Abstract
Objectives
Tobramycin inhalation solution (TIS; TOBI®) has improved forced expiratory volume in 1 second (FEV1) in cystic fibrosis (CF) trials. Using data from the Epidemiologic Study of CF (ESCF), we assessed the change in level and trend of FEV1 % predicted (pred) over a 2-year period associated with initiation of TIS during routine clinical practice.
Methods
Patients age 8–38 years and in ESCF for ≥2 years before treatment with TIS as a chronic therapy were selected if they remained on therapy for 2 years, defined as being on TIS for at least 3 months per year (C-TIS group). Comparator intervals age 8–38 years used TIS <10% of the time. For each interval, we estimated the level and trend (rate of decline) in FEV1 % pred before and after the index using a piecewise linear mixed-effects model adjusted for potential confounders.
Results
During the 2-year pre-index period the C-TIS group (n = 2,534) had a more rapid decline in FEV1 (−2.49 vs. −1.39 % pred/yr) and a lower FEV1 at index (62.6 vs. 74.7 % pred) than the comparator group (N = 17,656 intervals). After starting chronic TIS, the FEV1 trend line over the 2-year post-index period was higher, but the comparator group’s FEV1 was essentially unchanged (difference 2.22, P < 0.001). Change in slope was not different between groups (0.06, P = 0.82).
Conclusions
Initiating chronic TIS therapy in the routine clinical care of patients with CF was associated with improvement in FEV1 % pred but no change in rate of decline, which means that this benefit was sustained over the 2 years studied.
Keywords: cystic fibrosis, epidemiology, pulmonary function, tobramycin inhalation solution
INTRODUCTION
Clinical trials of tobramycin inhalation solution (TIS; TOBI®) in patients with cystic fibrosis (CF) with chronic Pseudomonas aeruginosa infection have shown acute improvement in lung function. Improvement in forced expiratory volume in 1 second (FEV1) has ranged from less than 2% predicted (pred)1 to a 10% relative improvement in FEV1 % pred.2 TIS is indicated for the treatment of patients with CF with P. aeruginosa, age ≥6 years, with FEV1 % pred between 25 and 75. TIS use has increased since its introduction in 1998.3 However, efficacy in clinical trials does not necessarily represent effectiveness in the routine clinical practice setting. Observational studies have the advantage of looking at real-world use of therapies, although they have other limitations, including indication bias.4
The Epidemiological Study of Cystic Fibrosis (ESCF) was a large, multicenter, prospective observational study of the clinical course of patients with CF in the United States and Canada from 1994 through 2005.5 Data collected on each patient included demographics, use of routine therapies, results of pulmonary function tests (PFTs), results of respiratory tract cultures, information on growth and nutrition, and details of use of antibiotics, anti-inflammatories, and other treatments. Informed consent was obtained based on decisions by a central or a local human subjects review board.
Previous analyses of ESCF data have addressed the impact on lung function of initiation of chronic therapies. Ren et al.6 showed an improvement in rate of decline of FEV1 % pred after initiation of inhaled corticosteroid therapy. Konstan et al.7 showed improvement in FEV1 % pred both overall (change in intercept) and in rate of decline (change in slope) after the introduction of dornase alfa. The dornase alfa analysis adjusted for age-specific deciles based on FEV1 % pred. As shown in the online supplement of the dornase alfa paper, the change in rate of decline before and after an arbitrary point in time varies according to the level of lung function at that time. By adjusting for the age-specific decile, the expected amount of change (positive or negative) is accounted for in the model.
In the current study, we use the same approach as previously used in the dornase alfa analysis7 to assess the relationship between the initiation of TIS therapy and lung function as measured by FEV1 % pred. We hypothesized that we would find an initial improvement in FEV1 from TIS similar to clinical trials, and we sought to establish whether this initial improvement would increase, decrease, or be sustained over the two years following initiation.
METHODS
Definition of Chronic TIS Use
Prior to the commercial availability of TOBI® in January 1998, patients were clinically treated with inhaled tobramycin, generally with doses lower than 300 mg bid.8 The ESCF case report forms (CRF) captured inhaled tobramycin from the inception of ESCF in 1994, including the dose prescribed, number of doses per day, start and stop dates, and the reason for use (treatment of exacerbation or prophylaxis). In November 1998, the CRF was modified to refer to inhaled tobramycin/TOBI®. For this version of the CRF, an inhaled tobramycin dose of 300 mg was assumed to be TOBI®. In 2003, the CRF was modified to specifically capture the chronic use of TOBI®, including dosing frequency, but instead of start and stop dates, TOBI use was recorded at each encounter.
In this analysis, we were interested in assessing the chronic use of TIS. In previous work assessing inhaled corticosteroids and dornase alfa, the definition of chronic use required the therapy to have been recorded at ≥80% of the clinical encounters.6,7 For encounters from 2003–2005 where a simple check-off was recorded at each clinical encounter, we used the same criterion of ≥80% of the clinical encounters. We needed to adapt this definition to the usual 28-day-on, 28-day-off pattern of TIS therapy for CRFs prior to 2003. We chose to require ≥25% of the available days be on therapy to be considered chronic (assuming up to half of the treatment courses might not be recorded if visits occurred quarterly or less frequently). The chronic use of TIS is hereafter referred to as C-TIS.
Definition of Patient Cohorts
The C-TIS group was compared to patients not chronically treated with tobramycin by inhalation (comparator group). The purpose of the comparator group was to understand the usual pattern of change in FEV1 % pred slope, not to act as matched controls. The initial population (i.e., “all patients”) consisted of patients who met inclusion criteria for either the C-TIS or comparator groups. We also defined a target population based on the labeled indication for TIS, which was limited to those patients with a positive pre-index culture for P. aeruginosa and an index FEV1 of 25% to 75% pred.
The C-TIS group included patients age 8 to 38 years enrolled in ESCF for at least 2 years before initiation of C-TIS. A PFT within 30 days of starting C-TIS (defined as the index PFT) was required to separate a 2-year pre-index period from a 2-year post-index period, but was not included in either period. The pre-index and post-index periods were each required to have at least one clinic encounter and at least three FEV1 values spanning at least 6 months. C-TIS patients were allowed to receive TIS no more than 10% of the time between the first pre-index FEV1 % pred and the index visit, and they could receive other inhaled antibiotics both prior to and following the index visit.
The comparator group included patients age 8 to 38 years not yet reported to have started C-TIS. The index PFT for comparator patients was defined as the PFT closest (within 30 days) to the first clinic encounter within one year following the eighth or subsequent even-numbered birthday (to achieve a random distribution during the year). As for the comparator group, the pre-index and post-index periods were each required to have at least one clinic encounter and at least three FEV1 % pred values spanning at least 6 months. In addition, comparator patients were allowed to receive TIS up to 10% of the time between the first pre-index FEV1 % pred and the index event, but also up to 10% of the time between the index event and the last post-index FEV1 % pred. (Any TIS use recorded on an encounter after the CRF change in 2003 was considered to exceed the 10% threshold.) C-TIS patients could contribute one or more sets of pre-index and post-index periods (intervals) to the comparator group if they had adequate data over a long enough period of time. Once included in the C-TIS group, patients could not contribute subsequently to either group.
In addition to the initial and target populations, four sensitivity analyses were performed on subpopulations of particular interest. We first limited the C-TIS group by including only patients meeting a more stringent definition of C-TIS: for CRFs prior to 2003 patients needed to be on TIS 40% of the time (i.e., 80% of every-other-month therapy) and on the 2003–2005 CRFs as on TIS at 100% of visits. The comparator group was not changed. In a second sensitivity analysis, the C-TIS group was limited to having no more than 10% of time on any other inhaled antibiotics during both the pre-index and post-index periods. The comparator patients in this second sensitivity analysis were similarly limited to no more than 10% of any inhaled antibiotic during the pre- or post-index periods. The third sensitivity analysis further limited both the C-TIS and comparator groups to patients with no use of any inhaled antibiotics in the pre-index period. As a fourth sensitivity analysis, the initial population was limited to those patients with a positive pre-index culture for P. aeruginosa. The analysis of the target population, reflecting criteria for the indicated use of TIS, is the subset of the fourth sensitivity analysis population restricted to patients with FEV1 between 25% and 75% pred.
Statistical Methods
The outcomes evaluated were change in FEV1 % pred (change in intercept) and change in rate of decline of FEV1 % pred (change in slope). Trend lines of FEV1 % pred values before and after the index PFT were calculated using slopes and intercepts estimated separately for each interval for each patient. This is a piecewise linear mixed-effects model with four random effects: intercept and slope before the index and the change in intercept and change in slope from before to after the index. Repeated use of patients in the comparator group (multiple intervals) was accounted for in the modeled covariance structure by allowing for within-patient correlation across intervals. Thus there are two components of within-patient longitudinal variation associated with the comparator intervals: the longitudinal measurements within the 2 years before and 2 years after the index PFT, which are used to estimate the four random effects within a 4-year birthday interval, and the longitudinal measurements across the 4-year birthday intervals for those patients with multiple intervals. Additional details on the model are in the body and online supplement of Konstan et al.7 and in the online supplement. Values for FEV1 % pred were calculated from Wang9 for males through age 17 years and for females through age 15 years and from the equations of Hankinson10 for patients over these ages.
The FEV1 % pred at the index PFT for each interval was categorized using age-specific deciles of FEV1 % pred, as defined in the online supplement of Konstan et al.7 The piecewise linear mixed model estimated separate effects by decile. Additionally, the model adjusted for age, gender, and time-dependent use of routine therapies (including other inhaled antibiotics) and pulmonary exacerbations treated with intravenous antibiotics. Routine therapies were based on data recorded at the closest visit prior to or within 14 days after each PFT.
Statistical analyses were performed using SAS 9.2 (SAS Institute, Inc., Cary, NC). All tests were two-sided and P values < 0.05 were considered statistically significant. No adjustments were made for multiple comparisons.
RESULTS
Patient Characteristics
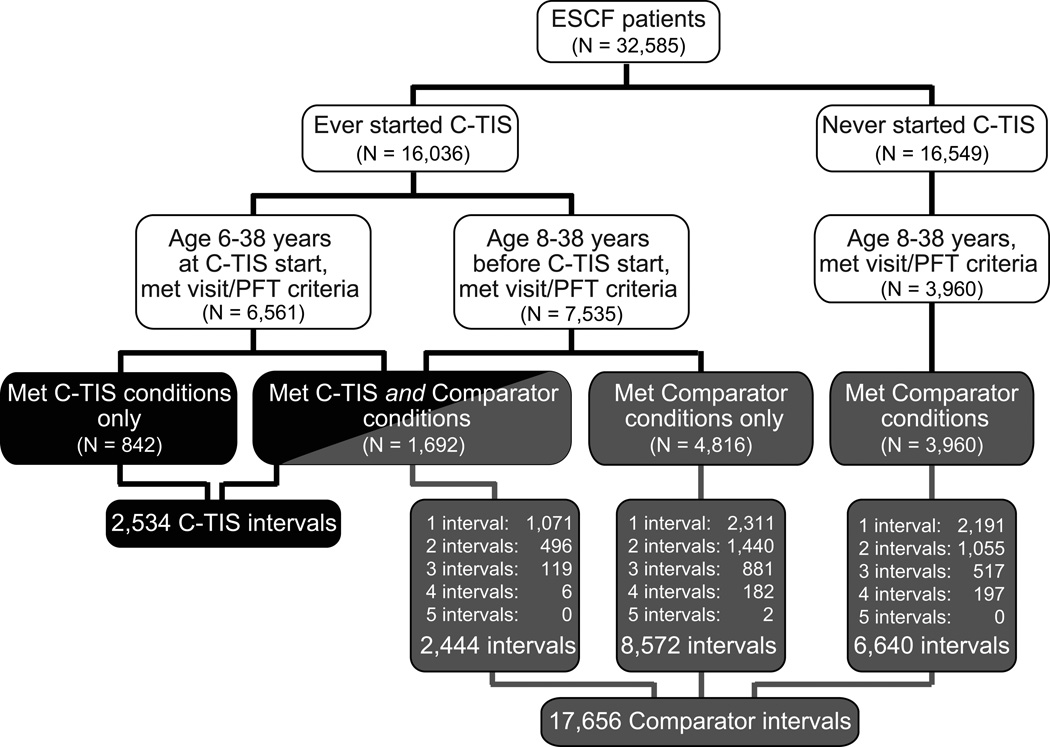
A total of 32,585 unique patients were enrolled in ESCF between 1994 and 2005. Of these, 16,549 patients never initiated C-TIS therapy; 3,960 met comparator conditions and contributed 6,640 comparator intervals. Of the 16,036 ever starting C-TIS, 842 met C-TIS conditions but not comparator conditions, 1,692 met both C-TIS and comparator conditions, and 4,816 met only comparator conditions. There were a total of 2,534 C-TIS intervals and 17,656 comparator intervals from 11,310 patients (Figure 1).
Fig. 1.
Identification of patients and intervals for the C-TIS and comparator groups. Comparator patients contribute up to 5 intervals each, as noted.
Demographic and clinical characteristics of C-TIS and target C-TIS groups at the time of the index PFT plus the corresponding information for the comparator and target comparator intervals are presented in Table 1. Lung function was worse for C-TIS patients at the time of their index PFT than for comparator intervals. Disease stage was less different between the target C-TIS and the target comparator intervals.
Table 1.
Demographic and Clinical Characteristics at Index PFT
| All C-TIS Patients |
All Comparator Intervals |
Target C-TIS Patients |
Target Comparator Intervals |
||
|---|---|---|---|---|---|
| N | 2,534 | 17,656 | 1,448 | 6,019 | |
| Age, mean (SD) | 17.5 (7.3) | 16.2 (7.2) | 19.0 (7.3) | 19.7 (7.7) | |
| Age group (yr), % | |||||
| 8–12 | 29.0 | 41.4 | 20.5 | 20.9 | |
| 13–17 | 29.8 | 23.4 | 28.3 | 21.8 | |
| 18–38 | 41.2 | 35.2 | 51.2 | 57.3 | |
| Female, % | 50.2 | 47.7 | 51.7 | 51.2 | |
| delta F508 homozygous*, % (n) | 57.5 (n = 1,713) | 53.3 (n = 11,514) | 55.7 (n = 912) | 54.6 (n = 3,519) | |
| Pseudomonas* positive, % (n) | 76.5 (n = 957) | 56.2 (n = 7,775) | 85.8 (n = 514) | 84.5 (n = 2,566) | |
| FEV1 (% pred), mean (SD) | 62.6 (24.2) | 74.7 (26.1) | 50.9 (13.9) | 51.8 (14.1) | |
| FEV1 groups, % | |||||
| <25% pred. | 4.5 | 3.0 | — | — | |
| 25–<50% pred. | 29.7 | 17.7 | 48.1 | 44.8 | |
| 50–<75% pred. | 33.2 | 25.2 | 51.9 | 55.2 | |
| ≥75% pred. | 32.5 | 54.1 | — | — | |
| Weight for age*, mean (SD) | 27.2 (25.1) | 32.4 (27.5) | 22.8 (23.0) | 24.7 (24.8) | |
| Pulmonary signs and symptoms* positive, % | |||||
| Sputum production | 84.1 | 71.1 | 90.3 | 89.2 | |
| Cough | 95.4 | 89.2 | 97.2 | 97.3 | |
| Crackles | 37.8 | 24.4 | 46.8 | 44.6 | |
| Wheeze | 8.5 | 7.1 | 9.4 | 9.8 | |
| Digital clubbing | 68.5 | 59.8 | 77.5 | 76.1 | |
Includes only patients with nonmissing data at index PFT. Approximately 1,500 and 800 intervals are missing weight for age and pulmonary signs and symptoms data from all and target populations, respectively. (n) indicates the denominator for that particular percentage.
The use of therapies before and after the index PFT for the C-TIS and comparator intervals is shown in Table 2. Overall, pre-index therapies were more frequent in the C-TIS group than comparator group. Most therapies increased after index in both groups. This increase in therapies was greater in the C-TIS group.
Table 2.
Average Proportion of PFT Visits at Which Therapies Were Recorded for All Patients
| Treatment Group | Pre-index Visit, Mean |
Post-index Visit, Mean |
|
|---|---|---|---|
| Dornase alfa | C-TIS | 0.74 | 0.80 |
| Comparator | 0.58 | 0.65 | |
| Oral bronchodilators | C-TIS | 0.11 | 0.07 |
| Comparator | 0.10 | 0.08 | |
| Inhaled bronchodilators | C-TIS | 0.88 | 0.91 |
| Comparator | 0.81 | 0.84 | |
| Oral corticosteroids | C-TIS | 0.15 | 0.18 |
| Comparator | 0.12 | 0.13 | |
| Inhaled corticosteroids | C-TIS | 0.34 | 0.44 |
| Comparator | 0.29 | 0.37 | |
| Mast cell stabilizers | C-TIS | 0.25 | 0.19 |
| Comparator | 0.24 | 0.20 |
P value for change from pre-index to post-index period within treatment group all < 0.001. P value comparing the group difference in change score (i.e., is the Difference Post- minus Pre- the same in the two treatment groups?) all <0.01 except for dornase alfa and inhaled bronchodilators (P > 0.10).
A total of 26,919 pre-index visits and 29,056 post-index visits exist for chronic TIS patients (N = 2,534).
A total of 140,836 pre-index visits and 153,954 post-index visits exist for comparator patients (N = 17,656).
Estimated Changes in FEV1 % Predicted by Age Group
Using a piecewise linear mixed model with adjustment only for deciles, the C-TIS group showed a significant, acute post-index improvement in FEV1 intercept (2.41% predicted, P < 0.001). In contrast, the comparator group showed a much smaller increase in FEV1 (0.19% predicted, P = 0.041) after the index PFT. The comparator group showed a significant worsening in slope (−0.50% pred/year, P < 0.001). Although the C-TIS group showed a similar worsening, it was not statistically significant (−0.44% pred/year, P = 0.058). The target C-TIS group also showed a significant post-index improvement in FEV1 intercept (1.78% pred, P < 0.001). The target comparator group showed no significant change in FEV1 after the index PFT (0.25% pred, P = 0.068). Both the target C-TIS group and the target comparator group showed a significant improvement in slope (0.58% pred/year, P = 0.027, 0.43% pred/year, P = 0.002).
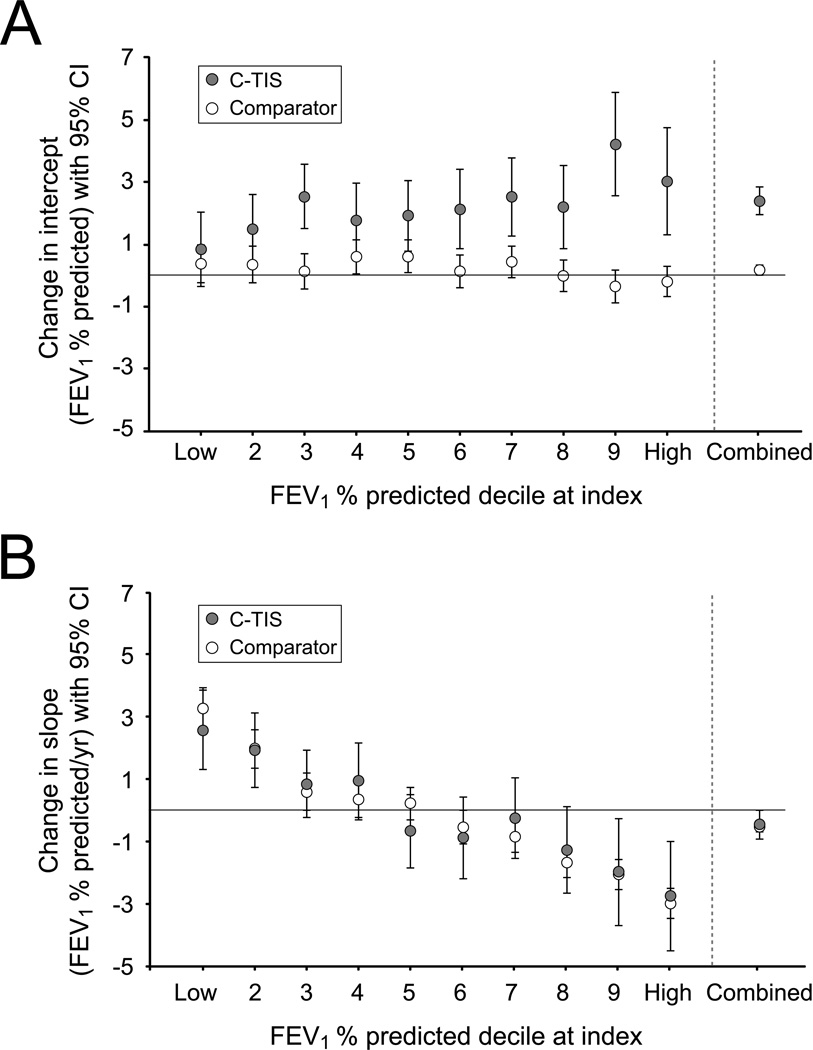
Figure 2 shows the results from the model adjusting for decile, age, gender, disease stage, pulmonary exacerbations treated with intravenous antibiotics, dornase alfa use, respiratory therapies (including other inhaled antibiotics), and nutritional supplements. The change in intercept for the comparator group was minimal in every decile (open circles in Figure 2A), whereas the change in intercept for the C-TIS group was consistently positive (filled circles in Figure 2A). The change in intercept is measured from the end of the line fitted during the pre-index period (69.8% pred; see Online Supplement) to the beginning of the line fitted during the post-index period (72.2% pred; see Online Supplement). Note that the index PFT measured at that time is substantially lower (62.6% pred; Table 1). The difference in intercept change (C-TIS minus comparator) by decile and overall is shown in the Online Supplement. The benefit of chronic TIS therapy tended to be greater in the patients with highest lung function.
Fig. 2.
Estimated change from before to after index PFT in intercept and slope of FEV1 % predicted by age-specific deciles of FEV1 % predicted. Error bars indicate 95% CI. (A) Change in FEV1 % predicted intercept for C-TIS and comparator patients. (B) Change in FEV1 % predicted slope for C-TIS and comparator patients.
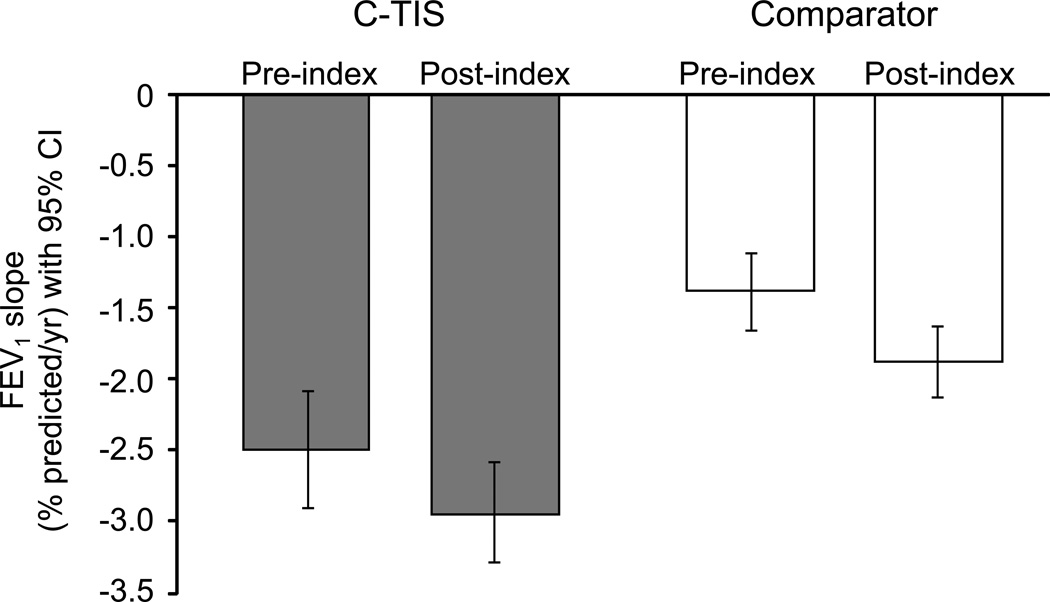
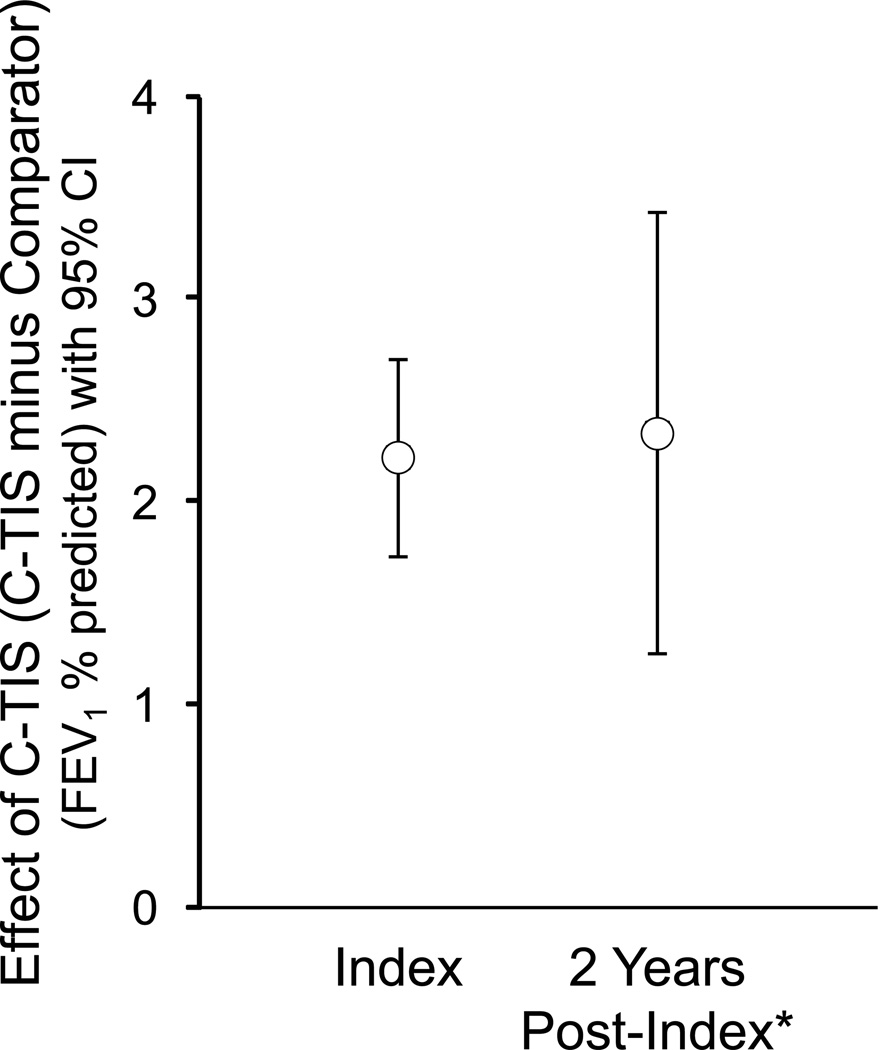
Figure 2B shows the change in slope for the comparator and C-TIS groups by decile and overall. Patients in the lowest decile (worst lung function) improved their rate of decline in FEV1 post-index compared to pre-index by about 3.3% pred/year for comparator patients and 2.6% pred/year for C-TIS patients. Patients in the highest decile (best lung function), regardless of group, had a worsening rate of decline. The Online Supplement shows the difference in slope change by decile and overall. Overall, the C-TIS group did not have a significant change in slope (−0.44% pred/year; P = 0.058). Figure 3 shows the rates of decline (slopes) pre- and post-index for the C-TIS and comparator groups. The groups have similar change in slope even though before the index PFT, C-TIS patients had a more rapid annual decline in FEV1 % pred than comparator patients (−2.49 vs. −1.39, P < 0.001). The effect of C-TIS therapy at the end of the 2 year post-index period can be estimated from the difference in the intercept change at index plus 2 times the difference in the slope change. Figure 4 shows the estimated effect at Index and 2 years later. The wider confidence intervals at 2 years reflect the additional uncertainty from estimating the difference in slopes.
Fig. 3.
Rate of FEV1 change (slope) pre- and post-index for the C-TIS and comparator groups (% pred/year). Error bars indicate 95% CI.
Fig. 4.
Effect of C-TIS therapy at index and 2 years later (C-TIS minus Comparator). Error bars indicate 95% CI.
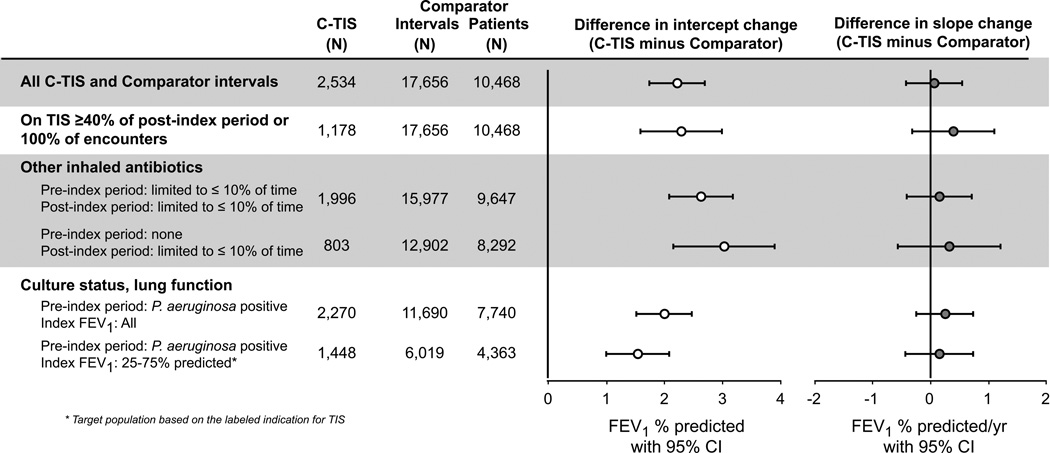
The evaluation of sensitivity analyses using alternative definitions of C-TIS and comparator groups, along with the initial and target populations, is summarized in Figure 5. The between-group difference in post-index change in intercept (C-TIS change minus comparator group change) ranged from 1.5 to 3.0 % pred, with larger values when the pre-index use of inhaled antibiotics was more limited. In all subpopulations, the between-group difference in change in annual rate of FEV1 decline from pre-index to post-index was positive but small and not statistically significant.
Fig. 5.
Difference (C-TIS minus Comparator) in change in intercept and change in slope for various subpopulations.
We chose to include in the comparator group patients who subsequently were started on C-TIS because it is potentially biasing to treat two otherwise identical patients differently based on whether or not they take C-TIS in the future, possibly the distant future. We also chose to include multiple intervals rather than choose only a single interval. Although for this analysis the additional statistical power from including multiple intervals is not essential, we used this approach in part to parallel our previous work with dornase alfa and in part because it is always valuable to maximize power even in the presence of robust results (for the change in intercept) or negative results (for the change in slope). To address potential concerns about these decisions, we repeated the decile-adjusted analysis using three subsets of Comparator data. First, we omitted from the comparator group those patients who were also included in the C-TIS group. Second, we used those comparator patients but used just the last eligible interval. Third, we omitted any patient who ever received C-TIS from the comparator group (but allowed multiple intervals). All three of these subset analyses produced results very similar to the original analysis, namely a substantial difference in change in intercept and no significant difference in change in slope (data not shown).
DISCUSSION
This study indicates that C-TIS use is associated with an improvement in FEV1 % pred in patients with CF across a range of disease stages, even after adjusting for other therapies. This finding is stronger when limiting the pre-index use of inhaled antibiotics in the analysis. The improvement identified here expands on the results from clinical trials of TIS by characterizing lung function improvement when chronic TIS therapy is initiated in clinical practice. In the pivotal trial of TIS, there was a 10% relative increase in FEV1 % pred at week 20, which corresponds to about a 5-point absolute increase in % pred.2 We found a smaller but sustained effect of 1.5- to 3.0-point absolute increase in FEV1 % pred depending on the subpopulation studied. The improvement we found may be less than the acute increase found in the clinical trial in part because it represents an averaged response over a longer period (including lung function during months off therapy) and in part because adherence is often lower in practice than in clinical trials.
The rate of decline in lung function did not change overall from pre- to post-initiation of C-TIS and the change was not different from the comparator group. The overall improvement in FEV1 % pred combined with the lack of change in slope means that the benefit of chronic TIS therapy persisted over the 2 years studied.
The C-TIS and comparator groups were similar but not closely matched because the purpose of the comparator group was to understand the usual pattern of change in FEV1 % pred slope about an arbitrary point in time rather than to act as a control group. We adjusted for concomitant medication use both pre- and post-index and partially adjusted for stage of disease by controlling for age-specific FEV1 deciles. The benefit of chronic TIS therapy tended to be greater in the patients with highest lung function.
Observational studies such as ESCF have the potential to demonstrate how real-world clinical use of CF therapies can affect lung function. However, observational studies have certain limitations. Physicians and patients are self-selected and each physician determines which therapies to prescribe for any given patient. Patients receiving C-TIS in this study received more CF-related therapies at the outset, and their use of these therapies increased after index relative to those patients who did not receive C-TIS. Although we used time-varying covariates to adjust for within-patient changes in pulmonary therapies, such adjustments are limited to available data. Additionally, although an epidemiologic study may have a large number of patients, the number of patients with adequate data for analysis may be limited. Finally, we looked only at patients treated chronically with TIS. Patients started on TIS and then discontinued for any reason would not be included. Therefore, the included C-TIS patients are more likely to have had a beneficial response to therapy.
Other observational studies have investigated TIS use in patients with CF. Rothman and Wentworth4 used a study of TIS to illustrate confounding by indication where sicker patients are more likely to be treated. They found that the crude death rate was 3.5 times greater for TIS (90% CI 3.0–4.2) in those who used TIS for more than four months. After control of confounders, the risk was reduced to 1.22 (90% CI 0.96–1.53). Sawicki et al.11 used longitudinal logistic regression to relate current-year TIS use to subsequent-year mortality, adjusting for multiple patient characteristics and known risk factors. They found that TIS use was associated with significantly reduced mortality (OR 0.79, 95% CI 0.72–0.88). The Sawicki study looked at up to 10 years after the index year and took account of TIS use in every year, in contrast to Rothman and Wentworth who studied a single follow-up year. The study by Rothman and Wentworth may have been affected by the tendency for newly introduced therapies to be initiated in patients late in their disease course, an effect that would diminish over time. Sawicki et al.11 also were able to control for more potential confounders. Although we did not evaluate mortality, our data support the Sawicki finding by showing lung function benefit from C-TIS is not only acute, as shown in clinical trials, but persists over the 2 years studied.
In conclusion, this study confirms that initiating chronic TIS therapy leads to an improvement in FEV1. Although small, this improvement is sustained over 2 years following initiation of therapy and the improvement is seen across a broad range of disease stages. This lung function benefit is consistent with the recent finding of reduced mortality associated with chronic TIS therapy.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the participation of the more than 400 site investigators and coordinators in ESCF in collecting this comprehensive database.
Disclosure of Conflict of Interest
This study is sponsored by Genentech, Inc. and Novartis Pharmaceuticals Corporation. Michael Konstan, Jeffrey Wagener, and Wayne Morgan have received honoraria from Genentech for serving as members of the Scientific Advisory Group for the Epidemiologic Study of Cystic Fibrosis (ESCF) and have served as consultants to Genentech. Michael Konstan and Wayne Morgan have served as consultants to Novartis. No compensation was provided to these authors in exchange for production of this manuscript. David Pasta and Stefanie Millar are employees of ICON Late Phase & Outcomes Research, which was paid by Genentech for providing analytical services for this study. Jeffrey Wagener was previously an employee of Genentech.
Funding source: Genentech, Inc., South San Francisco, California and Novartis Pharmaceuticals Corporation, East Hanover, New Jersey
REFERENCES
- 1.Murphy TD, Anbar RD, Lester LA, Nasr SZ, Nickerson B, Van Devanter DR, Colin AA. Treatment with tobramycin solution for inhalation reduces hospitalizations in young CF subjects with mild lung disease. Pediatr Pulmonol. 2004;38:314–320. doi: 10.1002/ppul.20097. [DOI] [PubMed] [Google Scholar]
- 2.Ramsey BW, Pepe MS, Quan JM, Otto KL, Montgomery AB, Williams-Warren J, Vasiljev KM, Borowitz D, Bowman CM, Marshall BC, Marshall S, Smith AL. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. N Engl J Med. 1999;340:23–30. doi: 10.1056/NEJM199901073400104. [DOI] [PubMed] [Google Scholar]
- 3.Moskowitz SM, Silva SJ, Mayer-Hamblett N, Pasta DJ, Mink DR, Mabie JA, Konstan MW, Wagener JS. Shifting patterns of inhaled antibiotic use in cystic fibrosis. Pediatr Pulmonol. 2008;43:874–881. doi: 10.1002/ppul.20873. [DOI] [PubMed] [Google Scholar]
- 4.Rothman KJ, Wentworth CE. Mortality of cystic fibrosis patients treated with tobramycin solution for inhalation. Epidemiology. 2003;14:55–59. doi: 10.1097/00001648-200301000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Morgan WJ, Butler SM, Johnson CA, Colin AA, FitzSimmons SC, Geller DE, Konstan MW, Light MJ, Rabin HR, Regelmann WE, Schidlow DV, Stokes DC, Wohl ME, Kaplowitz H, Wyatt MM, Stryker S. Epidemiologic study of cystic fibrosis: design and implementation of a prospective, multicenter, observational study of patients with cystic fibrosis in the U.S. and Canada. Pediatr Pulmonol. 1999;28:231–241. doi: 10.1002/(sici)1099-0496(199910)28:4<231::aid-ppul1>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 6.Ren CL, Pasta DJ, Rasouliyan L, Wagener JS, Konstan MK, Morgan WJ. Relationship between inhaled corticosteroid therapy and rate of lung function decline in children with cystic fibrosis. J Pediatr. 2008;153:746–751. doi: 10.1016/j.jpeds.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Konstan MW, Wagener JS, Pasta DJ, Millar SJ, Jacobs JR, Yegin A, Morgan WJ. Clinical use of dornase alfa is associated with slower rate of FEV1 decline in cystic fibrosis. Pediatr Pulmonol. 2011;46:545–553. doi: 10.1002/ppul.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Konstan MW, Butler SM, Schidlow DV, Morgan WJ, Julius JR, Johnson CA. Patterns of medical practice in cystic fibrosis: part II. Use of therapies. Pediatr Pulmonol. 1999;28:248–254. doi: 10.1002/(sici)1099-0496(199910)28:4<248::aid-ppul3>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Dockery DW, Wypij D, Fay ME, Ferris BG., Jr Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol. 1993;15:75–88. doi: 10.1002/ppul.1950150204. [DOI] [PubMed] [Google Scholar]
- 10.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 11.Sawicki GS, Signorovitch JE, Zhang J, Latremouille-Viau D, von Wartburg M, Wu EQ, Shi L. Reduced mortality in cystic fibrosis patients treated with tobramycin inhalation solution. Pediatr Pulmonol. 2012;47:44–52. doi: 10.1002/ppul.21521. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.