Abstract
P-glycoprotein (P-gp), a drug efflux pump, is known to alter the bioavailability of antiretroviral drugs at several sites, including the brain. We have previously shown that human immunodeficiency virus-1 (HIV-1) glycoprotein 120 (gp120) induces proinflammatory cytokine secretion and decreases P-gp functional expression in rat astrocytes, a cellular reservoir of HIV-1. However, whether P-gp is regulated in a similar way in human astrocytes is unknown. This study investigates the regulation of P-gp in an in vitro model of gp120-triggered human fetal astrocytes (HFAs). In this system, elevated levels of interleukin-6 (IL-6), IL-1β, and tumor necrosis factor-α were detected in culture supernatants. Pretreatment with CCR5 neutralizing antibody attenuated cytokine secretion, suggesting that gp120-CCR5 interaction mediated cytokine production. Treatment with gp120 (R5-tropic) resulted in reduced P-gp expression (64%) and function as determined by increased (1.6-fold) cellular accumulation of [3H]digoxin, a P-gp substrate. Exposure to R5 or R5/X4-tropic viral isolates led to a down-regulation in P-gp expression (75% or 90%, respectively), and treatment with IL-6 also showed lower P-gp expression (50%). Moreover, IL-6 neutralizing antibody blocked gp120-mediated P-gp downregulation, suggesting that IL-6 is a key modulator of P-gp. Gp120- or IL-6-mediated downregulation of P-gp was attenuated by SN50 (a nuclear factor-κB [NF-κB] inhibitor), suggesting involvement of NF-κB signaling in P-gp regulation. Our results suggest that, similarly to the case with rodent astrocytes, pathophysiological stressors associated with brain HIV-1 infection have a downregulatory effect on P-gp functional expression in human astrocytes, which may ultimately result in altered antiretroviral drug accumulation within brain parenchyma.
Keywords: astrocyte, HIV-1, gp120, interleukin-6, P-glycoprotein, inflammation, NF-κB
Neuroinflammation is a common immune response associated with human immunodeficiency virus-1 (HIV-1) infection. The sequestration of HIV-1 in brain cellular reservoirs (i.e., astrocytes, microglia) may allow the virus to evade antiretroviral (ARV) therapy, thereby prolonging HIV-1-associated inflammatory responses (i.e., production of proinflammatory cytokines such as tumor necrosis factor-α [TNF-α] and interleukin-6 [IL-6], chemokines, and other neurotoxins; Persidsky and Gendelman, 2003; Alexaki et al., 2008). Brain autopsy samples from HIV-1-infected individuals and tissue samples from HIV-1-inoculated animal models confirm elevation of inflammatory mediators during progression of HIV-1-associated neuroinflammation (Persidsky et al., 1997, 1999). The release of circulatory viral proteins and cytokines in brain parenchyma often results in neurologic impairment and neuronal cell loss that are associated with cognitive and motor disorders in patients with prolonged HIV-1 infection (Ances and Ellis, 2007; Kaul, 2009).
Poor penetration of ARV drugs across the blood–brain barrier (BBB) and subsequently into parenchymal cells remains a major obstacle in efficient suppression of HIV-1 infection in the brain (Ronaldson et al., 2008). Inadequate permeability of different ARV drugs, in particular HIV-1 protease inhibitors (PIs) and nucleoside-reverse transcriptase inhibitors (NRTIs), into different brain cellular compartments is due primarily to functional expression of ATP-binding cassette (ABC) efflux transporters (P-glycoprotein [P-gp], multidrug-resistance associated proteins [MRPs], and breast cancer resistance protein [BCRP]). P-gp is encoded by the multidrug resistance (MDR) gene, which has two isoforms in humans (i.e., MDR1, MDR2) and three isoforms in rodents (i.e., mdr1a, mdr1b, mdr2). P-gp substrates include various classes of ARV drugs, such as PIs (i.e., darunavir, ritonavir) and NRTIs (i.e., abacavir; Kis et al., 2010). Mdr1a/1b knockout animal models show increased central nervous system (CNS) accumulation of several PIs, which confirms a role for P-gp in ARV drug permeability into the brain (Salama et al., 2005; Spitzenberger et al., 2007). Localization of P-gp has been reported at the BBB in brain microvessel endothelial cells, adhesive pericytes, and astrocytes (Bendayan et al., 2006). Furthermore, we have also confirmed localization of P-gp in a rat microglia cell line, in cultured rat astrocytes, in a rat brain endothelial cell line, and in a human brain microvessel endothelial cell line (Lee et al., 2001b; Bendayan et al., 2002; Ronaldson et al., 2004a; Zastre et al., 2009).
Evidence described in the literature suggest that P-gp functional expression in the brain is altered during HIV-1 infection. Increased P-gp immunoreactivity in glial cells has been reported in brain autopsy tissues from patients with HIV-1 encephalitis (Langford et al., 2004; Persidsky et al., 2006). It has not yet been established whether the induced P-gp expression observed in postmortem brain tissue is due to the disease manifestation itself or to the therapeutic regimen. It is known that antiretrovirals, in particular, HIV-protease inhibitors, through their interaction with orphan nuclear receptors (i.e., PXR), may play an important role in the upregulation of P-gp expression (Chandler et al., 2003; Zastre et al., 2009; Chan et al., 2011). Therefore, in vitro systems have been used to delineate the cell-specific effect of viral proteins and cytokines. Hayashi et al. (2005) have reported HIV-1 viral protein Tat induced upregulation of P-gp expression in both murine brain micro-vessel endothelial cells and astrocytes. Our group has demonstrated that both gp120 and IL-6 can decrease P-gp expression in primary cultures of rat astrocytes, whereas, TNF-α or IL-1β exposure results in an enhancement in P-gp expression. Because of the expression of ABC drug transporters, astrocytes may act as a secondary barrier to drug permeability within brain parenchyma, resulting in altered drug distribution in the brain extracellular fluid (Lee et al., 2001a). Drug efflux transporters such as P-gp are known to limit the uptake of antiretroviral drugs in astrocytes, so the upregulation or downregulation of P-gp along with other ABC transporters can significantly alter the availability of these drugs within brain parenchyma.
Currently, much interest has focused on studying intracellular signaling systems/transcription factors that regulate P-gp. For example, a recent study showed that the transcription factor nuclear factor-κB (NF-κB) regulates TNF-α-induced upregulation of mdr1b promoter activity in a rat brain microvessel endothelial cell line (Yu et al., 2008). Although these studies provide insight into P-gp regulation in rodent or murine astrocytes, regulation of P-gp in human astrocytes in the context of HIV-1 infection has not yet been characterized. Therefore, this study sought to determine whether gp120 can induce an immune response in primary cultures of human fetal astrocytes and to examine how P-gp protein expression is regulated by viral isolates, gp120, and IL-6.
MATERIALS AND METHODS
Reagents
HIV-196ZM651 gp120 full-length protein (subtype C; R5-tropic), rabbit polyclonal anti-CCR5, and rabbit polyclonal anti-CXCR4 were obtained from the National Institutes of Health AIDS Research and Reference Reagent program (Bethesda, MD). HIV-1ADA gp120 (subtype B; R5-tropic) full-length protein was purchased from Immunodiagnostics (Woburn, MA). Murine monoclonal C219 antibody against P-gp was purchased from ID Laboratories (London, Ontario, Canada). Murine monoclonal AC-40 antibody against actin, horseradish peroxidase-conjugated anti-mouse and anti-rabbit, human IL-6, CXCR4, and CCR5 neutralizing antibody were obtained from Sigma-Aldrich (Oakville, Ontario, Canada). IL-6 neutralizing antibody was purchased from R&D Systems (Minneapolis, MN). NF-κB inhibitory peptide SN50 was purchased from EMD Biosciences (La Jolla, CA). [3H]digoxin (specific activity 23.5 Ci/mmol), a substrate for P-gp, was purchased from PerkinElmer Life and Analytical Sciences (Boston, MA).
Primary Cultures of Human Fetal Astrocytes
Human fetal brain tissue samples were collected from consenting patients undergoing elective pregnancy termination (between 10 and 14 weeks of gestation age). Ethics approval was obtained from the University Health Network (Toronto, Ontario, Canada). Primary cultures of human fetal astrocytes (HFAs) were established according to previously published protocol (Hammond et al., 2002), with few modifications. Briefly, tissue was collected in ice-cold medium (i.e., Dulbeccos’ modified Eagle’s medium [DMEM] supplemented with 5% fetal bovine serum [FBS] and 5% penicillin/streptomycin). After removal of meninges, tissue was triturated until disaggregated. The cell suspension was then centrifuged and resuspended in initial growth medium containing DMEM supplemented with 20% FBS and 0.025% penicillin/streptomycin. Cells were distributed in 75-cm2 tissue culture flasks and incubated overnight at 37°C under 5% CO2 and 95% air so that viable cells could adhere to culture flasks. The medium was then replaced with feeding medium (DMEM with 10% FBS and 0.025% penicillin/streptomycin), and cells were grown as a monolayer until confluent. HFAs were characterized by the detection of vimentin, a biochemical marker for fetal astrocytes.
Isolation of CCR5 HIV-1 ADA and CCR5/CXCR4 HIV-1 89.6 Viral Isolates
HIV-1 89.6, a macrophage/T-lymphocyte dual-tropic (R5/X4-tropic) viral isolate, was obtained from the AIDS Research and Reference Reagent Program, National institute of Allergy and Infectious Diseases. HIV-1 ADA, a macrophage-tropic (R5-tropic) strain, was isolated from peripheral blood mononuclear cells from an HIV-1-infected patient. Infected monocytes were cultured and maintained following previously published protocols (Ricardo-Dukelow et al., 2007).
Treatment With HIV-1 Isolates, HIV-1 gp120, and IL-6
Confluent HFA monolayers were treated with either R5 HIV-1 or R5/X4 HIV-1 virus (100× concentrated stocks) for 24 hr. Similarly, HFAs were treated with HIV-1 gp120 (1.0 nM) in the presence or absence of CXCR4 or CCR5 neutralizing antibodies (1 mg/ml) for 6 and 24 hr. Additionally, cells were treated with IL-6 (0.5 ng/ml or 10 ng/ml) or HIV-1 gp120 (1.0 nM) for the desired time (6, 12, and 24 hr) in the presence or absence of SN50 (1.0 μM), an NF-κB inhibitory peptide. IL-6 treatment (10 ng/ml) was also administered for longer time points (36 and 48 hr). HFAs were treated with HIV-1 gp120 in the presence or absence of IL-6 neutralizing antibody (0.5 μg/ml).
ELISA Analysis
IL-6, IL-1β, and TNF-α secretion from cultured HFAs in response to HIV-1 gp120 (1.0 nM) were measured using commercially available, ultrasensitive ELISA kits according to the manufacturer’s protocol (Pierce, Rockford, IL). Standard curves for all three cytokines (0–1,000 pg/ml) were generated using the appropriate recombinant human cytokines. For the IL-6 and IL-1β kits, the detection level ranged from 10.2 to 400 pg/ml, and, for the TNF-α kit, it ranged from 15.6 to 1,000 pg/ml.
Immunoblot Analysis
Protein expression of P-gp, CXCR4, and CCR5 was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) according to previously published protocols (Ronaldson et al., 2004b). Briefly, whole-cell lysates were isolated using radioimmunoprecipitation assay (RIPA) buffer. Proteins (50 μg) were resolved by SDS-PAGE and electrotransferred onto polyvinylidine fluoride (PVDF) membranes. The membranes were blocked overnight in TBS containing 0.1% tween and 5% skim milk. Then blots were incubated with appropriate primary antibody followed by horseradish peroxidase-conjugated secondary antibody. C219 (1:500 or 1:300), AC40 (1:500), anti-CXCR4 (1:1,000), and anti-CCR5 (1:1,000) antibodies were used to detect P-gp, actin, CXCR4, and CCR5, respectively. Finally, protein bands were detected using an enhanced chemiluminescence kit. Densitometric analysis was performed in AlphaDigiDoc RT2 software to quantify relative protein expression.
Transport Assay
Primary cultures of human fetal astrocytes were seeded on 48-well plates, and accumulation of [3H]digoxin was measured following previously published protocols (Ronaldson and Bendayan, 2006). Briefly, cells were washed and incubated at 37°C for 30 min in Hanks’ balanced salt solution, pH 7.4, containing 10 mM HEPES and 0.01% bovine serum albumin. The cells were then incubated for the desired time with [3H]digoxin (100 nM; specific activity 23.5 Ci/mmol). At the end of each time period, the incubation medium was aspirated, and the reaction was terminated with 1,000 μl ice-cold PBS. The cells were then solubilized with 200 μl of 1% Triton X-100 for 30 min. Radioactivity was measured by standard liquid scintillation counting. Cellular protein content was determined by the Bradford colorimetric method using bovine serum albumin as a standard.
Data Analysis
Each experiment was repeated at least three times on cultured astrocytes isolated from different tissues. Student’s t-test was used to determine statistical significance between two groups. Multiple comparisons were performed using ANOVA and Bonferroni’s post hoc analysis. P < 0.05 was considered statistically significant.
RESULTS
Interaction of Viral Protein gp120 (R5-Tropic) With Chemokine Receptor and Proinflammatory Cytokine Secretion
To characterize the inflammatory response mediated by gp120 in human astrocytes, we exposed HFAs to HIV-1 gp120 (R5-tropic strain) and observed a significant increase in the secretion of various proinflammatory cytokines (i.e., IL-6, IL-1β, TNF-α; Table I). When primary HFA cultures were exposed to gp120 in the presence of neutralizing antibodies directed against CXCR4 and CCR5, the CCR5 neutralizing antibody significantly decreased gp120-induced secretion of all three cytokines examined whether administered alone or in conjunction with CXCR4 neutralizing antibody. In contrast, administration of HIV-1 gp120 and CXCR4 neutralizing antibody failed to affect gp120-mediated cytokine secretion.
TABLE I.
Proinflammatory Cytokine Secretion in Primary Cultures of HFAs Treated With HIV-196ZM651 gp120†
| Treatment | IL-6 (pg/ml)
|
TNF-α (pg/ml)
|
IL-1β (pg/ml)
|
|||
|---|---|---|---|---|---|---|
| 6 hr | 24 hr | 6 hr | 24 hr | 6 hr | 24 hr | |
| Untreated | — | 182.36 ± 26.83 | — | 11.35 ± 3.69 | — | 2.64 ± 1.23 |
| gp120 | 431.57 ± 73.09★★★ | 336.33 ± 54.32★★★ | 679.50 ± 31.80★★★ | 401.37 ± 20.98★★★ | 296.46 ± 19.59★★★ | 211.81 ± 22.52★★★ |
| CXCR4 Nab ± gp120 | 452.40 ± 69.99★★★ | 347.52 ± 65.75★★★ | 735.85 ± 108.93★★★ | 464.13 ± 15.21★★★ | 274.07 ± 2.38★★★ | 210.71 ± 11.76★★★ |
| CCR5 Nab ± gp120 | 204.90 ± 18.55★★★ | 164.20 ± 10.59 | 12.09 ± 4.84 | 20.24 ± 16.82 | 6.81 ± 13.80 | 5.93 ± 8.46 |
| CXCR4 and CCR5 Nab ± gp120 | 189.65 ± 19.29★★★ | 167.59 ± 17.32 | 19.91 ± 13.04 | 26.08 ± 26.96 | 1.73 ± 1.75 | 1.98 ± 1.00 |
Cytokine secretion was detected by ELISA analysis in cell culture supernatants from cultured HFAs treated with HIV-1 gp120 (for 6 and 24 hr) in the presence or absence of CXCR4 and CCR5 neutralizing antibodies. Data points are expressed as mean ± SD of eight separate measurements obtained from different cultures. Nab, neutralizing antibody. For the 24 hr treatments, statistical comparisons are made between untreated controls and treated groups. For the 6 hr treatments, all the values are compared with the minimal detection limit of the kit. For the IL-6 and IL-1β kits, the detection level ranged from 10.2 to 400 pg/ml, and, for the TNF-α kit, it ranged from 15.6 to 1,000 pg/ml.
P < 0.001, significantly different from the control.
Effect of R5/X4 and R5-Tropic Viral Isolates on P-gp Protein Expression
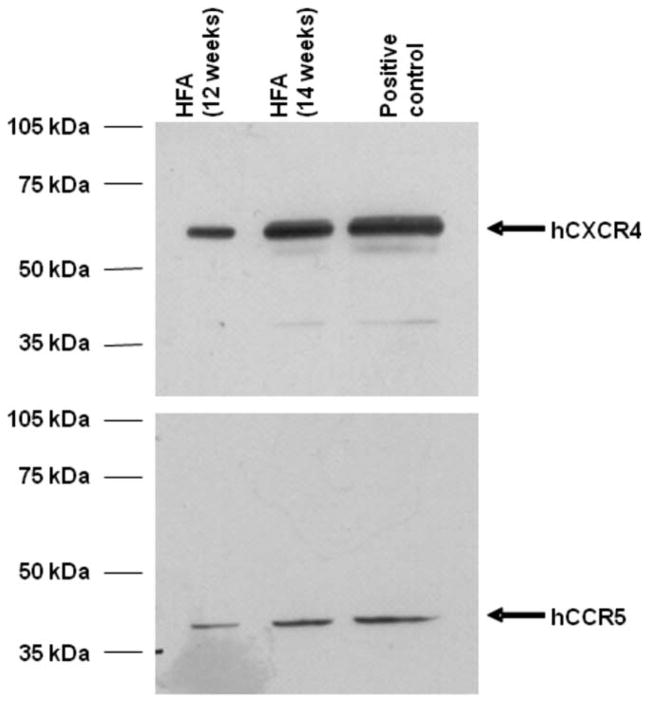
It is currently unknown whether interaction of intact HIV-1 virus with chemokine receptors in astrocytes can modify functional expression of drug transporters such as P-gp. In intact HIV-1 viral isolates, gp120 is expressed at the viral envelope and mediates attachment to chemokine receptors expressed at the surface of target cells. We detected protein expression of both CXCR4 and CCR5 in our HFA cultures (Fig. 1). To test whether HIV-1 can alter P-gp expression, primary cultures of HFAs were exposed to R5-tropic and R5/X4-tropic viral isolates. R5-tropic viruses are known to predominate in the brain, so we used HIV-1 ADA isolates. We also utilized HIV-1 89.6, an R5/X4 viral isolate, to test the effect of dual tropism on P-gp expression. Exposure to either R5-tropic or R5/X4-dual-tropic viral isolates for 24 hr resulted in decreased (P < 0.001) P-gp expression by up to 75% and 90% respectively (Fig. 2A).
Fig. 1.
Immunoblot analysis of CXCR4 and CCR5 in primary cultures of HFAs. Whole-cell lysates (50 μg) from primary cultures of HFAs and 3T3-CXCR4 and 3T3-CCR5 cells were resolved on a 10% SDS-polyacrylamide gel and transferred to PVDF membrane. Cell lysates prepared from 3T3-CXCR4 and 3T3-CCR5 cells were used as positive controls. CXCR4 and CCR5 were detected using the appropriate polyclonal antibody (anti-CXCR4, 1:1,000 dilution; anti-CCR5, 1:1,000 dilution).
Fig. 2.
Immunoblot and densitometric analysis of P-gp in primary cultures of HFAs after exposure to either CCR5-tropic HIV-1 ADA or CCR5/CXCR4 dual-tropic HIV-1 89.6 viral isolates (A), 1.0 nM gp120 (B), or IL-6 (0.5 or 10 ng/ml; C). Whole-cell lysates (50 μg) from primary cultures of HFAs were resolved on a 10% SDS-polyacrylamide gel and transferred to a PVDF membrane. MDR1-over-expressing cell lines (MDCK-MDR1 and MDA-MDR1) were used as positive control. P-gp was detected using the monoclonal antibody C219 (1:500 or 1:300 dilution), and actin was detected using AC40 antibody (1:500 dilution). Results are expressed as mean ± SD of three separate experiments. Asterisks represent data points that are significantly different from control (*P < 0.05, ***P < 0.001).
Effect of gp120 and IL-6 on P-gp Protein Expression
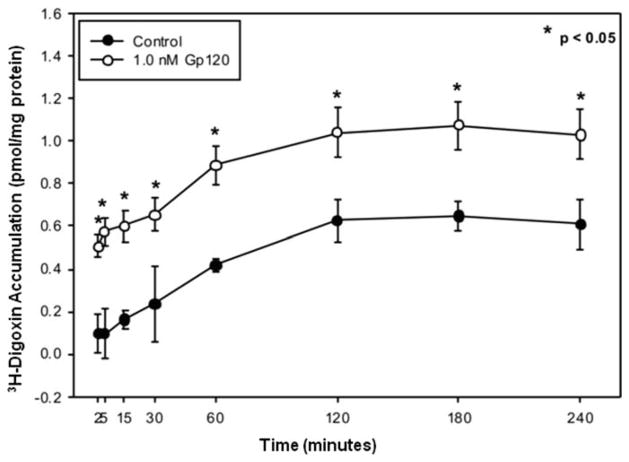
We have previously shown that gp120 exposure can significantly decrease P-gp protein expression in primary cultures of rat astrocytes. However, it was unknown whether gp120 has a similar effect on P-gp expression in human astrocytes. Immunoblot analysis showed that HIV-1 gp120 treatment resulted in a time-dependent decrease in P-gp protein expression (approximately 64% or 2.8-fold after 24 hr) compared with untreated cells (Fig. 2B). No significant change in protein expression was observed after 6 or 12 hr of treatment. Decreased P-gp protein expression correlated with a reduction in P-gp mediated drug transport activity. This was demonstrated by an increase in cellular [3H]digoxin accumulation, an established substrate for P-gp, in gp120-treated HFA cultures compared with untreated controls (Fig. 3).
Fig. 3.
Accumulation of [3H]digoxin by HFAs in the presence or absence of gp120. [3H]digoxin (100 nM) accumulation was measured at 37°C in cells treated with 1.0 nM gp120. Results are expressed as mean ± SD of three separate experiments, and each data point represents quadruplicate measurements. Asterisks represent data points that are significantly different from control (*P < 0.05).
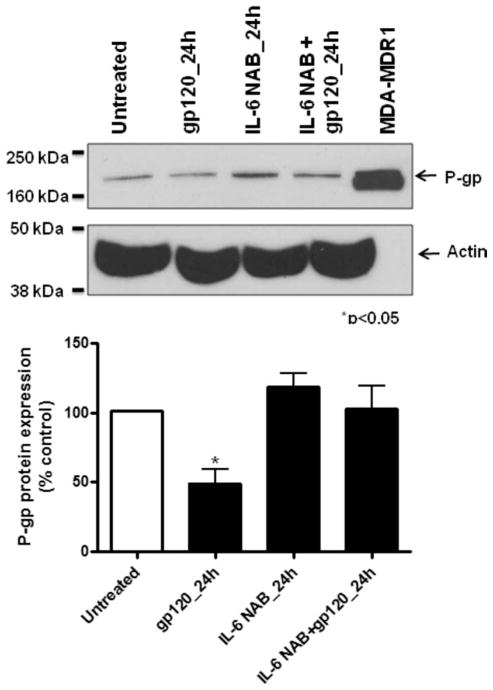
Previously, we have demonstrated that IL-6 was a key regulator of P-gp expression in rat astrocytes. Therefore, we investigated the effect of this cytokine in human astrocytes. IL-6 (0.5 and 10 ng/ml) decreased P-gp expression by up to 50% after 12 hr exposure (Fig. 2C). However, P-gp protein expression was not altered by longer exposure, up to 48 hr (data not shown). In the presence of IL-6-neutralizing antibody, P-gp protein expression was not significantly decreased by HIV-1 gp120 compared with HFAs treated with only HIV-1 gp120 (Fig. 4).
Fig. 4.
Immunoblot and densitometric analysis of P-gp in primary cultures of HFAs treated with 1.0 nM gp120 in the presence of 0.5 μg/ml IL-6 neutralizing antibody (NAB). Whole-cell lysates (50 μg) from primary cultures of HFAs were resolved on a 10% SDS-polyacrylamide gel and transferred to a PVDF membrane. An MDR1-overexpressing cell line (MDA-MDR1) was used as positive control. P-gp was detected using the monoclonal antibody C219 (1:300 dilution), and actin was detected using AC40 antibody (1:500 dilution). Results are expressed as mean ± SD of three separate experiments. Asterisks represent data points that are significantly different from control (*P < 0.05).
Involvement of NF-κB in the Regulation of P-gp Protein Expression
The role of NF-κB signaling in regulating P-gp expression in human astrocytes has not been demonstrated. Using immunoblot analysis, we investigated whether NF-κB can alter P-gp protein expression in HFAs exposed to gp120 or IL-6. Treatment with gp120 or IL-6 resulted in a significant decrease in P-gp expression (64% and 50%, respectively). However, in the presence of SN50, a peptidic inhibitor of NF-κB nuclear translocation, P-gp protein expression remained unchanged with exposure to gp120 or IL-6 in HFAs (Fig. 5).
Fig. 5.
Immunoblot and densitometric analysis of P-gp in primary cultures of HFAs treated with 1.0 nM gp120 (left) or IL-6 (0.5 or 10 ng/ml; right) in the presence of the NF-κB inhibitory peptide SN50 (1.0 μM). Whole-cell lysates (50 μg) from primary cultures of HFAs were resolved on a 10% SDS-polyacrylamide gel and transferred to a PVDF membrane. An MDR1-overexpressing cell line (MDCK-MDR1) was used as positive control. P-gp was detected using the monoclonal antibody C219 (1:500 dilution), and actin was detected using AC40 antibody (1:500 dilution). Results are expressed as mean ± SD of three separate experiments. Asterisks represent data points that are significantly different from control (*P < 0.05).
DISCUSSION
Pharmacotherapy for HIV-1 brain infection is limited. This, in part, is attributable to functional expression of ABC drug transporters such as P-gp actively pumping several xenobiotics, including ARV drugs, out of brain cellular compartments. Because astrocytes in brain parenchyma are able to harbor latent HIV-1 infection (Gorry et al., 2003), expression of P-gp in these cells is a major determinant of successful ARV drug cellular permeability and suppression of viral infection. Because the regulation of P-gp expression by HIV-1 in human astrocytes had not been investigated prior to the present study, we investigated the effect of HIV-1 isolates on P-gp protein expression in an in vitro model of human fetal astrocytes. With our primary cultures of HFAs, we observed a significant decrease in P-gp protein expression in the presence of R5-tropic and R5/X4-tropic HIV-1 viral isolates, suggesting a downregulatory effect of HIV-1 on P-gp expression. Previously, Gollapudi and Gupta (1990) reported altered expression of P-gp in an HIV-1-infected T-cell line and in a monocytic cell line. In contrast to our findings, they reported upregulation of P-gp expression in their leukocytic cell systems when subjected to HIV-1 infection. Compared with T cells or monocytes, astrocytes do not harbor productive HIV-1 infection and utilize different mechanisms of HIV-1 viral entry and sequestration (Sabri et al., 1999; Boutet et al., 2001; Liu et al., 2004). Therefore, it is possible that regulation of P-gp expression by HIV-1 is also cell type specific, which may account for the apparent discrepancies between our present study and the work of Gollapudi and Gupta (1990).
Shed viral proteins are also known to alter expression of ABC transporters. For example, viral protein Tat-induced P-gp expression has been reported both in cultured brain microvessel endothelial cells and in astrocytes (Hayashi et al., 2005), whereas we have previously demonstrated that gp120 exposure can significantly downregulate P-gp protein expression (4.7-fold) resulting in increased accumulation of saquinavir, an antiretroviral drug, in primary cultures of rat astrocytes (Ronaldson and Bendayan, 2006). HIV-1 Tat is an accessory protein that is critical for viral replication, whereas, gp120 is a structural protein that is part of the viral envelope and is essential for the viral binding to host cells that initiates HIV-1 cellular entry process. The mechanisms of action of the two proteins are different. Tat is known to cross the cell membrane and interact with transcription factors within a cell (Sune and Garcia-Blanco, 1995; Mahlknecht et al., 2008), whereas gp120 is known to interact with chemokine receptors on the cell surface (Wu et al., 1997; Ronaldson and Bendayan, 2006). Therefore, it is anticipated that these proteins may activate signaling pathways differently and, in turn, have different effects on P-gp expression. In the present study, we further demonstrate that gp120 exposure results in a 2.8-fold decrease in P-gp protein expression in human astrocytes. As expected, the magnitude of P-gp downregulation is less profound in HFAs exposed to viral protein compared with cells exposed to R5-tropic and dual-tropic HIV-1 viral isolates. In our hands, exposure to gp120 also results in a significant reduction in P-gp function, as shown by increased accumulation of digoxin (approximately 1.6-fold in gp120 treated cells compared with the control). Although the decrease in P-gp protein expression is less profound in HFAs than in cultured rat astrocytes (2.8-fold vs. 4.7-fold), the functional activity of P-gp was downregulated to a similar degree in both models (1.5-fold increase in digoxin accumulation in rat astrocytes vs. 1.6-fold increase in HFAs; Ronaldson and Bendayan, 2006). The comparable functional loss of P-gp in both human and rat astrocytes in the presence of gp120 suggests that, although the effect of this viral protein on the P-gp expression is different between two models, the effect on drug transport activity remains similar.
In this study, we have further characterized gp120-mediated cytokine secretion in HFAs. It is known that, during HIV-1 infection, secreted gp120 can interact with microglia/macrophages as well as astrocytes in the CNS and induce secretion of neurotoxic cytokines (Kaul and Lipton, 2006). Transgenic mice expressing gp120 show activation of glial cells and neuronal damage, suggesting a significant role for this viral protein in HIV-1-mediated neuroinflammation (Toggas et al., 1994). Previously, gp120-triggered cytokine secretion (IL-6, IL-1β, and TNF-α) has been characterized in primary cultures of rat astrocytes. Here, we have characterized cytokine secretion by gp120 in human astrocytes. Our findings show that R5-tropic gp120 induced secretion of IL-6, IL-1β, and TNF-α in HFAs, indicating that an inflammatory response can be triggered by gp120 in these cells. Neutralization of cell surface CCR5 chemokine receptor significantly diminished gp120-mediated cytokine production, suggesting that interaction of gp120 with CCR5 is responsible for cytokine release in these cells. Our findings for HFAs are in agreement with previously published findings from a rodent model and emphasize that the gp120-CCR5 interaction is a critical event in neuroinflammatory signaling within human brain parenchyma.
Ample literature evidence suggests alteration of P-gp expression by proinflammatory cytokines. In human hepatocytes, IL-6 exposure resulted in a reduced MDR1 gene expression and P-gp activity (Lee and Piquette-Miller, 2001). Recently, an IL-6 mediated decrease in P-gp expression has been reported for cultured human brain microvessel endothelial cells, further supporting the fact that P-gp expression can be altered by this cytokine (Poller et al., 2010). In rodent astrocytes, IL-6 treatment resulted in a continuous and profound decrease in P-gp expression from 6 to 24 hr (8.9-fold after 24 hr). Comparison of P-gp expression following IL-6 exposure in different cells suggests that this cytokine may have a down-regulatory effect on P-gp expression. Accordingly, treatment with IL-6 also results in a time-dependent decrease in P-gp protein expression in cultured HFAs. P-gp protein expression is 50% (2-fold) downregulated after 12 hr of treatment with either low or high concentration of IL-6. However, the downregulatory effect of IL-6 is lost during longer exposure (24 hr), and P-gp protein expression returned to the control levels. Time-dependent regulation of P-gp by other cytokines has been reported previously. Bauer et al. (2007) have shown that TNF-α-induced regulation of P-gp is time dependent; a transient exposure to TNF-α caused a decrease in P-gp expression, and a longer exposure increased the expression of this transporter in rat brain capillaries. Compared with the profound downregulation (8.9-fold) previously reported for rodent astrocytes (Ronaldson and Bendayan, 2006), the IL-6-mediated decrease in P-gp expression in HFAs is modest (2-fold). This finding may be explained by the difference in endogenous levels of IL-6 between the culture supernatants of rat and human astrocytes. IL-6 was undetectable in primary cultures of rat astrocytes. Although previous studies reported no evidence of IL-6 presence in untreated cultured HFAs (Lee et al., 1993), we detected IL-6 in HFA culture supernatant (approximately 182.36 pg/ml). Astrocytes are known to be the primary source of IL-6 cytokine during inflammation; however, elevated endogenous levels of IL-6 in HFAs may be related to the prenatal phenotype of these cells. Previously, a beneficial role of IL-6 has been reported in neuronal protection, implying that endogenous IL-6 secreted from HFAs may have a neuroprotective role during fetal brain development (Gadient and Otten, 1997). Since the HFAs are continuously exposed to endogenous IL-6 levels, it is likely that the IL-6-mediated decrease in P-gp expression differs substantially between human and rat astrocytes.
We have further examined the role of IL-6 in regulating P-gp expression by treating cultured HFAs with HIV-1 gp120 in the presence of IL-6-neutralizing antibody. Our results show that P-gp protein expression was not significantly altered under these conditions, confirming that IL-6 secretion is primarily responsible for the gp120-mediated downregulation observed in HFAs. These findings further support our findings in rodent astrocytes, in which IL-6 was also the principal cytokine responsible for P-gp downregulation (Ronaldson and Bendayan, 2006).
Viral proteins and/or cytokines are known to activate intracellular signaling pathways (Sluss et al., 1994; Kaul et al., 2005). Few studies have reported the activation of NF-κB in response to gp120 in various cell systems, including astrocytes (Saha and Pahan, 2007). Furthermore, NF-κB signaling is known to be involved in ABC drug transporter regulation. Using rat brain capillaries, Bauer et al. (2007) demonstrated that alteration of P-gp expression by TNF-α is NF-κB dependent. Previous work by our group showed that NF-κB is involved in the regulation of Mrp1, another drug efflux transporter, in gp120-treated cultured rat astrocytes, by inducing TNF-α secretion (Ronaldson et al., 2010). However, the role of NF-κB in P-gp regulation in HFAs triggered by gp120 or IL-6 has not been elucidated. Therefore, we investigated the involvement of NF-κB in our HFA model by inhibiting this transcription factor using the inhibitory peptide SN50. In HFAs, inhibition of NF-κB attenuated both gp120- and IL-6-mediated decreases in P-gp expression, whereas inhibition of this transcription factor did not affect endogenous P-gp expression. These findings demonstrate that NF-κB is involved in the downregulation of P-gp by gp120 or IL-6 in HFAs.
To date, limited numbers of studies have examined the expression of ABC transporters in adult human brain, and none of these studies has investigated P-gp expression in HIV-1-infected treatment-naive patients. Because of the limitations on obtaining appropriate controls (i.e., treatment-naive, age-matched), it is still unclear how HIV-1-associated neuroinflammation affects the functional expression of these transporters in adult human brain. Therefore, we have chosen to use an in vitro system to determine the effect of viral proteins and cytokines in HFAs. Our findings demonstrate for the first time that HIV-1 isolates, gp120, and/or IL-6 exposure can downregulate P-gp protein expression in primary cultures of HFAs and that the NF-κB signaling pathway is involved in this regulation. The present study performed with human astrocytes validates our previous data obtained from primary cultures of rat astrocytes by showing that gp120-induced inflammatory response and loss of P-gp function exist and remain comparable in the two models. Furthermore, our results suggest that astrocytes may be involved in ARV drug sequestration within brain parenchyma during HIV-1 infection. The availability of ARV drugs in astrocytes, in turn, may aid in limiting brain viral infection by preventing the latent virus from becoming infectious. However, in addition to P-gp, other transporters of the ABC family are also known to be involved in ARV drug transport. For example, MRP1 can transport several HIV-protease inhibitors used in ARV therapy (Dallas et al., 2004; Ronaldson et al., 2008). Using rodent astrocytes, we have previously demonstrated that gp120 exposure results in an up-regulation of Mrp1 expression in primary cultures of rat astrocytes (Ronaldson et al., 2010). Although we have not yet investigated the regulation of MRP1 or other MRP isoforms involved in ARV drug transport in human astrocytes, the regulation of these transporters during HIV-associated neuroinflammatory response will also contribute to the overall ARV drug permeability in astrocytes. Further work is needed to clarify the effect of downregulated P-gp in astrocytes on anti-HIV therapy efficacy. Overall, the results of our work emphasize that rodent astrocytes can be used as an effective glial culture model for understanding and elucidating mechanisms involved in inflammation and drug resistance in HIV-1 infection of human astrocytes.
Acknowledgments
Contract grant sponsor: Canadian Institutes of Health Research (CIHR); Contract grant number: MOP-56976 (to R.B.); Contract grant sponsor: Ontario HIV Treatment Network (OHTN; to R.B.); Contract grant sponsor: National Institutes of Health; Contract grant numbers: RO1 MH65151, RO1 AA017398 (to Y.P.).
The authors thank Dr. Michelle Farrugia (Mount Sinai Hospital, Toronto) for her remarkable assistance in obtaining human fetal brain tissue.
References
- Alexaki A, Liu Y, Wigdahl B. Cellular reservoirs of HIV-1 and their role in viral persistence. Curr HIV Res. 2008;6:388–400. doi: 10.2174/157016208785861195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ances BM, Ellis RJ. Dementia and neurocognitive disorders due to HIV-1 infection. Semin Neurol. 2007;27:86–92. doi: 10.1055/s-2006-956759. [DOI] [PubMed] [Google Scholar]
- Bauer B, Hartz AM, Miller DS. Tumor necrosis factor alpha and endothelin-1 increase P-glycoprotein expression and transport activity at the blood–brain barrier. Mol Pharmacol. 2007;71:667–675. doi: 10.1124/mol.106.029512. [DOI] [PubMed] [Google Scholar]
- Bendayan R, Lee G, Bendayan M. Functional expression and localization of P-glycoprotein at the blood–brain barrier. Microsc Res Techniq. 2002;57:365–380. doi: 10.1002/jemt.10090. [DOI] [PubMed] [Google Scholar]
- Bendayan R, Ronaldson PT, Gingras D, Bendayan M. In situ localization of P-glycoprotein (ABCB1) in human and rat brain. J Histochem Cytochem. 2006;54:1159–1167. doi: 10.1369/jhc.5A6870.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutet A, Salim H, Taoufik Y, Lledo PM, Vincent JD, Delfraissy JF, Tardieu M. Isolated human astrocytes are not susceptible to infection by M- and T-tropic HIV-1 strains despite functional expression of the chemokine receptors CCR5 and CXCR4. Glia. 2001;34:165–177. [PubMed] [Google Scholar]
- Chan GN, Tozammel HM, Cummins CL, Bendayan R. Regulation of P-glycoprotein by orphan nuclear receptors in human brain microvessel endothelial cells. J Neurochem. 2011;10:4159. doi: 10.1111/j.1471-4159.2011.07288.x. [DOI] [PubMed] [Google Scholar]
- Chandler B, Almond L, Ford J, Owen A, Hoggard P, Khoo S, Back D. The effects of protease inhibitors and nonnucleoside reverse transcriptase inhibitors on p-glycoprotein expression in peripheral blood mononuclear cells in vitro. J Acquir Immune Defic Syndr. 2003;33:551–556. doi: 10.1097/00126334-200308150-00001. [DOI] [PubMed] [Google Scholar]
- Dallas S, Ronaldson PT, Bendayan M, Bendayan R. Multidrug resistance protein 1-mediated transport of saquinavir by microglia. Neu-roreport. 2004;19:1183–1186. doi: 10.1097/00001756-200405190-00020. [DOI] [PubMed] [Google Scholar]
- Gadient RA, Otten UH. Interleukin-6 (IL-6)—a molecule with both beneficial and destructive potentials. Prog Neurobiol. 1997;52:379–390. doi: 10.1016/s0301-0082(97)00021-x. [DOI] [PubMed] [Google Scholar]
- Gollapudi S, Gupta S. Human immunodeficiency virus I-induced expression of P-glycoprotein. Biochem Biophys Res Commun. 1990;171:1002–1007. doi: 10.1016/0006-291x(90)90783-j. [DOI] [PubMed] [Google Scholar]
- Gorry PR, Ong C, Thorpe J, Bannwarth S, Thompson KA, Gatignol A, Vesselingh SL, Purcell DF. Astrocyte infection by HIV-1: mechanisms of restricted virus replication, and role in the pathogenesis of HIV-1-associated dementia. Curr HIV Res. 2003;1:463–473. doi: 10.2174/1570162033485122. [DOI] [PubMed] [Google Scholar]
- Hammond RR, Iskander S, Achim CL, Hearn S, Nassif J, Wiley CA. A reliable primary human CNS culture protocol for morphological studies of dendritic and synaptic elements. J Neurosci Methods. 2002;118:189–198. doi: 10.1016/s0165-0270(02)00126-7. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Pu H, Tian J, Andras IE, Lee YW, Hennig B, Toborek M. HIV-Tat protein induces P-glycoprotein expression in brain mi-crovascular endothelial cells. J Neurochem. 2005;93:1231–1241. doi: 10.1111/j.1471-4159.2005.03114.x. [DOI] [PubMed] [Google Scholar]
- Kaul M. HIV-1 associated dementia: update on pathological mechanisms and therapeutic approaches. Curr Opin Neurol. 2009;22:315–320. doi: 10.1097/WCO.0b013e328329cf3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Lipton SA. Mechanisms of neuroimmunity and neurode-generation associated with HIV-1 infection and AIDS. J Neuroimmune Pharmacol. 2006;1:138–151. doi: 10.1007/s11481-006-9011-9. [DOI] [PubMed] [Google Scholar]
- Kaul M, Zheng J, Okamoto S, Gendelman HE, Lipton SA. HIV-1 infection and AIDS: consequences for the central nervous system. Cell Death Differ. 2005;12(Suppl 1):878–892. doi: 10.1038/sj.cdd.4401623. [DOI] [PubMed] [Google Scholar]
- Kis O, Robillard K, Chan GN, Bendayan R. The complexities of antiretroviral drug–drug interactions: role of ABC and SLC transporters. Trends Pharmacol Sci. 2010;31:22–35. doi: 10.1016/j.tips.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Langford D, Grigorian A, Hurford R, Adame A, Ellis RJ, Hansen L, Masliah E. Altered P-glycoprotein expression in AIDS patients with HIV encephalitis. J Neuropathol Exp Neurol. 2004;63:1038–1047. doi: 10.1093/jnen/63.10.1038. [DOI] [PubMed] [Google Scholar]
- Lee G, Piquette-Miller M. Influence of IL-6 on MDR and MRP-mediated multidrug resistance in human hepatoma cells. Can J Physiol Pharmacol. 2001;79:876–884. [PubMed] [Google Scholar]
- Lee G, Dallas S, Hong M, Bendayan R. Drug transporters in the central nervous system: brain barriers and brain parenchyma considerations. Pharmacol Rev. 2001a;53:569–596. [PubMed] [Google Scholar]
- Lee G, Schlichter L, Bendayan M, Bendayan R. Functional expression of P-glycoprotein in rat brain microglia. J Pharmacol Exp Ther. 2001b;299:204–212. [PubMed] [Google Scholar]
- Lee SC, Liu W, Dickson DW, Brosnan CF, Berman JW. Cytokine production by human fetal microglia and astrocytes. Differential induction by lipopolysaccharide and IL-1 beta. J Immunol. 1993;150:2659–2667. [PubMed] [Google Scholar]
- Liu Y, Liu H, Kim BO, Gattone VH, Li J, Nath A, Blum J, He JJ. CD4-independent infection of astrocytes by human immunodeficiency virus type 1: requirement for the human mannose receptor. J Virol. 2004;78:4120–4133. doi: 10.1128/JVI.78.8.4120-4133.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahlknecht U, Dichamp I, Varin A, Van LC, Herbein G. NF-kappaB-dependent control of HIV-1 transcription by the second coding exon of Tat in T cells. J Leukoc Biol. 2008;83:718–727. doi: 10.1189/jlb.0607405. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Gendelman HE. Mononuclear phagocyte immunity and the neuropathogenesis of HIV-1 infection. J Leukoc Biol. 2003;74:691–701. doi: 10.1189/jlb.0503205. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Buttini M, Limoges J, Bock P, Gendelman HE. An analysis of HIV-1-associated inflammatory products in brain tissue of humans and SCID mice with HIV-1 encephalitis. J Neurovirol. 1997;3:401–416. doi: 10.3109/13550289709031186. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Ghorpade A, Rasmussen J, Limoges J, Liu XJ, Stins M, Fiala M, Way D, Kim KS, Witte MH, Weinand M, Carhart L, Gendelman HE. Microglial and astrocyte chemokines regulate monocyte migration through the blood–brain barrier in human immunodeficiency virus-1 encephalitis. Am J Pathol. 1999;155:1599–1611. doi: 10.1016/S0002-9440(10)65476-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persidsky Y, Ramirez SH, Haorah J, Kanmogne GD. Blood–brain barrier: structural components and function under physiologic and pathologic conditions. J Neuroimmune Pharmacol. 2006;1:223–236. doi: 10.1007/s11481-006-9025-3. [DOI] [PubMed] [Google Scholar]
- Poller B, Drewe J, Krahenbuhl S, Huwyler J, Gutmann H. Regulation of BCRP (ABCG2) and P-glycoprotein (ABCB1) by cytokines in a model of the human blood–brain barrier. Cell Mol Neurobiol. 2010;30:63–70. doi: 10.1007/s10571-009-9431-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricardo-Dukelow M, Kadiu I, Rozek W, Schlautman J, Persidsky Y, Ciborowski P, Kanmogne GD, Gendelman HE. HIV-1 infected monocyte-derived macrophages affect the human brain microvascular endothelial cell proteome: new insights into blood–brain barrier dysfunction for HIV-1-associated dementia. J Neuroimmunol. 2007;185:37–46. doi: 10.1016/j.jneuroim.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronaldson PT, Bendayan R. HIV-1 viral envelope glycoprotein gp120 triggers an inflammatory response in cultured rat astrocytes and regulates the functional expression of P-glycoprotein. Mol Pharmacol. 2006;70:1087–1098. doi: 10.1124/mol.106.025973. [DOI] [PubMed] [Google Scholar]
- Ronaldson PT, Bendayan M, Gingras D, Piquette-Miller M, Bendayan R. Cellular localization and functional expression of P-glycoprotein in rat astrocyte cultures. J Neurochem. 2004a;89:788–800. doi: 10.1111/j.1471-4159.2004.02417.x. [DOI] [PubMed] [Google Scholar]
- Ronaldson PT, Lee G, Dallas S, Bendayan R. Involvement of P-glycoprotein in the transport of saquinavir and indinavir in rat brain microvessel endothelial and microglia cell lines. Pharm Res. 2004b;21:811–818. doi: 10.1023/b:pham.0000026433.27773.47. [DOI] [PubMed] [Google Scholar]
- Ronaldson PT, Persidsky Y, Bendayan R. Regulation of ABC membrane transporters in glial cells: relevance to the pharmacotherapy of brain HIV-1 infection. Glia. 2008;56:1711–1735. doi: 10.1002/glia.20725. [DOI] [PubMed] [Google Scholar]
- Ronaldson PT, Ashraf T, Bendayan R. Regulation of multidrug resistance protein 1 by tumor necrosis factor alpha in cultured glial cells: involvement of nuclear factor-kappaB and c-Jun N-terminal kinase signaling pathways. Mol Pharmacol. 2010;77:644–659. doi: 10.1124/mol.109.059410. [DOI] [PubMed] [Google Scholar]
- Sabri F, Tresoldi E, Di SM, Polo S, Monaco MC, Verani A, Fiore JR, Lusso P, Major E, Chiodi F, Scarlatti G. Nonproductive human immunodeficiency virus type 1 infection of human fetal astrocytes: independence from CD4 and major chemokine receptors. Virology. 1999;264:370–384. doi: 10.1006/viro.1999.9998. [DOI] [PubMed] [Google Scholar]
- Saha RN, Pahan K. Differential regulation of Mn-superoxide dismutase in neurons and astroglia by HIV-1 gp120: implications for HIV-associated dementia. Free Radic Biol Med. 2007;42:1866–1878. doi: 10.1016/j.freeradbiomed.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama NN, Kelly EJ, Bui T, Ho RJ. The impact of pharmacologic and genetic knockout of P-glycoprotein on nelfinavir levels in the brain and other tissues in mice. J Pharm Sci. 2005;94:1216–1225. doi: 10.1002/jps.20344. [DOI] [PubMed] [Google Scholar]
- Sluss HK, Barrett T, Derijard B, Davis RJ. Signal transduction by tumor necrosis factor mediated by JNK protein kinases. Mol Cell Biol. 1994;14:8376–8384. doi: 10.1128/mcb.14.12.8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzenberger TJ, Heilman D, Diekmann C, Batrakova EV, Kabanov AV, Gendelman HE, Elmquist WF, Persidsky Y. Novel delivery system enhances efficacy of antiretroviral therapy in animal model for HIV-1 encephalitis. J Cereb Blood Flow Metab. 2007;27:1033–1042. doi: 10.1038/sj.jcbfm.9600414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sune C, Garcia-Blanco MA. Transcriptional trans activation by human immunodeficiency virus type 1 Tat requires specific coactivators that are not basal factors. J Virol. 1995;69:3098–3107. doi: 10.1128/jvi.69.5.3098-3107.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toggas SM, Masliah E, Rockenstein EM, Rall GF, Abraham CR, Mucke L. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature. 1994;367:188–193. doi: 10.1038/367188a0. [DOI] [PubMed] [Google Scholar]
- Wu L, LaRosa G, Kassam N, Gordon CJ, Heath H, Ruffing N, Chen H, Humblias J, Samson M, Parmentier M, Moore JP, Mackay CR. Interaction of chemokine receptor CCR5 with its ligands: multiple domains for HIV-1 gp120 binding and a single domain for chemo-kine binding. J Exp Med. 1997;186:1373–1381. doi: 10.1084/jem.186.8.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Argyropoulos G, Zhang Y, Kastin AJ, Hsuchou H, Pan W. Neuroinflammation activates Mdr1b efflux transport through NFkap-paB: promoter analysis in BBB endothelia. Cell Physiol Biochem. 2008;22:745–756. doi: 10.1159/000185558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zastre JA, Chan GN, Ronaldson PT, Ramaswamy M, Couraud PO, Romero IA, Weksler B, Bendayan M, Bendayan R. Up-regulation of P-glycoprotein by HIV protease inhibitors in a human brain microvessel endothelial cell line. J Neurosci Res. 2009;87:1023–1036. doi: 10.1002/jnr.21898. [DOI] [PubMed] [Google Scholar]