To the Editor
Opioid dependence is a risk factor for human immunodeficiency virus (HIV), sexually transmitted infections (STIs), and hepatitis C virus (HCV) infection.1 Opioid treatment programs, which provide medication-assisted treatment to over 300 000 opioid-dependent Americans,2 are well-positioned to offer testing for these infectious diseases to a high-risk population. They were among the first venues to offer HIV testing and are more likely to offer HIV, STI, and HCV testing than other drug treatment programs.1 Private for-profit opioid treatment programs are increasingly widespread and such programs offer on-site HIV testing less often than nonprofit and public programs.3 However, with the 2006 national recommendations for routine opt-out HIV testing,4 we hypothesized that the percent of programs offering on-site testing for HIV, STIs, and HCV would increase.
Methods
To determine the percent of opioid treatment programs offering on-site HIV, STI, and HCV testing over time, we analyzed the National Survey of Substance Abuse Treatment Services.5 The survey is sent to directors of all known drug treatment facilities (response rate 91.4–96.5%); survey questions had minor wording changes over time. We tabulated the percent of opioid treatment programs offering on-site HIV, STI, and HCV testing from 2000 to 2011, there was no survey in 2001. Next, we compared the percent of for-profit, nonprofit, and public (those owned and operated by local, state, tribal, or federal government) programs offering on-site testing over time. Programs with missing data (0.2–3.9% per year) were excluded. We calculated the relative difference and 95% confidence interval in programs offering on-site testing in 2000 and 2011 using SAS 9.3 (SAS Institute). P values were calculated using chi-square tests for trend. A two-sided P value ≤ 0.05 was considered significant. Because the survey is publicly available and contains no patient-level data, our affiliated IRB determined this study was not considered human research.
Results
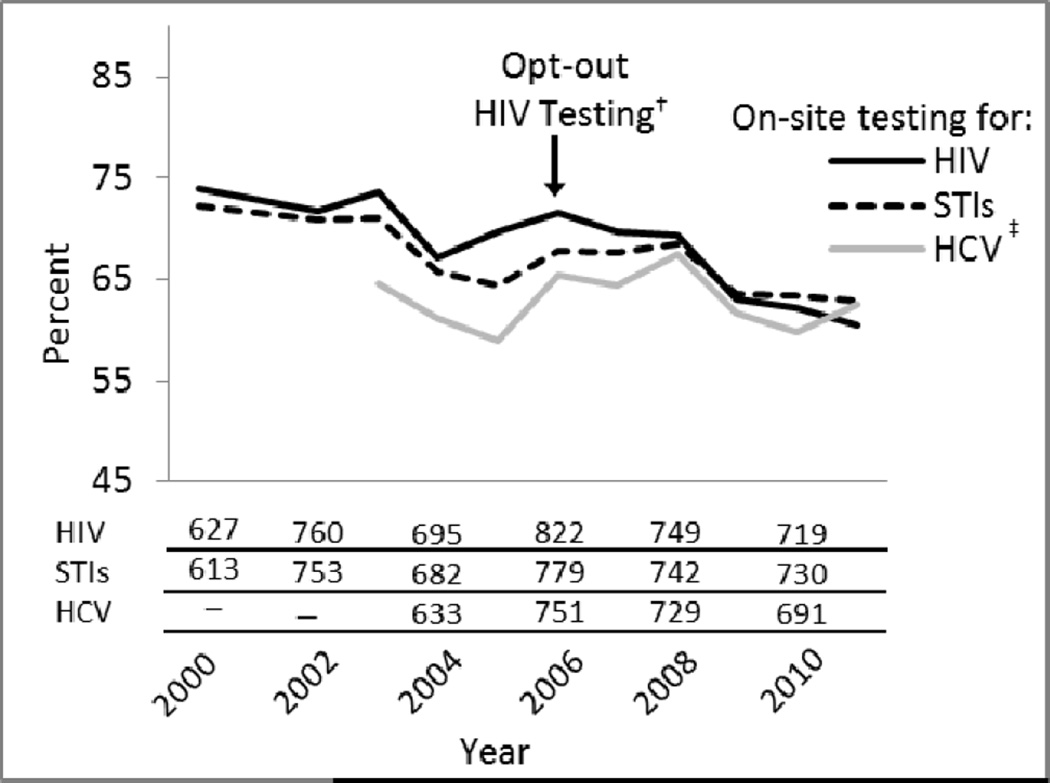
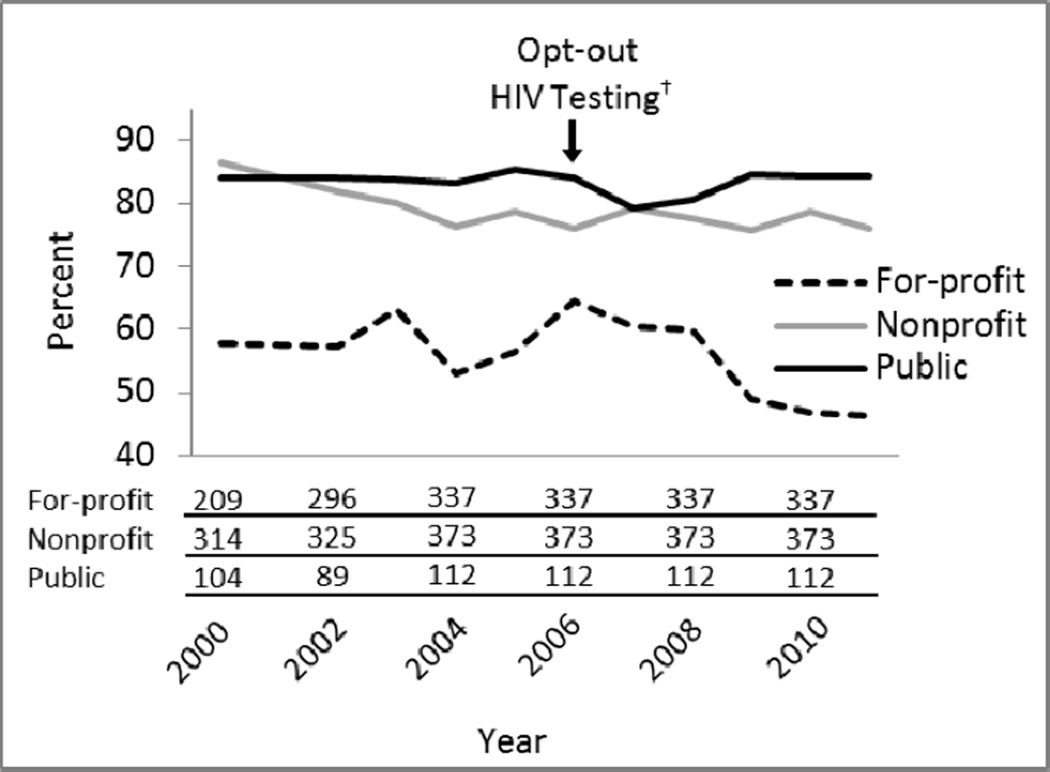
The number of U.S. opioid treatment programs increased from 849 in 2000 to 1175 in 2011. The percent of programs operating as for-profit businesses increased from 43% to 54%; nonprofits decreased from 43% to 36% and public programs decreased from 14% to 10%. From 2000 to 2011, the absolute number of programs offering testing for HIV, STIs, and HCV increased but the percent offering on-site HIV testing declined 18% (95% CI: 13–23%; P < 0.001), STI testing declined 13% (95% CI: 7–18%; P < 0.001), with no significant change in HCV testing (P = 0.63; Figure 1). Greater than 75% of public programs offered on-site testing for each infection, with no significant change over time. Offering on-site HIV testing declined 20% among for-profit programs (95% CI: 10–29%; P < 0.001) and 11% among nonprofit programs (95% CI: 6–17%; P < 0.001; Figure 2). Offering on-site STI testing declined 23% in for-profit programs (95% CI: 16–30%; P < 0.001). Offering HCV testing declined 13% in for-profit programs (95% CI: 3–22%; P = 0.002) and increased 14% in nonprofit programs (95% CI: 4–25%; P < 0.001).
Figure 1.
Percent of U.S. opioid treatment programs offering testing for HIV, sexually transmitted infections, and hepatitis C virus, 2000–2011*
STI = sexually transmitted infection; HCV = hepatitis C virus
*Lines show the percent of programs offering on-site testing for each infection; absolute numbers of programs offering on-site testing in 2000 and 2011 are below graph.
†Refers to revised recommendations from the Centers for Disease Control and Prevention in 2006 to screen all adults, adolescents, and pregnant women for HIV infection in all health-care settings, unless the patient declines5
‡On-site testing specifically for hepatitis C virus was recorded starting in 2003.
Figure 2.
Percent of for-profit, nonprofit, and publicly owned U.S. opioid treatment programs offering on-site HIV testing, 2000–2011*
*Lines show the percent of programs offering on-site testing for each infection; absolute numbers of programs offering on-site testing in 2000 and 2011 are below graph.
†Refers to revised recommendations from the Centers for Disease Control and Prevention in 2006 to screen all adults, adolescents, and pregnant women for HIV infection in all health-care settings, unless the patient declines5
Conclusion
The proportion of U.S. opioid treatment programs offering on-site testing for HIV and STIs declined substantially between 2000 and 2011, despite guidelines recommending routine opt-out HIV testing in all health-care settings, including substance abuse treatment facilities. Declines were most pronounced in for-profit programs, suggesting that persons enrolled in these programs may be at increased risk for delayed diagnosis and continued transmission of HIV, STIs, and HCV.
This study had limitations. Referral-based testing was not recorded; however, referral-based services often do not translate into patient utilization.6 Testing for STIs may be limited to syphilis, which is often required by health departments. The survey is cross-sectional with no identifiers linking responses over time. Because patient-level data are not available, patient requests, utilization, and opting out of testing cannot be determined.
Opioid treatment programs are important venues for offering testing to high-risk individuals. As the number of for-profit opioid treatment programs increases, and the opioid, HIV, and HCV epidemics continue to intersect, further work is needed to understand and reverse declines in offering on-site testing.
Acknowledgments
This study was supported by NIH R34DA031066, R01DA032110, R25DA023021, and the Center for AIDS Research at the Albert Einstein College of Medicine and Montefiore Medical Center (NIH AI-51519). The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Dr. Bachhuber had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis
Dr. Cunningham’s husband was recently employed by Pfizer Pharmaceuticals and is currently employed by Quest Diagnostics.
Footnotes
No other disclosures were reported.
Contributor Information
Marcus A. Bachhuber, Email: marcus.bachhuber@gmail.com.
Chinazo O. Cunningham, Email: ccunning@montefiore.org.
References
- 1.Substance Abuse and Mental Health Services Administration, Office of Applied Studies. Rockville, MD: 2010. Feb 25, The N-SSATS Report: Infectious Disease Screening. Available from: http://www.oas.samhsa.gov/2k10/227/227DiseaseScreen2k10.htm. [Google Scholar]
- 2.Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. Rockville, MD: 2013. Apr 23, The N-SSATS Report: Trends in the Use of Methadone and Buprenorphine at Substance Abuse Treatment Facilities: 2003 to 2011. [PubMed] [Google Scholar]
- 3.Pollack HA, D'Aunno T. HIV testing and counseling in the nation's outpatient substance abuse treatment system, 1995–2005. J Subst Abuse Treat. 2010;38(4):307–316. doi: 10.1016/j.jsat.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1–17. [PubMed] [Google Scholar]
- 5.Office of Applied Studies, Substance Abuse and Mental Health Services Administration. The DASIS Report: The National Survey of Substance Abuse Treatment Services (NSSATS) 2003 Dec 12;
- 6.Metsch LR, Feaster DJ, Gooden L, et al. Implementing rapid HIV testing with or without risk-reduction counseling in drug treatment centers: results of a randomized trial. Am J Public Health. 2012;102(6):1160–1167. doi: 10.2105/AJPH.2011.300460. [DOI] [PMC free article] [PubMed] [Google Scholar]