Abstract
In this study, we investigated the utility of antimicrobial blue light therapy for multidrug-resistant Acinetobacter baumannii infection in a mouse burn model. A bioluminescent clinical isolate of multidrug-resistant A. baumannii was obtained. The susceptibility of A. baumannii to blue light (415 nm)–inactivation was compared in vitro to that of human keratinocytes. Repeated cycles of sublethal inactivation of bacterial by blue light were performed to investigate the potential resistance development of A. baumannii to blue light. A mouse model of third degree burn infected with A. baumannii was developed. A single exposure of blue light was initiated 30 minutes after bacterial inoculation to inactivate A. baumannii in mouse burns. It was found that the multidrug-resistant A. baumannii strain was significantly more susceptible than keratinocytes to blue light inactivation. Transmission electron microscopy revealed blue light–induced ultrastructural damage in A. baumannii cells. Fluorescence spectroscopy suggested that endogenous porphyrins exist in A. baumannii cells. Blue light at an exposure of 55.8 J/cm2 significantly reduced the bacterial burden in mouse burns. No resistance development to blue light inactivation was observed in A. baumannii after 10 cycles of sublethal inactivation of bacteria. No significant DNA damage was detected in mouse skin by means of a skin TUNEL assay after a blue light exposure of 195 J/cm2.
Keywords: Blue light, Acinetobacter baumannii, burn, infection, multidrug resistance, mouse model
Infections are a common problem for military personnel wounded on the battlefield. Although the US military has provided rapid, highly effective care for casualties in Iraq and Afghanistan, infection outbreaks caused by multidrug-resistant organisms emerged as a problem early on in the course of military operations [1–3]. One of the most notorious pathogens is Acinetobacter baumannii [3], a member of a group of opportunistic bacteria that, in addition to their innate resistance to a significant number of antibiotics, are capable of developing drug resistance quickly. The only effective treatments available to fight these infections, in some cases, are highly toxic, older drugs (eg, colistin) that can cause additional severe harm to the patients [4]. There is consequently a pressing need for the development of new, preferably nonantibiotic [5] approaches to tackle multidrug-resistant A. baumannii wound infections.
As a nonantibiotic approach, light-based antimicrobial therapies, including antimicrobial photodynamic therapy (aPDT) and ultraviolet-C (UVC) irradiation therapy, have been extensively investigated as alternatives for localized infections [6, 7]. Advantages of light-based therapies include rapid action and equal inactivation effectiveness regardless of drug resistance [8–10]. However, one major disadvantage of aPDT is the challenge of introducing exogenous photosensitizers into the infecting bacteria and their less-than-perfect selectivity for these bacteria over host cells [11]. The use of UVC, on the other hand, has limitations because of its detrimental effects on host cells [12].
A novel light-based antimicrobial therapy, blue light therapy, has been attracting increasing attention because of its intrinsic antimicrobial effect without the involvement of exogenous photosensitizers [13–20]. In addition, it is well accepted that blue light is much less detrimental than UVC irradiation to host cells [21, 22]. The mechanism underlying the antimicrobial effect of blue light is still not fully understood. The commonly accepted hypothesis is that blue light excites the naturally occurring endogenous photosensitizing porphyrins in bacteria, which, in turn, leads to the production of cytotoxic reactive oxygen species [23–25].
However, the use of blue light for treatment of wound infections has not been established. The majority of the publications on the antimicrobial effect of blue light have been confined to in vitro studies [13–15, 17–20]. There have been (rather surprisingly) only 2 published reports (both from our laboratory) to demonstrate the use of blue light therapy for wound infections [26, 27]. We have demonstrated that blue light (415 nm) significantly reduced the bacterial burden (Pseudomonas aeruginosa or Staphylococcus aureus) in mouse wounds and burns and saved the lives of mice in the event of potentially lethal P. aeruginosa infections [26, 27]. In the present study, we investigated the usefulness of blue light therapy for multidrug-resistant A. baumannii infections in mouse burns.
MATERIALS AND METHODS
Blue-Light Source
Irradiation was performed using an Omnilux clear-U light-emitting diode array (Photo Therapeutics, Carlsbad, CA) with a central wavelength of 415 nm and a full-width half maximum of 20 nm. The irradiance of blue light on the target surface, which was measured using a PM100D power/energy meter (Thorlabs, Newton, NJ), was adjusted to 19.5 mW/cm2 for cell culture experiments and to 14.6 mW/cm2 for in vivo experiments.
A. baumannii Strain and Culture Condition
The A. baumannii strain was a clinical isolate recovered from an infected US service member deployed to Iraq. The strain demonstrated multidrug resistance according to susceptibility tests performed at the US Army Institute of Surgical Research (Table 1). The luxCDABE operon, which was contained in plasmid pMF 385, was cloned into the A. baumannii strain as described previously [28]. This allowed real-time monitoring of the extent of infection in mice by using bioluminescence imaging [29]. A. baumannii cells were grown in brain heart infusion (BHI) medium supplemented with 50 µg/mL kanamycin in an orbital shaking incubator (at 37°C and 100 rpm) to an optical density of 0.6–0.8 at 600 nm, which corresponds to 108 colony-forming units (CFU)/mL. This suspension was then centrifuged, washed with phosphate-buffered saline (PBS), and resuspended in PBS at designated cell density for experimental use.
Table 1.
Results of Antibiotic Susceptibility Testing for the Acinetobacter baumannii Strain Used in the Study
| Antibiotic | Susceptibility |
|---|---|
| Amikacin | Resistant |
| Levofloxacin | Resistant |
| Chloramphenicol | Resistant |
| Ceftazidime | Intermediate |
| Cefoperazone | Resistant |
| Ciprofloxacin | Resistant |
| Meropenem | Resistant |
| Ceftriaxone | Resistant |
| Cefotaxime | Resistant |
| Mezlocillin | Resistant |
| Gentamicin | Resistant |
| Piperacillin | Resistant |
| Aztreonam | Resistant |
| Colistin | Susceptible |
| Minocycline | Susceptible |
| Imipenem | Resistant |
| Tobramycin | Resistant |
| Doxycycline | Resistant |
| Ampicillin-sulbactam | Resistant |
| Tetracycline | Resistant |
| Ticarcillin | Resistant |
| Ticarcillin-clavulanate | Resistant |
| Piperacillin-tazobactam | Resistant |
Keratinocytes and Culture Condition
The human keratinocyte cell line (HaCaT) [30] was cultured in 75-cm3 tissue culture flasks in 20 mL of Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated fetal bovine serum, penicillin (100 units/mL), and streptomycin (100 µg/mL; Sigma, St. Louis, MO). Cells were incubated at 37°C in 95% air and 5% CO2 in a humidified incubator for 2–3 days until the cell monolayer became confluent. Upon reaching at least 70% confluence, the cells were washed with PBS and trypsinized for 10 minutes at 37°C with 0.25% trypsin and 0.02% ethylenediamine tetraacetic acid (Sigma). The cell suspension was centrifuged, washed with PBS, and resuspended in HEPES buffer (catalog no. A14291 DJ; Life Technologies, Grand Island, NY) to a cell density of 106 cell/mL (measured by a hemocytometer) for experimental use.
Blue Light Inactivation of A. baumannii In Vitro
A 3-mL A. baumannii suspension at approximately 108 CFU/mL in PBS was placed into 35-mm petri dishes at room temperature (21°C). The suspension was exposed to blue light at an irradiance of 19.5 mW/cm2, with the lid of the petri dish removed. During light irradiation, the bacterial suspension was stirred by a minimagnetic bar at 20 rpm. Aliquots of 40 µL of the suspension were withdrawn at 0, 12, 24, 36, 48, and 60 minutes respectively, when 0, 14.0, 28.1, 42.1, 56.2, and 70.2 J/cm2 blue light had been delivered. CFU were then determined by serial dilution on BHI agar plates, using the method described by Jett et al [31]. Colonies were allowed to grow for 18–24 hours at 37°C. The experiments were performed in triplicate.
Blue Light Irradiation of Keratinocytes In Vitro
A 3-mL keratinocyte suspension at 106 cell/mL in HEPES buffer was placed into 35-mm petri dishes at room temperature. The suspension was exposed to blue light at an irradiance of 19.5 mW/cm2, with the lid of the petri dish removed. During light irradiation, the keratinocyte suspension was stirred by a minimagnetic bar (20 rpm). Aliquots of 40 µL of the suspension were withdrawn at 0, 24, 48, 72, 96, 120, and 144 minutes, respectively, when 0, 28.0, 56.2, 84.2, 112.3, 140.4, and 168.5 J/cm2 blue light had been delivered. Viable counts were determined immediately by mixing each sample with an equal volume of 0.4% (w/v) trypan blue [32], and the mixture was transferred to a hemocytometer. The percentage of cells that survived was calculated as the ratio of the number of viable cells (unstained cells) to the total number of cells. The experiments were performed in triplicate.
Transmission Electron Microscopy (TEM)
Untreated or blue light–treated A. baumannii cells were fixed in 2.5% glutaraldehyde plus 2% paraformaldehyde immediately after blue light exposure and stored overnight at 4°C. After spinning down (at 1200 rpm for 10 minutes) and decanting the fixative, 0.1 M sodium cacodylate buffer (pH 7.2) was added to the pellets. After fixation, hot agar (2% in distilled water, heated to boiling) was immediately added to each pellet. Once the agar had hardly solidified, the cell pellets were then processed routinely for TEM. The cell pellets were postfixed in 2% OsO4 in sodium cacodylate buffer, dehydrated in a graded alcohol series, and embedded in Epon t812 (Tousimis, Rockville, MD). Ultrathin sections were cut on a Reichert-Jung Ultracut E microtome (Vienna, Austria), collected on uncoated 200 mesh copper grids, stained with uranyl acetate and lead citrate, and examined on a Philips CM-10 transmission electron microscope (Eindhoven, the Netherlands). The negatives were scanned on an Epson Perfection 3200 photoscanner. Multiple parasite sections were microscopically analyzed, and images representing the most-typical morphologies observed were presented in the study.
Fluorescence Spectroscopy
To identify the presence of endogenous porphyrins within A. baumannii cells, an overnight A. baumannii culture was centrifuged, washed with PBS, and centrifuged again, and the supernatant was removed. The A. baumannii pellets were added to 1 mL of a mixture of 0.1 M NaOH/1% sodium dodecyl sulfate (SDS) stored in the dark for 1 day. Fluorescence of the dissolved pellets in NaOH/SDS (in a 1-cm thick cuvette) was measured on a fluorometer (Fluoromax 3, SPEX Industries, Edison, NJ), with excitation at 405 nm and emission scanned from 580 to 700 nm.
A. baumannii Burn Infection in Mice
Female BALB/c mice, aged 6–7 weeks and weighing 17–19 g, were purchased from Charles River Laboratories (Wilmington, MA). The animals were housed 2 per cage, with access to food and water ad libitum; a 12-hour light/dark cycle, a temperature of <21°C, and a relative humidity range of 30% to 70% were maintained. All animal procedures were approved by the Institutional Animal Care and Use Committee at the Massachusetts General Hospital and were in accordance with the guidelines of the National Institutes of Health.
Before the incurrence of burns, mice were anesthetized by intraperitoneal injection of a ketamine-xylazine cocktail, and their dorsal surface was shaved. Burns were incurred by applying a preheated brass block (temperature, approximately 95°C) to the dorsal surface of each mouse for 7 seconds, resulting in nonlethal, full-thickness, and third-degree burns measuring approximately 1.2 cm × 1.2 cm. Five minutes after burn incurrence, a 60-µL suspension containing 107 CFU of A. baumannii was topically applied to the eschar of each burn.
Bioluminescence Imaging
The bioluminescence imaging setup consists of an ICCD camera (C2400-30H, Hamamatsu Photonics, Bridgewater, NJ), a camera controller, a specimen chamber, an image processor (C5510-50, Hamamatsu Photonics), and a color monitor (PVM 1454Q, Hamamatsu Photonics). Light-emitting diodes are mounted inside the specimen chamber and supply the light required for obtaining dimensional imaging of the sample. In photo-counting mode, a clear image can be obtained even under extremely low levels of light by detecting and integrating individual photons one by one.
Before imaging, mice were anesthetized by intraperitoneal injections of a ketamine/xylazine cocktail. Mice were then placed on a height-adjustable stage in the specimen chamber, and the infected burns were positioned directly under the camera. A gray-scale background image of each mouse burn was made, followed by a photon count of the same region. This entire burn photon count was quantified in terms of relative luminescence units and was displayed in a false color scale ranging from pink (most intense) to blue (least intense).
Blue Light–Inactivation of A. baumannii in Infected Mouse Burns
A group of 11 mice with infected burns were exposed to blue light. In addition, a group of 9 mice with infected burns but no exposure to blue light were used as untreated controls. Blue light was initiated 30 minutes after bacterial inoculation. Mice were exposed to a total light exposure of up to 55.8 J/cm2 (total illumination duration, 62 minutes; irradiance, 14.6 mW/cm2) in aliquots with bioluminescence imaging taking place after each aliquot of light. To record the time course of the extent of infection, bacterial luminescence from mouse burns was measured daily after blue light exposure until the infections were cured (characterized by the disappearance of bacterial luminescence).
Repeated Sublethal Blue Light–Inactivation of A. baumannii In Vitro and Bacterial Regrowth
We tested whether this multidrug-resistant strain of A. baumannii could develop resistance to blue light inactivation by performing 10 consecutive cycles of sublethal inactivation of bacteria in vitro, followed by bacterial regrowth. In each inactivation-growth cycle, 3 independent cultures were tested. For each culture, a 3-mL bacterial suspension containing 108 CFU/mL in PBS was placed into a 35-mm petri dish. The suspension was then exposed to blue light at an irradiance of 19.5 mW/cm2. During blue light irradiation, the bacterial suspension was stirred with a miniature magnetic bar (at 20 rpm). In the first inactivation-growth cycle, blue light exposure was adjusted to leave about 0.01% bacterial survival (about 4-log10 inactivation) after blue light inactivation (approximately 70.2 J/cm2), and the same light exposure was then used throughout the successive cycles. Bacterial CFU were determined by serial dilution on BHI agar plates [31]. The surviving bacterial cells (colonies from the agar plates exposed to 70.2 J/cm2 blue light) were collected and recultured for the next inactivation-growth cycle. This procedure was repeated until the tenth cycle was reached. Bacteria survival rates for different cycles were compared using a one-way analysis of variance test.
TUNEL Assay
TUNEL staining was used to examine blue light–induced DNA fragmentation in mouse skin cells. Mouse skin was exposed to blue light at a single exposure of 195 J/cm2. Skin biopsy specimens were taken immediately before and 0, 24, and 48 hours after blue light exposure, respectively. The biopsy specimens were preserved in 10% phosphate-buffered formalin for 18–24 hours, processed, and then embedded in paraffin. Serial tissue sections of 4 µm in thickness were subjected to a TUNEL assay, using the FragEL DNA Fragmentation Detection Kit (EMD Millipore, MA) according to the manufacturer's instructions. Stained samples were observed by confocal microscopy (FV1000-MPE, Olympus, Tokyo, Japan) by using fluorescein isothiocyanate as the fluorochrome dye and DAPI as the nuclear counterstain.
Research Ethics Declaration
The research described here complied with the Animal Welfare Act regulations and other US federal statutes relating to animals and experiments involving animals and adhered to the principles set forth in the 1996 National Research Council Guide for Care and Use of Laboratory Animals.
RESULTS
A. baumannii Was Significantly More Susceptible Than Keratinocytes to Blue Light Inactivation In Vitro
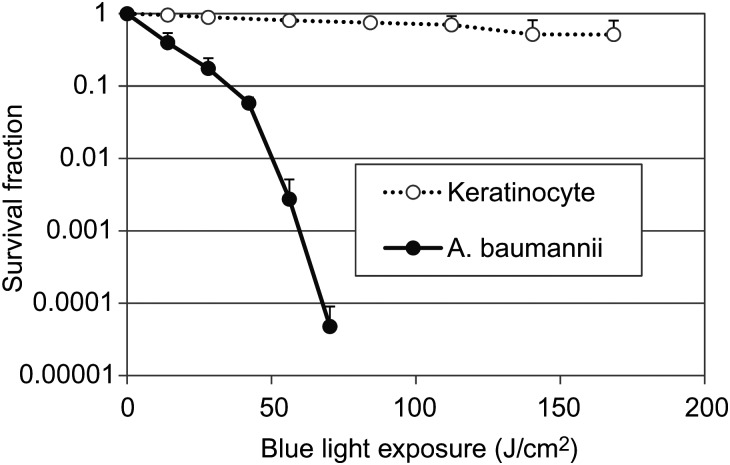
As illustrated in Figure 1, >4 log10 A. baumannii CFU were inactivated after a single exposure of 70.2 J/cm2 blue light. While under the same light exposure, only approximately 0.1-log10 viability loss was observed in keratinocytes. The mean rates of blue light–induced inactivation among A. baumannii and keratinocytes were 0.003 log10 CFU per J/cm2 and 0.064 log10 CFU per J/cm2, respectively (P = .006).
Figure 1.
Blue light inactivation of Acinetobacter baumannii and keratinocytes in vitro. Bars denote SDs.
TEM Revealed Blue Light–Induced Ultrastructural Damage in A. baumannii Cells
TEM showed ultrastructural damage to A. baumannii cells after a single exposure of blue light at 86.4 J/cm2 had been delivered (Figure 2). Severe cell wall damage was found with leakage of intracellular substances (Figure 2B). Cytoplasmic vacuoles (Figure 2C) were found in a large number of bacterial cells, and intracellular structures were disrupted and discontinuous (Figure 2D). Many bubbles were observed around the cell walls (Figure 2C).
Figure 2.
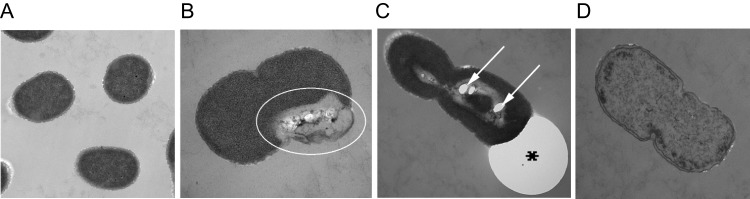
Transmission electron microscope images of Acinetobacter baumannii cells. A, Untreated A. baumannii cells. B–D, Blue light–treated A. baumannii cells: severe cell wall damage (B; oval); cytoplasmic vacuole formation (C; arrows), intracellular structural discontinuation, bubble formation around the cell wall (C; asterisk); and significant leakage of intracellular substances (D).
Intracellular Porphyrins May Be Responsible for Blue Light Inactivation of A. baumannii
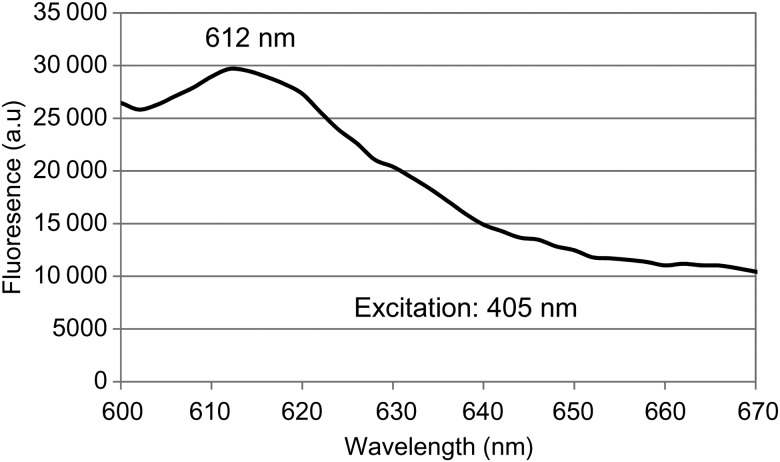
The fluorescence spectrum (excitation at 405 nm) of the A. baumannii cells dissolved in NaOH/SDS is shown in Figure 3. The spectrum peaked at 612 nm, which is characteristic for the typical fluorescence emissions of porphyrins (coproporphyrin III) at the same excitation of 405 nm [25], suggesting that endogenous porphyrins within the A. baumannii cells were the photosensitizing chromophores responsible for the antimicrobial effect of blue light.
Figure 3.
Fluorescence spectrum of Acinetobacter baumannii cell pellets from overnight culture dissolved in NaOH/sodium dodecyl sulfate. The excitation wavelength was 405 nm. Abbreviation: a.u, arbitrary units.
Blue Light Significantly Reduced Bacterial Burden in Mouse Burns Infected With A. baumannii
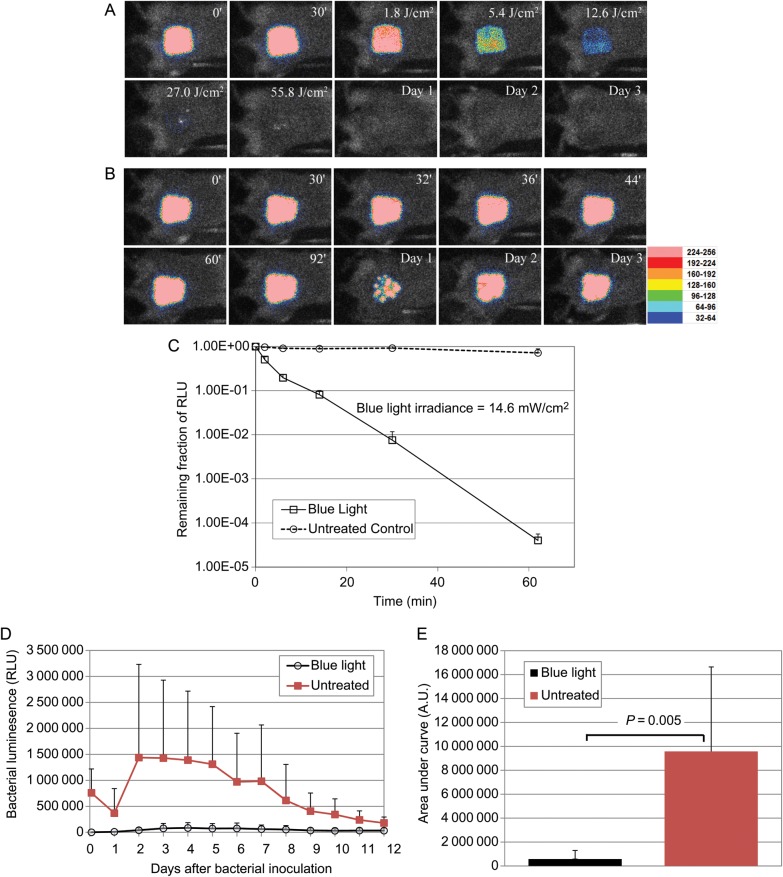
Figure 4 shows the successive bioluminescence images of representative mouse burns infected with 107 CFU of bioluminescent A. baumannii, with (Figure 4A) and without (Figure 4B) blue light exposure. Blue light was delivered 30 minutes after bacterial inoculation. It can be seen that bacterial luminescence was completely eliminated after a single exposure of 55.8 J/cm2 blue light, while in the untreated mouse burn, luminescence remained unchanged during same period. In the blue light–treated mouse, no reoccurrence of infection was observed during the following days, whereas in the untreated mouse, the extent of infection remained steady.
Figure 4.
A and B, Successive bacterial luminescence images of representative mouse burns infected with 107 colony-forming unites (CFU) of luminescent Acinetobacter baumannii, with (A) and without (B) blue light exposure. The blue light irradiance was 14.6 mW/cm2. Blue light was delivered 30 minutes after bacterial inoculation. In panel A, the 0′ bacterial luminescence image was taken immediately after bacterial inoculation; the 30′ image was taken 30 minutes after bacterial inoculation and just before blue light irradiation; the 1.80 J/cm2, 5.40 J/cm2, 12.6 J/cm2, 27.0 J/cm2, and 55.8 J/cm2 images were taken immediately after 1.80 J/cm2, 5.40 J/cm2, 12.6 J/cm2, 27.0 J/cm2, and 55.8 J/cm2 blue light had been delivered, respectively; and the day 1, day 2, and day 3 images were taken 24 hours, 48 hours, and 72 hours after bacterial inoculation, respectively. In panel B, the 0′, 30′, 32′, 36′, 44′, 60′, and 92′ images were taken at the corresponding time points (in minutes) after bacterial inoculation, respectively; and the day 1, day 2, and day 3 images were taken 24 hours, 48 hours, and 72 hours after bacterial inoculation, respectively. C, Dose responses of mean bacterial luminescence of mouse burns infected with 107 CFU of A. baumannii, with (n = 11 mice) and without (n = 9 mice) blue light exposure. Blue light was delivered 30 minutes after bacterial inoculation. Bars denote SDs. D, Time courses of mean bacterial luminescence of the infected mouse burns with (n = 11 mice) and without (n = 9 mice) blue light exposure. Bars denote SDs. E, Mean areas under the bacterial luminescence versus time curves in the 2-dimensional coordinate system in panel D, representing the overall bacterial burden of infected mouse burns. Bars denote SDs. Abbreviations: A.U, arbitrary units; RLU, relative luminescence units.
Figure 4C shows the average reduction in bacterial luminescence among 11 mice that were exposed to blue light and 9 mice that were untreated. The in vivo bacterial inactivation curves approximated first-order kinetics [33]. After a single exposure of 55.8 J/cm2 blue light, an average reduction in bacterial luminescence of 4.4 log10 was achieved in a light dose–dependent manner. In the untreated mice, a reduction of only approximately 0.14 log10 was observed during the same period (P < .00001).
Figure 4D shows the time courses of the relative luminescence units of the mean bacterial luminescence from days 1 to 12 among the 11 blue light–treated mice and 9 untreated mice. In the untreated mice, there was a decrease of bacterial luminescence from days 0 to 1. However, the bacterial luminescence significantly increased back from days 1 to 2, and the infection was sustained for >10 days. The mean areas under the curve (AUC) of the bioluminescence time course were 5.80 × 106 and 9.60 × 107 for blue light–treated and untreated mice, respectively (P = .005; Figure 4E), indicating the blue light exposure resulted in an approximately 16.5-fold reduction of the AUC (or of the bacterial burden, in infected mouse burns).
No Evidence of Resistance Development by A. baumannii to Blue Light Inactivation Was Observed After 10 Consecutive Cycles of Sublethal Inactivation of Bacteria In Vitro
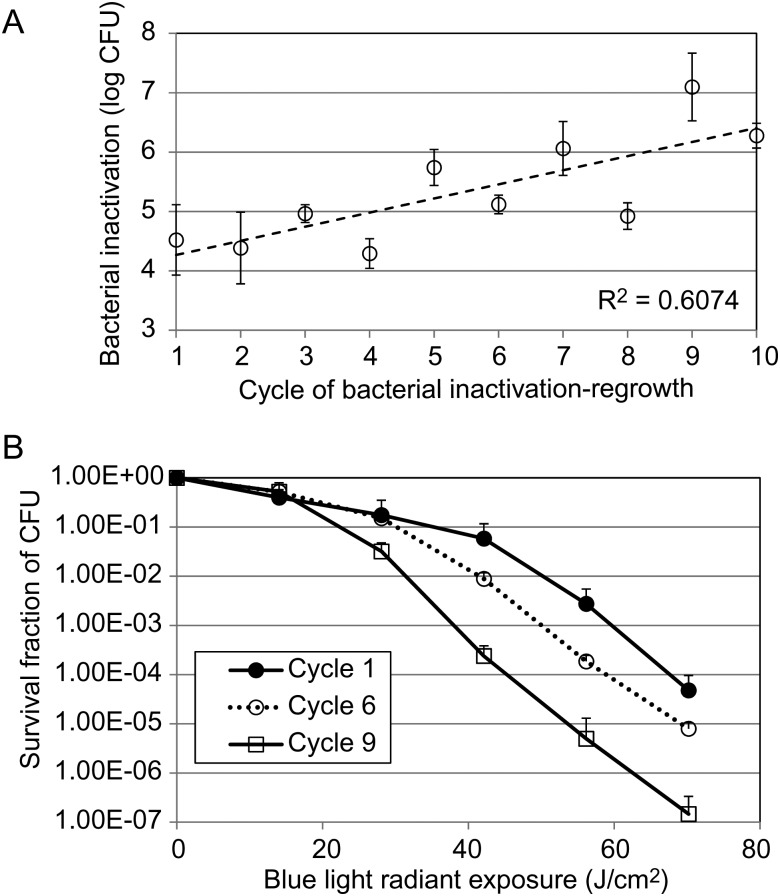
Figure 5A shows the extent of bacterial inactivation by blue light under the same exposure (70.2 J/cm2) in different inactivation-regrowth cycles from cycles 1 to 10. A statistically significant increase (rather than a decrease) in the extent of bacterial inactivation was observed between the first (4.52 ± 0.59 log10 CFU) and the tenth cycle (6.28 ± 0.21 log10 CFU; P = .04). As can also be seen from Figure 5A, there was a tendency toward increased susceptibility (or increased extent of bacterial inactivation by blue light) of the A. baumannii strain to blue light inactivation in vitro with the the number of cycles. Correlation analysis of the extent of bacterial inactivation and the cycles showed a correlation coefficient of 0.78 (P = .008). Figure 5B shows the A. baumannii inactivation curves by blue light for 3 representative cycles (cycles 1, 6, and 9).
Figure 5.
A, efficiency of blue light inactivation of bacteria (expressed log10 colony-forming units [CFU]) in different cycles of sublethal inactivation of bacteria by blue light, followed by bacterial regrowth. B, Bacterial inactivation curves of representative cycles (cycles 1, 6, and 9). Bars denote SDs.
No Significant or Irreversible DNA Damage Was Observed in the Mouse Skin Exposed to a High exposure of Blue Light
Figure 6 shows the results of a TUNEL assay of a representative skin biopsy specimen from a mouse exposed to blue light at a high exposure of 195 J/cm2. A blue light exposure of 195 J/cm2 led to almost no apoptotic cells in the epidermis immediately after blue light exposure (only 1 TUNEL-positive cell was observed in the confocal image). Similarly, lack of TUNEL-positive epidermal cells was observed after 24 or 48 hours (no TUNEL-positive cells were observed in each confocal image).
Figure 6.
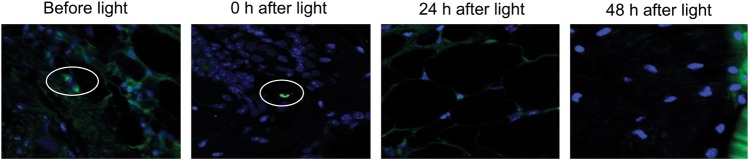
TUNEL analyses of DNA damage in mouse skin biopsy specimens exposed to blue light at a single exposure of 195 J/cm2 (original magnification, 100×). Skin samples were taken before and 0 hours, 24 hours, and 48 hours after blue light exposure. Immunofluorescence of fluorescein and DAPI are represented by green and blue pseudocolor, respectively. DAPI was used for nuclear counterstain. Ovals denote TUNEL-positive cells.
DISCUSSION
In this preclinical study, we investigated antimicrobial blue light therapy for multidrug-resistant A. baumannii infection in a mouse burn model. In vitro study showed that the multidrug-resistant A. baumannii strain isolated from a military patient was significantly more susceptible than human keratinocytes to blue light inactivation. The mean inactivation rate of A. baumannii by blue light was approximately 21-fold faster than that of keratinocytes. This finding indicated that there exists a therapeutic window in which A. baumannii can be selectively inactivated by blue light and the host cells can be preserved. In the in vivo study using mouse burns infected with A. baumannii, blue light was initiated at 30 minutes after inoculation. It was demonstrated that a single exposure of 55.8 J/cm2 blue light significantly reduced the bacterial burden (by approximately 16.5-fold) in mouse burns over a 12-day observation period, in comparison to untreated mouse burns. Fluorescence spectroscopy supported the hypothesis that endogenous porphyrins are the intracellular photosensitizing chromophores responsible for the antimicrobial effect of blue light.
To use blue light for inactivation of bacteria, one question that will have to be addressed is whether bacteria can develop resistance to blue light inactivation. We investigated this by performing 10 repeated cycles of sublethal inactivation of bacteria followed by bacterial regrowth. The multidrug-resistant A. baumannii strain failed to develop resistance to blue light inactivation. On the contrary, the susceptibility of bacteria to blue light inactivation tended to increase with the number of cycles, suggesting that at least 1 mutation that favors the bacterial susceptibility to blue light inactivation might occur. Further studies are warranted to elucidate the mechanism of the increased susceptibility of A. baumannii to blue light–induced inactivation after repeated light exposures.
We also evaluated the extent of blue light–induced premutagenic effect on mouse skin cells. At a single exposure of blue light at 195 J/cm2, which corresponded to a therapeutic ratio of 195/55.8, or approximately 3.5, almost no blue light–induced DNA damage in mouse skin was observed up to 48 hours after blue light exposure. This finding indicated that blue light therapy may have significant potential to be a safe approach for wound infections.
The use of blue light for combat-related wound infections is compelling because it is a nonantibiotic approach that is noninjurious to host cells and tissue. Blue light sources can be easily militarized for portable and lightweight applications. The blue-light source is also convenient to operate (it is battery powered) and can even be provided to each service member and used with limited medical training. This technology could be implemented on the battlefield and could delay the onset or progression of infection until medical intervention is available. This is particularly suitable for combat casualty care, in which a majority of injured service members have to be transported by medevac to receive care and transportation is often delayed because of combat conditions.
The application of blue light for infections is of importance for civilian medicine, as well. Antimicrobial resistance is now a global problem, causing bacterial infections that cannot be treated with existing antibiotics. Recently, a dangerous new enzyme (New Delhi metallo-β-lactamase 1) that makes some bacteria resistant to carbapenems, which are the antibiotics used as a last resort when treatment with common antibiotics has failed, is being found in patients in the United States [34]. Many physicians are concerned that several bacterial infections soon may become untreatable [35]. As a result, there is a pressing need for the development of alternative treatment regimens, to which bacteria will not be easily able to develop resistance (eg, antimicrobial blue light therapy), for multidrug-resistant wound infections.
Notes
Acknowledgments. We are grateful to Dr Tayyaba Hasan at Wellman Center for her helpful discussion and her co-mentorship for Y. Zhu.
T. D., C. K. M., M. S. V., and M. R. H. designed the study. Y. Zhang, A. G., and T. D. did the experiment. T. D., Y. Zhang, Y. Zhu, A. G., M. E. S., and M. R. H. collected and analyzed the data. T. D., A. G., Y. Zhu, and M. R. H. interpreted the data. T. D., Y. Zhu, A. G., and M. R. H. wrote the report. Y. H. prepared the keratinocyte cultures and assisted with the corresponding experiment. D. G. B. collected the A. baumannii strain and tested the strain's susceptibility to various antibiotics.
Disclaimer. The views expressed herein are those of the authors and do not reflect the official policy or position of the Department of the Army, the Department of Defense, or the US government.
Financial support. This work was supported by the Center for Integration of Medicine and Innovative Technology (CIMIT) under a US Army Medical Research Acquisition Activity cooperative agreement (CIMIT no. 13-1033 to T. D.), COTA/Smith and Nephew (grant 2012-16 to T. D.), the Airlift Research Foundation (Extremity Trauma Research Grant 109421 to T. D.), and the National Institutes of Health (grant RO1AI050875 to M. R. H.).
Potential conflicts of interest. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Vento TJ, Cole DW, Mende K, et al. Multidrug-resistant gram-negative bacteria colonization of healthy US military personnel in the US and Afghanistan. BMC Infect Dis. 2013;13:68. doi: 10.1186/1471-2334-13-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calhoun JH, Murray CK, Manring MM. Multidrug-resistant organisms in military wounds from Iraq and Afghanistan. Clin Orthop Relat Res. 2008;466:1356–62. doi: 10.1007/s11999-008-0212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scott P, Deye G, Srinivasan A, et al. An outbreak of multidrug-resistant Acinetobacter baumannii-calcoaceticus complex infection in the US military health care system associated with military operations in Iraq. Clin Infect Dis. 2007;44:1577–84. doi: 10.1086/518170. [DOI] [PubMed] [Google Scholar]
- 4.Crane DP, Gromov K, Li D, et al. Efficacy of colistin-impregnated beads to prevent multidrug-resistant A. baumannii implant-associated osteomyelitis. J Orthop Res. 2009;27:1008–15. doi: 10.1002/jor.20847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bush K, Courvalin P, Dantas G, et al. Tackling antibiotic resistance. Nat Rev Microbiol. 2011;9:894–6. doi: 10.1038/nrmicro2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dai T, Huang YY, Hamblin MR. Photodynamic therapy for localized infections--state of the art. Photodiagnosis Photodyn Ther. 2009;6:170–88. doi: 10.1016/j.pdpdt.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai T, Vrahas MS, Murray CK, Hamblin MR. Ultraviolet C irradiation: an alternative antimicrobial approach to localized infections? Expert Rev Anti Infect Ther. 2012;10:185–95. doi: 10.1586/eri.11.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maisch T. A new strategy to destroy antibiotic resistant microorganisms: antimicrobial photodynamic treatment. Mini Rev Med Chem. 2009;9:974–83. doi: 10.2174/138955709788681582. [DOI] [PubMed] [Google Scholar]
- 9.Wainwright M, Phoenix DA, Laycock SL, Wareing DR, Wright PA. Photobactericidal activity of phenothiazinium dyes against methicillin-resistant strains of Staphylococcus aureus. FEMS Microbiol Lett. 1998;160:177–81. doi: 10.1111/j.1574-6968.1998.tb12908.x. [DOI] [PubMed] [Google Scholar]
- 10.Conner-Kerr TA, Sullivan PK, Gaillard J, Franklin ME, Jones RM. The effects of ultraviolet radiation on antibiotic-resistant bacteria in vitro. Ostomy Wound Manage. 1998;44:50–6. [PubMed] [Google Scholar]
- 11.Wainwright M. Photodynamic antimicrobial chemotherapy (PACT) J Antimicrob Chemother. 1998;42:13–28. doi: 10.1093/jac/42.1.13. [DOI] [PubMed] [Google Scholar]
- 12.Dai T, Gupta A, Huang YY, et al. Blue light rescues mice from potentially fatal Pseudomonas aeruginosa burn infection: efficacy, safety, and mechanism of action. Antimicrob Agents Chemother. 2013;57:1238–45. doi: 10.1128/AAC.01652-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enwemeka CS, Williams D, Enwemeka SK, Hollosi S, Yens D. Blue 470-nm light kills methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Photomed Laser Surg. 2009;27:221–6. doi: 10.1089/pho.2008.2413. [DOI] [PubMed] [Google Scholar]
- 14.Enwemeka CS, Williams D, Hollosi S, Yens D, Enwemeka SK. Visible 405 nm SLD light photo-destroys methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Lasers Surg Med. 2008;40:734–7. doi: 10.1002/lsm.20724. [DOI] [PubMed] [Google Scholar]
- 15.Maclean M, MacGregor SJ, Anderson JG, Woolsey G. Inactivation of bacterial pathogens following exposure to light from a 405-nanometer light-emitting diode array. Appl Environ Microbiol. 2009;75:1932–7. doi: 10.1128/AEM.01892-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDonald R, Macgregor SJ, Anderson JG, Maclean M, Grant MH. Effect of 405-nm high-intensity narrow-spectrum light on fibroblast-populated collagen lattices: an in vitro model of wound healing. J Biomed Opt. 2011;16:048003. doi: 10.1117/1.3561903. [DOI] [PubMed] [Google Scholar]
- 17.Guffey JS, Wilborn J. In vitro bactericidal effects of 405-nm and 470-nm blue light. Photomed Laser Surg. 2006;24:684–8. doi: 10.1089/pho.2006.24.684. [DOI] [PubMed] [Google Scholar]
- 18.Lipovsky A, Nitzan Y, Gedanken A, Lubart R. Visible light-induced killing of bacteria as a function of wavelength: implication for wound healing. Lasers Surg Med. 2010;42:467–72. doi: 10.1002/lsm.20948. [DOI] [PubMed] [Google Scholar]
- 19.Murdoch LE, McKenzie K, Maclean M, Macgregor SJ, Anderson JG. Lethal effects of high-intensity violet 405-nm light on Saccharomyces cerevisiae, Candida albicans, and on dormant and germinating spores of Aspergillus niger. Fungal Biol. 2013;117:519–27. doi: 10.1016/j.funbio.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 20.McKenzie K, Maclean M, Timoshkin IV, Endarko E, Macgregor SJ, Anderson JG. Photoinactivation of bacteria attached to glass and acrylic surfaces by 405 nm light: potential application for biofilm decontamination. Photochem Photobiol. 2013;89:927–35. doi: 10.1111/php.12077. [DOI] [PubMed] [Google Scholar]
- 21.Kleinpenning MM, Smits T, Frunt MH, van Erp PE, van de Kerkhof PC, Gerritsen RM. Clinical and histological effects of blue light on normal skin. Photodermatol Photoimmunol Photomed. 2010;26:16–21. doi: 10.1111/j.1600-0781.2009.00474.x. [DOI] [PubMed] [Google Scholar]
- 22.Liebmann J, Born M, Kolb-Bachofen V. Blue-light irradiation regulates proliferation and differentiation in human skin cells. J Invest Dermatol. 2010;130:259–69. doi: 10.1038/jid.2009.194. [DOI] [PubMed] [Google Scholar]
- 23.Hamblin MR, Viveiros J, Yang C, Ahmadi A, Ganz RA, Tolkoff MJ. Helicobacter pylori accumulates photoactive porphyrins and is killed by visible light. Antimicrob Agents Chemother. 2005;49:2822–7. doi: 10.1128/AAC.49.7.2822-2827.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maclean M, Macgregor SJ, Anderson JG, Woolsey GA. The role of oxygen in the visible-light inactivation of Staphylococcus aureus. J Photochem Photobiol B. 2008;92:180–4. doi: 10.1016/j.jphotobiol.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Ashkenazi H, Malik Z, Harth Y, Nitzan Y. Eradication of Propionibacterium acnes by its endogenic porphyrins after illumination with high intensity blue light. FEMS Immunol Med Microbiol. 2003;35:17–24. doi: 10.1111/j.1574-695X.2003.tb00644.x. [DOI] [PubMed] [Google Scholar]
- 26.Dai T, Gupta A, Huang YY, et al. Blue light eliminates community-acquired methicillin-resistant Staphylococcus aureus in infected mouse skin abrasions. Photomed Laser Surg. 2013;31(11):531–8. doi: 10.1089/pho.2012.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dai T, Gupta A, Huang YY, et al. Blue light rescues mice from potentially fatal Pseudomonas aeruginosa burn infection: efficacy, safety, and mechanism of action. Antimicrob Agents Chemother. 2013;57:1238–45. doi: 10.1128/AAC.01652-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dai T, Tegos GP, Lu Z, et al. Photodynamic therapy for Acinetobacter baumannii burn infections in mice. Antimicrob Agents Chemother. 2009;53:3929–34. doi: 10.1128/AAC.00027-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamblin MR, O'Donnell DA, Murthy N, Contag CH, Hasan T. Rapid control of wound infections by targeted photodynamic therapy monitored by in vivo bioluminescence imaging. Photochem Photobiol. 2002;75:51–7. doi: 10.1562/0031-8655(2002)075<0051:rcowib>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 30.Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–71. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jett BD, Hatter KL, Huycke MM, Gilmore MS. Simplified agar plate method for quantifying viable bacteria. Biotechniques. 1997;23:648–50. doi: 10.2144/97234bm22. [DOI] [PubMed] [Google Scholar]
- 32.Zeina B, Greenman J, Corry D, Purcell WM. Cytotoxic effects of antimicrobial photodynamic therapy on keratinocytes in vitro. Br J Dermatol. 2002;146:568–73. doi: 10.1046/j.1365-2133.2002.04623.x. [DOI] [PubMed] [Google Scholar]
- 33.Xiong R, Xie G, Edmondson AE, Sheard MA. A mathematical model for bacterial inactivation. Int J Food Microbiol. 1999;46:45–55. doi: 10.1016/s0168-1605(98)00172-x. [DOI] [PubMed] [Google Scholar]
- 34.Detection of Enterobacteriaceae isolates carrying metallo-beta-lactamase—United States, 2010. MMWR Morb Mortal Wkly Rep. 2010;59:750. [PubMed] [Google Scholar]
- 35.Arias CA, Murray BE. Antibiotic-resistant bugs in the 21st century—a clinical super-challenge. N Engl J Med. 2009;360:439–43. doi: 10.1056/NEJMp0804651. [DOI] [PubMed] [Google Scholar]