Abstract
Mutations in the aristaless-related homeobox (ARX) gene result in a spectrum of structural and functional nervous system disorders including lissencephaly, movement disorders, intellectual disabilities, and epilepsy. Some patients also have symptoms indicative of hypothalamic dysfunction, however, little is known about the role of ARX in diencephalic development. To begin evaluating diencephalic defects we examined the expression of a panel of known genes and gene products that label specific diencephalic nuclei in two different Arx mutant mouse lines. Male mice engineered to have a polyalanine expansion mutation (Arx(GCG)7/Y) revealed no expression differences in any diencephalic nucleus when compared to wildtype littermates. In contrast, mice null for Arx (Arx−/Y) lost expression of specific markers of the thalamic reticular nucleus (TRN) and zona incerta (ZI), while retaining expression in other thalamic nuclei and in the hypothalamus. Tyrosine hydroxylase, a marker of the ZI’s dopaminergic A13 sub-nucleus, was among those lost, suggesting a requirement for Arx in normal TRN and ZI development, and for A13 dopaminergic fate, specifically. Since the ZI and A13 regions make connections to several hypothalamic nuclei, such mis-specification may contribute to the “hypothalamic dysfunction” observed in some patients.
Keywords: Arx, zona incerta, thalamic reticular nucleus, A13, dopaminergic fate, diencephalon
Introduction
The aristaless-related homeobox gene, ARX, is an evolutionarily conserved transcription factor found on the X-chromosome that is required for normal forebrain development (1,2, reviewed in 3 and 4). A variety of mutations in this gene have been described in human patients, typically males, resulting in a diverse array of phenotypes that range from relatively mild intellectual disability, various types of epilepsy, and social impairments, to severe structural malformations such as agenesis of the corpus collosum and lissencephaly (reviewed in 3 and 4). Mutations in highly conserved amino acids of the homeodomain, large deletions, and truncations frequently give rise to severe structural abnormalities, intractable seizures, ambiguous genitalia, and death in the neonatal period (1,5). In contrast, mutations that result in an expansion of the gene’s first polyalanine tract, such as those found in patients with Ohtahara or West Syndrome, typically do not lead to obvious structural changes, but do result in infantile spasms or other severe epilepsies, accompanied by intellectual disabilities later in life (6–8).
Interestingly, XLAG (X-linked lissencephaly with ambiguous genitalia) patients with loss-of-function mutations as described above, also present with impaired temperature and circadian rhythm regulation, both symptoms referable to hypothalamic dysfunction (9–11). While it is known that Arx is expressed as early as E12.5 in the mouse diencephalon, including regions destined to become specific ventral thalamic nuclei and hypothalamic nuclei (1,12,13), its precise role in the patterning and specification of these regions is largely unknown.
Given the expression of Arx in the anlagen for the hypothalamus and the hypothalamic dysregulation observed in patients, as well as the implicated involvement of the thalamus in several forms of epilepsy, particularly infantile spasms (14,15), we hypothesized that Arx would have a role in normal diencephalic development and function. To begin testing this hypothesis we have examined the expression of various markers in specific diencephalic nuclei during development using immunohistochemistry and in situ hybridization. Furthermore, we have evaluated these nuclei in two Arx mutant mouse models. One model includes an expansion of the first polyalanine track (Arx(GCG)7/Y). Patients with this mutation often survive past early childhood and typically do not exhibit features of hypothalamic dysregulation, though they do exhibit progressive epilepsy and learning deficits (6,7,16). In contrast, patients with a deletion or loss of function, comparable to our second mouse model (Arx−/Y), frequently succumb in the perinatal period with symptoms of hypothalamic dysregulation. Interestingly, this mouse model and XLAG patients exhibit similar brain morphological and functional abnormalities. We now report that the hypothalamic nuclei appear intact in both mouse models, however, the Arx−/Y mice lack expression of specific markers in the thalamic reticular nucleus (TRN) and zona incerta (ZI). Additionally, the A13 subnucleus of the zona incerta lacks the expected dopaminergic neuronal marker, tyrosine hydroxylase. These findings suggest that while Arx is not required for diencephalic patterning, it is essential for the proper development and specification of discreet sub-populations of neurons in the thalamus.
Materials and Methods
Mice
All animal experiments were performed in accordance with the Children’s Hospital of Philadelphia Animal Care and Use Committee. Male mice carrying a seven alanine expansion within the first polyalanine tract of Arx (Arx(GCG)7/Y) were generated and provided by Dr. K. Kitamura(16). The Arx−/Y mice were generated from mice carrying a floxed allele (17) by crossing homozygous floxed females with males homozygous for E2a-Cre, which expresses CRE ubiquitously in early embryos, prior to implantation (#003724, Jackson Laboratories). The resultant compound heterozygous females, effectively Arx−/X, were back-crossed to wild type C57B6 males to eliminate the E2a-Cre. As expected, based upon the previously described Arx−/Y mice generated in 2002 by Kitamura et al. (1), Arx−/Y mice died shortly after birth, had visibly smaller brains, and abnormal fiber tracts (unpublished observations).
Tissue Processing and Microscopy
For all experiments, E18.5 male mice were obtained from crosses between heterozygous females (Arx(GCG)7/X or Arx−/X) and wild type C57B6 males. Pregnant dams were euthanized, the embryos removed from the uterus and decapitated in cold HBSS (Gibco, Carlsbad CA), and the heads fixed in 4% paraformaldehyde in 1× PBS overnight. Heads were washed in 1× PBS, cryoprotected in 30% sucrose overnight, and then frozen in embedding medium for cryosectioning. Coronal or sagittal serial sections were cut 16 µm thick, and mounted on positively charged slides such that every eighth section shared a slide. All labeling protocols were therefore carried out on adjacent sections, with sample sizes ranging from 3–7 animals for each genotype and stain/antibody/probe. Genotypes and gender were determined from tail tissue DNA using established primers and PCR protocols (16,17). All pictures were taken on a Leica DM6000B microscope equipped with a Leica DFC420 camera (Buffalo Grove IL).
In Situ Hybridization
Probe generation
Digoxigenin-labeled RNA probes for Nr5a1, Pomc (kindly provided by Dr. D. Epstein, University of Pennsylvania in Philadelphia, PA), Six3 (kindly provided by Dr. P. Gray, Washington University in St. Louis, MO(18)), Dlx2 (AddGene #15537, Cambridge MA), and Gad67 (kindly provided by Dr. N. Tillakaratne, University of California, Los Angeles) were all generated from pBluescript plasmids with DNA template inserts. Briefly, plasmids were linearized with the appropriate restriction enzyme, cleaned with phenol:cholorform and concentrated, then the RNA probe labeled and amplified with T7 or T3 polymerase in a 1× mixture of digoxigenin-labeled dNTPs, RNasin, DTT, and enzyme buffer. The DNA template was digested away with DNaseI, and the probes concentrated, cleaned, and resuspended in RNase-free water for in situ hybridzation.
In situ staining
All slides were post-fixed in 4% PFA for 10 min, washed in PBT (0.1% Tween-20 in 1× PBS), permeabilized in 0.5% proteinase K, and acetylated in 0.1 M TEA (1.86% triethanolamine, 0.4% 10N NaOH, 0.5% acetic anhydride). Slides were incubated with probe in hybridization solution (50% formamide, 5× SSC, 2% blocking reagent (Roche, Mannheim, Germany; 11096176001), 0.1% Triton, 0.15 CHAPS, 1mg/ml tRNA from yeast, 5mM EDTA, 50ug/ml heparin) overnight at 65°C in a box humidified with a solution of 50% formamide and 5× SSC. Following an incubation in 1× SSC/50% formamide for 30 minutes at 65°C, slides were treated with RNaseA, and placed through a series of washes with SSC and MABT (100mM maleic acid, 150mM NaCl, 0.1% Tween-20, pH 7.5). After blocking for 1 hour at room temperature in 2% blocking reagent (Roche, Indianapolis IN) and 20% goat serum, slides were incubated overnight at 4°C with anti-DIG antibody (1:2500; Roche #11093274910, Indianapolis IN) in 2% blocking reagent and 5% goat serum. Slides were washed with five 1 hour washes in MABT and one 10min wash in NTM (100mM Tris pH 9.5, 100mM NaCl, 50mM MgCl2) before developing in the dark overnight with BM Purple (Roche, 11442074001, Indianapolis IN) at room temperature. Finally, slides were rinsed with NTM, washed 3× in 1× PBS, fixed in PFA for 30min, dehydrated, and mounted with Permount.
Immunohistochemistry
Detection of Islet-1 (Isl-1) and tyrosine hydroxylase (TH) expression was carried out using standard immunohistochemical protocols. Briefly, slides were baked at 65°C for 20min and either washed with 1× PBS for 15 min and permeabilized with PBS-T (0.5% TritonX-100 in 1× PBS) with two 5 min incubations (for TH), or autoclaved in antigen retrieval solution (Vector Laboratories #H3300, Burlingame CA) for 20 min at 105°C (for Isl-1). Endogenous peroxidase activity was blocked with 0.3% hydrogen peroxide in methanol for 20 min, slides washed twice with PBS-T, and blocked first in M.O.M. blocking reagent (for Isl-1, Vector Labs BMK-2202 kit, Burlingame CA) and then in 10% normal goat serum in PBS-T for 30 min (for both Isl-1 and TH). Slides were then incubated overnight in either mouse anti-Islet1 (5mg/ml in 10% goat serum, Developmental Studies Hybridoma Bank #40.2D6, Iowa City IA) at 4°C, or in rabbit anti-TH (1:500 in 1% goat serum, Millipore #AB152, Billerica MA) at room temperature. Slides were washed twice, incubated in biotin-conjugated secondary antibody for 30min, followed by two more washes and then a 30 min incubation with ABC solution (Vector Laboratories #PK-6100, Burlingame CA). Slides were washed twice more and developed with ImmPACT DAB (Vector Laboratories #SK-4105, Burlingame CA) for 2 min in the dark. Slides were then dehydrated and mounted with Permount.
Results
Hypothalamic nuclei are intact in Arx(GCG)7/Y and Arx−/Y mice
To evaluate the role of Arx in the development of the hypothalamus, we employed a panel of markers known to express in specific hypothalamic regions and nuclei. We began with markers specific for the ventromedial hypothalamus (VMH) and the arcuate nucleus (ARC), known to regulate satiety and appetite respectively (19,20). In situ hybridization for Nr5a1, which demarcates the VMH (21), was used to assess the nucleus’s general morphology and placement, while Pomc, was employed to examine these same characteristics in the ARC (22). In both the Arx(GCG)7/Y and Arx−/Y mice, Nr5a1 was detected in the expected location of the VMH and at visually similar intensity and morphology to wild type controls, suggesting proper development of this nucleus despite altered Arx function (Figure 1). Likewise, Pomc expression reliably demarcated the arcuate nucleus in both transgenic mouse models, as it did in wild type, an indication that this nucleus also remains intact (Figure 2).
Figure 1. Nr5a1 demarcates the VMH in WT and Arx transgenic mice.
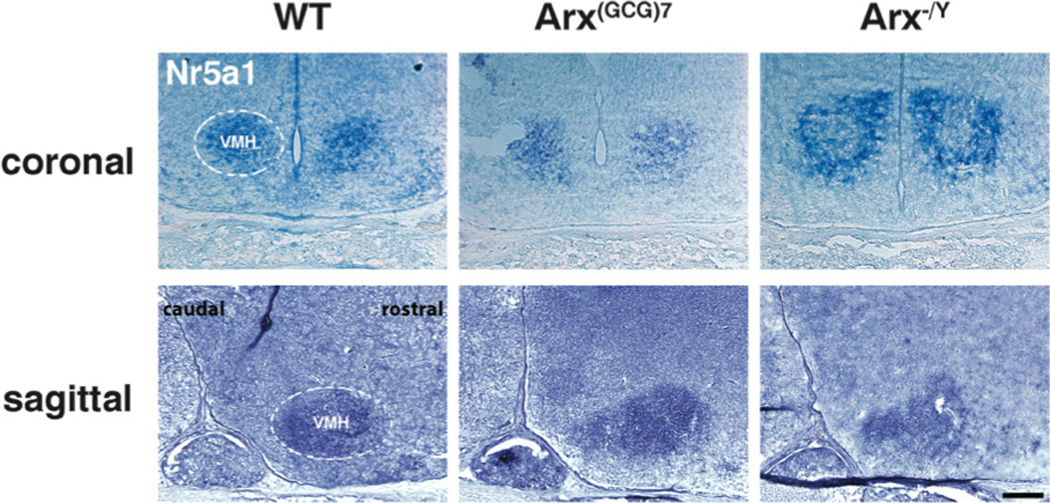
Representative coronal and sagittal sections, probed with Nr5a1, are shown for wild type (left panels), Arx(GCG)7/Y (middle panels) and Arx−/Y (right panels) mice. For reference, the left ventromedial hypothalamic nucleus (VMH) is roughly enclosed by dotted lines in the wild type images, but is present in all sections probed. Scale bar = 200 µm.
Figure 2. Pomc demarcates the ARC and pituitary in WT and Arx transgenic mice.
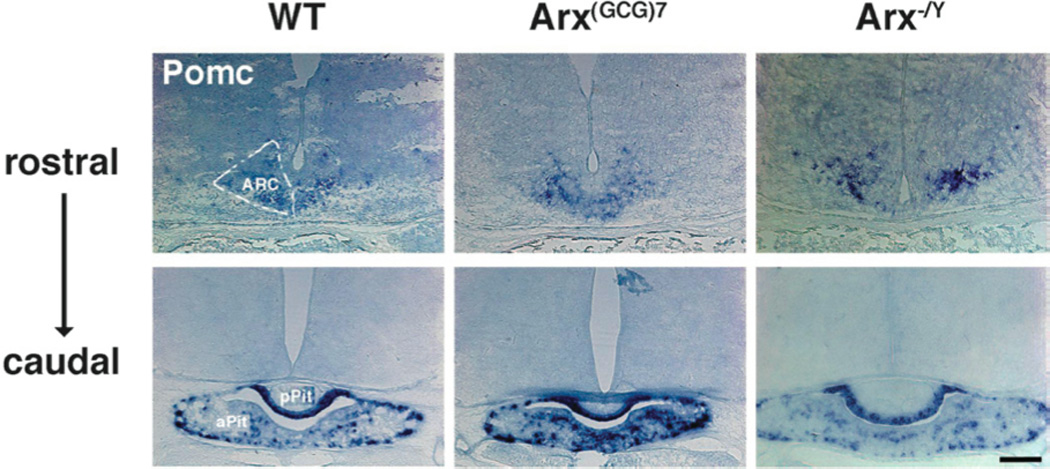
Representative coronal sections, probed with Pomc, are shown for wild type (left panels), Arx(GCG)7/Y (middle panels) and Arx−/Y (right panels) mice. In the wild type images, the approximate outline of the left arcuate nucleus (ARC) is illustrated by dotted lines in the top, more rostral section, whereas the anterior and posterior pituitary (aPit and pPit, respectively) are labeled in the bottom, more caudal section. These nuclei are also normally expressing Pomc in the Arx transgenic animals. Scale bar = 200 µm.
In addition to the ARC, Pomc is also expressed in the pituitary (23), which releases hormones in response to signals from various hypothalamic centers. As was observed for the ARC, no differences in expression pattern were detected in the pituitary of either Arx(GCG)7/Y or Arx−/Y mice as compared to wild type (Figure 2).
We next evaluated the suprachiasmatic nucleus (SCN). This nucleus controls the body’s circadian rhythms, and XLAG patients have difficulty maintaining sleep/wake cycles (9,11). We assessed location and morphology of the SCN with Six3 expression, as this transcription factor is required for SCN development and remains expressed into adulthood (18). Similar to our hypothalamic nuclei results for Nr5a1 and Pomc, expression of Six3 was unchanged in the SCN of both Arx(GCG)7/Y and Arx−/Y mice when compared to wild type mice (Figure 3).
Figure 3. Six3 demarcates the PVN and SCN in Arx transgenic mice.
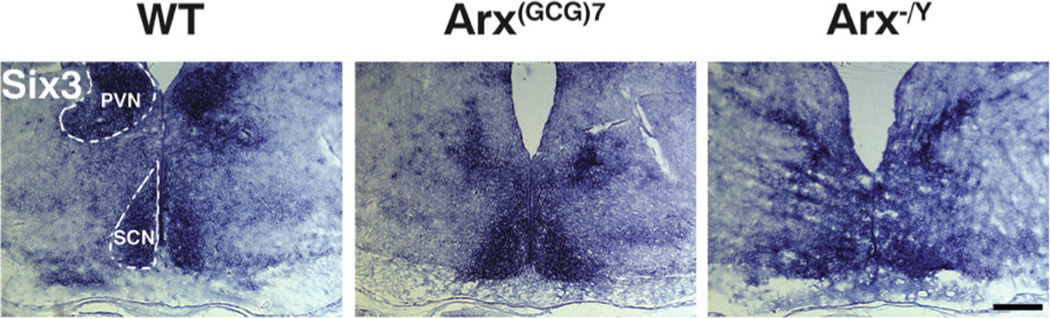
Representative coronal sections at the level of the optic chiasm and probed with Six3, are shown for wild type (left panels), Arx(GCG)7/Y (middle panels) and Arx−/Y (right panels) mice. For reference, the left periventricular nucleus (PVN) and suprachiasmatic nucleus (SCN) are enclosed by dotted lines in the wild type image. These nuclei are also normally expressing Six3 in the Arx transgenic animals. Scale bar = 200 µm.
Arx−/Y mice lack specific marker expression in the zona incerta and reticular nucleus of the thalamus
In addition to marking the SCN, Six3 is also expressed in several other diencephalic nuclei, including the hypothalamic periventricular (PVN) and anterodorsal (AD) nuclei, as well as the thalamic zona incerta (ZI) and reticular nucleus (TRN) (24). Although the SCN, PVN, and AD stained robustly for Six3 in all genotypes examined (Figures 3, 4), Arx−/Y mice showed a complete absence of expression in the expected thalamic nuclei and subnuclei (Figure 4). Arx(GCG)7/Y mice, however, maintained Six3 expression in all expected hypothalamic and thalamic nuclei.
Figure 4. Six3 and Isl-1 are not expressed in TRN or ZI of Arx−/Y mice.
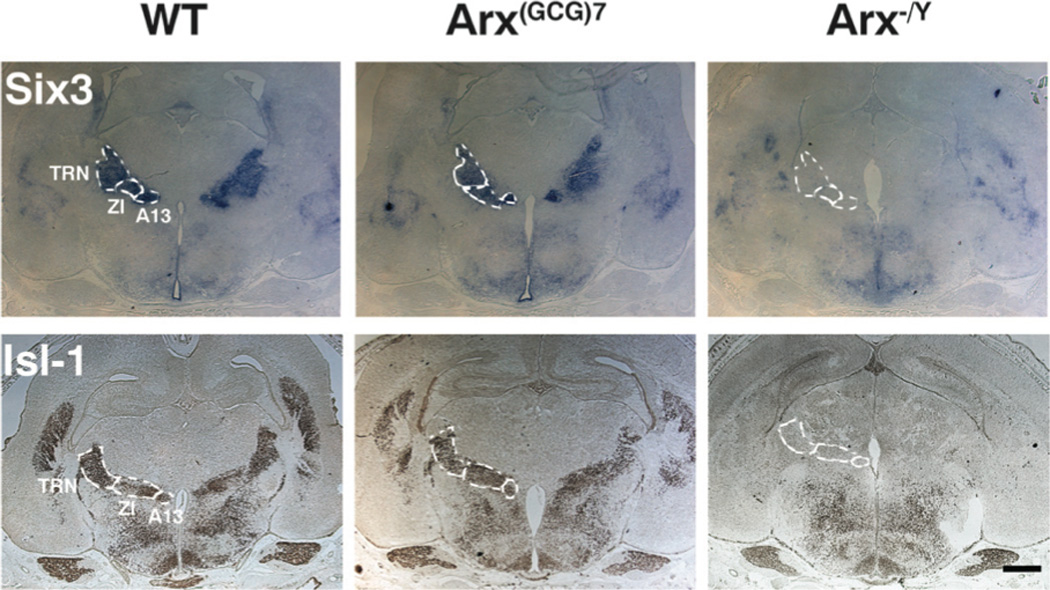
Representative coronal sections are shown from wild type (left panels), Arx(GCG)7/Y (middle panels), and Arx−/Y (right panels), that were probed with Six3 (top panels) or stained for Isl-1 (bottom panels). The approximate boundaries of the thalamic reticular nucleus (TRN), majority of the zona incerta (ZI) and the ZI’s A13 subnucleus (A13) are illustrated with dotted lines in all images. Staining is present for both markers within these regions in the wild type and Arx(GCG)7/Y sections, but is absent in the Arx−/Y sections. Scale bar = 500 µm.
Given the lack of expression of Six3 in the TRN, the ZI, and the ZI’s A13 dopaminergic subnucleus, we sought to further define expression patterns in this region. We began by examining the expression of Isl-1, a LIM domain transcription factor that is highly expressed in these regions (25). Just as with Six3, Isl-1 was detected in the entire ZI and TRN of both wild type and Arx(GCG)7/Y mice, but was completely absent from Arx−/Y mice (Figure 4).
Zona incerta and reticular nucleus are present Arx−/Y mice
To address whether the loss of Six3 and Isl-1 expression in the ZI and TRN was due to complete loss of these nuclei in Arx−/Y mice, we probed for Gad67, which detects all GABAergic interneurons. Although the ZI contains a variety of different neuron types, inhibitory interneurons account for more than 80% of the ventral ZI (26), and the TRN consists entirely of GABAergic neurons (27,28). As expected, Gad67 was detected in the TRN and ZI (but not the dopaminergic A13 region) of both wild types and Arx(GCG)7/Y mice. Interestingly, and in contrast to our findings for Six3 and Isl-1, Gad67 was also detected in these regions on Arx−/Y sections (Figure 5), although the level of expression appeared slightly reduced and perhaps restricted, as compared to wild type. Despite this potential decrease, the presence of Gad67 expression clearly indicates that although the normal gene expression pattern is altered in the ZI and TRN, these nuclei are present and remain GABAergic.
Figure 5. Gad67 and Dlx2 are expressed in TRN and ZI of Arx transgenic mice.
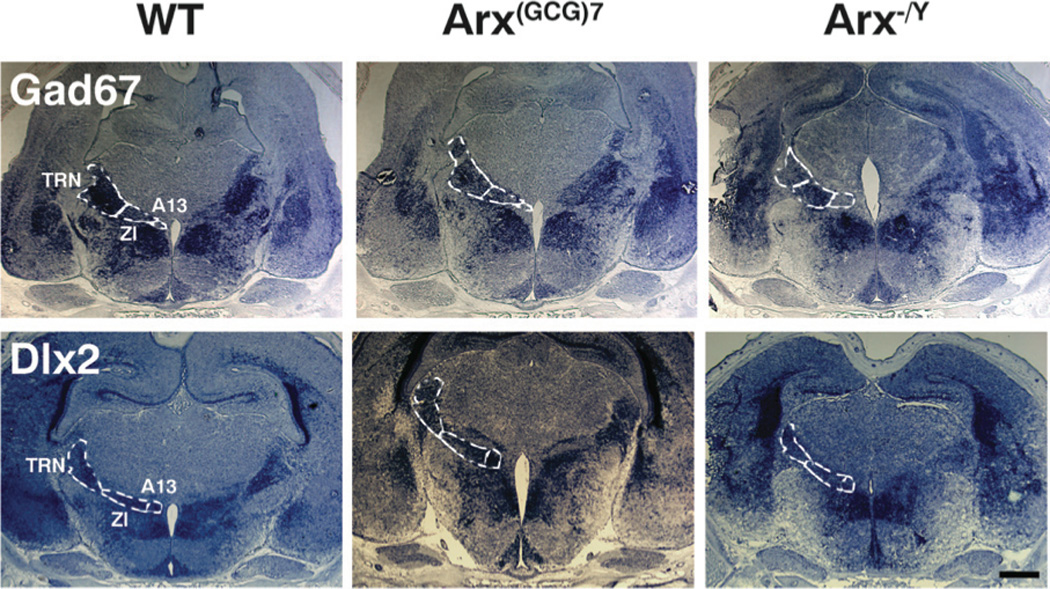
Representative coronal sections are shown from wild type (left panels), Arx(GCG)7/Y (middle panels), and Arx−/Y (right panels), that were probed with Gad67 (top panels) or Dlx2 (bottom panels). The approximate boundaries of the thalamic reticular nucleus (TRN), majority of the zona incerta (ZI) and the ZI’s A13 subnucleus (A13) are illustrated with dotted lines in all images. Gad67 is expressed in the TRN and majority of ZI, but not the A13 dopaminergic subnucleus of all three genotypes, whereas Dlx2 is expressed in all three regions for all three genotypes. Scale bar = 500 µm.
The A13 subnucleus of the ZI contains dopaminergic neurons and not GABAergic neurons, and so Gad67 does not label this nucleus in wild type mice (Figure 5). Dlx2, however, is a known regulator of Isl-1 and dopaminergic fate in the developing ventral thalamus, appearing as early as E10–11, and continues to be expressed in the ZI and TRN until at least P0 (29–31). Dlx2 is also required for Arx expression and may play a role in GABAergic fate specification in other regions of the developing forebrain (32–34), placing it upstream of any changes in expression that may be due to Arx deletion, thus making it an ideal marker for the presence or absence of the ZI’s A13 subnucleus. As expected, Dlx2 was detected in the appropriate regions for all three nuclei and subnuclei, regardless of genotype, thus confirming the presence of the ZI and TRN in the Arx−/Y mice and demonstrating that the A13 subnucleus exists (Figure 5).
Arx loss of function results in loss of dopaminergic neurons in ZI
Since GABAergic neurons were detected in the ZI and TRN of Arx−/Y mice, despite loss of Six3 and Isl-1 expression, we then examined whether the dopaminergic neurons of the A13 subnucleus also were also conserved. Previous studies have demonstrated a requirement of Dlx2 for the dopaminergic cell fate in the ventral thalamus, such that loss of Dlx2 eliminates expression not only of Isl-1, but also of tyrosine hydroxylase (TH), an enzyme essential to the synthesis of dopamine, and frequently used as a marker of dopaminergic neurons (29). We therefore hypothesized that loss of Arx not only results in loss of Six3 and Isl-1 in the A13 subnucleus, but also TH expression, and hence dopaminergic fate. Immunohistochemical staining for TH confirmed this, as it was easily detected in the A13 subnucleus of both wild type and Arx(GCG)7/Y mice, but not Arx−/Y mice (Figure 6). This implies a requirement for Arx in establishing the dopaminergic fate of these A13 neurons.
Figure 6. Arx−/Y mice lack TH expression in the ZI’s A13 dopaminergic subnucleus.
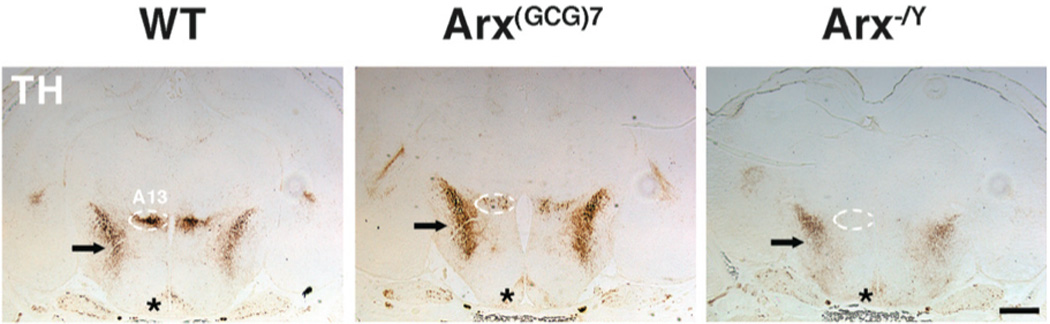
Representative coronal sections, stained for tyrosine hydroxylase (TH), are shown for wild type (left panel), Arx(GCG)7/Y (middle panel) and Arx−/Y (right panel) mice. The approximate boundaries of the A13 subnucleus of the zona incerta (ZI) are demarcated by dotted white lines. TH expression is apparent in this region for both wild type and Arx(GCG)7/Y, but is absent from Arx−/Y sections. Other areas of expected TH staining, such as the arcuate nucleus (*) and the retrochiasmatic projection fibers from the median forebrain bundle (arrow) maintained TH expression. Scale bar = 500 µm.
Discussion
Immunohistochemistry and in situ hybridization were used to assess the role of Arx in diencephalic development, by determining specific marker expression patterns in transgenic mouse models. Two mouse lines, which model Ohtahara Syndrome and XLAG, were employed to determine if alteration of Arx function in the diencephalon could explain phenotypic components of these syndromes. Although we found no obvious changes to the hypothalamic nuclei in either model, mice with a complete loss of Arx function, Arx−/Y, also lost expression of Six3 and Isl-1 in the thalamic reticular nucleus (TRN) and zona incerta (ZI), resulting in the loss of dopaminergic fate in the A13 subregion of the ZI (see Figure 7 for a schematized depiction of our findings). These findings establish a requirement for Arx expression in the dopaminergic lineage of this discreet population of neurons, and suggest a possible alteration in cell fate for other regions of the ZI and TRN.
Figure 7. Schematized summary of marker expression within the TRN and ZI of wild type and Arx−/Y mice.
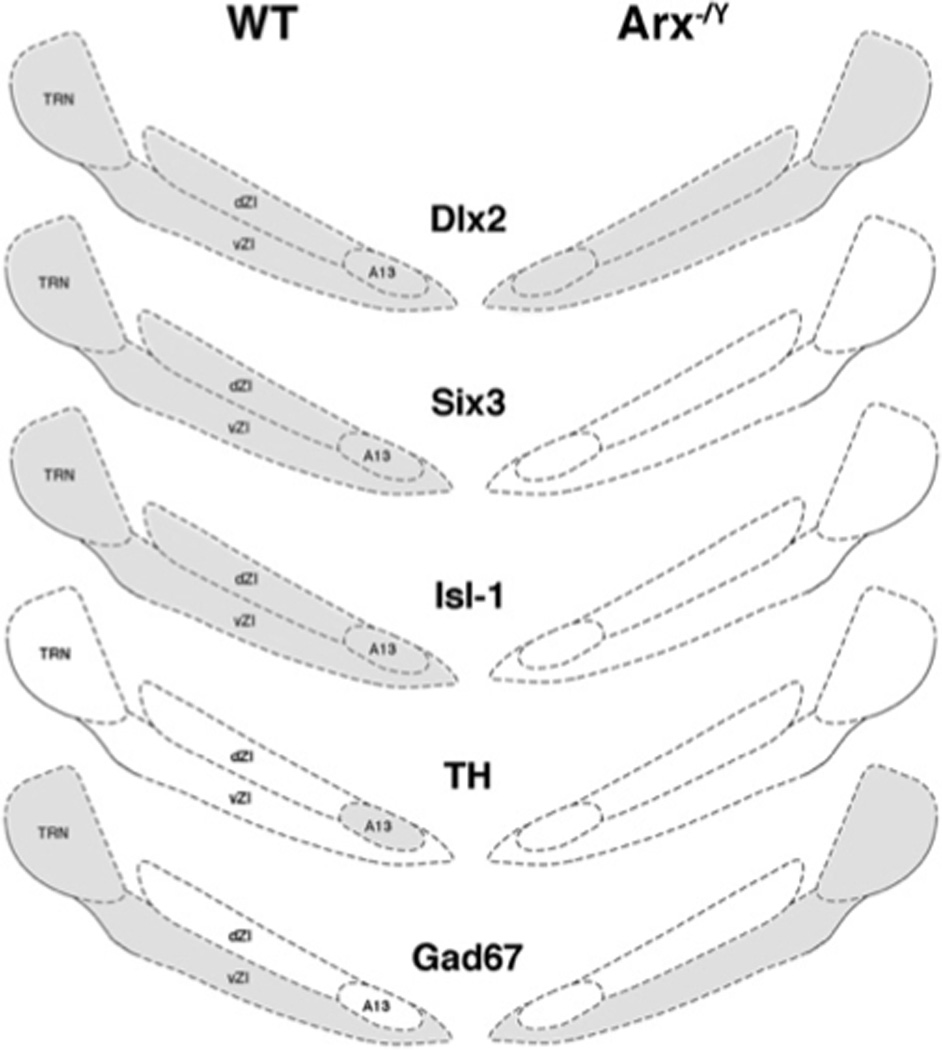
The expected and observed expression patterns within the wild type thalamic nuclei are depicted on the left, with the corresponding observed expression pattern for the Arx−/Y mice depicted on the right. Since Dlx2 is upstream of Arx, its expression pattern is unchanged by loss of Arx. In contrast, Six3, Isl-1, and TH, previously demonstrated to require Dlx2 for expression in these areas, are lost upon loss of Arx. This indicates that Arx is also required for their expression, but places it downstream of Dlx2 in the cascade. Gad67 expression is essentially unchanged in the Arx−/Y, indicating that Arx is not required for Gad67 expression in these ventral forebrain regions. Drawings are modified, with permission, from Paxinos et al.’s Atlas of the Developing Mouse Brain at P0 (2007) (45).
One of our major findings is the loss of dopamine neurons from the A13 subnucleus of the ZI in Arx−/Y mice, as indicated by the absence of TH staining (Figure 6). Since Dlx2 staining was still detected, these findings suggest a role for Arx in dopaminergic fate determination. This is significant, for although the precise function of this region is still being elucidated, the A13 dopaminergic neurons have been shown to both receive and send projections to several regions of the hypothalamus and thalamus, and may be important in the regulation of autonomic, endocrine, and motor functions (34, reviewed in 35). Altering the function of these neurons would be expected to significantly impact their normal activity, potentially contributing to some of the “hypothalamic” attributable symptoms observed in XLAG patients.
Similarly, the requirement of Arx for expression of Six3 and Isl-1 in both the TRN and ZI is a novel finding of this study. Like its’ A13 subnucleus, the precise function of the ZI is controversial, though its’ connections are wide, varied, and suggestive of a role in integrating sensory input with arousal, attention, postural, or visceral output (reviewed in 36). The role of the TRN is also ambiguous, however, it is generally considered to be an important “gate” that directs cortical attention, and has recently been shown to receive connections from the ZI (37,38). Therefore, it is worth speculating as to the significance of losing both Isl-1 and Six3 expression in these nuclei. Since microarray and promoter-occupancy studies have failed to show any significant alteration in expression or Arx promoter-binding of either Isl-1 or Six3, it is unlikely that these transcription factors are directly regulated by Arx (17,39,40). Furthermore, since both the TRN and ZI nuclei still contained GABAergic neurons in Arx−/Y mice, as evidenced by Gad67 staining, it is difficult to predict the end effect that loss of these transcription factors would have on the functions of these nuclei. However, particular subtypes of GABAergic interneurons have distinct electrical properties and connections, and whether the appropriate interneuron subtype composition remains in the TRN and ZI of Arx−/Y mice is still unknown, as expression of subtype specific markers do not appear until later ages. Considering that the overall expression of Gad67 in these areas appeared reduced in Arx−/Y mice, and that similar mouse models exhibit both reduced numbers and mislocalization of specific interneuron subtypes in other brain regions (1), it is possible that loss of Arx function in these regions has a similar effect. Such alterations to this delicate balance of GABAergic subtypes would presumably lead to functional consequences, which may also contribute to some of the phenotypes observed in XLAG patients.
Dlx1/2 has been shown to be required for specifying dopaminergic fate in the developing ventral thalamus (29). Detected as early as E10.5, knocking out both Dlx1 and Dlx2 results in loss of expression of Pax6, Isl-1, and TH from the primitive A13 region at E12.5. Since Arx is a direct target of Dlx2 (32,33), Pax6 is known to be regulated by Six3 in other areas of the developing forebrain (41), and TH is thought to be regulated by Isl-1 (42), the loss of Six3, Isl-1, and TH staining from this same region in our Arx−/Y mice at E18.5 is entirely consistent with the findings in Dlx1/2 compound knockouts. Furthermore, since Dlx2 expression was unaltered in the Arx−/Y mice, our findings are the first to demonstrate that Arx is required for Six3 and Isl-1 expression in mouse, as well as dopaminergic fate in this region.
Recent studies in zebrafish have revealed a similar role for Arx in establishing dopaminergic cell fate. Using morphants of Arx and Isl-1 have indicated that while Isl-1 is required at later stages for terminal differentiation of dopamine neurons, Arx is required for earlier patterning and for the expression of Pax6a, a homolog of the mouse Pax6 (43). This is consistent with our findings in the mouse, placing Arx upstream of Six3, Isl-1 and TH expression (Figure 7). In the zebrafish studies, knockdown of either Arx or Isl-1 resulted in reduced TH staining, however, contrary to our results, neither Isl-1 or Arx expression was altered when the other was knocked down, indicating two independent pathways (43). These findings suggest that while the necessary gene expression patterns for dopaminergic fate in this region are evolutionarily conserved, the precise pathways involved in establishing these patterns differ between species. Further studies in both species will be useful to identify the intermediate players and their respective roles in determining dopaminergic fate.
It is worth noting that all expression changes reported here were only observed in the Arx−/Y mice, and not in the Arx(GCG)7/Y mice. This is not particularly surprising, considering our previous data indicating that only a subset of gene targets are misregulated by polyalanine-expanded Arx, as compared to the complete loss of function in Arx−/Y mice (44). Our data is also consistent with observations from patients with polyalanine expansion mutations, who do not exhibit any outward diencephalic dysfunction.
In conclusion, our findings are the first to describe a requirement for Arx in the proper specification of the TRN and ZI in mouse. Furthermore, we illustrate for the first time that the dopaminergic fate of the A13 region depends upon functional Arx expression. Taken together, these studies provide further evidence for the specificity of functional consequences for specific Arx mutations, and suggest another potential mechanism for the hypothalamic dysregulation observed in patients with severe Arx mutations.
Supplementary Material
Acknowledgements
The authors would like to thank George Clement and Almedia McCoy for their assistance with genotyping and mouse colony management, and Dr. Stewart Anderson for the Dlx2 probe. This research was supported by the NIH (NS46616, HD26979, GM081259, KO2NS065975).
References
- 1.Kitamura K, Yanazawa M, Sugiyama N, Miura H, Iizuka-Kogo A, Kusaka M, et al. Mutation of ARX causes abnormal development of forebrain and testes in mice and X-linked lissencephaly with abnormal genitalia in humans. Nature genetics. 2002;32(3):359–369. doi: 10.1038/ng1009. [DOI] [PubMed] [Google Scholar]
- 2.Miura H, Yanazawa M, Kato K, Kitamura K. Expression of a novel aristaless related homeobox gene “Arx” in the vertebrate telencephalon, diencephalon and floor plate. Mechanisms of development. 1997;65(1–2):99–109. doi: 10.1016/s0925-4773(97)00062-2. [DOI] [PubMed] [Google Scholar]
- 3.Friocourt G, Poirier K, Rakić S, Parnavelas JG, Chelly J. The role of ARX in cortical development. The European journal of neuroscience. 2006;23(4):869–876. doi: 10.1111/j.1460-9568.2006.04629.x. [DOI] [PubMed] [Google Scholar]
- 4.Friocourt G, Parnavelas JG. Mutations in ARX Result in Several Defects Involving GABAergic Neurons. Frontiers in cellular neuroscience. 2010;4:4. doi: 10.3389/fncel.2010.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dobyns WB, Berry-Kravis E, Havernick NJ, Holden KR, Viskochil D. X-linked lissencephaly with absent corpus callosum and ambiguous genitalia. American journal of medical genetics. 1999;8;86(4):331–337. [PubMed] [Google Scholar]
- 6.Cossée M, Faivre L, Philippe C, Hichri H, De Saint-Martin A, Laugel V, et al. ARX polyalanine expansions are highly implicated in familial cases of mental retardation with infantile epilepsy and/or hand dystonia. American journal of medical genetics. 2011;155A:98–105. doi: 10.1002/ajmg.a.33785. [DOI] [PubMed] [Google Scholar]
- 7.Kato M, Saitoh S, Kamei A, Shiraishi H, Ueda Y, Akasaka M, et al. A longer polyalanine expansion mutation in the ARX gene causes early infantile epileptic encephalopathy with suppression-burst pattern (Ohtahara syndrome) The American Journal of Human Genetics. 2007;81:361–366. doi: 10.1086/518903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guerrini R, Moro F, Kato M, Barkovich AJ, Shiihara T, McShane MA, et al. Expansion of the first PolyA tract of ARX causes infantile spasms and status dystonicus. Neurology. 2007;69:427–433. doi: 10.1212/01.wnl.0000266594.16202.c1. [DOI] [PubMed] [Google Scholar]
- 9.Miyata R, Hayashi M, Miyai K, Akashi T, Kato M, Kohyama J. Analysis of the hypothalamus in a case of X-linked lissencephaly with abnormal genitalia (XLAG) Brain & development. 2009;31(6):456–460. doi: 10.1016/j.braindev.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Hahn a, Gross C, Uyanik G, Hehr U, Hügens-Penzel M, Alzen G, et al. X-linked lissencephaly with abnormal genitalia associated with renal phosphate wasting. Neuropediatrics. 2004;35(3):202–205. doi: 10.1055/s-2004-817955. [DOI] [PubMed] [Google Scholar]
- 11.Bonneau D, Toutain A, Laquerrie A, Saugier-veber P, Barthez M, Radi S, et al. X-Linked Lissencephaly with Absent Corpus Callosum and Ambiguous Genitalia (XLAG): Clinical, Magnetic Resonance Imaging, and Neuropathological Findings. 2002:340–349. doi: 10.1002/ana.10119. [DOI] [PubMed] [Google Scholar]
- 12.Cobos I, Calcagnotto ME, Vilaythong AJ, Thwin MT, Noebels JL, Baraban SC, et al. Mice lacking Dlx1 show subtype-specific loss of interneurons, reduced inhibition and epilepsy. Nature neuroscience. 2005;8(8):1059–1068. doi: 10.1038/nn1499. [DOI] [PubMed] [Google Scholar]
- 13.Shimogori T, Lee Da, Miranda-Angulo A, Yang Y, Wang H, Jiang L, et al. A genomic atlas of mouse hypothalamic development. Nature neuroscience. Nature. 2010;13(6):767–775. doi: 10.1038/nn.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mccormick DA. Cortical and subcortical generators of normal and abnormal rhythmicity. International review of neurobiology. 2002;49:99–114. doi: 10.1016/s0074-7742(02)49009-5. [DOI] [PubMed] [Google Scholar]
- 15.Steriade M, Timofeev I. Generators of ictal and interictal electroencephalograms associated with infantile spasms: intracellular studies of cortical and thalamic neurons. International review of neurobiology. 2002;49:77–98. doi: 10.1016/s0074-7742(02)49008-3. [DOI] [PubMed] [Google Scholar]
- 16.Kitamura K, Itou Y, Yanazawa M, Ohsawa M, Suzuki-Migishima R, Umeki Y, et al. Three human ARX mutations cause the lissencephaly-like and mental retardation with epilepsy-like pleiotropic phenotypes in mice. Human molecular genetics. 2009;18(19):3708–3724. doi: 10.1093/hmg/ddp318. [DOI] [PubMed] [Google Scholar]
- 17.Fulp CT, Cho G, Marsh ED, Nasrallah IM, Labosky Pa, Golden JA. Identification of Arx transcriptional targets in the developing basal forebrain. Human molecular genetics. 2008;17(23):3740–3760. doi: 10.1093/hmg/ddn271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.VanDunk C, Hunter La, Gray Pa. Development, maturation, and necessity of transcription factors in the mouse suprachiasmatic nucleus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(17):6457–6467. doi: 10.1523/JNEUROSCI.5385-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.King BM. The rise, fall, and resurrection of the ventromedial hypothalamus in the regulation of feeding behavior and body weight. Physiology & Behavior. 2006;87:221–244. doi: 10.1016/j.physbeh.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki K, Simpson KA, Minnion JS, Shillito JC, Bloom SR. The role of gut hormones and the hypothalamus in appetite regulation. Endocrine Journal. 2010;57:359–372. doi: 10.1507/endocrj.k10e-077. [DOI] [PubMed] [Google Scholar]
- 21.Tran PV, Lee MB, Marín O, Xu B, Jones KR, Reichardt LF, et al. Requirement of the orphan nuclear receptor SF-1 in terminal differentiation of ventromedial hypothalamic neurons. Molecular and Cellular Neuroscience. 2003;22(4):441–453. doi: 10.1016/S1044-7431(03)00027-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Padilla SL, Reef D, Zeltser LM. Defining POMC neurons using transgenic reagents: impact of transient Pomc expression in diverse immature neuronal populations. Endocrinology. 2012;153(3):1219–1231. doi: 10.1210/en.2011-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lugo DI, Roberts JL, Pintar JE. Analysis of proopiomelanocortin gene expression during prenatal development of the rat pituitary gland. Molecular Endocrinology. 1989;3:1313–1324. doi: 10.1210/mend-3-8-1313. [DOI] [PubMed] [Google Scholar]
- 24.Conte I, Morcillo J, Bovolenta P. Comparative analysis of Six 3 and Six 6 distribution in the developing and adult mouse brain. Developmental dynamics : an official publication of the American Association of Anatomists. 2005;234(3):718–725. doi: 10.1002/dvdy.20463. [DOI] [PubMed] [Google Scholar]
- 25.Allen Developing Mouse Brain Atlas [database online] 2012 Available from: http://developingmouse.brain-map.org/experiment/show/100092412. [Google Scholar]
- 26.Kolmac C, Mitrofanis J. Distribution of various neurochemicals within the zona incerta: an immunocytochemical and histochemical study. Anatomy and embryology. 1999;199(3):265–280. doi: 10.1007/s004290050227. [DOI] [PubMed] [Google Scholar]
- 27.Clemence AE, Mitrofanis J. Cytoarchitectonic heterogeneities in the thalamic reticular nucleus of cats and ferrets. Journal of Comparative Neurology. 1992;322:167–180. doi: 10.1002/cne.903220203. [DOI] [PubMed] [Google Scholar]
- 28.Houser CR, Vaughn JE, Barber RP, Roberts E. GABA neurons are the major cell type of the nucleus reticularis thalami. Brain research. 1980;200:341–354. doi: 10.1016/0006-8993(80)90925-7. [DOI] [PubMed] [Google Scholar]
- 29.Andrews GL, Yun K, Rubenstein JL, Mastick GS. Dlx transcription factors regulate differentiation of dopaminergic neurons of the ventral thalamus. Molecular and Cellular Neuroscience. 2003;23(1):107–120. doi: 10.1016/s1044-7431(03)00016-2. [DOI] [PubMed] [Google Scholar]
- 30.Jones EG, Rubenstein JLR. Expression of regulatory genes during differentiation of thalamic nuclei in mouse and monkey. The Journal of comparative neurology. 2004;477(1):55–80. doi: 10.1002/cne.20234. [DOI] [PubMed] [Google Scholar]
- 31.Eisenstat DD, Liu JENK, Mione M, Zhong W, Yu G, Anderson SA, et al. Dlx-1, Dlx-2, and Dlx-5 Expression Define Distinct Stages of Basal Forebrain Differentiation. Journal of Comparative Neurology. 1999;237:217–237. doi: 10.1002/(sici)1096-9861(19991115)414:2<217::aid-cne6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 32.Colasante G, Collombat P, Raimondi V, Bonanomi D, Ferrai C, Maira M, et al. Arx is a direct target of Dlx2 and thereby contributes to the tangential migration of GABAergic interneurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28(42):10674–10686. doi: 10.1523/JNEUROSCI.1283-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cobos I, Broccoli V, Rubenstein JLR. The vertebrate ortholog of Aristaless is regulated by Dlx genes in the developing forebrain. The Journal of comparative neurology. 2005;483(3):292–303. doi: 10.1002/cne.20405. [DOI] [PubMed] [Google Scholar]
- 34.Long JE, Swan C, Liang WS, Cobos I, Potter GB, Rubenstein JLR. Dlx1&2 and Mash1 transcription factors control striatal patterning and differentiation through parallel and overlapping pathways. The Journal of comparative neurology. 2009;512(4):556–572. doi: 10.1002/cne.21854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wagner CK, Eaton MJ, Moore KE, Lookingland KJ. Efferent projections from the region of the medial zona incerta containing A13 dopaminergic neurons: a PHA-L anterograde tract-tracing study in the rat. Brain research. 1995;677(2):229–237. doi: 10.1016/0006-8993(95)00128-d. [DOI] [PubMed] [Google Scholar]
- 36.Mitrofanis J. Some certainty for the “zone of uncertainty”? Exploring the function of the zona incerta. Neuroscience. 2005;130(1):1–15. doi: 10.1016/j.neuroscience.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 37.Pinault D. The thalamic reticular nucleus: structure, function and concept. Brain research reviews. 2004;46:1–31. doi: 10.1016/j.brainresrev.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 38.Cavdar S, Onat F, Cakmak YO, Saka E, Yananli HR, Aker R. Connections of the zona incerta to the reticular nucleus of the thalamus in the rat. Journal of anatomy. 2006;209(2):251–258. doi: 10.1111/j.1469-7580.2006.00600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quillé M-L, Carat S, Quéméner-Redon S, Hirchaud E, Baron D, Benech C, et al. High-throughput analysis of promoter occupancy reveals new targets for Arx, a gene mutated in mental retardation and interneuronopathies. PloS one. 2011;6(9):e25181. doi: 10.1371/journal.pone.0025181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Friocourt G. Identification of Arx targets unveils new candidates for controlling cortical interneuron migration and differentiation. Frontiers in Cellular Neuroscience. 2011 Dec;5:1–12. doi: 10.3389/fncel.2011.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu W, Lagutin OV, Mende M, Streit A, Oliver G. Six3 activation of Pax6 expression is essential for mammalian lens induction and specification. The EMBO journal. 2006;25(22):5383–5395. doi: 10.1038/sj.emboj.7601398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thor S, Thomas JB. The Drosophila islet Gene Governs Axon Pathfinding and Neurotransmitter Identity. Neuron. 1997;18:397–409. doi: 10.1016/s0896-6273(00)81241-6. [DOI] [PubMed] [Google Scholar]
- 43.Filippi A, Jainok C, Driever W. Analysis of transcriptional codes for zebrafish dopaminergic neurons reveals essential functions of Arx and Isl1 in prethalamic dopaminergic neuron development. Developmental biology. 2012;369(1):133–149. doi: 10.1016/j.ydbio.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 44.Nasrallah MP, Cho G, Simonet JC, Putt ME, Kitamura K, Golden Ja. Differential effects of a polyalanine tract expansion in Arx on neural development and gene expression. Human molecular genetics. 2012;21(5):1090–1098. doi: 10.1093/hmg/ddr538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paxinos G, Halliday G, Watson C, Koutcherov Y, Wang H. Atlas of the Developing Mouse Brain at E17.5, P0 and P6. 2007 Figure 69. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


