Abstract
A high prevalence of infection with Borrelia burgdorferi in ixodid ticks is correlated with a high incidence of Lyme disease. The transmission of B. burgdorferi to humans can be disrupted by targeting 2 key elements in its enzootic cycle: the reservoir host and the tick vector. In a prospective 5-year field trial, we show that oral vaccination of wild white-footed mice resulted in outer surface protein A–specific seropositivity that led to reductions of 23% and 76% in the nymphal infection prevalence in a cumulative, time-dependent manner (2 and 5 years, respectively), whereas the proportion of infected ticks recovered from control plots varied randomly over time. Significant decreases in tick infection prevalence were observed within 3 years of vaccine deployment. Implementation of such a long-term public health measure could substantially reduce the risk of human exposure to Lyme disease.
Keywords: Oral vaccine, Borrelia burgdorferi, Lyme disease, enzootic cycle, transmission, wildlife reservoir
Borrelia burgdorferi, the etiologic agent of Lyme disease, is widely distributed throughout temperate zones of the Northern Hemisphere [1]. In some areas, the Lyme disease incidence is rapidly increasing, as documented by the doubling of newly diagnosed cases in the United States over the past decade. Disrupting pathogen transmission between the tick vector and reservoir hosts is regarded as a promising strategy to reduce human exposure to this zoonosis [2–6]. The enzootic cycle of B. burgdorferi provides a unique opportunity to reduce transmission to humans because vector ticks (Ixodes organisms) must acquire B. burgdorferi from wildlife reservoirs, mainly the white-footed mouse (Peromyscus leucopus); there is no transovarial transmission [7]. Injectable vaccines based on the outer surface protein A (OspA) of B. burgdorferi have been shown to protect humans [8], dogs [9], and mice [10, 11] against infection. When these injectable vaccines were administered to trapped wildlife over 1 summer in a noncontinuous basis, they modestly decreased tick acquisition of B. burgdorferi from infected white-footed mice the following year [4]. However, the challenge remains to develop a vaccine that can be easily deployed in natural ecosystems and takes into consideration the high-population density and rapid turnover of the reservoir host. Practical vaccine delivery and effective immunization of wildlife can be achieved using thermostable vaccines delivered via oral bait. These vaccines are powerful tools to reduce B. burgdorferi prevalence and, thus, human Lyme disease risk. We and others have developed OspA-based transmission-blocking, oral, reservoir-targeted vaccines (RTVs) against B. burgdorferi [5, 12]. Our vaccine vehicle is a safe, intestinal commensal bacterium that can be delivered to reservoir hosts that naturally transmit B. burgdorferi to feeding ticks during its enzootic cycle.
In northeastern North America, B. burgdorferi cycles between arthropod vectors and small-sized, ground-dwelling vertebrate hosts in an enzootic cycle spanning at least 2 consecutive years, depending on the life cycle of the tick vector, Ixodes scapularis. Newly hatched larval ticks take a blood meal during the summer of the first year, during which they can acquire infection, and after molting into a nymph they take a second blood meal the following year, during which they can transmit infection. The vector life cycle stage most important for transmission of B. burgdorferi to humans is the nymphal stage. We hypothesized that prolonged and continuous treatment of the most competent reservoir host for B. burgdorferi (white-footed mice) with an oral OspA-based RTV should lead to increased seropositivity that correlates with reductions of B. burgdorferi infection in nymphal ticks and results in a reduced risk of human exposure to Lyme disease.
MATERIALS AND METHODS
Animal experimentation guidelines were followed in compliance with the University of Tennessee Health Science Center Institutional Animal Care and Use Committee (IACUC; 13-010, 1741) and the Cary Institute of Ecosystem Studies (CIES) IACUC (07-021, 10-01III).
Study Design
The field study was conducted at the CIES in Dutchess County, New York (Figure 1). Seven plots were included: 4 plots (NY1, NY2, NY3, and NY4) received OspA/RTV bait, and 3 control plots (Ctrl1, Ctrl2, and Ctrl3) did not receive RTV. Ideally, an Escherichia coli vehicle without the vaccine should have been used in the control plots. However, additional production and deployment would have made the cost of the study prohibitive. We deployed crimped oats only in the control plots. The RTV was deployed in NY1 from 2007 until 2011; in NY2 from 2008 to 2011; and in NY3 and NY4 from 2009 until 2011. Treatment and control plots were monitored from 2007 through 2011. We matched control plots for basic vegetative structure and small-mammal community. All plots contain similar oak-dominated forests and understory vegetation, as well as physical characteristics, such as soil type, slope, and drainage, that are typical of Lyme disease–endemic areas in the northeastern regions of the United States [13]. The area of each plot was 1.1 hectare and consisted of an 8 by 8 array of Sherman live traps, with 15 m between traps. Plots were separated by at least 500 m, a distance sufficient to ensure biological and statistical independence. The total number of mice that moved from one of our plots to another at any point in the study was 11, or 0.29% of the 3791 mice captured. The bait was produced daily, using oatmeal, water, and 200 mg of E. coli expressing OspA [14]; this constituted an RTV unit. Each trap was set with 1 RTV unit in the late afternoon for 5 consecutive nights per week from mid May until mid September. The following morning, all traps were checked by 10 am, and the level of vaccine consumed was recorded. At first capture all mice were provided with uniquely numbered ear tags, and for all captures we recorded date, plot, tag number, trap location, sex, age, and body mass. During the peak questing activity period for larval ticks (ie, August and September), we randomly selected mice and brought them to the laboratory (10 per grid) for collection of a 100-µL blood specimen. Given that the average mouse lifespan is <1 year, each mouse had blood sampled once. Determination of anti-OspA antibody levels was performed as described [14]. Considering our minimum RTV effective dose (5 RTV units [14]), we determined the cutoff for anti-OspA seropositivity as 3 SDs above the mean of antibody levels (OD450) in blood from mice that consumed <4 RTV units in 2 plots, NY1 and NY2 in 2008 (seropositivity was defined as an OD450 of >0.27). In the spring (May and June), host-seeking nymphal ticks were collected by drag sampling in each field plot along a series of 80-m linear transects [13]. Drag cloths were examined, and all ticks were removed every 30 m along the transects. At least 2 dragging sessions were conducted each year. B. burgdorferi DNA was extracted from 16–150 ticks per site per year [15], and real-time polymerase chain reaction (PCR) analysis was performed to assess tick infection rates and RTV efficacy [16].
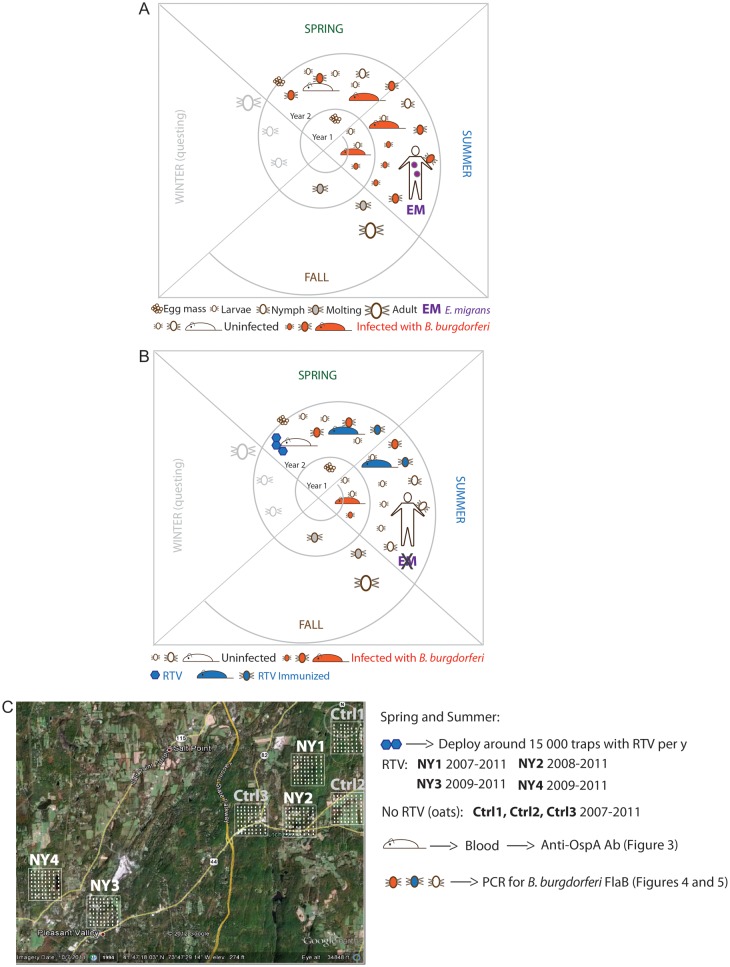
Figure 1.
Proposed strategy to break the enzootic cycle of the Lyme disease spirochete. A, The triad which comprises the enzootic cycle: the tick vector (Ixodes scapularis), the major reservoir host (white footed-mouse), and Borrelia burgdorferi. B, Hypothesis: immunizing wild white-footed mice with oral reservoir-targeted vaccine (RTV) breaks the enzootic cycle of B. burgdorferi. C, Field study design and methods. Abbreviations: Ab, antibody; Ctrl, control plot; FlaB, Flagellin B; OspA, outer surface protein A; PCR, polymerase chain reaction.
Statistical Analysis
The RTV effect on anti-OspA antibody and on nymphal infection prevalence were determined by generalized linear mixed model (GLMM), using the glm and lmer packages in R [17]. The effect of the vaccine on levels of OspA antibody was determined using a GLMM accounting for the main effects of the treatment and calendar year as fixed factors and grid as a random factor, using a Gaussian error. The GLMM analyses control for natural variation among years and among grids while assessing the main effect of treatment on OspA antibody levels. Duration of treatment and the interaction between treatment and treatment duration were also investigated to assess cumulative effects of treatment on OspA antibody. Anti-OspA antibody levels were not normally distributed about the mean (P < .002 for all grids, by the Shapiro-Wilkes test for normality). Log transformation of OspA antibody levels resulted in the normally distributed data (P > .13) necessary to use a Gaussian error term. The effect of the vaccine on the seroprevalence of OspA in field mice was analyzed using a contingency test (ie, χ2 analysis). The effect of the vaccine on the nymphal infection prevalence was assessed using a GLMM accounting for the main effects of the treatment and duration of treatment, the interaction between these 2 fixed factors, calendar year as a fixed factor, and grid as a random factor, using a binomial error term and a logit link, for logistic regression. The GLMM analyses control for natural variation among years and among grids while assessing main cumulative effects of treatment over nymphal infection prevalence over multiple enzootic cycles. The Tukey honest significant difference test was used to test for unplanned pairwise comparisons of differences in nymphal infection prevalence between the control plots and each experimental plot separately while preserving family-wise type I error.
RESULTS
Field Study Design
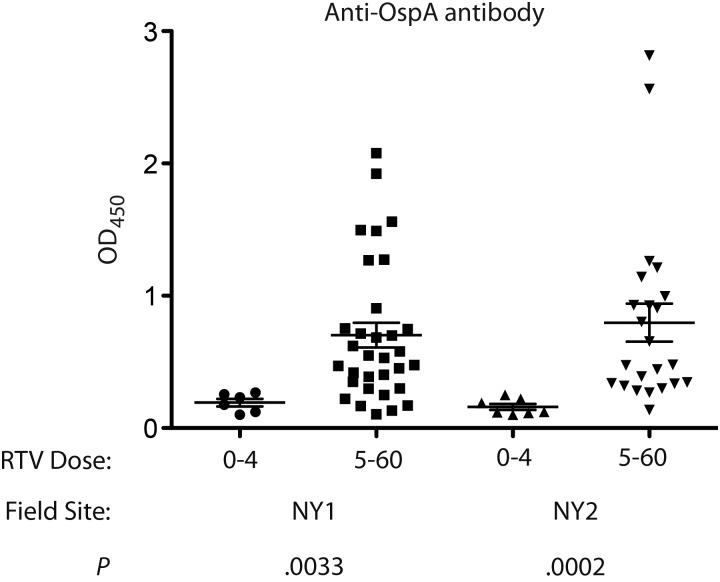
Of the seven 1-hectare study plots, 4 were assigned for RTV deployment and 3 were controls in which we deployed crimped oats only (without RTV) (Figure 1). During the study, we deployed a total of 100 608 baits in Sherman live traps, 79 104 of which were RTV units, and we captured 3791 individual white-footed mice, 2259 of which were captured in the plots treated with RTV, for an average deployment of approximately 35 RTVs per mouse. Most mice were captured repeatedly, with the average number of recaptures approximately 6.5 per mouse across all sites and years (Table 1). Bait consumption records from 277 mice gathered in 2007, 2008, and 2009 in 2 plots (NY1 and NY2) treated with RTV show that 140 mice (51%) consumed 5–60 units of RTV and that 137 (49%) consumed 0–4 units of RTV. We analyzed the antibody response to OspA as a function of the minimum RTV effective dose in 68 mice captured in NY1 and NY2 in 2008 (Figure 2) [14]. Consumption of ≥5 RTV units correlated with production of higher levels of anti-OspA antibodies in 2 plots in which the RTV was deployed.
Table 1.
Number of White-Foot Mouse (WFM) Captures in the Field
| Study Year | Unique WFM Captured, No. | Nights of Trap Use, No. | Total WFM Captures, No. | WFM Trapabilitya |
|---|---|---|---|---|
| 2007 | 700 | 9472 | 6043 | 8.63 |
| 2008 | 240 | 13 824 | 1647 | 6.86 |
| 2009 | 716 | 26 112 | 5399 | 7.75 |
| 2010 | 877 | 27 136 | 3806 | 4.34 |
| 2011 | 1258 | 24 064 | 6078 | 4.83 |
| Overall | 3791 | 100 608 | 22 973 | 6.48 |
Data are for mice recovered from plots in which reservoir-targeted vaccine was deployed and control plots.
a Defined as the mean no. of captures per WFM.
Figure 2.
Analysis of the antibody response to outer surface protein A (OspA) as a function of the minimum effective dose (ie, 5 reservoir-targeted vaccine [RTV] units) in white-footed mice trapped in 2 distinct plots in suburban New York in 2008 (38 mice in plot NY1 and 30 mice in plot NY2). P values were determined by the Mann–Whitney U test.
Anti-OspA Seroprevalence
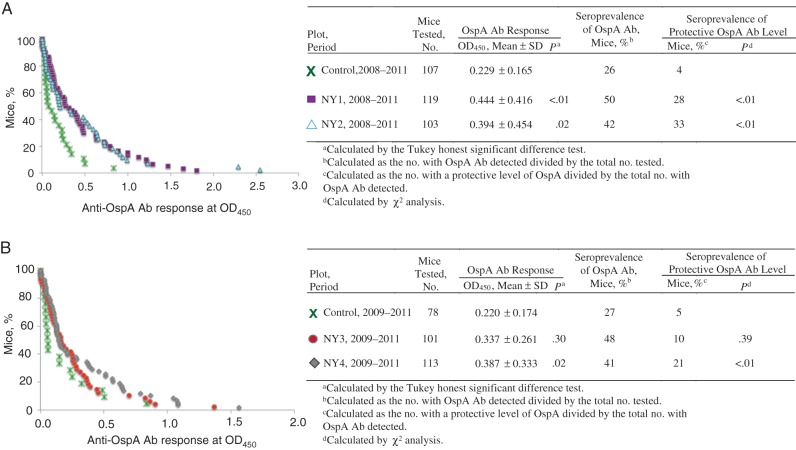
Between April and September from 2008 through 2011, we collected blood from populations of white-footed mice trapped in the field for quantitative analysis of the OspA-specific antibody response among vaccinated and control groups by ELISA (OD450; Figure 3). We observed that mice captured in vaccinated plots had a mean antibody response (±SD) of 0.391±0.366, compared with 0.229±0.165 for mice captured in control plots. Statistical analysis of the log-transformed data (to make them normally distributed over the mean) by using a GLMM (to control for effects of plot and year) revealed a statistically significant increase in anti-OspA antibodies in mice captured in the vaccine-deployed plots (P = .002). A Tukey honest significant difference post-hoc test to assess pairwise differences among all the plots revealed that differences in OspA-specific antibody levels between mice captured in the vaccine plots and those captured in the control plots were statistically significant for 3 plots (NY1, NY2, and NY4) but not for a fourth (NY3). Additionally, we evaluated differences in OspA seroprevalence among mice from plots in which vaccine was deployed, compared with those from control plots, using a cutoff for a positive anti-OspA antibody response at an OD450 of ≥ 0.27. All antibody to OspA accumulating in the system should contribute to future reductions of B. burgdorferi in the field. Nevertheless, we evaluated a putative correlate of protection based on our previous findings in the laboratory [14] (ie, the proportion of mice with OspA-specific antibodies, defined by an OD450 of approximately 1.0). The prevalence of a protective anti-OspA Ab response was significantly higher among mice from the vaccine-deployed plots NY1 (28%, during 2008–2011), NY2 (33%, during 2008–2011), and NY4 (21%, during 2009–2011) than among those from NY3 (10%, during 2009–2011) or the control plots (approximately 5%, during 2009–2011; Figure 3).
Figure 3.
Anti–outer surface protein A (OspA) antibody (Ab) distribution between plots with and plots without deployment of reservoir-targeted vaccine (RTV). Blood specimens from trapped mice were tested for the presence of OspA-specific Ab by enzyme-linked immunosorbent assay. A, Mice were trapped in RTV-deployed plots NY1 and NY2 and untreated control plots during 2008–2011. B, Mice were trapped in RTV-deployed plots NY3 and NY4 and untreated control plots during 2009–2011. Vaccination resulted in a statistically significant increase in the anti-OspA Ab response after control for natural variation among plots and years, using a generalized linear mixed model (P = .002). Multiple pairwise comparisons of means by use of the Tukey honest significant difference post-hoc test revealed that differences in anti-OspA–specific Ab levels between mice captured in the vaccine plots and those captured in the control plots were statistically significant for all plots except NY3. Statistical analysis of the protective seroprevalence data was done using the χ2 test. 2010 data were not included for NY3 because both values were 0.
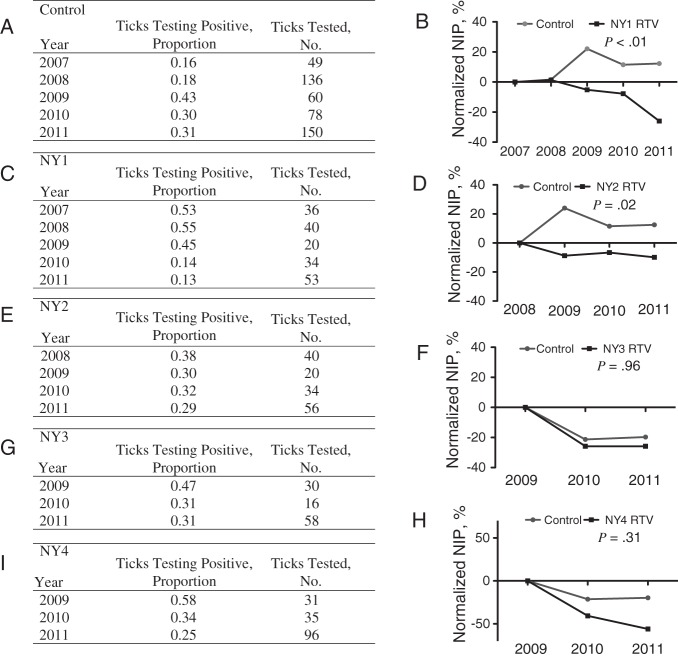
Reductions Over Time in B. burgdorferi Infection Among Nymphal I. scapularis Ticks From Field Sites in Which Oral RTV Was Deployed
Given the 2-year life cycle of the tick and the potential for a self-reinforcing feedback loop between lower nymphal infection prevalence and lower host infection prevalence, we hypothesized that the efficacy of the vaccine should be cumulative over time. We collected ticks from 4 vaccinated and 3 unvaccinated plots and determined the proportion of host-seeking nymphal ticks infected with B. burgdorferi over 2 consecutive enzootic cycles (2–5 calendar years) of the spirochete by PCR (Figure 4). In the 3 control plots, the average nymphal infection prevalence, calculated as the number of infected nymphs divided by the total number tested, was 0.16 in 2007, 0.43 in 2009, and 0.31 in 2011. There was no statistical evidence of a temporal trend in the 3 control plots. In contrast, in all vaccinated plots, the nymphal infection prevalence showed a declining trend over time. The cumulative reduction in nymphal infection prevalence over the years was significant in the field plots with RTV deployed for the longest periods (NY1 [5 years], P < .0001; and NY2 [4 years], P = .0244). Differences in nymphal infection prevalence gradually distance themselves from the initial nymphal infection prevalence, compared with random variation in B. burgdorferi infection in the control.
Figure 4.
Borrelia burgdorferi infection in nymphal Ixodes scapularis ticks in plots where an oral reservoir-targeted vaccine (RTV) for wild white-footed mice were deployed. A, C, E, G, and I, Proportion of ticks infected with B. burgdorferi from control plots (A); from the NY1 plot, in which RTV was deployed for 5 consecutive years (C); from the NY2 plot, in which RTV was deployed for 4 years (E); and from the NY3 plot (G) and the NY4 plot (I), in which RTV was deployed for 3 years. B, D, F, and H, Nymphal infection prevalence (NIP) normalized against the baseline vs the control for the same years of treatment. Given that RTV is expected to exert an effect 1 year after deployment, the baseline NIP is the NIP recorded in the first year of the study. P values were determined by the χ2 test.
Breaking the Enzootic Cycle of B. burgdorferi
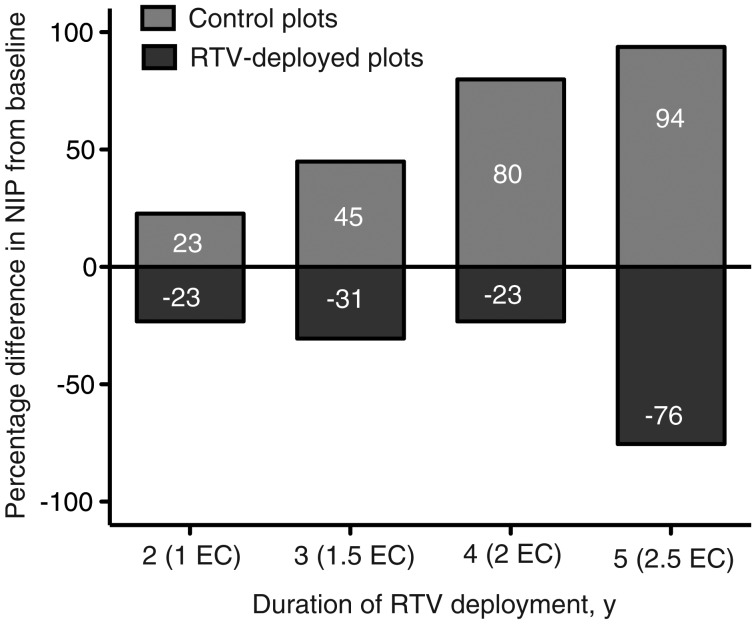
We averaged the differences in nymphal infection prevalence from baseline in all field plots after 2, 3, 4, and 5 years of RTV deployment (Figure 5). In the plots where the vaccine was deployed for 2 consecutive years (1 enzootic cycle), the mean prevalence of B. burgdorferi among nymphal ticks declined by 23% (average between plots NY1, NY2, NY3, and NY4), whereas it increased by 23% at the same time in the control plots. In the plots that received the RTV for 3 consecutive years (1.5 enzootic cycles), the prevalence of B. burgdorferi in nymphal ticks declined by 31% (average between plots NY1, NY2, NY3, and NY4), whereas it increased by 45% at the same time in the control plots. In the plots that received RTV for 4 consecutive years (2 enzootic cycles), the prevalence of B. burgdorferi among nymphal ticks declined by 23% (average between plots NY1 and NY2), whereas it increased by 80% at the same time in the control plots. In the plot that received RTV for 5 consecutive years (2.5 enzootic cycles; NY1), the prevalence of B. burgdorferi in nymphal ticks declined by 76%, whereas it increased by 94% at the same time in the control plots.
Figure 5.
Percentage difference from baseline in the nymphal infection prevalence (NIP) after long-term deployment of reservoir-targeted vaccine (RTV), by duration of deployment. For 2-year deployment, NIP was calculated as the difference between the average proportions for years 1 and 2 (for plot NY1, 2008 and 2007; for plot NY2, 2009 and 2008; for plot NY3, 2010 and 2009; and for plot NY4, 2010 and 2009); for 3-year deployment, the difference between the average proportions for years 1 and 3 was used (for plot NY1, 2009 and 2007; for plot NY2, 2010 and 2008; for plot NY3, 2011 and 2009; and for plot NY4, 2011 and 2009); for 4-year deployment, the difference between the average proportions for years 1 and 4 was used (for plot NY1, 2010 and 2007; and for plot NY2, 2011 and 2008); and for 5-year deployment, the difference between the average proportions for years 1 and 5 was used (for plot NY1, 2011 and 2007). P = 4.7×10−5, by generalized linear mixed modeling. Abbreviation: EC, enzootic cycle.
Despite our efforts to match control and experimental sites according to habitat features, such as tree and understory community composition, the control and experimental sites were not well matched with respect to initial nymphal infection prevalence levels. Thus, the natural variation in nymphal infection prevalence seen in the control fields could have influenced our comparison of treated and control plots. To overcome this natural variation, we analyzed the effect of vaccine treatment on the nymphal infection prevalence over time, using a GLMM including treatment (vaccine vs control), duration of treatment, and calendar year as fixed explanatory factors and plot as a random explanatory factor. This analysis accounts for natural interannual and among-plot variation to assess cumulative effects of the treatment over multiple enzootic cycles. The interaction between treatment and duration of treatment—the combination of factors that accounts for the rate at which nymphal infection prevalence changes over time—was statistically significant (P = 4.4×10−5). Although all plots treated with RTV had a decreasing nymphal infection prevalence over time, this effect was not significant after the first or second year the RTV was deployed (P > .05). The RTV effect was significant only after 3 years of application (P = 4.7×10−5; Table 2).
Table 2.
Factors in mixed-model ANOVA analyses correlated with OspA antibody and tick infection prevalence.
| Model | Estimate | Error | z | Pr(>|z|) |
|---|---|---|---|---|
| OspA antibody: fixed effects | ||||
| Intercept | −1.1092 | 0.5798 | −1.9130 | 0.0557 |
| TRTMT | 1.5056 | 0.5848 | 2.5750 | 0.0100 |
| TIMETREATED | 0.0252 | 0.2148 | 0.1170 | 0.9066 |
| Year2008 | −0.9428 | 0.3072 | −3.0926 | 0.0018 |
| Year2009 | −1.0102 | 0.3102 | −3.2570 | 0.0011 |
| Year2010 | −1.2789 | 0.3543 | −3.6090 | 0.0003 |
| Year2011 | −1.0635 | 0.4156 | −2.5590 | 0.0105 |
| TRTMT: TIMETREATED | −0.2238 | 0.2178 | −1.0280 | 0.3042 |
| Tick infection prevalence: fixed effects | ||||
| Intercept | −1.5200 | 0.4104 | −3.7040 | 0.0002 |
| TRTMTVACC | 1.4446 | 0.5601 | 2.5790 | 0.0099 |
| TIMETREATED1 | 0.4442 | 0.6293 | 0.7060 | 0.4802 |
| TIMETREATED2 | 1.1655 | 0.6635 | 1.7570 | 0.0790 |
| TIMETREATED3 | 1.3891 | 1.0200 | 1.3810 | 0.0287 |
| TIMETREATED4 | 1.7841 | 0.7416 | 2.4060 | 0.0161 |
| Year2008 | −0.1739 | 0.4612 | −0.3770 | 0.7061 |
| Year2009 | −0.0055 | 0.4737 | −0.0120 | 0.9908 |
| Year2010 | −0.5851 | 0.5385 | −1.0870 | 0.2772 |
| Year2011 | −1.0263 | 0.6054 | −1.6950 | 0.0901 |
| TRTMTVACC: TIMETREATED1 | −0.7341 | 0.5909 | −1.2420 | 0.2141 |
| TRTMTVACC: TIMETREATED2 | −1.1512 | 0.6067 | −1.8970 | 0.0578 |
| TRTMTVACC: TIMETREATED3 | −2.0173 | 0.9737 | −2.2230 | 0.0182 |
| TRTMTVACC: TIMETREATED4 | −2.7591 | 0.6783 | −4.0680 | 4.74×10−5 |
Abbreviation: OspA, outer surface protein A.
DISCUSSION
We demonstrate that an OspA-based oral bait vaccine delivered to wild white-footed mice over time resulted in an increase in anti-OspA seroprevalence. This increase in antibodies was associated with reduced rates of B. burgdorferi infection among nymphal ticks as early as 2 years after RTV deployment (23%). Moreover, 5 years of consecutive RTV use appeared to cause a substantial disruption in the enzootic cycle of B. burgdorferi, with a reduction of 76% in the nymphal infection prevalence. Such decreases in the prevalence of infected I. scapularis vectors could reduce the risk to humans and other accidental mammalian hosts (dogs) of acquiring Lyme disease.
Our bacteria-based RTV was previously tested in the laboratory in the white-footed mouse, the most competent reservoir host for B. burgdorferi in much of its North American range [18, 19]. The minimum effective vaccine dose in laboratory studies was 5 units of oral RTV, and natural temperature or humidity conditions to which a baited wildlife RTV would be subjected did not adversely affect vaccine efficacy [14]. We also devised a series of immunization schedules that resulted in development of protective levels of anti-OspA antibodies in mice for an entire year [14]. In the field study reported here, we reproduced our best immunization schedule by delivering an average of approximately 35 RTVs per mouse and observed that >50% of field mice consumed >5 RTV units, the minimum number of units needed to induce protective immunity [14]. These observations were also consistent with a separate study of different formulations of vaccine-free rhodamine-laced bait, which showed that more than half of resident mice (approximately 55%) ingested bait deployed in nest boxes under field conditions [20].
OspA seroprevalence is one of our metrics of RTV effect. Analysis of anti-OspA specific antibodies among vaccinated and unvaccinated populations revealed that a low percentage of resident mice have low levels of antibodies to OspA. Given that OspA expression is downregulated as B. burgdorferi infects the vertebrate host [21, 22], the presence of anti-OspA antibodies in mice primarily represents vaccine immunity. It has been reported that OspA expression is upregulated during prolonged infections [23], which could explain the low levels of anti-OspA antibodies observed in unvaccinated mice.
Deployment of the RTV resulted in a higher prevalence of protective levels of antibodies to OspA in the NY1 plot (28%) and the NY2 plot (33%) from 2008 to 2011, which correlated with significantly lower infection rates of nymphal ticks in these plots. The significant differences in levels of protective antibodies from 2009 to 2011 in the NY4 plot (approximately 20%) correlated with a declining trend in nymphal infection prevalence. In NY3, neither the prevalence of protective antibodies (10%) nor the nymphal infection prevalence trends were significantly different from values for the control. Thus, we observed a very strong association between the prevalence of mice with protective anti-OspA antibody levels and the reduction of infection in nymphal I. scapularis ticks in plots treated with the RTV. The lack of a definitive RTV effect in the NY3 and NY4 plots can be explained by the shorter time these 2 plots were treated with RTV (3 years), in contrast to 4 years in the NY2 plot and 5 years in the NY1 plot.
There is no evidence from similar live-trapping studies that the small amount of bait provided in traps has any influence on rodent population densities [24, 25]. In our study, all sites had similar small-mammal communities, dominated primarily by white-footed mice and secondarily by eastern chipmunks (Tamias striatus) and short-tailed shrews (Blarina brevicauda). Plot NY2 had the highest proportion of nonmouse captures (mean, 0.16) averaged across the years of the study. The 3 other sites averaged <0.08 nonmouse captures throughout the study. Thus, it does not appear that higher abundances of nontarget species [4, 18] are responsible for differences between treated plots.
The risk of infection with B. burgdorferi among humans is higher in areas abundant in infected ixodid ticks [26, 27]. Tick-to-host transmission rates depend on infection prevalence in nymphal ticks, and host-to-tick transmission rates depend on host infection prevalence [28]. Although eliminating B. burgdorferi from its natural enzootic cycle seems unrealistic, diminishing its threat to humans via reduction of the tick infection prevalence is an achievable goal with remarkable public health ramifications [4, 29]. A field study gauging the effect of an RTV in Lyme disease risk was published previously [4]. There are 2 crucial differences between the former study and the study reported here: vaccine formulation and duration of vaccine treatment. Unlike the former study, we used an oral vaccine delivered to trapped mice imbibed in bait for their ad libitum consumption. Because mice do not need to be trapped and injected individually, the vaccination technology is potentially feasible for field use. The second fundamental difference between these studies was the duration of treatment. In contrast to a single summer deployment of RTV, we planned a long-term (5-year) deployment of the oral vaccine, because disruption of the 2-year enzootic cycle of B. burgdorferi could only be achieved if we treated the field sites for a minimum of 3 years. As previously reported [4], we observed modest reductions in the prevalence of nymphal ticks infected with B. burgdorferi the year following the first vaccine deployment (approximately 23%). Furthermore, we observed that efficacy of the RTV in reducing infection of nymphal ticks was significant only after 3 years of treatment (P = 4.7 × 10−5), indicating that vaccine effect is time dependent and cumulative across time. This was supported by the fact that 4 years of treatment in plot NY1 produced similar decreases in nymphal infection prevalence as 4 years of treatment in plot NY2 but another year of RTV treatment in the NY1 plot led to a substantial decrease in nymphal infection prevalence (approximately 76%) in this field plot. These data indicate that cumulative decreases in nymphal infection prevalence are likely to be maximized by continued long-term deployment of RTV. These data are consistent with a projection modeling study suggesting that reductions in current nymphal infection prevalence due to vaccinations reinforce lower future nymphal infection prevalence by increasing the proportion of mice that can be vaccinated before exposure to a B. burgdorferi–infected tick, thus establishing a self-reinforcing feedback loop for Lyme disease protection [30].
The risk of contracting Lyme disease is a function of the probability of being bitten by an infected tick [31]. Thus, if all other factors remain constant, a reduction in nymphal infection prevalence is expected to lead to a directly proportional decrease in the risk of contracting Lyme disease. Greater decreases in the risk of exposure to Lyme disease can be expected following sustained deployment of RTV to all potential reservoir species over time [32].
Lyme disease in the United States is reported to the Centers for Disease Control and Prevention (CDC) at the rate of 30 000 cases per year. Recently, the CDC revised the number of probable infections 10-fold (to 300 000 new cases/per year) to account for widespread underreporting [33]. Our results suggest that prevention of Lyme disease can be shifted from the current standard of direct vaccination of humans to an indirect strategy of containment of transmission, as the pathogen moves through its natural enzootic cycle, before it spins out into a zoonotic human disease. Strategic implementation of the intervention reported in this study would ultimately protect human populations from contracting B. burgdorferi in geographic regions where the Lyme disease risk is high.
Notes
Acknowledgments. We thank Godefroy Devevey, Marshall (Buck) West, Parris Humphrey, and Chris Neil, for their dedication to this project over time and logistics coordination in the field; Carolyn Livensperger, Katie Renwick, Elizabeth Lund, Laura Bendernagle, Stephanie Locke, Amy Viscito, James Burtis, Dan Becker, Mia Strauss, Sarah Szymanski, Kimberly Harle, Ian Hannigan, Rob Cimitile, Trang Nguyen, James Duguay, Erica Arnold, Kim Lewis, Alexandra Title, Fred Elslager, and John Darcy, who deployed the vaccine and collected ticks in our fields over the 5 years of the study; Vera Neves, Miguel Aroso, Beatriz del Rio, Larisa Ivanova, Charity Brannen, and Ana Vieira, for technical support in the laboratory; our 10 high school students, who helped split the RTV doses; Drs Ben Beard and Joe Piesman at the National Center for Emerging and Zoonotic Infectious Diseases, CDC, for development of a cooperative agreement that provided substantial financial support for these studies; and Dr Thomas Monath, for his insightful review of the manuscript.
Financial support. This work was supported by the Center for Disease Control and Prevention (grant UO1 CK000107 to M. G. S.); the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grants R43 AI072810 and R44 AI058364, to M. G. S., and RO1 AI076342, to D. B); and the National Science Foundation (grant DEB 0949702 to R. S. O.).
Potential conflicts of interest. M. G. S. has relevant patents (assignees, Biopeptides and Stony Brook University) that might pose a conflict of interest. M. G. S. is chair of the scientific advisory board of and a shareholder in US Biologic. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Kurtenbach K, Hanincova K, Tsao JI, Margos G, Fish D, Ogden NH. Fundamental processes in the evolutionary ecology of Lyme borreliosis. Nat Rev Microbiol. 2006;4:660–9. doi: 10.1038/nrmicro1475. [DOI] [PubMed] [Google Scholar]
- 2.Dolan MC, Maupin GO, Schneider BS, et al. Control of immature Ixodes scapularis (Acari: Ixodidae) on rodent reservoirs of Borrelia burgdorferi in a residential community of southeastern Connecticut. J Med Entomol. 2004;41:1043–54. doi: 10.1603/0022-2585-41.6.1043. [DOI] [PubMed] [Google Scholar]
- 3.Scheckelhoff MR, Telford SR, Hu LT. Protective efficacy of an oral vaccine to reduce carriage of Borrelia burgdorferi (strain N40) in mouse and tick reservoirs. Vaccine. 2006;24:1949–57. doi: 10.1016/j.vaccine.2005.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsao JI, Wootton JT, Bunikis J, Luna MG, Fish D, Barbour AG. An ecological approach to preventing human infection: vaccinating wild mouse reservoirs intervenes in the Lyme disease cycle. Proc Natl Acad Sci U S A. 2004;101:18159–64. doi: 10.1073/pnas.0405763102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gomes-Solecki MJ, Brisson DR, Dattwyler RJ. Oral vaccine that breaks the transmission cycle of the Lyme disease spirochete can be delivered via bait. Vaccine. 2006;24:4440–9. doi: 10.1016/j.vaccine.2005.08.089. [DOI] [PubMed] [Google Scholar]
- 6.Hoen AG, Rollend LG, Papero MA, et al. Effects of tick control by acaricide self-treatment of white-tailed deer on host-seeking tick infection prevalence and entomologic risk for Ixodes scapularis-borne pathogens. Vector Borne Zoonotic Dis. 2009;9:431–8. doi: 10.1089/vbz.2008.0155. [DOI] [PubMed] [Google Scholar]
- 7.Patrican LA. Absence of Lyme disease spirochetes in larval progeny of naturally infected Ixodes scapularis (Acari:Ixodidae) fed on dogs. J Med Entomol. 1997;34:52–5. doi: 10.1093/jmedent/34.1.52. [DOI] [PubMed] [Google Scholar]
- 8.Steere AC, Sikand VK, Meurice F, et al. Vaccination against Lyme disease with recombinant Borrelia burgdorferi outer-surface lipoprotein A with adjuvant. Lyme Disease Vaccine Study Group. N Engl J Med. 1998;339:209–15. doi: 10.1056/NEJM199807233390401. [DOI] [PubMed] [Google Scholar]
- 9.Chang YF, Appel MJ, Jacobson RH, et al. Recombinant OspA protects dogs against infection and disease caused by Borrelia burgdorferi. Infect Immun. 1995;63:3543–9. doi: 10.1128/iai.63.9.3543-3549.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fikrig E, Barthold SW, Kantor FS, Flavell RA. Long-term protection of mice from Lyme disease by vaccination with OspA. Infect Immun. 1992;60:773–7. doi: 10.1128/iai.60.3.773-777.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsao J, Barbour AG, Luke CJ, Fikrig E, Fish D. OspA immunization decreases transmission of Borrelia burgdorferi spirochetes from infected Peromyscus leucopus mice to larval Ixodes scapularis ticks. Vector Borne Zoonotic Dis. 2001;1:65–74. doi: 10.1089/153036601750137705. [DOI] [PubMed] [Google Scholar]
- 12.Fikrig E, Barthold SW, Kantor FS, Flavell RA. Protection of mice from Lyme borreliosis by oral vaccination with Escherichia coli expressing OspA. J Infect Dis. 1991;164:1224–7. doi: 10.1093/infdis/164.6.1224. [DOI] [PubMed] [Google Scholar]
- 13.Ostfeld RS, Canham CD, Oggenfuss K, Winchcombe RJ, Keesing F. Climate, deer, rodents, and acorns as determinants of variation in lyme-disease risk. PLoS Biol. 2006;4:e145. doi: 10.1371/journal.pbio.0040145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meirelles Richer L, Aroso M, Contente-Cuomo T, Ivanova L, Gomes-Solecki M. Reservoir targeted vaccine for lyme borreliosis induces a yearlong, neutralizing antibody response to OspA in white-footed mice. Clin Vaccine Immunol. 2011;18:1809–16. doi: 10.1128/CVI.05226-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crowder CD, Matthews HE, Schutzer S, et al. Genotypic variation and mixtures of Lyme Borrelia in Ixodes ticks from North America and Europe. PLoS One. 2010;5:e10650. doi: 10.1371/journal.pone.0010650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodzic E, Feng S, Freet KJ, Borjesson DL, Barthold SW. Borrelia burgdorferi population kinetics and selected gene expression at the host-vector interface. Infect Immun. 2002;70:3382–8. doi: 10.1128/IAI.70.7.3382-3388.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.R Development CoreTeam. R: A language and environment for statistical computing. Vienna, Austria: Foundation for Statistical Computing; 2012. http://www.R-project.org . Accessed 10 September 2013. [Google Scholar]
- 18.LoGiudice K, Ostfeld RS, Schmidt KA, Keesing F. The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk. Proc Natl Acad Sci U S A. 2003;100:567–71. doi: 10.1073/pnas.0233733100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bunikis J, Tsao J, Luke CJ, Luna MG, Fish D, Barbour AG. Borrelia burgdorferi infection in a natural population of Peromyscus Leucopus mice: a longitudinal study in an area where Lyme Borreliosis is highly endemic. J Infect Dis. 2004;189:1515–23. doi: 10.1086/382594. [DOI] [PubMed] [Google Scholar]
- 20.Telford SR, III, Cunningham JA, Waltari E, Hu L. Nest box-deployed bait for delivering oral vaccines to white-footed mice. Ticks Tick Borne Dis. 2011;2:151–5. doi: 10.1016/j.ttbdis.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwan TG, Piesman J, Golde WT, Dolan MC, Rosa PA. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci U S A. 1995;92:2909–13. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Silva AM, Telford SR, III, Brunet LR, Barthold SW, Fikrig E. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J Exp Med. 1996;183:271–5. doi: 10.1084/jem.183.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalish RA, Leong JM, Steere AC. Association of treatment-resistant chronic Lyme arthritis with HLA-DR4 and antibody reactivity to OspA and OspB of Borrelia burgdorferi. Infect Immun. 1993;61:2774–9. doi: 10.1128/iai.61.7.2774-2779.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boutin S. Food supplementation experiments with terrestrial vertebrates patterns, problems, and the future. Can J Zool. 1990;68:203–20. [Google Scholar]
- 25.Gilbert BS, Krebs CJ. Effects of extra food on Peromyscus and Clethrionomys populations in the southern Yukon. Oecologia. 1981;51:326–31. doi: 10.1007/BF00540901. [DOI] [PubMed] [Google Scholar]
- 26.Diuk-Wasser MA, Hoen AG, Cislo P, et al. Human risk of infection with Borrelia burgdorferi, the Lyme disease agent, in eastern United States. Am J Trop Med Hyg. 2012;86:320–7. doi: 10.4269/ajtmh.2012.11-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stafford KC, III, Cartter ML, Magnarelli LA, Ertel SH, Mshar PA. Temporal correlations between tick abundance and prevalence of ticks infected with Borrelia burgdorferi and increasing incidence of Lyme disease. J Clin Microbiol. 1998;36:1240–4. doi: 10.1128/jcm.36.5.1240-1244.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ostfeld RS. Lyme disease: the ecology of a complex system. 1st ed. New York: Oxford University Press; 2011. p. 216. [Google Scholar]
- 29.Clark RP, Hu LT. Prevention of Lyme disease and other tick-borne infections. Infect Dis Clin North Am. 2008;22:381–96. doi: 10.1016/j.idc.2008.03.007. vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Voordouw MJ, Tupper H, Onder O, et al. Reductions in human Lyme disease risk due to the effects of oral vaccination on tick-to-mouse and mouse-to-tick transmission. Vector Borne Zoonotic Dis. 2013;13:203–14. doi: 10.1089/vbz.2012.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ginsberg HS. Transmission risk of Lyme disease and implications for tick management. Am J Epidemiol. 1993;138:65–73. doi: 10.1093/oxfordjournals.aje.a116778. [DOI] [PubMed] [Google Scholar]
- 32.Brisson D, Dykhuizen DE, Ostfeld RS. Conspicuous impacts of inconspicuous hosts on the Lyme disease epidemic. Proc Biol Sci. 2008;275:227–35. doi: 10.1098/rspb.2007.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Young JD. Underreporting of Lyme disease. N Engl J Med. 1998;338:1629. doi: 10.1056/NEJM199805283382216. [DOI] [PubMed] [Google Scholar]