Abstract
Bone marrow transplantation (BMT) substantially improves 10-day survival after total body irradiation (TBI), consistent with an effect on intestinal radiation death. Total body irradiation, in addition to injuring the intestinal epithelium, also perturbs the mucosal immune system, the largest immune system in the body. This study focused on how transplanted bone marrow cells (BMCs) help restore mucosal immune cell populations after sublethal TBI (8.0 Gy). We further evaluated whether transplanted BMCs: (a) home to sites of radiation injury using green fluorescent protein labeled bone marrow; and (b) contribute to restoring the mucosal barrier in vivo. As expected, BMT accelerated recovery of peripheral blood (PB) cells. In the intestine, BMT was associated with significant early recovery of mucosal granulocytes (P = 0.005). Bone marrow transplantation did not affect mucosal macrophages or lymphocyte populations at early time points, but enhanced the recovery of these cells from day 14 onward (P = 0.03). Bone marrow transplantation also attenuated radiation-induced increase of intestinal CXCL1 and restored IL-10 levels (P = 0.001). Most importantly, BMT inhibited the post-radiation increase in intestinal permeability after 10 Gy TBI (P = 0.02) and modulated the expression of tight junction proteins (P = 0.01–0.05). Green fluorescent protein-positive leukocytes were observed both in intestinal tissue and in PB. These findings strongly suggest that BMT, in addition to enhancing general hematopoietic and immune system recovery, helps restore the intestinal immune system and enhances intestinal mucosal barrier function. These findings may be important in the development and understanding of strategies to alleviate or treat intestinal radiation toxicity.
INTRODUCTION
Injuries to the bone marrow and gastrointestinal (GI) tract are critical determinants of lethality after total body irradiation (TBI). Radiation causes inflammation, loss of mucosal barrier function and immune imbalance. Typically, humans exposed to radiation doses in the range of 0.7–4 Gy develop symptoms that are secondary to hematopoietic and immune system damage (1). Moreover, alteration of the mucosal immune system occurs at doses that do not cause symptoms of radiation sickness and mucosal permeability increases at doses as low as 1–2 Gy. This loss of mucosal barrier integrity can lead to bacterial translocation and/or the release of nonmicrobial gut-derived factors that potentiate the development of a septic state, one of the overwhelming causes of mortality after exposure to ionizing radiation.
The predominant cause of death within 10 days of radiation exposure has traditionally been attributed to GI injury. Interestingly, replacing or shielding part of the bone marrow substantially increases 10 day survival rates seemingly without changing the level of epithelial injury (2), suggesting that local and/or remote immune mechanisms play a role. In fact, damage to the hematopoietic/lymphopoietic system also occurs over a similar time period (3) and radiation exposure also leads to complete perturbation of the mucosal immune system (4), the largest and most complex immune system in the body.
Bone marrow transplantation (BMT) has become a powerful adjunct in the treatment of hematological disorders, congenital immunodeficiencies, autoimmune diseases and malignant tumors (5). The ability of stem cells to divide and differentiate allows them to act as a repair system for the body (6). Bone marrow cells (BMCs) have been reported to modulate epithelial regeneration (7, 8), home to sites of injury or inflammation (9–11) and play a direct role in vasculogenesis (12). Therefore, it is important to gain an understanding of whether immune cell reconstitution, mechanisms related to endothelial cells and/or vasculogenesis and/or epithelial regeneration or any combination of these mechanisms, are the important variable that helps protect the intestine after exposure to ionizing radiation.
This study was undertaken to investigate whether transplanted BMCs: (a) help restore intestinal immune cell populations after a sublethal dose of TBI; (b) home to sites of radiation injury in the gut; and (c) contribute to the restoration of post-TBI intestinal mucosal barrier integrity. BMT was associated with significant early recovery of mucosal granulocytes, with subsequent recovery of mucosal macrophage or lymphocyte populations, as well as with attenuation of post-TBI changes in the levels of certain chemokines and cytokines. Despite the observation of BMC homing to the injured gut, BMT did not appear to influence the level of structural mucosal injury. However, BMT significantly enhanced mucosal barrier integrity, thus suggesting a mechanism by which BMCs may reduce GI radiation-induced death without altering crypt survival or mucosal architecture.
MATERIALS AND METHODS
Animals
The experimental protocol was reviewed and approved by the Institutional Animal Care and Use Committees (IACUC) at the University of Arkansas for Medical Sciences (UAMS) and the Central Arkansas Veterans Healthcare System (CAVHS). Male CD2F1 mice, with an initial body weight of 23–25 g, were obtained from Harlan Sprague Dawley®(Indianapolis, IN). In addition, C57BL/6 mice and GFP-transgenic (Tg) mice [C57BL/6-Tg (CAG-EGFP) 131Osb/LeySopJ], 7–8 weeks old, were obtained from the Jackson Laboratory (Bar Harbor, ME). Animals were housed in conventional cages under standardized conditions, with controlled temperature and humidity and a 12/12 h, day/night light cycle. Animals had free access to water and chow (Harlan Teklad laboratory diet 7012, Purina Mills, St. Louis, MO).
To study the effect of bone marrow transplantation on radiation-induced intestinal immune cell populations, mice received a single dose of TBI (8.0 Gy, unless otherwise specified), and groups of mice were euthanized at set time points after irradiation [0 h (nonirradiated), 1 day, 3.5, 7, 14, 21 and 30 days], with n = 8–10 mice per time point. Previous experiments in CD2F1 mice have shown that 8.0 Gy induces substantial intestinal and hematopoietic injury but with adequate 30 day survival (13). Green fluorescent protein (GFP) transgenic mice were used to investigate the ability of BMCs to home to areas of radiation-induced injury in the gut.
Chemicals and Media
Calcium- and magnesium-free PBS, RPMI-1640, FBS, penicillin-streptomycin, L-glutamine, β-mercaptoethanol, Percoll (1:130 ± 0.005 g/ml) and Dulbecco’s phosphate-buffered saline (DPBS) were purchased from Sigma-Aldrich (St. Louis, MO). RPMI-1640 was supplemented with FBS (10% v/v), 100 U/ml penicillin-streptomycin, 2 mM L-glutamine and 5 × 10−5 M β-mercaptoethanol. A stock solution of 0.5 M ethylenediaminetetraacetic acid (EDTA) and dithiothreitol (DTT) were purchased from Sigma-Aldrich.
Irradiation and Dosimetry
Irradiation was performed as previously described (13). Briefly, after confirmation of dose uniformity by thermoluminescence dosimetry, irradiation was performed in a Shepherd Mark I, model 25, Cs-137 irradiator (J.L. Shepherd & Associates, San Fernando, CA). The average dose rate was 1.35 Gy/min. Irradiation was performed on a turntable rotating at 6 rpm, without anesthesia, and with the mice held in a pie-shaped container with one compartment for each animal.
Generation of Bone Marrow Transplanted Mice
The procedure for generation of bone marrow transplanted mice was based on previously published studies, with minor modifications (14). Briefly, donor mice (CD2F1 or GFP) were euthanized and BMCs were collected from the tibias and femurs by flushing with sterile PBS. Recipient mice were subjected to a dose of 8.0 or 10.0 Gy TBI for the assessment of in vivo permeability (see below). Within 4–6 h after irradiation, recipient mice received an intravenous (i.v.) injection of 2 × 107 BMCs supplemented with 1 × 107 cells from the spleen to serve as an immediate source of immune cells. In the homing experiment, recipient mice received BMCs only.
Immunohistochemical and Immunofluorescent Detection
Immunohistochemical staining for myeloperoxidase (MPO, neutrophils), macrophages, CD45R (B-lymphocytes), and CD4 (T-lymphocytes) on proximal segments of jejunum obtained at 0 h, 4 h, 1 day, 3.5, 7, 14, 21 and 30 days (with and without BMT) was performed as previously described (4). Quantitative assessment of immunoreactivity was performed using computerized image analysis (Image-Pro Plus, Media Cybernetics Inc., Silver Springs, MD) as described and validated previously (15). Cells positive for MPO, CD45R, CD4 or macrophages were identified by color thresholding. The number of positive cells per 10 fields (40× objective) was considered a single value for statistical analysis.
For immunofluorescent detection of CD68, proximal segments of jejunum samples were obtained and flash frozen at 7 days after BMT from GFP mice. The tissues were embedded in optimal cutting temperature (OCT) media and were stored at −80°C until use. Briefly, 6 μm sections after fixation in pre-cold acetone for 5 min at room temperature and air drying for 2 min, were incubated with blocking solution (10% FBS in PBS) for 1 h. Sections were then incubated with rat anti-CD68 (Abcam, Cambridge, MA) overnight at 4°C in a humidified chamber (1:500). After washes in PBS, CD68 were detected and visualized with goat anti-rat IgG-H&L (DyLight A®594) (1:250) for 1 h at room temperature. To detect the eGFP protein, Alexa Fluor 488-labeled rabbit anti-GFP (Invitrogen) was used (1:800) for 2 h at room temperature for double immunostaining. Upon completion of the staining protocols, coverslips were placed over the slides using the Prolong Gold antifade reagent with DAPI (Invitrogen). Slides were scanned with Aperio Scanscope XT (Aperio Technologies Inc., Vista, CA).
RNA Extraction and Quantitative Reverse Transcription PCR (qRT-PCR)
Total RNA was purified from frozen tissue using RNeasy Plus Mini Kit (Qiagen, Valencia, CA) as instructed by the manufacturer after homogenizing the samples in TRIzol® Reagent (Life Technologies, Grand Island, NY). cDNA was synthesized using a cDNA reverse transcription kit (Applied Biosystems®) after treating with RQ-DNase I (Promega, Madison, WI). Predesigned Taqman assay (Applied Biosystems®) for mouse gene: CXCL1, Mm04207460_m1; CXCL9, Mm00434946_m1; IL-12, Mm0043 4200_m1; IL-10, Mm0043 9614_ m1; F oxp3, Mm00475162_m1; CTLA-4, Mm00486849_m1; Claudin-2, Mm00516703_s1; Claudin-4, Mm00515514_s1; Claudin-10, Mm01226326_g1; Claudin-11, Mm00500915_m1; Mylk, Mm00653039_m1; Occludin, Mm00500912_m1 and 18s rRNA, Hs99999901_s1 was used. The mRNA levels were normalized to eukaryotic 18s rRNA and calculated relative to control mice, using the standard ΔΔCt method.
Preparation of Intestinal Tissue Homogenates
To prepare intestinal homogenates for protein analysis, tissues obtained at different time points were homogenized in 600 μl of PBS supplemented with a complete protease inhibitor cocktail tablet (Roche Diagnostics, Indianapolis, IN) and 160 U/ml RNase inhibitor (Promega, Madison, WI) using a polytron homogenizer (Brinkmann Instruments, Delran, NJ). Subsequently, homogenates were centrifuged at 14,000 rpm for 15 min at 4°C to pellet membrane material and supernatants were removed and stored at −80°C until multiplex cytokine microbead array analysis, described below.
Multi-Analyte Microbead Array to Detect Proinflammatory Mediator Expression
To assess inflammatory mediators in whole tissue homogenates of the proximal jejunum, a mouse 20-plex cytokine microbead array system was used according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA). Results were analyzed using a Bioplex Workstation (Bio-Rad Laboratories, Hercules, CA) and normalized against the amount of total protein extracted from the intestinal tissues. The level of sensitivity for each microbead cytokine standard curve ranged from 1–40 pg/ml.
Assessment of Radiation Injury
Mucosal surface area (MSA)
Intestinal mucosal surface area, a well-validated, sensitive parameter of intestinal radiation injury that, at sublethal radiation doses, mainly reflects changes in the villus epithelium, was measured in vertical H&E–stained sections of the jejunum at baseline and 3.5 days, 7 days and 14 days after TBI, using a projection/cycloid method as described by Baddeley et al. (16). The method has previously been validated by us specifically for surface area determination of the intestinal mucosa after irradiation (17) and has been used extensively both with localized intestinal irradiation as well as after exposure to TBI (13).
Plasma citrulline levels
The plasma level of citrulline is a well-validated biomarker for functional enterocyte mass. While there is excellent correlation between plasma citrulline levels and more conventional markers of intestinal radiation injury, including mucosal surface area and the crypt colony survival assay (18), plasma citrulline, by reflecting enterocyte mass (both villus and crypt cells), is more sensitive than the crypt colony assay at sublethal doses of radiation. Because citrulline levels can be determined in as little as 10 μl of plasma, the citrulline assay is an attractive, minimally invasive longitudinal marker of radiation-induced bowel injury.
Whole blood was collected into EDTA-coated tubes (Fisher Scientific, Pittsburgh, PA.). Plasma was obtained by centrifugation (12,000 rpm, 5 min, 4°C) and stored at −80°C until analyzed. Citrulline was determined in plasma using a validated LC-MS method as described before (19). Briefly, plasma samples were protein-precipitated for assay in 96-well Strata Impact 2-ml filtration plates (Phenomenex, Torrance, CA). Samples (10 μL) were treated with 490 μL acetonitrile:water:formic acid (85:14.8:0.2 v/v) containing internal standard (2 μM). After mixing gently, the plate was covered, allowed to stand for 5 min, and the filtrate was collected under vacuum. The LC-MS system was an Acquity UPLC system interfaced to a Quattro Premier triple quadrupole mass spectrometer (Waters Corp., Milford, MA), and chromatographic separation was achieved using a Phenomenex 1.7 μM Kinetex Diol analytical column (50 × 2.1 mm i.d.). Mass spectrometric detection was performed in the multiple-reaction-monitoring (MRM) mode using the precursor→product ions 176.2→158.9 Th and 181.2→163.8 Th for citrulline and citrulline+5, respectively.
Peripheral Blood Cell Count
Whole blood was collected into EDTA-coated tubes from mice with or without BMT at 0 h, 4 h, 1 day, 3.5 days, 7 days, 14 days, 21 days and 30 days after 8.0-Gy TBI. Peripheral blood cell counts were obtained using a veterinary hemocytometer (Hematrue System, Heska Corporation, Loveland, CO) according to the manufacturer’s instructions.
In Vivo Intestinal Permeability Assay
Intestinal permeability was examined on day 7 post-TBI in mice exposed to 10 Gy, with or without BMT, by using an in vivo fluorescein isothiocyanate (FITC)-labeled dextran method. Briefly, the mice were anesthetized with isoflurane inhalation, a midline laparotomy was performed, and the renal artery and vein were ligated bilaterally. A 10-cm small intestine segment, located 5 cm distal to the ligament of Treitz, was isolated and tied off. One hundred μl of 4-kDa fluorescein isothiocyanate-conjugated dextran (FITC-dextran, 25 mg/ml in PBS) was injected into the isolated intestine using a 30-gauge needle, and the abdominal incision was closed. Blood samples were collected retro-orbitally at 90 min after infusion of FITC-dextran. Plasma was separated by centrifuging (8000 rpm, 10 min, 4°C), and was analyzed for FITC-dextran concentration using a fluorescence spectrophotometer (Synergy HT, Bio-Tek Instruments, Winooski, VT) at an excitation wavelength of 480 nm and an emission wavelength of 520 nm. Standard curves to calculate FITC-dextran concentration in the plasma samples were prepared from dilutions of FITC-dextran in PBS.
Intestinal Intraepithelial Leukocyte Preparation and Flow Cytometry Examination
For detecting the percentage of GFP-positive intestinal intraepithelial leukocyte (i-IELs) by FACS, i-IELs were isolated as described by Montufar-Solis and Klein (20). Confirmation of IELs was carried out by staining cells with APC-conjugated anti-CD45 and analyzing by FACSCalibur flow cytometry. A gate was set up according to SSC and FSC signals to exclude cell debris and clumps. Data were analyzed using FCS Express V3 software (De Novo™).
Statistical Analysis
Data are expressed as the mean ± the standard error of the mean (SEM). Statistical analysis was performed with NCSS 2007 (NCSS, Kaysville, UT) and GraphPad Prism 5.0 (La Jolla, CA). Comparison among multiple means was performed by analysis of variance (ANOVA) and comparison of groups was performed by either Mann Whitney U test or two-tailed t tests and difference of P < 0.05 was considered statistically significant.
RESULTS
BMT Accelerated Peripheral Blood Counts
The 8.0 Gy dose was well tolerated up to 30 days after radiation, with only two deaths noted on day 29 after TBI in mice without BMT. Irradiation caused a significant decrease in the numbers of circulating leukocytes, erythrocytes and thrombocytes. For some cell types (particularly lymphocytes and monocytes) significant decreases were already observed 4 h postirradiation. As expected, BMT significantly accelerated blood cell recovery (data not shown).
BMT Enhanced the Recovery of Intestinal Immune Cell Populations in Jejunum Mucosa
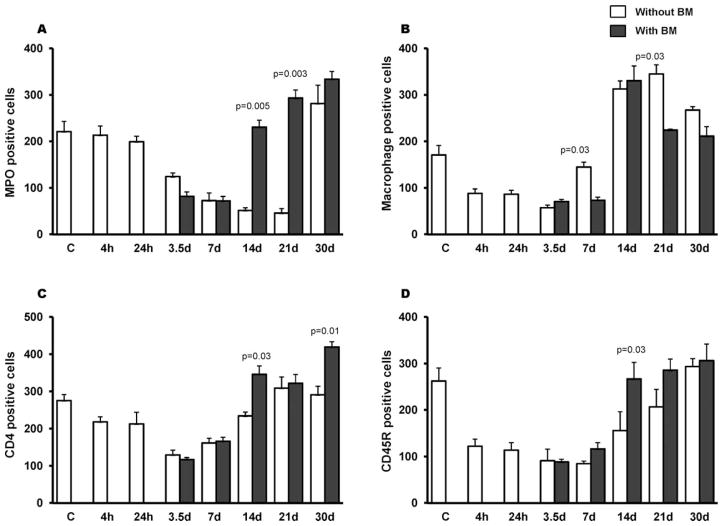
Immunohistochemistry was used to evaluate the influence of BMT on the distribution of various types of immune cells in the intestinal mucosa. Radiation alone induced a time-dependent decrease in MPO-positive cells (mainly neutrophils), which was statistically significant at 3.5 days (P = 0.01) and persisted until 21 days (P = 0.001). Interestingly, in the intestine BMT was associated with significant recovery of mucosal granulocytes by day 14 (P = 0.005), which persisted until day 21 (P = 0.003). By day 30 after TBI, animals with and without BMT had reached baseline levels (Fig. 1A).
FIG. 1.
Effects of BMT on intestinal immune cell populations. Sublethal TBI (8 Gy) induced a time-dependent decrease in numbers of MPO-positive cells (panel A), macrophages (panel B), CD45R-positive cells (B-lymphocytes) (panel C) and CD4-positive cells (T-lymphocytes) (panel D). BMT significantly enhanced the recovery of mucosal granulocytes (P = 0.005), T-lymphocytes (P = 0.03) and B-lymphocytes (P = 0.03) by day 14. However, there was no difference in macrophage number in groups with or without BMT. Ten fields per section with a 40× objective; average ± SEM, n = 4–6.
TBI also caused a reduction in the number of macrophages between 4 h and 3.5 days (Fig. 1B) while CD45R-positive cells (B-lymphocytes) and CD4-positive cells (mainly T-lymphocytes) remained reduced up to 7 days after TBI (Fig. 1C–D). Although BMT did not affect the numbers of mucosal macrophages, B-lymphocytes or T-lymphocytes at early time points (Fig. 1B–D), recovery of these cells in transplanted mice was enhanced from day 14 onward (P = 0.03). However, macrophages unlike the other immune cells were noted to recover equally in both groups (with or without BMT).
BMT did not Affect Structural Radiation Injury of the Intestinal Epithelium
It is well known that postirradiation crypt survival is not affected by BMT or bone marrow sparing (2, 3). However, it is not known if this also applies to other compartments of the intestinal epithelium. Therefore, structural radiation injury of the gut was assessed using intestinal mucosal surface area and plasma citrulline levels.
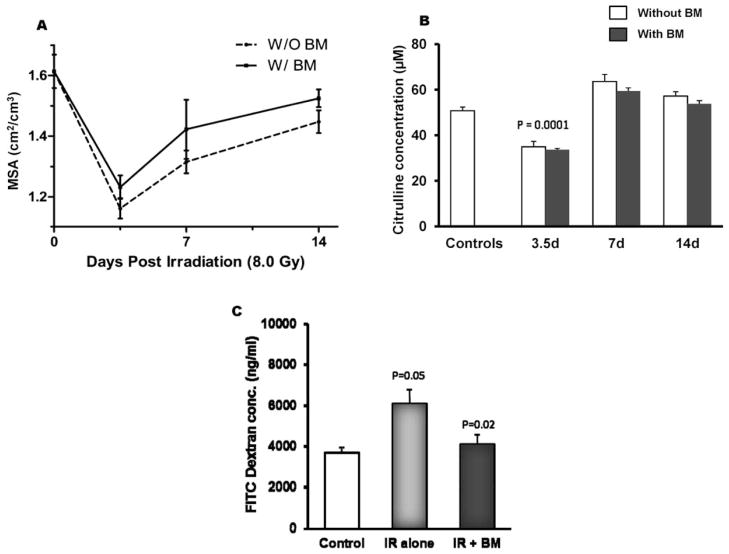
Mucosal surface area primarily reflects changes in the villus epithelium. Exposure to 8.0 Gy TBI resulted in a significant decrease in mucosal surface area in groups with or without BMT at 3.5 days postirradiation. A trend toward improved mucosal area was observed from day 7 forward in mice with BMT compared with irradiated mice that were not transplanted, although a level of statistical significance was not reached (Fig. 2A).
FIG. 2.
Effect of BMT on post-TBI mucosal surface area, plasma citrulline and intestinal permeability. Panel A: BMT did not significantly improve postirradiation mucosal surface area 3.5, 7 or 14 days after 8 Gy TBI when compared to groups without transplantation. Average ± SEM, n = 4–6. Panel B: TBI (8 Gy) induced a significant reduction in plasma citrulline at 3.5 days postirradiation (P = 0.0001). BMT did not prevent the effect of TBI on plasma citrulline. Mean ± SEM, n = 8 mice per group. Panel C: Compared to sham-irradiated controls, 10 Gy TBI induced a significant increase in intestinal permeability (P = 0.05) at day 7, while BMT significantly reduced FITC-dextran levels (P = 0.02). Average ± SEM, 6–8 mice per group.
To study the effect of BMT on overall enterocyte mass, plasma levels of citrulline were measured in sham-irradiated mice and on days 3.5, 7 and 14 in mice exposed to 8.0 Gy TBI with or without BMT. TBI induced a significant decrease in plasma citrulline levels on day 3.5 (P = 0.0001) (Fig. 2B), but levels had returned to baseline values by day 7. No significant difference was noted between groups with or without BMT.
BMT was Associated with Reduced Intestinal Permeability
In vivo assessment of intestinal permeability showed that, compared to sham-irradiated controls, permeability increased significantly (P = 0.05) 7 days after mice were exposed to 10 Gy TBI. In contrast, transplanted mice exposed to 10 Gy TBI showed significantly lower FITC-dextran levels at day 7 postirradiation. Permeability levels in irradiated BMT mice were similar to those of sham-irradiated control mice, and irradiated BMT mice exhibited a statistically significant reduction (P = 0.02) compared with the irradiated non-transplanted group (Fig. 2C).
Effect of BMT on Radiation-Induced Alterations in Cytokines and Chemokines on Intestinal Tissue
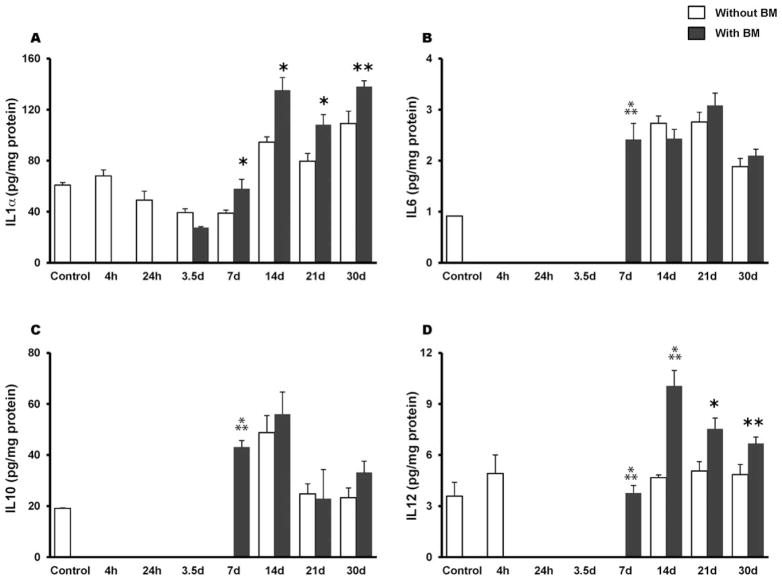
A mouse 20-plex cytokine microbead assay was used to assess levels of inflammatory mediators in whole tissue homogenates of proximal jejunum in mice with or without BMT. As shown in Fig. 3A, radiation exposure caused a decrease in IL-1α in the early postirradiation phase (24 h to 7 days). BMT induced a significant increase in IL-1α (P = 0.01) by day 7 compared with non-transplanted animals and remained consistently high until day 30. Both IL-6 and IL-10 were greatly reduced during the early phase of irradiation, to such an extent that their levels were below the detection limit of the assay. BMT increased their availability at day 7 postirradiation (P = 0.0003 and P = 0.0001, respectively) (Fig. 3B–C). Likewise, tissue IL-12 levels were also below the detection limit as early as 24 h postirradiation and this persisted until day 7 (Fig. 3D), whereas BMT increased the availability of IL-12 at day 7 (P = 0.0001). Interestingly, by day 14 forward, the transplanted group exhibited IL-12 levels that were significantly higher than in the non-transplanted groups (day 14, P = 0.001; day 21, P = 0.01; day 30, P = 0.04) (Fig. 3D). Further, at day 14 post-TBI, MIG was significantly induced (P = 0.0004) whereas FGF-2 exhibited a significant attenuation (P = 0.0008) in the BMT group (Fig. 4A–B).
FIG. 3.
Effect of BMT on radiation-induced (8 Gy TBI) alterations in cytokines in whole tissue homogenates of the jejunum. Panel A: BMT induced a significant increase in IL-1α at 7 days, which remained consistently high until day 30 postirradiation. Panel B: IL-6 levels decreased after 8.0 Gy TBI (4 h to 3.5 day) while BMT significantly induced IL-6 levels at day 7. Similarly, BMT induced (panel C) IL-10 and (panel D) IL-12 significantly at day 7 postirradiation. *P = 0.01; **P = 0.05;
 P = 0.001 compared to non-transplanted groups. Average ± SEM, n = 3–6 mice per group.
P = 0.001 compared to non-transplanted groups. Average ± SEM, n = 3–6 mice per group.
FIG. 4.
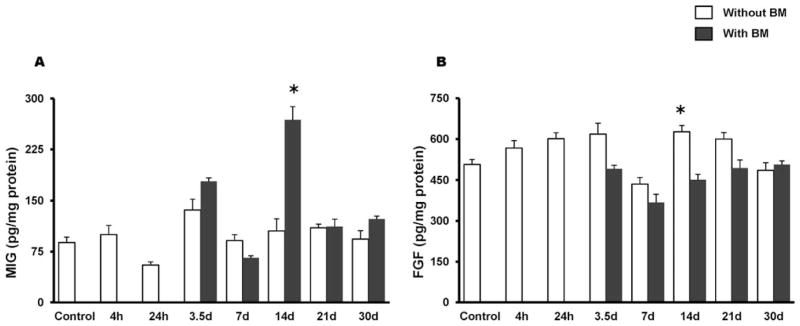
Effect of BMT on radiation-induced (8 Gy TBI) alteration in MIG and FGF-2 in whole tissue homogenates of the jejunum. Panel A: MIG was significantly induced in transplanted mice at day 14 and reached baseline levels by day 21. Panel B: TBI induced a significant increase in FGF-2 (4 h to 3.5 days) while BMT reduced FGF-2 at 3.5 and 14 days. *P = 0.001 compared to non-transplanted group. Average ± SEM, n = 3–6 mice per group.
BMT Limits the Radiation-Induced Increased Production of CXCL1 and CXCL9 and Restores IL-10 and IL-12 in Jejunum Mucosa
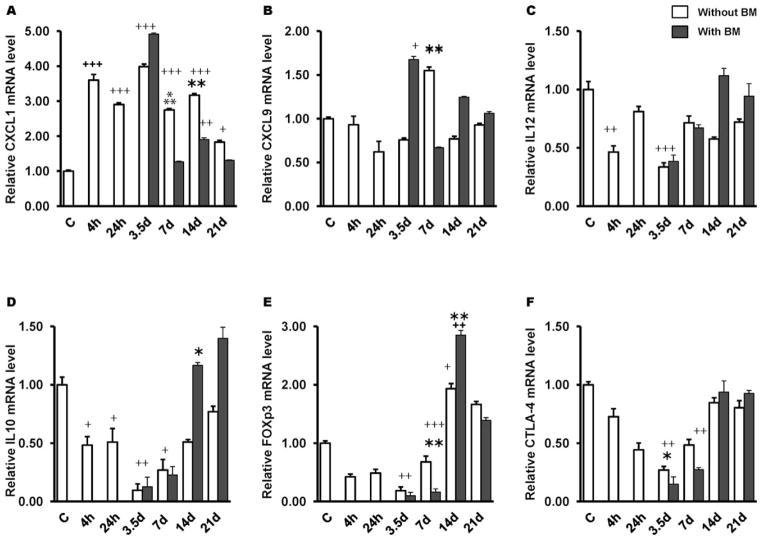
Radiation-induced increase of CXCL1 mRNA levels in the jejunum was noted as early as 4 h postirradiation and was maintained until 21 days (Fig. 5A), however, BMT attenuated this increase at day 7 post-TBI (P = 0.001). On the contrary, BMT restored radiation-induced decrease in CXCL9 and IL-12 by day 14 post-TBI (Fig. 5B–C). While IL-10 is required for the maintenance of noninflammatory homeostasis in the intestine, Foxp3 and CTLA-4 have been identified as critical for Tregs function in inflammatory models, we therefore examined these in jejunum mucosa. Both IL-10 and Foxp3 expression were significantly repressed as early as 4 h postirradiation and remained persisted until day 7 (Fig. 5D–E). CTLA-4 expression evolved similarly (Fig. 5F). By day 14 post-TBI, Foxp3 levels were significantly elevated compared to controls (P = 0.05) in both groups (with or without BMT), whereas CTLA-4 expression reached the baseline (Fig. 5F). Further, IL-10 levels were restored in BMT group by day 14 post-TBI (P = 0.05).
FIG. 5.
BMT ameliorates CXCL1 and restores IL-10 and IL-12 in the jejunum. Irradiation (8 Gy TBI) significantly induced mRNA levels of CXCL1 (panel A) and reduced CXCL9 (panel B), IL-12 (panel C), IL-10 (panel D), Foxp3 (panel E) and CTLA-4 (panel F). BMT reduced CXCL1 expression at day 7 post-TBI and restored IL-10 and Il-12 at day 14. *P = 0.05; **P = 0.03;
 P = 0.001 compared to non-transplanted group; +P = 0.03; ++P = 0.05; +++P = 0.001 compared to controls. Average ± SEM, n = 4 mice per group.
P = 0.001 compared to non-transplanted group; +P = 0.03; ++P = 0.05; +++P = 0.001 compared to controls. Average ± SEM, n = 4 mice per group.
BMT Modulated the Expression of Tight Junctions in Jejunum Mucosa
Real time quantitative PCR revealed that a sublethal dose of TBI induced a significant increased expression of Claudin-2 mRNA in the jejunum at early time points (4 h, 24 h, 3.5 days). However, by day 7 post-TBI the level returned to baseline (Fig. 6A). Likewise, the mRNA levels of Claudin-4 (Fig. 6B) initially showed an increased expression at 4 h post-TBI (P = 0.01). However, a time-dependent decrease was noted after 24 h, which persisted until day 21 (latest time point examined in this study). Conversely, Claudin-11 (Fig. 6C) showed an increased expression at all time points examined. However, by day 7 post-TBI, BMT appears to have restored both claudin-4 and claudin-11 mRNA level in the jejunum. Further, myosin light chain kinase (MYLK), an important regulator of intestinal barrier function, exhibited a decreased mRNA level at 4 h post-TBI (P = 0.01). However, by day 7, baseline levels were reached in both groups (with or without BMT) (data not shown). Relative mRNA levels of Claudin-10 and Occludin in groups with or without BMT were also not significantly different from controls (data not shown).
FIG. 6.
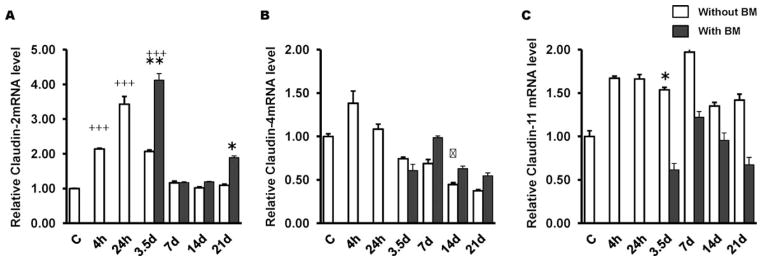
Effects of BMT on intestinal tight junction-related genes in the jejunum. Irradiation (8 Gy TBI) significantly induced mRNA levels of Claudin-2 (panel A) and Claudin-4 (panel B), while Claudin-11 (panel C) were induced at all time points.. BMT restored mRNA levels of Claudin-4 and Claudin-11 to baseline by day 7 post-TBI. *P = 0.05; **P = 0.003;
 P = 0.001 compared to non-transplanted group; +++P = 0.001 compared to controls. Average ± SEM, n = 4 mice per group.
P = 0.001 compared to non-transplanted group; +++P = 0.001 compared to controls. Average ± SEM, n = 4 mice per group.
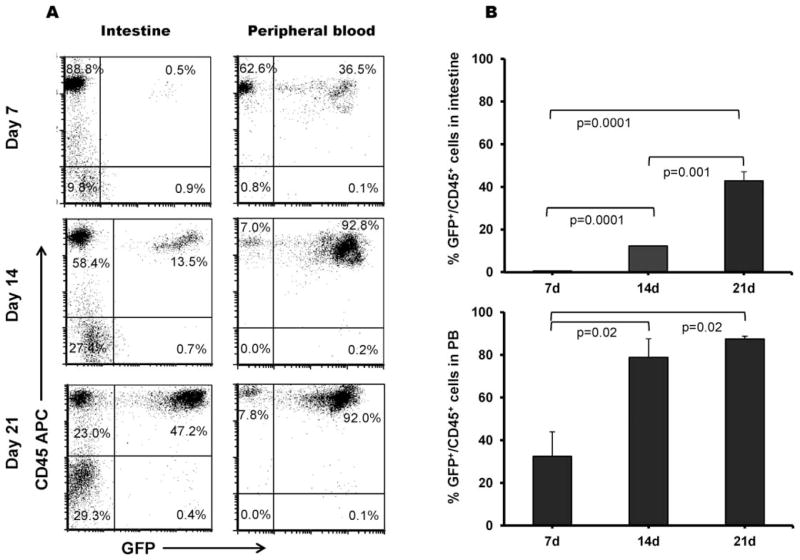
Homing of Donor BMCs to Recipient Intestine
Using flow cytometry, we quantitatively analyzed the percentage of GFP-positive and CD45 positive cells (i.e. GFP-positive leukocytes) both in intestine and peripheral blood. On day 7 post-TBI, the percentage of GFP-positive and CD45 positive cells in the gut were almost negligible compared with GFP-positive and CD45 positive cells in the peripheral blood. However, by day 14 post-TBI, the GFP-positive and CD45 positive cells were significantly increased in both the intestine and the peripheral blood (P =0.0001 and P = 0.02, respectively) (Fig. 7A–B).
FIG. 7.
Homing of GFP-positive BMCs in the intestine and peripheral blood after 8 Gy TBI. Panel A: Flow cytometry analysis of iIELs and peripheral blood leukocytes to show GFP-positive and CD45-positive cells and (panel B) its quantitative analysis of percentage of GFP-positive and CD45-positive cells in the intestine and peripheral blood. Average ± SEM, n = 4–6 mice per group.
Immunofluorescent studies revealed the presence of GFP-positive cells in the gut on day 7 post-TBI (the earliest time point examined in these studies). To further determine the characteristics of these accumulated cells, an immunofluorescent labeling technique was applied to detect the expression of macrophages (CD68-positive). As shown in Supplementary Fig. S1 (http://dx.doi.org/10.1667/RR13548.1S1), cells were positive for both GFP and CD68 were mainly localized in the intestinal lamina propria.
DISCUSSION
The mucosal surface of the intestine represents one of the largest and most complex parts of the immune system (21). Although changes in intestinal immune cell populations and in the expression levels of cytokines, chemokines and transcription factors after localized and abdominal irradiation (22, 23) and/or total body irradiation have been well documented (4, 24), relatively little is known about the role of BMCs in restitution of these immune cell populations and about the possible functional implications thereof.
Recently, bone marrow-derived stem cells (BMDSCs) have garnered a great deal of attention from various research groups even though the high degree of plasticity of BMDSCs is somewhat controversial (25, 26). The ability of bone marrow stem cells (or progenitor cells) to home to areas of injury, including the heart, liver, brain, kidney and pancreas suggest that the bone marrow may contribute to organ repair after cellular injury. BMCs have also been shown to have the ability to differentiate into various nonhematopoietic cells (27, 28), such as epithelial cells that can repopulate damaged intestinal epithelium (29, 30) and vasculogenic cells (12). These studies highlight the importance of BMDSCs to the body’s healing process.
The mechanisms by which BMCs attenuate mucosal inflammation are poorly understood. However, it is well recognized that the epithelial barrier coordinates immune responses to the myriad of microorganisms that colonize the gut and, if dysregulated, might contribute to intestinal inflammation (31). The intestinal epithelial barrier is maintained by intracellular junctional complexes, such as tight junction, adherent junctions and desmosomes. Numerous studies have demonstrated that decrease of tight junction complexes may disturb the epithelial barrier and increase intestinal permeability. We and others have shown that ionizing radiation increases intestinal permeability through a combination of epithelial tight junction disruption and insufficient replacement of villus epithelium (32, 33). It is also possible that BMCs, through enhancing vasculogenesis (12), help counteract both acute (34) and delayed (35) radiation effects in the gut.
The current study demonstrates that intestinal “leakage”, which was significantly increased in animals exposed to radiation, was markedly reduced by BMT. Analogously, bone marrow-derived mesenchymal stem cells have also been reported to improve intestinal permeability after ischemia-reperfusion intestinal injuries (36). Further, we demonstrated that radiation was associated with increased intestinal expression of Claudin-2 and Claudin-11, and also showed decreased expression of Claudin-4, similar to in ulcerative colitis (37). Restoration of Claudin-4 and Claudin-11 by BMT post-TBI day 7 accompanied by decreased gut permeability suggest a possible role of Claudins in intestinal barrier function. These findings strongly suggest that BMCs contribute to restore the mucosal barrier after injury caused by irradiation. Moreover, since there was no evidence of enhanced epithelial regeneration by the citrulline or mucosal surface area assays, our study suggests that the benefit of BMT is derived from immune reconstitution (directly or indirectly) rather than from enterocyte regeneration. More detailed studies with various amounts of purified hematopoietic and bone marrow stromal cells and different doses of radiation (lethal, as well as sublethal) are needed to address the questions of whether BMCs influence the bowel (directly or indirectly through resident cells), as well as which component(s) of the bone marrow is critical for the beneficial effect.
The profile of cytokine secretion reflects the functional integrity of the immune system. Results from this study showed that BMT increased the intestinal tissue levels of IL-1α, IL-6, IL-12 and IL-10, all interleukins that are known to stimulate hematopoiesis. Previous studies have shown that IL-12 in synergy with IL-1 can enhance hematopoietic effects due to its ability to synergize with hematopoietic growth factors to increase the number and size of hematopoietic colonies (38). The fact that IL-1 and TNF, both potent inducers of IL-6, have been reported to increase in mice after a sublethal dose of irradiation (4), suggests that hematopoietic regeneration may be partially mediated by the induction of endogenous IL-6 by release of endogenous IL-1 and TNF. Interestingly, results from some experiments even suggest that IL-6 may protect the intestinal mucosa from the consequences of systemic inflammation (39). Furthermore, CD4+FOXP3+CD25+ Tregs also play an important role in immune regulation in the intestine. Reduced expression of various signaling molecules such as Foxp3 (recognized marker of Tregs), microenvironmental factors (CTLA-4), and anti-inflammatory cytokines (as IL-10) may contribute to impairment of Treg function. In our study, the reduced intestinal expression of IL-10, Foxp3 and CTLA-4 post-TBI suggest a defect of Tregs, which is inconsistent with results of studies with abdominal irradiation (40). Interestingly, BMT enhanced the recovery of IL-10 by day 14 post-TBI, suggesting that BMCs may play a role in maintaining mucosal homeostasis. In addition, IL-10 has also been reported to be highly implicated in maintaining Treg function in the intestine (41). Most importantly, we observed that BMT decreased the radiation-induced production of inflammatory mediators such as CXCL1.
Both in animal models as well as in patients, BMCs have been reported to be involved in the healing process after intestinal injury (42). Therefore, to investigate the homing potential of BMCs to sites of radiation-induced injury, BMT mice were generated by using BMCs from GFP donor mice. Analysis of trafficked GFP-expressing cells within the intestine was performed 7, 14 and 21 days post-TBI. Many GFP-positive leukocytes from the donor’s bone marrow could be found in the recipient intestine at different time points after transplantation and were noted as early as 7 days post-BMT. These observations suggest that donor BMCs do home to the injured gut and possibly participate in the physiological recovery/healing process.
To obtain further evidence, the fluorescent labeling technique for GFP and CD68 was applied. A majority of the GFP positive cells were positive for CD68, located primarily in the lamina propria of the intestine. Macrophages are known to be actively recruited to sites of injury (43). Reports have shown that monocytes/macrophages may contribute to wound healing after vascular injury, myocardial infarction and notably, gastrointestinal mucositis (44). Donor-derived intraepithelial cells in the small intestine also showed a significant time-dependent increase after transplantation. This may partly explain the healing of mucosal damage observed in our mouse model.
In summary, we observed that BMT, in addition to enhancing general hematopoietic and immune system recovery, also enhances the local intestinal immune system and restores mucosal barrier function after sublethal irradiation. Future studies should be performed to determine whether bone marrow transplantation protects against mucosal barrier dysfunction because of immune cell reconstitution, epithelial cell regeneration, through micro-vascular mechanisms, or by a combination of these mechanisms. Understanding how bone marrow cells participate in shaping the immune response and promote repair of the damaged tissue could be important for the development of new therapies to treat intestinal radiation toxicity.
Supplementary Material
http://dx.doi.org/10.1667/RR13548.1.S1; Homing of transplanted BMCs as detected by presence of GFP-positive macrophages in the mouse small intestine on day 7 postirradiation. CD68-positive cells were mainly seen in the lamina propria of villi. Most if not all GFP-positive cells were CD68-positive (as shown by arrows). Panel A: GFP-positive cells were shown by Alexa Fluor 488. Panel B: CD68-positive cells shown by Dylight 594. Panel C: Nuclei stained with DAPI. Panel D: Merged image of A, B and C. Bar = 50 μm.
Acknowledgments
Assistance with tissue processing by Jennifer D. James of the UAMS Experimental Pathology Core laboratory, with the citrulline assay by Dr. Howard P. Hendrickson of the UAMS Biodosimetry Diagnostic Core, and with the Luminex assays by Dr. Gregory D. Sempowski of the Duke University Immune Monitoring Core is gratefully acknowledged. This work was supported by the National Institutes of Health (grants AI67798 and CA71382) and by the Veterans Administration.
Footnotes
Editor’s note. The online version of this article (DOI: 10.1667/RR13548.1) contains supplementary information that is available to all authorized users.
References
- 1.Mettler FA, Jr, Voelz GL. Major radiation exposure—what to expect and how to respond. N Engl J Med. 2002;346:1554–61. doi: 10.1056/NEJMra000365. [DOI] [PubMed] [Google Scholar]
- 2.Terry NH, Travis EL. The influence of bone marrow depletion on intestinal radiation damage. Int J Radiat Oncol Biol Phys. 1989;17:569–73. doi: 10.1016/0360-3016(89)90108-9. [DOI] [PubMed] [Google Scholar]
- 3.Mason KA, Withers HR, McBride WH, Davis CA, Smathers JB. Comparison of the gastrointestinal syndrome after total-body or total-abdominal irradiation. Radiat Res. 1989;117:480–8. [PubMed] [Google Scholar]
- 4.Garg S, Boerma M, Wang J, Fu Q, Loose DS, Kumar KS, et al. Influence of sublethal total-body irradiation on immune cell populations in the intestinal mucosa. Radiat Res. 2010;173:469–78. doi: 10.1667/RR1742.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ikehara S. Bone marrow transplantation: a new strategy for intractable diseases. Drugs Today (Barc) 2002;38:103–11. doi: 10.1358/dot.2002.38.2.820106. [DOI] [PubMed] [Google Scholar]
- 6.Theise ND, Henegariu O, Grove J, Jagirdar J, Kao PN, Crawford JM, et al. Radiation pneumonitis in mice: a severe injury model for pneumocyte engraftment from bone marrow. Exp Hematol. 2002;30:1333–8. doi: 10.1016/s0301-472x(02)00931-1. [DOI] [PubMed] [Google Scholar]
- 7.Rizvi AZ, Swain JR, Davies PS, Bailey AS, Decker AD, Willenbring H, et al. Bone marrow-derived cells fuse with normal and transformed intestinal stem cells. Proc Natl Acad Sci U S A. 2006;103:6321–5. doi: 10.1073/pnas.0508593103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J, Gong J, Zhang W, Zhu W, Li J. Effects of transplanted bone marrow mesenchymal stem cells on the irradiated intestine of mice. J Biomed Sci. 2008;15:585–94. doi: 10.1007/s11373-008-9256-9. [DOI] [PubMed] [Google Scholar]
- 9.Hu KX, Sun QY, Guo M, Ai HS. The radiation protection and therapy effects of mesenchymal stem cells in mice with acute radiation injury. Br J Radiol. 2010;83:52–8. doi: 10.1259/bjr/61042310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kudo K, Liu Y, Takahashi K, Tarusawa K, Osanai M, Hu D, et al. Transplantation of mesenchymal stem cells to prevent radiation-induced intestinal injury in mice. J Radiat Res. 2010;51:73–9. doi: 10.1269/jrr.09091. [DOI] [PubMed] [Google Scholar]
- 11.Semont A, Mouiseddine M, Francois A, Demarquay C, Mathieu N, Chapel A, et al. Mesenchymal stem cells improve small intestinal integrity through regulation of endogenous epithelial cell homeostasis. Cell Death Differ. 2010;17:952–61. doi: 10.1038/cdd.2009.187. [DOI] [PubMed] [Google Scholar]
- 12.Milovanova TN, Bhopale VM, Sorokina EM, Moore JS, Hunt TK, Hauer-Jensen M, et al. Lactate stimulates vasculogenic stem cells via the thioredoxin system and engages an autocrine activation loop involving hypoxia-inducible factor 1. Mol Cell Biol. 2008;28:6248–61. doi: 10.1128/MCB.00795-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu Q, Berbee M, Boerma M, Wang J, Schmid HA, Hauer-Jensen M. The somatostatin analog SOM230 (pasireotide) ameliorates injury of the intestinal mucosa and increases survival after total-body irradiation by inhibiting exocrine pancreatic secretion. Radiat Res. 2009;171:698–707. doi: 10.1667/RR1685.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui Y, Hisha H, Yang G, Fan T, Jin T, Li Q, et al. Optimal protocol for total body irradiation for allogeneic bone marrow transplantation in mice. Bone Marrow Transplant. 2002;30:843–9. doi: 10.1038/sj.bmt.1703766. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Zheng H, Sung CC, Richter KK, Hauer-Jensen M. Cellular sources of transforming growth factor-beta isoforms in early and chronic radiation enteropathy. Am J Pathol. 1998;153:1531–40. doi: 10.1016/s0002-9440(10)65741-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baddeley AJ, Gundersen HJ, Cruz-Orive LM. Estimation of surface area from vertical sections. J Microsc. 1986;142:259–76. doi: 10.1111/j.1365-2818.1986.tb04282.x. [DOI] [PubMed] [Google Scholar]
- 17.Langberg CW, Sauer T, Reitan JB, Hauer-Jensen M. Relationship between intestinal fibrosis and histopathologic and morphometric changes in consequential and late radiation enteropathy. Acta Oncol. 1996;35:81–7. doi: 10.3109/02841869609098484. [DOI] [PubMed] [Google Scholar]
- 18.Lutgens LC, Deutz NE, Gueulette J, Cleutjens JP, Berger MP, Wouters BG, et al. Citrulline: a physiologic marker enabling quantitation and monitoring of epithelial radiation-induced small bowel damage. Int J Radiat Oncol Biol Phys. 2003;57:1067–74. doi: 10.1016/s0360-3016(03)00781-8. [DOI] [PubMed] [Google Scholar]
- 19.Gupta PK, Brown J, Biju PG, Thaden J, Deutz NE, Kumar S, et al. Development of high-throughput HILIC-MS/MS methodology for plasma citrulline determination in multiple species. Anal Methods. 2011;3:1759. [Google Scholar]
- 20.Montufar-Solis D, Klein JR. An improved method for isolating intraepithelial lymphocytes (IELs) from the murine small intestine with consistently high purity. J Immunol Methods. 2006;308:251–4. doi: 10.1016/j.jim.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Brandtzaeg P, Halstensen TS, Kett K, Krajci P, Kvale D, Rognum TO, et al. Immunobiology and immunopathology of human gut mucosa: humoral immunity and intraepithelial lymphocytes. Gastroenterology. 1989;97:1562–84. doi: 10.1016/0016-5085(89)90406-x. [DOI] [PubMed] [Google Scholar]
- 22.Gremy O, Benderitter M, Linard C. Caffeic acid phenethyl ester modifies the Th1/Th2 balance in ileal mucosa after gamma-irradiation in the rat by modulating the cytokine pattern. World J Gastroenterol. 2006;12:4996–5004. doi: 10.3748/wjg.v12.i31.4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linard C, Marquette C, Mathieu J, Pennequin A, Clarencon D, Mathe D. Acute induction of inflammatory cytokine expression after gamma-irradiation in the rat: effect of an NF-kappaB inhibitor. Int J Radiat Oncol Biol Phys. 2004;58:427–34. doi: 10.1016/j.ijrobp.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 24.Pecaut MJ, Nelson GA, Gridley DS. Dose and dose rate effects of whole-body gamma-irradiation: I. Lymphocytes and lymphoid organs In Vivo. 2001;15:195–208. [PubMed] [Google Scholar]
- 25.Vogel G. Cell biology. Stem cells: new excitement, persistent questions. Science. 2000;290:1672–4. doi: 10.1126/science.290.5497.1672. [DOI] [PubMed] [Google Scholar]
- 26.Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297:2256–9. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- 27.Grove JE, Bruscia E, Krause DS. Plasticity of bone marrow-derived stem cells. Stem Cells. 2004;22:487–500. doi: 10.1634/stemcells.22-4-487. [DOI] [PubMed] [Google Scholar]
- 28.Huttmann A, Li CL, Duhrsen U. Bone marrow-derived stem cells and “plasticity”. Ann Hematol. 2003;82:599–604. doi: 10.1007/s00277-003-0713-2. [DOI] [PubMed] [Google Scholar]
- 29.Okamoto R, Matsumoto T, Watanabe M. Regeneration of the intestinal epithelia: regulation of bone marrow-derived epithelial cell differentiation towards secretory lineage cells. Hum Cell. 2006;19:71–5. doi: 10.1111/j.1749-0774.2006.00010.x. [DOI] [PubMed] [Google Scholar]
- 30.Okamoto R, Yajima T, Yamazaki M, Kanai T, Mukai M, Okamoto S, et al. Damaged epithelia regenerated by bone marrow-derived cells in the human gastrointestinal tract. Nat Med. 2002;8:1011–7. doi: 10.1038/nm755. [DOI] [PubMed] [Google Scholar]
- 31.Rescigno M. The intestinal epithelial barrier in the control of homeostasis and immunity. Trends Immunol. 2011;32:256–64. doi: 10.1016/j.it.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Solheim KE, Laerum F, Stordahl A, Aase S. Urinary excretion of iohexol after enteral administration in rats with radiation injury of the small intestine. Scand J Gastroenterol. 1991;26:1097–106. doi: 10.3109/00365529109003962. [DOI] [PubMed] [Google Scholar]
- 33.Nejdfors P, Ekelund M, Westrom BR, Willen R, Jeppsson B. Intestinal permeability in humans is increased after radiation therapy. Dis Colon Rectum. 2000;43:1582–7. doi: 10.1007/BF02236743. discussion 1587–8. [DOI] [PubMed] [Google Scholar]
- 34.Paris F, Fuks Z, Kang A, Capodieci P, Juan G, Ehleiter D, et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293:293–7. doi: 10.1126/science.1060191. [DOI] [PubMed] [Google Scholar]
- 35.Richter KK, Fink LM, Hughes BM, Sung CC, Hauer-Jensen M. Is the loss of endothelial thrombomodulin involved in the mechanism of chronicity in late radiation enteropathy? Radiother Oncol. 1997;44:65–71. doi: 10.1016/s0167-8140(97)00063-7. [DOI] [PubMed] [Google Scholar]
- 36.Jiang H, Qu L, Li Y, Gu L, Shi Y, Zhang J, et al. Bone marrow mesenchymal stem cells reduce intestinal ischemia/reperfusion injuries in rats. J Surg Res. 2011;168:127–34. doi: 10.1016/j.jss.2009.07.035. [DOI] [PubMed] [Google Scholar]
- 37.Oshima T, Miwa H, Joh T. Changes in the expression of claudins in active ulcerative colitis. J Gastroenterol Hepatol. 2008;23 (Suppl 2):S146–50. doi: 10.1111/j.1440-1746.2008.05405.x. [DOI] [PubMed] [Google Scholar]
- 38.Jacobsen SE, Veiby OP, Smeland EB. Cytotoxic lymphocyte maturation factor (interleukin 12) is a synergistic growth factor for hematopoietic stem cells. J Exp Med. 1993;178:413–8. doi: 10.1084/jem.178.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rollwagen FM, Yu ZY, Li YY, Pacheco ND. IL-6 rescues enterocytes from hemorrhage induced apoptosis in vivo and in vitro by a bcl-2 mediated mechanism. Clin Immunol Immunopathol. 1998;89:205–13. doi: 10.1006/clin.1998.4600. [DOI] [PubMed] [Google Scholar]
- 40.Billiard F, Buard V, Benderitter M, Linard C. Abdominal gamma-radiation induces an accumulation of function-impaired regulatory T cells in the small intestine. Int J Radiat Oncol Biol Phys. 2011;80:869–76. doi: 10.1016/j.ijrobp.2010.12.041. [DOI] [PubMed] [Google Scholar]
- 41.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Godoi DF, Cardoso CR, Ferraz DB, Provinciatto PR, Cunha FQ, Silva JS, et al. Hematopoietic SCT modulates gut inflammation in experimental inflammatory bowel disease. Bone Marrow Transplant. 2010;45:1562–71. doi: 10.1038/bmt.2010.6. [DOI] [PubMed] [Google Scholar]
- 43.Pull SL, Doherty JM, Mills JC, Gordon JI, Stappenbeck TS. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc Natl Acad Sci U S A. 2005;102:99–104. doi: 10.1073/pnas.0405979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takaba J, Mishima Y, Hatake K, Kasahara T. Role of bone marrow-derived monocytes/macrophages in the repair of mucosal damage caused by irradiation and/or anticancer drugs in colitis model. Mediators Inflamm. 2010;2010:634145. doi: 10.1155/2010/634145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
http://dx.doi.org/10.1667/RR13548.1.S1; Homing of transplanted BMCs as detected by presence of GFP-positive macrophages in the mouse small intestine on day 7 postirradiation. CD68-positive cells were mainly seen in the lamina propria of villi. Most if not all GFP-positive cells were CD68-positive (as shown by arrows). Panel A: GFP-positive cells were shown by Alexa Fluor 488. Panel B: CD68-positive cells shown by Dylight 594. Panel C: Nuclei stained with DAPI. Panel D: Merged image of A, B and C. Bar = 50 μm.