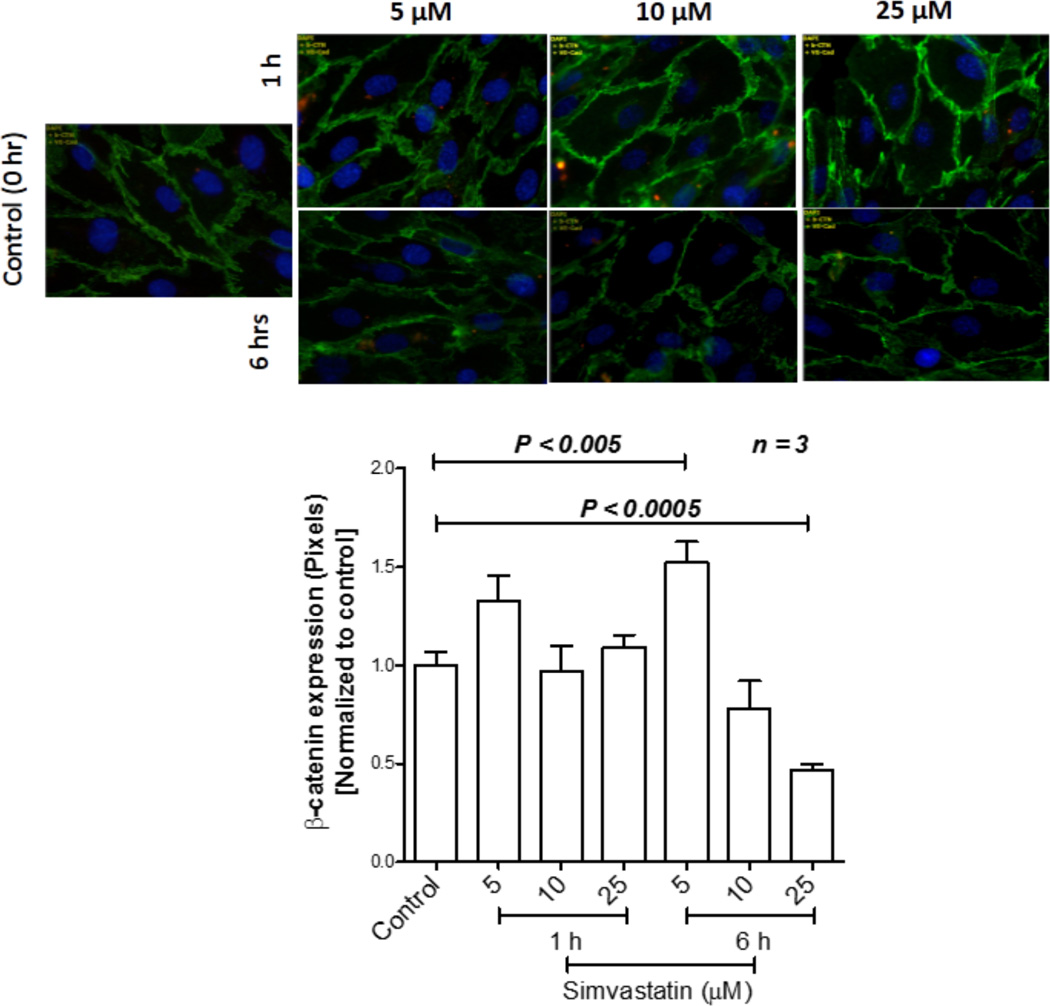
Simvastatin enhances endothelial cell-barrier integrity via βcatenin stabilization
Next, we determined the direct effects of simvastatin on vascular endothelial-barrier junctions. To investigate this, we performed immunocytochemistry analysis of endothelial monolayer subjected for dose- and time-dependent effects of simvastatin, with antibodies specific for βcatenin. βcatenin expression analysis of HMVECs treated with simvastatin for 6 h indicated a significantly elevated βcatenin expression in HMVEC-barrier junctions with 5 µM simvastatin with modest, but significant reduction in expression βcatenin with 25 µM simvastatin (Figure 6 A and B). These results indicated that simvastatin elicits vascular protective effects via endothelial-barrier enhancement. However, our results also provide the need for caution at the use of very high doses of simvastatin for prostate cancer treatment as higher doses can be toxic to endothelial cells and normal vasculature.
Figure 6.
Simvastatin stabilizes the endothelial-barrier in mediating normalization of the tumor vasculature. (A) Fluorescent microscopic images of HMVEC monolayer stained with adherens junction protein β-catenin. (B) Bar graph of the above data indicating changes in expression with 5, 10 and 25 µM doses of simvastatin.
Discussion
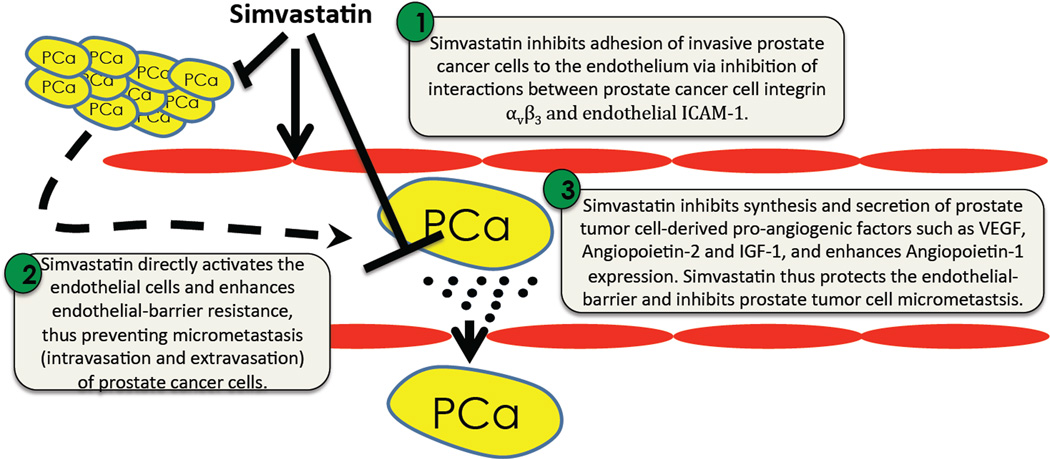
The process of micrometastasis involving intravasation and extravasation of cancer cells is an essential pre-requisite for the cancer cells to metastasize to distant tissues (Steffen et al., 1994). Our current study demonstrates the potential benefits of simvastatin in the inhibition of prostate cancer metastasis (Figure 7). We first showed that simvastatin treatment inhibited transendothelial migration of highly invasive PC3 cells in an ECIS array equipment in vitro. Our gene array experiment, followed by Western analysis identified a decrease in VEGF-A expression and increase in Angiopietin-1 expression by PC3 cells in response to simvastatin treatment. WOW-1 binding assay that measures the surface expression of activated integrin αvβ3 in PC3 cells and fibrinogen binding assay demonstrated the effect of simvastatin on significantly inhibiting the affinity of integrin αvβ3 to bind to its ligands. Co-treatment with AP7.4, integrin αvβ3 activating antibodies, rescued simvastatin-mediated inhibition of PC3 cell micrometastasis. While pre-treatment with simvastatin significantly impaired the ability of PC3 cells to bind to the endothelial cell surface, adhesion of PC3 cells to soluble ICAM-1, adhesion molecules that are abundantly expressed in endothelial cells (Dustin et al., 1986) often implicated in mediating transendothelial migration of inflammatory cells (Steiner et al., 2010) and cancer cells (Conrad et al., 2009) was significantly blunted by treatment with simvastatin. Finally, simvastatin treatment stabilized the vascular adherens junctions and protected endoethelial-barrier by enhancing β-catenin expression. Collectively, our data demonstrates the potential therapeutic benefits of simvastatin in preventing prostate cancer micrometastasis.
Figure 7.
Schematic representation of the working hypothesis.
Much of the effects of simvastatin on prostate cancer micrometastasis have been attributed to its ability to differentially modulate cell signaling in normal cells at rest and malignant cells. While statins have been previously shown to inhibit proliferation of vascular smooth muscle cells in atherosclerotic plaques (Liao, 2005) and inhibition of cell motility and migration of various cancer cells (Goc et al., 2012d), including prostate cancer cells (Goc et al., 2011, Goc et al., 2012b), vascular endothelium is protected by statins via activation of Akt-eNOS pathway (Kureishi et al., 2000). Hence, while statins protect the vasculature, it inhibits tumor growth and may prevent micrometastasis. This ability of statins to normalize the tumor cells and the cells in the microenvironment provides the benefit of improving the efficacy of chemotherapeutic drugs when treated in combinations with statins.
Our previous results related to the effects of simvastatin on prostate cancer cells in vitro and tumor xenograft growth in vivo are completely in agreement with the general perception that statins normalize the deregulated signaling pathways in malignant cells. We showed that while simvastatin inhibited prostate cancer cell proliferation, migration and colony formation in vitro, it impaired the growth of tumor xenografts in vivo (Kochuparambil et al., 2011). In addition, simvastatin induced apoptosis in prostate cancer cells via inhibition of intrinsic cell survival as well as activation of extrinsic death-receptor pathways (Goc et al., 2012b). Results from the current study on the specific effects of simvastatin on micrometastasis of prostate cancer cells in vitro further strengthens our overall idea of utilizing statins for prostate cancer therapy either alone (for prevention) or in combination with chemotherapeutic drugs such as Taxotere®(for therapy). Our studies suggest that dual effects of simvastatin on inhibition of interactions between the prostate cancer cells and the endothelium, and on the modulation of tumor-derived factors such as VEGF and angiopoietins will provide a net stabilizing effect on the tumor vasculature, by reducing the vascular permeability, an important feature of tumor vasculature (Goel et al., 2011).
A very recent study has also demonstrated the effect of atorvastatin on VEGF-A expression by the human small non-cell lung carcinoma cell lines (Chen et al., 2012). While our study confirms this report to be true also in the case of prostate cancer cells, we provide additional information on the inhibitory effect of simvastatin on the expression of angiopoietin-2, a signaling companion of VEGF in the modulation of tumor vascular permeability (Gale et al., 2002), and enhance the expression of angiopoietin-1, a growth factor that stabilizes the vascular endothelial-barrier junctions, thus stabilizing the endothelial-barrier and reducing vascular permeability (Gale et al., 2002). Other prominent pro-angiogeneic growth factors or signaling molecules identified in the gene array that are regulated by simvastatin in PC3 cells include TEK-Receptor tyrosine kinase, Insulin like growth factor-1 (IGF-1), integrins α4, β3 and β5 and FGF Receptor-2, all of which are inhibited by simvastatin. Since IGF-1 has been implicated in the development of castration resistant prostate cancer (Koutsilieris et al., 2000) and integrins are involved in micrometastasis, simvastatin treatment appears to be an effective treatment strategy to prevent recurrence after prostatectomy and prevent further spread as has been shown recently (Hamilton et al., 2010). Furthermore, staining of endothelial-barrier junctions for βcatenin, a predominant adherens junction protein indicated enhanced endothelial-barrier protection upon treatment with simvastatin, accompanied by enhanced barrier-resistance. Interestingly, these changes in gene and protein expression of angiogenic modulators by simvastatin did not have a net effect on tumor angiogenesis in vivo, indicating that simvastatin provides a normalizing and stabilizing effect on the tumor endothelium. This priming effect on the tumor vasculature is a feature considered essential for anti-angiogenic therapy (Al-Husein et al., 2012).
Impaired micrometastasis of PC3 cells pre-treated with simvastatin in an ECIS array suggested that factors other than tumor derived factors may be involved in the modulation of simvastatin-mediated prostate cancer micrometastasis. An important mechanism by which circulating blood cells and cancer cells transmigrate the endothelial-barrier is via a direct heterophilic interaction with the endothelium mediated through cell-surface integrins and cellular adhesion molecules (Steiner et al., 2010, Conrad et al., 2009). Statins have previously shown to impair transendothelial migration of inflammatory cells via inhibition of their interaction with the endothelium (Steiner et al., 2010). Our previous studies have shown that Akt and Rac pathways enhance prostate cancer micrometastasis involving integrin αvβ3 (Goc et al., 2012a, Goc et al., 2012d), which are extracellular matrix (ECM) receptors abundantly expressed in prostate cancer cells and have been implicated to be necessary for prostate cancer metastasis (McCabe et al., 2007). Since Akt and RhoGTPases are important targets of simvastatin in cancer cells (Kochuparambil et al., 2011, Ghosh et al., 1999) we postulated that simvastatin-mediated inhibition of PC3 micrometastasis may involve integrin αvβ3. Our findings supported this hypothesis and demonstrated that while simvastatin treatment resulted in impaired inside-out activation of integrin αvβ3 and thus reduced their affinity for specific ligands such as fibrinogen. Furthermore, co-treatment of PC3 cells with simvastatin and AP7.4 (integrin αvβ3 activating antibodies) rescued the impaired micrometastasis, once again confirming that simvastatin has a direct effect on integrin αvβ3 on reducing its ligand affinity.
Cell-surface integrins on circulating blood cells and cancer cells often interact with cell adhesion molecules expressed on endothelial cells such as VCAMs and ICAMs (Steiner et al., 2010). Among these, interaction between integrin αvβ3 and ICAM-1 is the best characterized (Conrad et al., 2009). Our data indicated that pre-treatment with simvastatin significantly impaired the ability of PC3 cells to recognize and adhere to human soluble ICAM-1, thus demonstrating the direct effect of simvastatin on inhibiting interactions of prostate cancer cells with cellular adhesion molecules. Although not tested in the current study, statins have been previously reported to reduce the expression of cellular adhesion molecules such as ICAMs and VCAMs on endothelial cell surface (Dustin et al., 1986). Hence, simvastatin is expected to elicit a much more potent inhibitory effect on prostate cancer micrometastasis in vivo as compared to our unidirectional approach in the in vitro experiments. Altogether, this study identifies simvastatin as a potent inhibitor of prostate cancer micrometastasis via inhibition of tumor derived factor expression, inhibition of prostate cancer cell transendothelial migration and by stabilizing the endothelial-barrier. These results combined with the anti-inflammatory and endothelial-barrier stabilizing effects of simvastatin suggest that statins could be re-purposed for the management of prostate cancer either alone or in combination with chemotherapeutic regimen.
Acknowledgments
Contract Grant Sponsor: University of Georgia Research Foundation
Contract Grant Sponsor: Wilson Pharmacy Foundation
Contract Grant Sponsor: National Institutes of Health grant
Contract Grant Number: R01HL103952
Contract Grant Sponsor: American Heart Association Scientist Development Grant
Contract Grant Number: 0830326N
Footnotes
Conflict of interest
None
References
- Al-Husein B, Abdalla M, Trepte M, Deremer DL, Somanath PR. Antiangiogenic Therapy for Cancer: An Update. Pharmacotherapy. 2012 doi: 10.1002/phar.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts AW, Chen J, Kuron G, Hunt V, Huff J, Hoffman C, Rothrock J, Lopez M, Joshua H, Harris E, Patchett A, Monaghan R, Currie S, Stapley E, Albers-Schonberg G, Hensens O, Hirshfield J, Hoogsteen K, Liesch J, Springer J. Mevinolin: a highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent. Proc Natl Acad Sci U S A. 1980;77:3957–3961. doi: 10.1073/pnas.77.7.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M, Hart C, Tawadros T, Ramani V, Sangar V, Lau M, Clarke N. The differential effects of statins on the metastatic behaviour of prostate cancer. Br J Cancer. 2012;106:1689–1696. doi: 10.1038/bjc.2012.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Liu B, Yuan J, Yang J, Zhang J, An Y, Tie L, Pan Y, Li X. Atorvastatin reduces vascular endothelial growth factor (VEGF) expression in human non-small cell lung carcinomas (NSCLCs) via inhibition of reactive oxygen species (ROS) production. Mol Oncol. 2012;6:62–72. doi: 10.1016/j.molonc.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Somanath PR, Razorenova O, Chen WS, Hay N, Bornstein P, Byzova TV. Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nat Med. 2005;11:1188–1196. doi: 10.1038/nm1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad F, Zhu X, Zhang X, Chalkley RJ, Burlingame AL, Marks JD, Liu B. Human antibodies targeting cell surface antigens overexpressed by the hormone refractory metastatic prostate cancer cells: ICAM-1 is a tumor antigen that mediates prostate cancer cell invasion. J Mol Med (Berl) 2009;87:507–514. doi: 10.1007/s00109-009-0446-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin ML, Rothlein R, Bhan AK, Dinarello CA, Springer TA. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1) J Immunol. 1986;137:245–254. [PubMed] [Google Scholar]
- Gale NW, Thurston G, Davis S, Wiegand SJ, Holash J, Rudge JS, Yancopoulos GD. Complementary and coordinated roles of the VEGFs and angiopoietins during normal and pathologic vascular formation. Cold Spring Harb Symp Quant Biol. 2002;67:267–273. doi: 10.1101/sqb.2002.67.267. [DOI] [PubMed] [Google Scholar]
- Gauthaman K, Manasi N, Bongso A. Statins inhibit the growth of variant human embryonic stem cells and cancer cells in vitro but not normal human embryonic stem cells. Br J Pharmacol. 2009;157:962–973. doi: 10.1111/j.1476-5381.2009.00241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh PM, Ghosh-Choudhury N, Moyer ML, Mott GE, Thomas CA, Foster BA, Greenberg NM, Kreisberg JI. Role of RhoA activation in the growth and morphology of a murine prostate tumor cell line. Oncogene. 1999;18:4120–4130. doi: 10.1038/sj.onc.1202792. [DOI] [PubMed] [Google Scholar]
- Goc A, Abdalla M, Al-Azayzih A, Somanath PR. Rac1 activation driven by 14-3-3zeta dimerization promotes prostate cancer cell-matrix interactions, motility and transendothelial migration. PLoS One. 2012a;7:e40594. doi: 10.1371/journal.pone.0040594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goc A, Al-Husein B, Kochuparambil ST, Liu J, Heston WW, Somanath PR. PI3 kinase integrates Akt and MAP kinase signaling pathways in the regulation of prostate cancer. Int J Oncol. 2011;38:267–277. [PubMed] [Google Scholar]
- Goc A, Kochuparambil ST, Al-Husein B, Al-Azayzih A, Mohammad S, Somanath PR. Simultaneous modulation of the intrinsic and extrinsic pathways by simvastatin in mediating prostate cancer cell apoptosis. BMC Cancer. 2012b;12:409. doi: 10.1186/1471-2407-12-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goc A, Kochuparambil ST, Al-Husein B, Al-Azayzih A, Mohammad S, Somanath PR. Simultaneous modulation of the intrinsic and extrinsic pathways by simvastatin in mediating prostate cancer cell apoptosis. BMC Cancer. 2012c;12:409. doi: 10.1186/1471-2407-12-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goc A, Liu J, Byzova TV, Somanath PR. Akt1 mediates prostate cancer cell microinvasion and chemotaxis to metastatic stimuli via integrin beta(3) affinity modulation. Br J Cancer. 2012d;107:713–723. doi: 10.1038/bjc.2012.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, Jain RK. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev. 2011;91:1071–1121. doi: 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton RJ, Banez LL, Aronson WJ, Terris MK, Platz EA, Kane CJ, Presti JC, Jr, Amling CL, Freedland SJ. Statin medication use and the risk of biochemical recurrence after radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) Database. Cancer. 2010;116:3389–3398. doi: 10.1002/cncr.25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarsten P, Cipriano M, Josefsson A, Stattin P, Egevad L, Granfors T, Fowler CJ. Phospho-Akt Immunoreactivity in Prostate Cancer: Relationship to Disease Severity and Outcome, Ki67 and Phosphorylated EGFR Expression. PLoS One. 2012;7:e47994. doi: 10.1371/journal.pone.0047994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstein SA, Knapp HR, Clamon GH, Murry DJ, Hohl RJ. Pharmacodynamic effects of high dose lovastatin in subjects with advanced malignancies. Cancer Chemother Pharmacol. 2006;57:155–164. doi: 10.1007/s00280-005-0013-8. [DOI] [PubMed] [Google Scholar]
- Ii M, Losordo DW. Statins and the endothelium. Vascul Pharmacol. 2007;46:1–9. doi: 10.1016/j.vph.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Jakobisiak M, Golab J. Statins can modulate effectiveness of antitumor therapeutic modalities. Med Res Rev. 2010;30:102–135. doi: 10.1002/med.20162. [DOI] [PubMed] [Google Scholar]
- Kochuparambil ST, Al-Husein B, Goc A, Soliman S, Somanath PR. Anticancer efficacy of simvastatin on prostate cancer cells and tumor xenografts is associated with inhibition of Akt and reduced prostate-specific antigen expression. J Pharmacol Exp Ther. 2011;336:496–505. doi: 10.1124/jpet.110.174870. [DOI] [PubMed] [Google Scholar]
- Koutsilieris M, Mitsiades C, Sourla A. Insulin-like growth factor I and urokinase-type plasminogen activator bioregulation system as a survival mechanism of prostate cancer cells in osteoblastic metastases: development of anti-survival factor therapy for hormone-refractory prostate cancer. Mol Med. 2000;6:251–267. [PMC free article] [PubMed] [Google Scholar]
- Kureishi Y, Luo Z, Shiojima I, Bialik A, Fulton D, Lefer DJ, Sessa WC, Walsh K. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med. 2000;6:1004–1010. doi: 10.1038/79510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak BR, Mulhaupt F, Mach F. Atherosclerosis: anti-inflammatory and immunomodulatory activities of statins. Autoimmun Rev. 2003;2:332–338. doi: 10.1016/s1568-9972(03)00049-1. [DOI] [PubMed] [Google Scholar]
- Liao JK. Effects of statins on 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition beyond low-density lipoprotein cholesterol. Am J Cardiol. 2005;96:24F–33F. doi: 10.1016/j.amjcard.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol. 2005;45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccabe NP, De S, Vasanji A, Brainard J, Byzova TV. Prostate cancer specific integrin alphavbeta3 modulates bone metastatic growth and tissue remodeling. Oncogene. 2007;26:6238–6243. doi: 10.1038/sj.onc.1210429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N Engl J Med. 2012;367:1792–1802. doi: 10.1056/NEJMoa1201735. [DOI] [PubMed] [Google Scholar]
- Papadopoulos G, Delakas D, Nakopoulou L, Kassimatis T. Statins and prostate cancer: molecular and clinical aspects. Eur J Cancer. 2011;47:819–830. doi: 10.1016/j.ejca.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Somanath PR, Chen J, Byzova TV. Akt1 is necessary for the vascular maturation and angiogenesis during cutaneous wound healing. Angiogenesis. 2008;11:277–288. doi: 10.1007/s10456-008-9111-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen BJ, Butcher EC, Engelhardt B. Evidence for involvement of ICAM-1 and VCAM-1 in lymphocyte interaction with endothelium in experimental autoimmune encephalomyelitis in the central nervous system in the SJL/J mouse. Am J Pathol. 1994;145:189–201. [PMC free article] [PubMed] [Google Scholar]
- Steiner O, Coisne C, Cecchelli R, Boscacci R, Deutsch U, Engelhardt B, Lyck R. Differential roles for endothelial ICAM-1, ICAM-2, and VCAM-1 in shear-resistant T cell arrest, polarization, and directed crawling on blood-brain barrier endothelium. J Immunol. 2010;185:4846–4855. doi: 10.4049/jimmunol.0903732. [DOI] [PubMed] [Google Scholar]
- Takemoto M, Liao JK. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Arterioscler Thromb Vasc Biol. 2001;21:1712–1719. doi: 10.1161/hq1101.098486. [DOI] [PubMed] [Google Scholar]
- Tantivejkul K, Kalikin LM, Pienta KJ. Dynamic process of prostate cancer metastasis to bone. J Cell Biochem. 2004;91:706–717. doi: 10.1002/jcb.10664. [DOI] [PubMed] [Google Scholar]
- USPSTF. [Accessed 12/02/2012];USPSTF Prostate Cancer Screening Recommendation: What are the benefits and harms of prostate cancer screening? 2012 [Online]. Available: http://www.uspreventiveservicestaskforce.org/prostatecancerscreening.htm. [Google Scholar]
- Vainio P, Lehtinen L, Mirtti T, Hilvo M, Seppanen-Laakso T, Virtanen J, Sankila A, Nordling S, Lundin J, Rannikko A, Oresic M, Kallioniemi O, Iljin K. Phospholipase PLA2G7, associated with aggressive prostate cancer, promotes prostate cancer cell migration and invasion and is inhibited by statins. Oncotarget. 2011;2:1176–1190. doi: 10.18632/oncotarget.397. [DOI] [PMC free article] [PubMed] [Google Scholar]