Abstract
Chikungunya virus (CHIKV) is a mosquito-borne alphavirus that causes major epidemics of rash, fever, and debilitating arthritis. Currently, there are no vaccines or antivirals available for prevention or treatment. We therefore generated 2 live-attenuated vaccine candidates based on the insertion of a picornavirus internal ribosome entry site (IRES) sequence into the genome of CHIKV. Vaccination of cynomolgus macaques with a single dose of either vaccine produced no signs of disease but was highly immunogenic. After challenge with a subcutaneous inoculation of wild-type CHIKV, both vaccine candidates prevented the development of detectable viremia. Protected animals also exhibited no significant changes in core body temperature or cardiovascular rhythm, whereas sham-vaccinated animals showed hyperthermia, followed by sustained hypothermia, as well as significant changes in heart rate. These CHIKV/IRES vaccine candidates appear to be safe and efficacious, supporting their strong potential as human vaccines to protect against CHIKV infection and reduce transmission and further spread.
Keywords: Chikungunya virus, nonhuman primates, viral vaccine
Chikungunya virus (CHIKV), an alphavirus in the family Togaviridae, is one of the most important arthropod-borne viruses (arboviruses) that infect humans [1–3]. The virus periodically emerges from enzootic, sylvatic African transmission cycles to initiate urban transmission and epidemics. This emergence involves switches from nonhuman primate enzootic hosts to humans as amplification hosts, and from arboreal mosquito vectors to the urban, anthropophilic species Aedes aegypti and A. albopictus, which transmit among humans. Once the urban cycle is initiated, it can spread dramatically via human travel, leading to major outbreaks. Historically, such urban emergence has involved movement into African cities, followed by spread to Asia and even Europe [1]. CHIKV transmission has not yet been documented in the Americas. However, the frequent travel to the western hemisphere of infected persons during recent Asian epidemics [4] highlights the risk that CHIKV could become endemic in the New World in many locations where the human population is completely naive and the epidemic mosquito vectors are abundant.
Infection by CHIKV typically leads to chikungunya fever (CHIK), a self-limiting disease characterized by fever, severe arthralgia, and often a rash [5]. The arthralgia can be highly debilitating and chronic; for example, over 72% of Indian patients suffered from arthralgia one month after onset [6], and estimates of the mean duration of arthritic pain as high 89 days have been reported [7]. In La Reunion, joint pain persisted in many patients at least 18 months after infection [8]. This severe and chronic arthralgia can also result in major economic losses from disability, as indicated by the approximately 2/3 of all DALYs attributed to CHIKV infection during an Indian outbreak [9].
Vaccines often provide the optimal means to prevent and control infectious diseases, including arboviral diseases such as yellow fever [10]. Since the 1970s, many attempts have been made to develop a vaccine to protect against CHIK. Early vaccine candidates included inactivated [11], and live-attenuated CHIKV [12, 13], and more recently, chimeric live-attenuated [14, 15], subunit [16], DNA [17, 18], and virus-like particle [19] approaches have been described. Of these, only the live-attenuated strain 181/clone25 was tested in clinical trials, where it proved highly immunogenic but mildly reactogenic [12]. This reactogenicity probably reflected instable attenuation resulting from the presence of only 2 attenuating point mutations in the 181/clone25 strain [20].
We recently developed a novel, live-attenuated vaccine to protect against chikungunya fever that takes advantage of a picornavirus internal ribosome entry site (IRES) to reduce expression of the CHIKV structural protein genes. This approach also eliminates the potential for transmission by virtue of the relative inability of this IRES to initiate translation in insect cells, rendering the vaccine strain unable to infect mosquitoes. This vaccine candidate, CHIKV/IRES, is highly attenuated in murine models, induces a strong neutralizing antibody response, and protects mice after a single dose from fatal CHIKV challenge [21]. Its inability to infect mosquito vectors is also an important safety feature for a live-attenuated arbovirus, especially for use in nonendemic locations. The IRES-based attenuation strategy has also proved useful in the development of vaccines against other alphaviral diseases including eastern [22] and Venezuelan equine encephalitis [23, 24] and is more stable than traditional, point mutation-based attenuation [25].
Here, we describe the development of a new CHIK vaccine based on a modification of the original IRES-based attenuation approach, and further evaluations of this new (CHIKV/IRESv2) as well as the original CHIKV/IRESv1 vaccine for preclinical safety, immunogenicity, and efficacy. We tested the vaccines in cynomolgus macaques, which represent an excellent nonhuman primate (NHP) model for human CHIK [26], and telemetry was employed to sensitively measure adverse events following vaccination as well as disease outcome following challenge.
METHODS
Vaccine Design and Generation
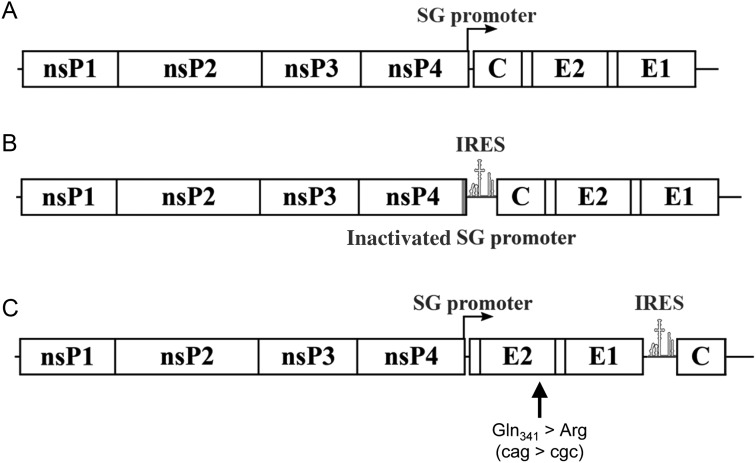
Two versions of the CHIKV/IRES vaccine strain were tested in separate cohorts (Figure 1); the first version (CHIKV/IRESV1) has been described elsewhere [21]. A second version (CHIKV/IRESV2) was constructed in a similar fashion using an infectious clone of CHIKV strain La Reunion (CHIKV-LR) [27]. The capsid gene was removed, and a start codon was introduced upstream of the E3 envelope glycoprotein gene via polymerase chain reaction (PCR) mutagenesis. The IRES/capsid fused DNA was then amplified from the original CHIKV/IRES infectious clone and, using fusion PCR, introduced into the untranslated genome region (3′UTR) of the virus (Figure 1). The rescued virus was passaged 10 times using a multiplicity of infection 0.1 plaque-forming units (PFU)/cell on Vero cells and then sequenced. An adaptive mutation encoding a substitution of amino acid 341 of the E2 envelope glycoprotein was identified and introduced into the original clone (Figure 1). The amino acid substitution associated with this mutation was further stabilized by introducing a second nucleotide mutation in the same codon.
Figure 1.
Schematic representation of (A) wild-type CHIKV-La Reunion strain (CHIKV-LR), (B) CHIKV/IRESv1, and (C) CHIKV/IRESv2. A, Diagram showing the locations of the 2 open reading frames encoding the nonstructural proteins (nsP1–4) and the subgenomic message encoding the capsid (C) as well as the envelope glycoproteins E2 and E1. B, Inactivation of the subgenomic promoter and insertion of the sequence of the encephalomyocarditis virus (ECMV) internal ribosome entry site (IRES) to drive expression of the structural proteins from the genomic RNA. C, The subgenomic promoter was unaltered, but was directly followed by an inserted start codon and the E3 gene. The capsid gene was transferred to the 3′ end of the E1 gene and fused directly behind the EMCV IRES sequence. A Vero cell culture adaptive mutation was also introduced in the E2 gene. Abbreviation: CHIKV, chikungunya virus.
Each vaccine strain as well as the wild-type (WT) CHIKV strain La Reunion (CHIKV-LR) was produced by electroporation of Vero cells (African green monkey kidney cells, American Type Culture Collection, Bethesda, MD) with RNA transcribed from linearized plasmid DNA. All virus titers were determined by plaque assay on Vero cell monolayers [28].
RNA Replication
Vero cells were infected on 35 mm2 6-well plates at a multiplicity of 20 PFU/cell. The medium was removed 18 hours after infection and replaced with 0.8 mL of complete medium with 1 µg/mL of actinomycin D (Sigma, St. Louis, MO), and 30 µCI of [5,6–3H] uridine from Moravak Biochemicals (Brea, CA). The cells were then incubated for 8 hours, and RNA was extracted with TRIzol (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. The RNA was incubated in a sodium phosphate buffer containing dimethyl sulfoxide (DMSO) and glyoxal at 50°C for 1 hour and then was electrophoresed on a 1% agarose gel at 150 v for 3–4 hours. The gel was washed twice in methanol for 30 minutes, placed into a 2.5% diphenyloxazole and methanol solution overnight. The gel was washed with deionized water to precipitate the PPO and the dried and exposed with X-OMAT AR film (Kodak), at −80°C for 16 hours.
Antibody Assays
Neutralizing antibodies were assayed using 50% and 80% plaque reduction neutralization tests (PRNTs), and enzyme-linked immunosorbent assays (ELISA) were used to measure immunoglobulin G (IgG) and immunoglobulin M (IgM) responses [28].
Animals
Cynomolgus macaques (Macaca fascicularis) of similar age (>3 yr) and weighing 3–6 kg, free of simian immunodeficiency virus (SIV), simian type D retrovirus (SRV), simian T-lymphotropic virus (STLV), and alphavirus antibodies against western (WEEV), Venezuelan (VEEV), eastern equine encephalitis (EEEV), Sindbis (SINV), Semliki Forest (SFV), and CHIKV (assayed by hemagglutination inhibition), were used. The study was approved by the Institutional Animal Care and Use Committee at Tulane University, and all animals were handled in accordance with guidance from the American Association for Accreditation of Laboratory Animal Care.
Vaccinations and Challenges
CHIKV/IRESv1 and CHIKV/IRESv2 were randomly assigned to macaque cohorts; one (n = 4) was vaccinated subcutaneously with CHIKV/IRESv1, and a second (n = 4) was vaccinated intradermally with the same vaccine candidate. A third cohort (n = 4) was vaccinated subcutaneously with CHIKV/IRESv2, and a fourth (n = 7) was sham-vaccinated subcutaneously with saline. Anesthetized animals were vaccinated either subcutaneously or intradermally in the upper deltoid with a single inoculation of either saline or 5.0 log10 PFU of vaccine in a volume of 100 μL. The animals were observed for signs of any clinically recognizable adverse responses and bled on days 1–3, 15, and 50 after vaccination.
On day 52 after vaccination, anesthetized macaques were challenged with a single subcutaneous inoculation in the upper deltoid of WT CHIKV-LR (5.0 log10 PFU in a volume of 100 μL). Blood was collected on days 1–3, 6, 9, 13, and 35 after challenge, when the experiment was terminated and necropsies were performed. Tissues were placed in 10% zinc-formalin for histopathological analysis. Some tissues were also frozen for viral titration by plaque assay: axillary, bronchial, and inguinal lymph nodes.
Telemetry
Subcutaneous radio telemetry transmitters combined with sensors capable of detecting biopotential signals of an electrocardiogram (ECG) as well as thermistor type sensors capable of detecting temperature signals (T34G-8; Konigsberg Instruments [KI], Inc., Pasadena, CA) were surgically implanted, under aseptic conditions in 15 of the study animals at least 2 weeks prior to vaccination. Following surgical implantation, animals were housed in rooms and cages specifically designed with cage-mounted antennas (TR38–1FG; Konigsberg Instruments, Inc) configured to receive and transmit signals to a KI data acquisition base station. Data collection was continuously recorded in 48–72 hour intervals for 16 days using the CA Recorder (Data Integrated Scientific Systems, Dexter, MI). Data parameters and analysis results recorded over the course of the study included: (1) ECG Online (EOL) in which ECG signal amplitudes and various intervals are measured and recorded for heart rate, and (2) mean analysis for body temperature measurements.
Telemetric parameters, including heart rate and core body temperature, were reported in hourly means for 1-hour observation intervals, with each animal serving as its own control. Baseline control data averages were generated from each subject for a minimum of 6 days prior to vaccination. Control data were aligned by the time of day over a 24-hour period and averaged for hourly means to establish detection thresholds for each animal. These thresholds of detection of significant events were defined to be >1.5 times the maximum standard deviation of the control averages. Individual postexposure data were then aligned by time and compared against control values for each animal. An assessment of fever (hyperthermia) hours, hypothermia hours, and fever intensity was then performed for each animal.
Statistics
Statistical analyses were performed on postexposure data defined as significant by the established detection thresholds described above for fever intensity, fever (hyperthermia) hours, and hypothermia hours. Fever intensity, indicated as the maximum change in temperature, fever hours, hypothermia hours, and antibody titers among treatment groups were compared using the Kruskal-Wallis test. When the results of Kruskal-Wallis test indicated a significant difference at the P < .05 level among the groups examined, Tukey multiple comparison test was then used to identify groups that differed at the P < .05 significance level.
RESULTS
Genetic Modifications
Analysis of viral RNA produced in Vero cells infected with WT CHIKV-LR, from which our vaccines were derived, as well as CHIKV/IRESv1 and CHIKV/IRESv2, revealed the expected results (Figure 2). Compared to WT CHIKV, CHIKV/IRESv1 produced no detectable subgenomic RNA, whereas CHIKV/IRESv2 produced a slightly larger subgenomic RNA reflecting the addition of the IRES between the envelope glycoprotein and capsid open reading frames. The CHIKV/IRESv2 subgenomic RNA appeared to be produced in slightly larger amounts relative to genomic RNA, which was not surprising considering the varied genomic:subgenomic ratios found among alphaviruses [29].
Figure 2.
Viral RNA present in Vero cells 18 hours after infection with wild-type CHIKV strain La Reunion (CHIKV-LR), CHIKV/IRESv1, and CHIKV/IRESv2. For CHIKV/IRESv1, the subgenomic promoter was inactivated using 13 synonymous mutations to preserve the wild-type amino acid sequence of nsP4. The resultant virus, rescued by electroporation of in vitro-transcribed RNA into Vero cells, contained a nonfunctional subgenomic promoter as indicated by the absence of subgenomic RNA within infected cells. For CHIKV/IRESv2, the slight differences in genomic and subgenomic RNA sizes when compared to the CHIKV-LR strain reflected the addition to the subgenomic RNA of the IRES in CHIKV/IRESv2. Abbreviations: CHIKV, chikungunya virus; IRES, internal ribosome entry site.
Attenuation
The attenuation of the CHIKV/IRES vaccine candidates was evaluated by assessing viremia and telemetric measures of disease after vaccination. Viremia was only detected in 1 vaccinated macaque (IT85), with a titer of only 10 PFU/mL on only 1 time point (day 2 after immunization). Although the amount of virus in this sample was insufficient for genomic amplification and sequencing without further passage, to assess vaccine stability, mouse-to-mouse passages of CHIKV/IRESv1 have demonstrated much greater phenotypic and sequence stability than the 181/25 vaccine strain (K. Plante, unpublished). Results of the telemetric monitoring showed no discernible clinical change or adverse physiological response during this time for any vaccinated animals. Overall, these results corroborate previous attenuation data for CHIKV/IRESv1 generated using mouse models [21].
Immunogenicity
Independent of vaccine strain or route of administration, all vaccinated macaques developed neutralizing antibodies to CHIKV, which were first detected on day 15 postvaccination (Table 1). Animals IV13 and IV15, which received CHIKV/IRESv1 vaccine subcutaneously, did not develop a detectable PRNT80 response on day 15; however, PRNT50 titers of 1:80 and 1:320, respectively, were detected. In all cases, PRNT titers increased by day 50. Among the 3 vaccination cohorts, the only significant difference among mean neutralizing antibody titers was on day 15 after vaccination, when CHIKV/IRESv2 was significantly more immunogenic than CHIKV/IRESv2 via the subcutaneous route. These data are consistent with the greater immunogenicity expected from CHIKV/IRESv2 based on its generation of a subgenomic RNA to express great amounts of the envelope proteins (Figure 1).
Table 1.
Immunogenicity of CHIKV/IRES Vaccine Candidates in Cynomolgus Macaques
| Vaccine (Route of Vaccination) | Animal no. | PRNT80 Titer |
PRNT50 Titer |
||
|---|---|---|---|---|---|
| Day 15 Post Vaccination | Day 50 Post Vaccination | Day 15 Post Vaccination | Day 50 Post Vaccination | ||
| CHIKV/IRESv1 (subcutaneous) | IV04 | 20 | 80 | 40 | 160 |
| IV13 | <20 | 80 | 20 | 160 | |
| IV15 | <20 | 320 | 40 | 640 | |
| IT85 | 80 | 640 | 160 | 1280 | |
| Mean ± SD | 30 ± 34 | 280 ± 230 | 65 ± 64 | 560 ± 531 | |
| CHIKV/IRESv1 (intradermal) | IV17 | 80 | 160 | 160 | 320 |
| IV18 | 40 | 160 | 80 | 320 | |
| IT82 | 40 | 80 | 80 | 160 | |
| IT96 | 40 | 80 | 80 | 160 | |
| Mean ± SD | 50 ± 20 | 120 ± 46 | 100 ± 40 | 240 ± 93 | |
| CHIKV/IRESv2 (subcutaneous) | IV02 | 80 | 160 | 160 | 640 |
| IV05 | 40 | 80 | 160 | 320 | |
| IV27 | 80 | 640 | 160 | 1280 | |
| IT92 | 80 | 320 | 320 | 640 | |
| Mean ± SD | 70 ± 20 | 300 ± 248 | 200 ± 80 | 720 ± 403 | |
Abbreviations: CHIKV, chikungunya virus; IRES, internal ribosome entry site; PRNT, plaque reduction neutralization test; SD, standard deviation.
Both IgM and IgG titers were measured following vaccination and challenge (Figure 3). For all vaccine groups, IgM was detected on day 15 postvaccination and decreased slightly by day 50, prior to challenge (Figure 3A). Following challenge, there was a slight increase in IgM titers, peaking between days 6 and 9 postchallenge, before decreasing by day 13. CHIKV challenge induced a very strong IgM response in sham-vaccinated macaques, nearly a 2-fold increase when compared to the vaccinated groups. For all vaccinated animals, IgG was detected on day 15 postvaccination and increased slightly by day 50 (Figure 3B). Following challenge, there was a strong IgG memory response, where CHIKV/IRESv2 induced stronger titers than CHIKV/IRESv1. CHIKV induced a very strong IgG response in sham-vaccinated macaques, which continued to increase by day 35 postchallenge, when the experiment was terminated. Based on these findings, both vaccine candidates effectively induced both IgM and IgG responses in macaques, but as expected, they were not as immunogenic as WT CHIKV due to attenuation. Overall, there was a consistent trend of greater immunogenicity of CHIKV/IRESv2 compared to CHIKV/IRESv1, but most individual comparisons were not significant.
Figure 3.
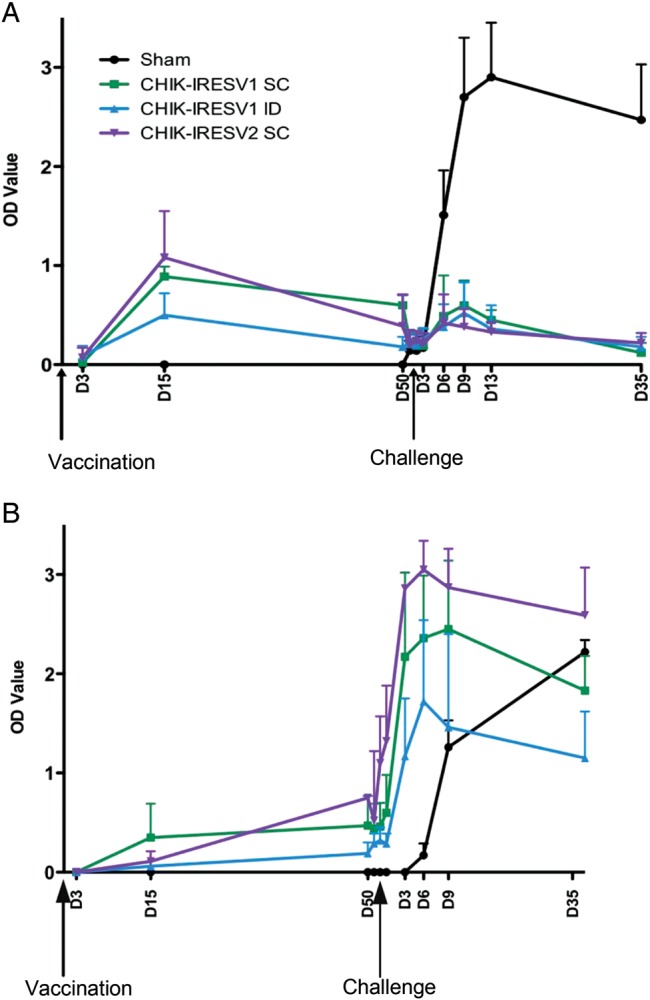
IgM (A) and IgG (B) antibody responses of cynomolgus macaques after vaccination and challenge. Macaques were vaccinated subcutaneously (SC) with saline (sham) or CHIKV/IRESv1 (CHIK-IRESv1 SC), vaccinated intradermally (ID) with CHIKV/IRESv1 (CHIK-IRESv1 ID), or vaccinated SC with CHIKV/IRESv2 (CHIK-IRESv2 SC). On day 52 after vaccination, the macaques were challenged with a single 105 PFU SC inoculation of wild-type CHIKV strain La Reunion (CHIKV-LR). Bars indicate standard deviations. Abbreviations: CHIKV, chikungunya virus; IgG, immunoglobulin G; IgM, immunoglobulin M; IRES, internal ribosome entry site.
Efficacy
The efficacies of the 2 CHIK vaccines were assessed following challenge with WT CHIKV approximately 7 weeks after a single vaccination. The primary indicator of efficacy was protection from viremia (measured on days 1–3 postchallenge; Table 2); secondary endpoints included changes in physiologic response (telemetric parameters; Figure 4). Both CHIKV/IRES vaccines provided highly significant protection from acute viremia, with none of the 12 animals in the 2 vaccine cohorts developing measurable viremia after challenge (<1.0 log10 PFU/mL). All (7/7) sham-vaccinated NHPs developed viremia, with peak titers on day 2 postchallenge of approximately 5 log10 PFU/mL (Table 2).
Table 2.
Viremia Titers of Vaccinated Cynomolgus Macaques After Challenge With CHIKV
| Vaccine (Route of Vaccination) | Animal no. | Viremia (log10 PFU/mL) |
||
|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | ||
| Sham | H78 | 4.3 | 5.0 | <1.0 |
| H82 | 3.6 | 5.0 | <1.0 | |
| H84 | 3.3 | 5.0 | <1.0 | |
| IV10 | 4.6 | 5.0 | 3.3 | |
| IV11 | 4.0 | 4.6 | <1.0 | |
| IV16 | 2.8 | 4.3 | <1.0 | |
| IT87 | 5.2 | 5.3 | 3.5 | |
| CHIKV/IRESv1 (subcutaneous) | IV04 | <1.0 | <1.0 | <1.0 |
| IV13 | <1.0 | <1.0 | <1.0 | |
| IV15 | <1.0 | <1.0 | <1.0 | |
| IT85 | <1.0 | <1.0 | <1.0 | |
| CHIKV/IRESv1 (intradermal) | IV17 | <1.0 | <1.0 | <1.0 |
| IV18 | <1.0 | <1.0 | <1.0 | |
| IT82 | <1.0 | <1.0 | <1.0 | |
| IT96 | <1.0 | <1.0 | <1.0 | |
| CHIKV/IRESv2 (subcutaneous) | IV02 | <1.0 | <1.0 | <1.0 |
| IV05 | <1.0 | <1.0 | <1.0 | |
| IV27 | <1.0 | <1.0 | <1.0 | |
| IT92 | <1.0 | <1.0 | <1.0 | |
Abbreviations: CHIKV, chikungunya virus; IRES, internal ribosome entry site; PFU, plaque-forming units; SD, standard deviation.
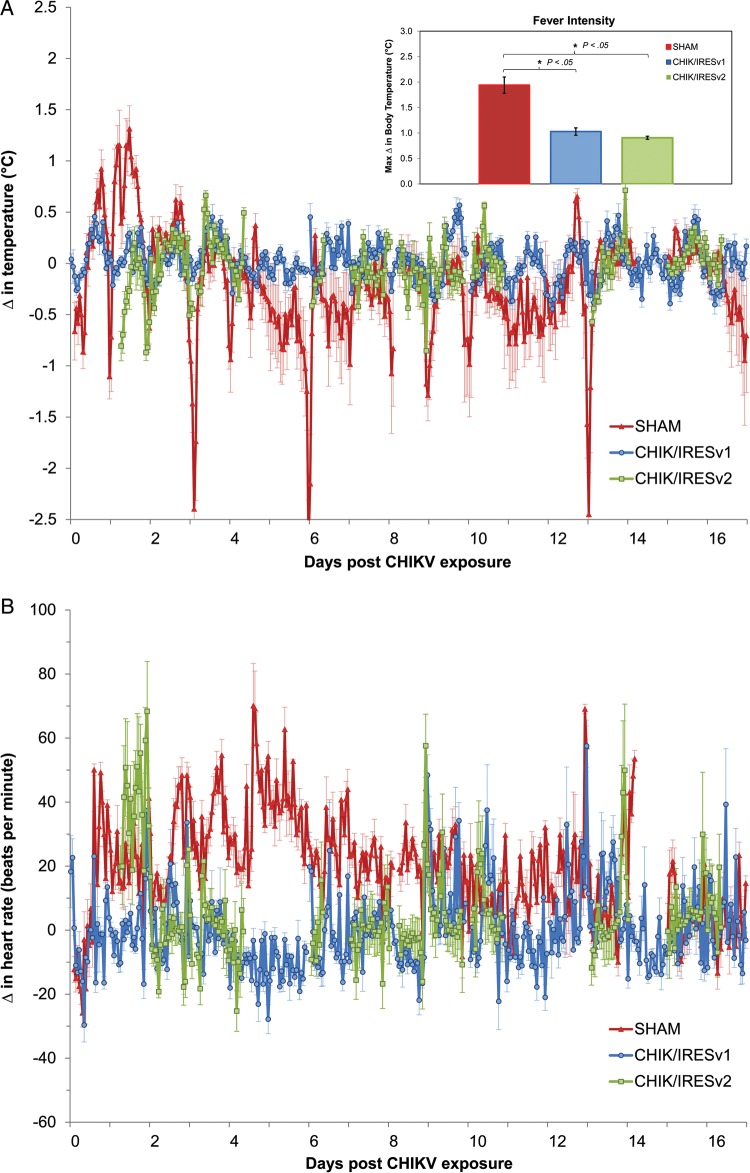
Figure 4.
Physiological responses of vaccinated cynomolgus macaques to CHIKV challenge. Changes (Δ) in body temperature (°C) (A) and heart rate (beats per minute) (B) from baseline following challenge with wild-type CHIKV strain La Reunion (CHIKV-LR), displayed as hour means (mean ± SD) for animals vaccinated with saline (red), CHIKV/IRESv1 (blue), and CHIKV/IRESv2 (green). Gaps in the data are a result of transient loss of signal in the telemetry system. The extended gaps in the CHIKV/IRESv2 group data, in particular, are a result of an intermittent malfunction of the acquisition software. The bar graph inset in (A) details the results of fever intensity analysis among groups. Significant changes among vaccination groups were compared statistically (Kruskal-Wallis). Further post hoc analysis was performed (Tukey HSD) to highlight differences between the SHAM vaccinated group and the 2 vaccines cohorts. Bars indicate standard deviations and significant results with P < .05 are indicated on the bar graph inset. Abbreviations: CHIKV, chikungunya virus; IRES, internal ribosome entry site; SD, standard deviation.
Histopathologic analysis was performed on tissues collected at the end of the study from all animals. There were no apparent lesions in any of the specimens (data not shown). Infectious virus was not detected in the axillary, bronchial, and inguinal lymph nodes or in the serum at the time of necropsy.
Efficacy of the vaccines was also assessed using telemetric data for core body temperature (Figure 4A) and heart rate (Figure 4B). Sham-vaccinated animal IV11 was excluded from the final telemetry data analysis summary based on observations of abnormal control data findings and problems with signal acquisition. Additionally, telemetry data were not available for animal IV02 from the CHIKV/IRESv2 group due to an implant issue. However, dramatic physiological changes occurred after CHIKV infection of the 6 sham-vaccinated animals, which experienced significant increases in core temperature acutely (6/6, 100%; Figure 4A) postexposure when compared to individualized control values (data not shown). Sham-vaccinated animals also exhibited hypothermia beginning 3–4 days postchallenge (5/6, 83%), which was sustained for the duration of the study in 4/6 (67%) of the animals (Figure 4A). In contrast, only 2/5 (40%) of implanted animals in the CHIK/IRESv1 group and 1/3 (33%) of implanted animals in CHIK/IRESv2 group experienced any fever after challenge, which was milder than that in sham-vaccinated animals, postchallenge. The lack of a hypothermic response in the vaccinated groups was similar, with 1/5 (20%) and 1/3 (33%) animals in the CHIK/IRESv1 and CHIK/IRESv2 groups, respectively, showing any change from control values. Analysis of group differences in acute hyperthermic response measured as fever hours (mean ± SD) did not show significant differences among any of the groups: control (0.250 ± 0.109 fever hours/total measured hours, n = 6), CHIK/IRESv1 (0.064 ± 0.043 fever hours/total measured hours, n = 5), and CHIK/IRESv2 (0.120 ± 0.071 fever hours/total measured hours, n = 3) with P = .118. Analysis of group differences in hypothermic response also did not show significance among groups; sham (0.196 ± 0.103 hypothermia hours/total measured hours, n = 6), CHIK/IRESv1 (0.053 ± 0.018 hypothermia hours/total measured hours, n = 5), and CHIK/IRESv2 (0.135 ± 0.073 hypothermia hours/total measured hours, n = 3) with P = .733. Analysis of group differences in fever intensity did reveal significant protection by vaccination; Control (+2.150 ± 0.287°C, n = 6), CHIK/IRESv1 (+1.028 ± 0.145°C, n = 5), CHIK/IRESv2 (+0.907 ± 0.062°C, n = 3) with P = .016. Further analysis of the fever intensity (Figure 4A; inset) revealed significant differences in responses between the CHIK/IRESv1 compared to the sham group with P < .05, and CHIK/IRESv2 compared to sham with P < .05. However, no significant differences in fever intensity were found among the vaccine cohorts (Figure 4A; inset).
Significant changes in heart rate were observed in sham-vaccinated animals following challenge (6/6, Figure 4B). In contrast, there was little measurable change in heart rate in the vaccinated groups, with 2/5 (40%) and 1/3 (33%) animals in the CHIK/IRESv1 and CHIK/IRESv2 groups, respectively, showing any sustained changes from control values.
DISCUSSION
Although several CHIK vaccine candidates have been developed since the 1980s, most if not all have significant drawbacks for the vaccination of populations in endemic regions that are principally found in developing countries [30]. Inactivated virus, DNA, and virus-like particle vaccines offer major advantages in safety, but their multiple dose requirements and, in some cases, expensive manufacture costs are not ideal. Live-attenuated vaccines offer rapid, single-dose protection and long-lived immunity, but traditional attenuation approaches can be unstable, leading to adverse events following administration [12] that are probably related to the reversion of the attenuating point mutations [20]. Also, live-attenuated vaccines including the CHIKV 181/clone25 strain [31] can be capable of infecting mosquitoes, a major safety concern for their use in nonendemic regions when the potential exists for revisions of attenuating point mutations.
To overcome these safety issues, we developed a rational attenuation strategy to yield a vaccine that retained the advantages of live-attenuated vaccines yet improved upon safety. The resultant vaccine strains, CHIKV/IRESv1 and CHIKV/IRESv2, are attenuated via the down-regulation of structural protein expression and unable to infect mosquitoes due to the inefficient IRES-dependent translation in insect cells.
By all measures, both CHIKV/IRES vaccine candidates were highly attenuated and efficacious in the cynomolgus macaque model. Only one case of very minimal viremia was associated with inoculation with either vaccine candidate. Consistent with the results from murine models of CHIK [24], the CHIKV/IRES vaccine candidates provided protection in NHPs against disease following challenge with WT CHIKV. Because CHIKV does not typically cause fatal disease, vaccine efficacy testing relied on the measurement of physiological parameters, primarily core body temperature and heart rate. Based on telemetric monitoring, both vaccine candidates protected against all measures of disease. Unlike the sham-vaccinated controls, there was also no detectable viremia in the vaccinated animals following challenge. This finding was independent of vaccine candidate (CHIKV/IRESv1, CHIKV/IRESv2) or route of inoculation in the case of CHIKV/IRESv1. Although slight changes in temperature and heart rate occurred in a few vaccinated animals after challenge, our challenge dose of 105 PFU was far greater that CHIKV amounts inoculated by infected mosquitoes, typically less than 103 PFU [28].
Two different vaccine candidates differing slightly in their attenuation approach, CHIKV/IRESv1 and v2, were compared for their efficacy and immunogenicity. Similar vaccines applied to the TC-83 vaccine [14, 25] and a WT VEEV subtype IE strain [24] indicated that the v2 approach is more immunogenic in that alphavirus backbone. Based on our CHIKV PRNT titers, CHIKV/IRESv2 appeared to be more immunogenic than CHIKV/IRESv1. In addition, 2 different routes of inoculation were performed with CHIKV/IRESv1 to determine whether subcutaneous administration is more immunogenic than intradermal administration. However, the lack of a significant difference in mean PRNT titers following these administration routes indicated no major difference.
Neither version of the CHIK/IRES vaccine caused overt signs of disease or physiologic changes in macaques following immunization. Telemetry has been useful in evaluating other alphavirus vaccines in NHPs, including the VEE vaccines TC-83 and C-84 [32]. They provide greater precision to detect physiological changes associated with vaccination and challenge, which can often be overlooked based on clinical observation. After viral challenge with CHIKV, the groups immunized with either CHIKV/IRES version showed little physiological response change from control baseline data. Thus, these data augmented the endpoints that could be achieved with such a challenge model and were highly informative regarding the quality of immune protection. The distinct pattern of hyperthermia (from acute viral infection) followed by a period of sustained hypothermia in the sham-vaccinated, challenged animals was a novel finding as it relates to physiologic monitoring of NHPs. Classifying fever hours and hypothermia hours in addition to fever intensity in postchallenge responses provided ideal parameters to test differences in physiologic response between vaccinees and controls. Although comparison of hypothermic and fever hours did not yield a statistically significant differences among vaccinee groups and controls, trends in these differences (reduction in fever hours) were apparent. Comparison of fever intensity as measured by the maximum change in temperature revealed significant differences in response between vaccines and sham-vaccinated controls, with a significant increase in the fever intensity in unvaccinated controls, revealing the protective efficacy of the vaccines.
The cynomolgus macaque model of CHIK was recapitulated for use in this vaccine efficacy trial. Unprotected NHPs exhibited hallmarks of CHIK described in prior modeling efforts as well as unique physiological responses (core temperature changes) that have to date not been thoroughly described. All animals receiving either version of the CHIKV/IRES vaccine were significantly protected from acute viremia. Furthermore, disease associated with viral challenge, manifesting in physiological changes, was significantly reduced when compared to controls. These results indicate that both CHIKV/IRES vaccine candidates, which appear to have equivalent efficacy, deserve further preclinical evaluation and are highly promising as human vaccines.
Notes
Acknowledgments. The authors thank Kasi Russell-Lodridge of the Veterinary Medicine Division of the TNPRC for surgical implantation of the radiotelemetry devices and Rose Langsjoen for statistical consultation.
Financial support. This work was supported by a grant from the National Institute of Allergy and Infectious Disease (NIAID) through the Western Regional Center of Excellence for Biodefense and Emerging Infectious Disease Research (WRCE), National Institutes of Health (NIH) grant U54 AI057156. A. P. A. was supported by a NIH K08 grant AI077796, and R. L. S. was supported by a NIH T32 grant AI007536. This work was supported in part through the NIH/OD grant OD-011104-51 (Tulane National Primate Research Center Base grant).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Tsetsarkin KA, Chen R, Sherman MB, Weaver SC. Chikungunya virus: evolution and genetic determinants of emergence. Curr Opin Virol. 2011;1:310–7. doi: 10.1016/j.coviro.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Powers AM. Chikungunya. Clin Lab Med. 2010;30:209–19. doi: 10.1016/j.cll.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Powers AM, Logue CH. Changing patterns of chikungunya virus: re-emergence of a zoonotic arbovirus. J Gen Virol. 2007;88:2363–77. doi: 10.1099/vir.0.82858-0. [DOI] [PubMed] [Google Scholar]
- 4.Lanciotti RS, Kosoy OL, Laven JJ, et al. Chikungunya virus in US travelers returning from India. Emerg Infect Dis. 2006;13:764–72007. doi: 10.3201/eid1305.070015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith DW, Mackenzie JS, Weaver SC. Alphaviruses. In: Richman DD, Whitley RJ, Hayden FG, editors. Clinical virology. Washington, DC: ASM Press; 2009. pp. 1241–74. [Google Scholar]
- 6.Vijayakumar KP, et al. Clinical profile of chikungunya patients during the epidemic of 2007 in Kerala, India. J Glob Infect Dis. 2011;3:221–6. doi: 10.4103/0974-777X.83526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Andrade DC, Jean S, Clavelou P, Dallel R, Bouhassira D. Chronic pain associated with the chikungunya fever: long lasting burden of an acute illness. BMC Infect Dis. 2010;10:31. doi: 10.1186/1471-2334-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerardin P, et al. Perceived morbidity and community burden after a chikungunya outbreak: the TELECHIK survey, a population-based cohort study. BMC Med. 2011;9:5. doi: 10.1186/1741-7015-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krishnamoorthy K, Harichandrakumar KT, Krishna Kumari A, Das LK. Burden of chikungunya in India: estimates of disability adjusted life years (DALY) lost in 2006 epidemic. J Vector Borne Dis. 2009;46:26–35. [PubMed] [Google Scholar]
- 10.Monath TP, Cetron MS, Teuwen DE. Yellow fever vaccine. In: Plotkin S, Orenstein WA, Offit PA, editors. Vaccines. Philadelphia, PA: Saunders; 2008. pp. 959–1056. [Google Scholar]
- 11.Harrison VR, Eckels KH, Bartelloni PJ, Hampton C. Production and evaluation of a formalin-killed chikungunya vaccine. J Immunol. 1971;107:643–7. [PubMed] [Google Scholar]
- 12.Edelman R, et al. Phase II safety and immunogenicity study of live chikungunya virus vaccine TSI-GSD-218. Am J Trop Med Hyg. 2000;62:681–5. doi: 10.4269/ajtmh.2000.62.681. [DOI] [PubMed] [Google Scholar]
- 13.Levitt NH, et al. Development of an attenuated strain of chikungunya virus for use in vaccine production. Vaccine. 1986;4:157–62. doi: 10.1016/0264-410x(86)90003-4. [DOI] [PubMed] [Google Scholar]
- 14.Wang D, et al. A complex adenovirus vaccine against chikungunya virus provides complete protection against viraemia and arthritis. Vaccine. 2011;29:2803–9. doi: 10.1016/j.vaccine.2011.01.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang E, et al. Chimeric alphavirus vaccine candidates for chikungunya. Vaccine. 2008;26:5030–9. doi: 10.1016/j.vaccine.2008.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Metz SW, et al. Functional processing and secretion of chikungunya virus E1 and E2 glycoproteins in insect cells. Virol J. 2011;8:353. doi: 10.1186/1743-422X-8-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muthumani K, et al. Immunogenicity of novel consensus-based DNA vaccines against chikungunya virus. Vaccine. 2008;26:5128. doi: 10.1016/j.vaccine.2008.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mallilankaraman K, et al. A DNA vaccine against chikungunya virus is protective in mice and induces neutralizing antibodies in mice and nonhuman primates. PLoS Negl Trop Dis. 2011;5:e928. doi: 10.1371/journal.pntd.0000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akahata W, et al. A virus-like particle vaccine for epidemic chikungunya virus protects nonhuman primates against infection. Nat Med. 2010;16:334–8. doi: 10.1038/nm.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorchakov R, et al. Attenuation of chikungunya virus vaccine strain 181/clone 25 is determined by two amino acid substitutions in the e2 envelope glycoprotein. J Virol. 2012;86:6084–96. doi: 10.1128/JVI.06449-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plante K, et al. Novel chikungunya vaccine candidate with an IRES-based attenuation and host range alteration mechanism. PLoS Pathog. 2011;7:e1002142. doi: 10.1371/journal.ppat.1002142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pandya J, Gorchakov R, Wang E, Leal G, Weaver SC. A vaccine candidate for eastern equine encephalitis virus based on IRES-mediated attenuation. Vaccine. 2012;30:1276–82. doi: 10.1016/j.vaccine.2011.12.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paessler S, et al. Recombinant Sindbis/Venezuelan equine encephalitis virus is highly attenuated and immunogenic. J Virol. 2003;77:9278–86. doi: 10.1128/JVI.77.17.9278-9286.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rossi SL, et al. IRES-based Venezuelan equine encephalitis vaccine candidate elicits protective immunity in mice. Virol. 2013;87:2475–88. doi: 10.1016/j.virol.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guerbois M, et al. IRES-driven expression of the capsid protein of the Venezuelan equine encephalitis virus TC-83 vaccine strain increases its attenuation and safety. PLoS Negl Trop Dis. 2013;7:e2197. doi: 10.1371/journal.pntd.0002197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Labadie K, et al. Chikungunya disease in nonhuman primates involves long-term viral persistence in macrophages. J Clinical Invest. 2010;120:894–906. doi: 10.1172/JCI40104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsetsarkin K, et al. Infectious clones of chikungunya virus (La Reunion isolate) for vector competence studies. Vector Borne Zoonotic Dis. 2006;6:325–37. doi: 10.1089/vbz.2006.6.325. [DOI] [PubMed] [Google Scholar]
- 28.Beaty BJ, Calisher CH, Shope RE. Arboviruses. In: Lennete ET, Lennete DA, editors. Diagnostic procedures for viral, rickettsial and chlamydial infections. 7th edition. Washington, DC: American Public Health Association; 1995. pp. 189–212. [Google Scholar]
- 29.Strauss JH, Strauss EG. The alphaviruses: gene expression, replication, and evolution. Microbiol Rev. 1994;58:491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weaver SC, Osorio JE, Livengood JA, Chen R, Stinchcomb DT. Chikungunya virus and prospects for a vaccine. Expert Rev Vaccines. 2012;11:1087–101. doi: 10.1586/erv.12.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turell MJ, Malinoski FJ. Limited potential for mosquito transmission of a live, attenuated chikungunya virus vaccine. Am J Trop Med Hyg. 1992;47:98–103. doi: 10.4269/ajtmh.1992.47.98. [DOI] [PubMed] [Google Scholar]
- 32.Reed DS, et al. Genetically engineered, live, attenuated vaccines protect nonhuman primates against aerosol challenge with a virulent IE strain of Venezuelan equine encephalitis virus. Vaccine. 2005;23:3139–47. doi: 10.1016/j.vaccine.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 33.Dubrulle M, Mousson L, Moutailler S, Vazeille M, Failloux AB. Chikungunya virus and Aedes mosquitoes: saliva is infectious as soon as two days after oral infection. PLoS One. 2009;4:e5895. doi: 10.1371/journal.pone.0005895. [DOI] [PMC free article] [PubMed] [Google Scholar]