Abstract
Background. Prospective evaluation of the antifungal drug, voriconazole, is needed to determine whether drug toxicity correlates with CYP2C19 genotype or serum concentrations of voriconazole or its metabolites.
Methods. We conducted a prospective study of 95 patients to determine voriconazole toxicity and its relationship to genotype and serum levels of voriconazole and its two metabolites. Efficacy was not evaluated because, in most cases, the drug was given for empirical or prophylactic therapy.
Results. Hallucinations occurred in 16 patients (16.8%), visual changes in 17 (17.9%), photosensitivity in 10 (10.5%), and hepatotoxicity in 6 (6.3%). There was no correlation between photosensitivity or hepatotoxicity and levels of voriconazole or metabolites. Patients with hallucinations had higher average voriconazole levels (4.5 vs 2.5 μg/mL) but with extensive overlap. The recommended oral dose of 200 mg did not provide consistently detectable serum voriconazole levels in adults. CYP2C19 and CYP2C9 genotypes had a minor influence over levels, though the 4 patients homozygous for the 2C19*2 genotype had higher average levels for voriconazole (4.3 vs 2.5 μg/mL) and lower N-oxide levels (1.6 vs 2.5 μg/mL).
Conclusions. CYP2C19 and 2C9 genotypes were not major determinants of voriconazole metabolism. No toxic serum level of voriconazole or its metabolites could be identified.
Keywords: voriconazole, metabolites, CYP2C19, toxicity
Voriconazole is a broad-spectrum triazole antifungal that is frequently used in immunosuppressed patients with known or suspected aspergillosis. Toxicity is difficult to determine in immunosuppressed patients because of numerous comorbidities and other toxic treatments. Some retrospective studies have related toxicity to voriconazole blood levels above 5.0 or 5.5 μg/mL [1, 2]. Determining a safe blood level has been complicated by remarkable variability in intra- and interpatient blood levels. How much interpatient variability is due to genotype is an open question, though in normal volunteers voriconazole levels are higher in persons homozygous to the CYP2C19 nonfunctional alleles *2, *3, and *4 and are lower in CYP2C9 *17, a rapid metabolizer genotype (reviewed in [3]). Nothing is known of the role voriconazole metabolites play in toxicity, though it is known that both the N-oxide and hydroxymetabolites of voriconazole appear in serum [4, 5].
In the present study of primarily white patients, we attempted to determine whether prospectively evaluated toxicity correlated with serum levels of voriconazole or its metabolites and whether CYP2C9 or 2C19 genotypes were important determinants of voriconazole metabolism and toxicity.
MATERIALS AND METHODS
Subjects
Ninety-five patients at the National Institutes of Health Clinical Center were enrolled between 2005 and 2007 in a prospective study of voriconazole pharmacology and toxicity after informed consent was obtained under a protocol (06-I-0077) approved by the National Institute of Allergy and Infectious Diseases Institutional Review Board. Patients eligible for the protocol were aged >12 years who had been prescribed voriconazole by their physician. Characteristics of the study population are given in Table 1. Toxicity was assessed by a structured interview at weekly intervals or during outpatient visits, as well as by laboratory tests ordered by the patients’ physicians (Supplementary Table 1). Trough serum samples were obtained weekly beginning on day 5 but not until after day 7 in 15 patients. If toxicity was encountered on days 2–4 of the first week, additional samples were obtained. Under terms of the protocol, serum voriconazole levels were not reported to the clinician or used in dosing. Physicians could obtain voriconazole levels from the routine laboratory if they wished, but that was rarely done during the study period. A total of 318 serum samples were obtained from 94 of the 95 patients (range, 1–17 samples/patient) and analyzed for voriconazole. The 2 main voriconazole metabolites, 4-hydroxyvoriconazole and the N-oxide of voriconazole, were analyzed on 164 sera from the first 60 patients.
Table 1.
Characteristics of the Patient Population
| Age, y (average, range) | 43 (13–76) | |
| Sex (% males) | 63 | |
| Ethnicity (%) | ||
| White, non-Latino | 59 | |
| White, Latino | 22 | |
| African American | 16 | |
| Asian | 3 | |
| Diagnosis (number of cases) | ||
| Hematologic malignancy | 44 | |
| Post-allogeneic stem cell transplant | 31 | |
| Aplastic anemia | 18 | |
| Post-allogeneic stem cell transplant | 11 | |
| Solid tumor with autologous stem cell transplant | 13 | |
| Chronic granulomatous disease | 6 | |
| Job's syndrome | 2 | |
| Cryptococcosis | 2 | |
| Coccidioidomycosis | 2 | |
| System lupus erythematosus | 2 | |
| Sickle cell anemia | 2 | |
| Primary immunodeficiency | 2 | |
| Allergic bronchopulmonary aspergillosis | 1 | |
| Mycobacteriosis other than Mycobacterium tuberculosis | 1 | |
| Total | 95 | |
| Weeks of therapy (avg., median, range) | 10.6, 5, 1–80 | |
| Oral therapy at start of study (number patients) | 51 | |
Genotyping
DNA samples adequate for genotyping were obtained from 92 of the 95 patients using peripheral blood; in the case of hematopoietic stem cell transplant recipients, buccal cells were used. DNA was extracted from peripheral blood mononuclear cells and buccal swabs using the QIAamp DNA Blood Mini Kit (Qiagen, Valencia, CA) and amplified with appropriate primers (Supplementary Table 2). Products were purified and then sequenced with 1 of the primers used in the amplification. The sequence of the 2CYP19 open reading frame was compared with the published sequence (GenBank locus AY796203; http://www.ncbi.nlm.nih.gov/nuccore/AY796203). The promoter of 2CYP19 surrounding −806 and −3402 was also sequenced to identify patients with the CYP2C19*17 genotype (GenBank locus AL583836; http://www.ncbi.nlm.nih.gov/nuccore/AL583836). The promoter area was not sequenced in 14 patients because of insufficient DNA. CYP2C9 was sequenced in 66 patients and compared with *2 and *3 genotypes [6].
Assays
Voriconazole N-oxide (>99% purity) was supplied by Pfizer (Sandwich, UK). 4-Hydroxyvoriconazole was prepared in our laboratory as described [7]. Serum concentrations of voriconazole were determined by high- performance liquid chromatography (HPLC) at the Fungus Testing Laboratory at the University of Texas, San Antonio [6]. Metabolites were assayed by HPLC using our method described previously [7, 8].
Ten voriconazole levels that were detectable but below 0.2 μg/mL, the limit of accurate quantitation, were given the level of 0.1 μg/mL for analysis in order to distinguish them from 13 sera with undetectable voriconazole concentrations.
Statistical Analysis
Spearman rank correlation and the Mann-Whitney test were performed with the program Prism, version 5 (GraphPad Software, San Diego, CA). Random intercepts regression analysis was performed on SAS software [9]. Variability in this model comes from 2 sources: between patients and within patients. The percentage of the variance that is from within patients was calculated by dividing the mean square error by the Restricted Maximum Likelihood (REML) variance component estimate (total variance).
RESULTS
Voriconazole Concentrations
Variability of Voriconazole Concentrations
The correlation between voriconazole trough levels and voriconazole dose was low, considering either intravenous or oral dosing (Figure 1A). Concomitant drugs that might have played a role were examined in patients with sudden changes or extreme levels; only an interaction with phenytoin, a strong inducer of 2C19 metabolism, was detected. One patient's voriconazole level on day 3 of a 10-day course of intravenous phenytoin fell to <0.2 μg/mL and was still <0.2 μg/mL 13 days after the patient's anticonvulsant therapy was changed to levetirecetam. The 2 low values were excluded from all analyses. When checked 4 weeks after phenytoin discontinuation, the voriconazole level had returned to 1.04 μg/mL, similar to the patient's prior values.
Figure 1.
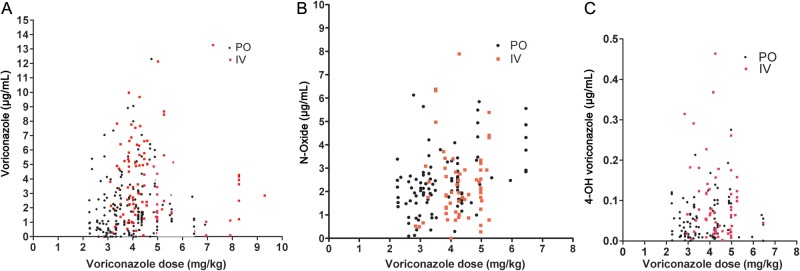
A, Serum concentrations of voriconazole in 94 patients receiving oral or intravenous drug. Serum concentrations of voriconazole N-oxide (B) and 4-hydroxyvoriconazole (C) in 60 patients receiving oral (PO) or intravenous (IV) voriconazole. Doses given in mg/kg body weight.
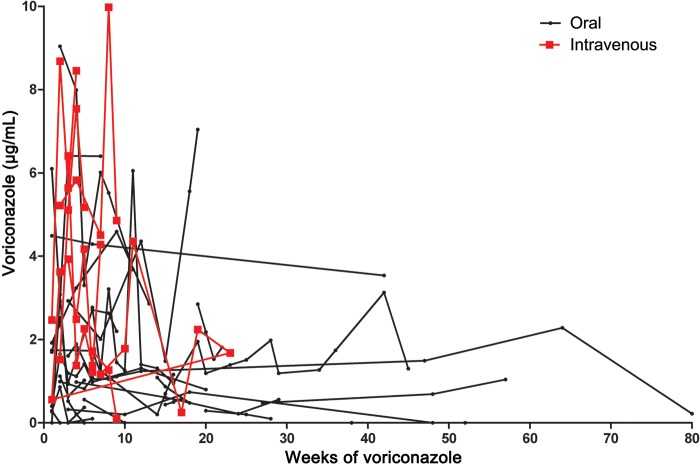
An analysis was conducted to determine if voriconazole levels changed over time. Patients were selected who received the same dose of voriconazole by the same route over a period of at least 14 days and had at least 3 consecutive serum voriconazole levels obtained over that period, excluding values during the first 5 days, which might have been prior to steady state. With oral therapy, 28 patients had 123 voriconazole levels over an average of 25 weeks (range, 2–80 weeks) and 7 patients receiving intravenous therapy had 34 blood levels drawn over an average of 8 weeks (range, 3–23 samples; Figure 2). Random intercepts regression analysis indicated that voriconazole levels decreased very slightly over time, but the decrease was not statistically significant for either the oral (P = .4811) or the intravenous (IV; P = .5038) treatment groups [8]. Using this analysis, there was no evidence that voriconazole blood levels changed during prolonged administration. However, variability over time within the same patient receiving the same dose was large (63% for IV voriconazole, 26% for oral voriconazole).
Figure 2.
Serum concentrations for individual patients who received the same dose by the same route for at least 3 doses.
Undetectable Voriconazole
Ten patients had 13 undetectable serum levels. At the times when the undetectable levels were found, 7 levels were from inpatients and 6 were from outpatients. No patient had received voriconazole for fewer than 5 days. Mean dose was 3.2 mg/kg (range, 2.6–4.7 mg/kg), given orally in 9 patients and 1 being treated intravenously. The oral dose was 200 mg twice daily for 11 of the assays. Nine of the 10 patients had other blood levels that were also low but detectable on this dose. N-oxide levels were detectable on all 8 sera assayed for the N-oxide, a mean of 1.16 μg/mL (range, 0.009–2.13). Among the 10 patients with undetectable serum voriconazole levels, the 2C19 type was *1/*1 in 9 patients and *1/*15 in the tenth. The data indicate that the recommended oral dose of 200 mg twice daily provided no detectable drug in at least 1 blood sample of 10 of 33 patients who received that dose. Although noncompliance of outpatients could have been a factor, undetectable values were not seen with other outpatient doses. The N-oxide was detectable in all sera, and presence of low voriconazole levels in the same patients at other time points indicated that in our population, compliance was not the major issue. Rather, poor absorption or rapid metabolism probably prevented the recommended twice daily 200-mg dose from providing a consistently detectable drug concentration in nonobese adults (average weight 64 kg).
Dose Response of Voriconazole Metabolite Levels
As can be seen in Figure 1B and 1C, there was a poor correlation between voriconazole doses and the blood concentrations of voriconazole metabolites. There was slightly better correlation of serum voriconazole N-oxide levels and voriconazole dose (r = 0.26, P = .01) than voriconazole levels and voriconazole dose (r = 0.14, P < .05). The mean serum level of voriconazole N-oxide was almost identical to the mean voriconazole level: voriconazole (2.5 ± 2.4 µg/mL, mean ± standard error) and voriconazole N-oxide (2.3 ± 1.3 µg/mL). The mean concentration (±standard error) of 4-hydroxyvoriconazole in blood samples was far lower than the N-oxide (0.067 ± 0.072 µg/mL). The correlation was low between the 4-hydroxyvoriconazole concentration and either the intravenous voriconazole dose (r = 0.16) or the oral dose (r = −0.09; Figure 1C).
Metabolic Ratios
The ratio between the serum concentrations of metabolites and voriconazole reflects drug metabolism. The ratio of both the N-oxide and 4-hydoxyvoriconazole to voriconazole fell abruptly to a constant value above a voriconazole level of about 2 μg/mL, which is consistent with saturation of metabolism (Supplementary Figure 1). This may help explain the known nonlinearity of the dose response curve [10]
Genotype CYP2C19
As shown in Table 2, only 4 patients (4%) were homozygous for the slow metabolizer (*2/*2) phenotype of CYP2C19. Eighteen were heterozygotes (*1/*2) and 63 were *1/*1. Trough concentrations of voriconazole and its 2 metabolites are displayed by serum concentration in Table 1 but are shown by both serum values and individual patient mean concentration in Figure 3A–C. There appeared to be a clear trend for the voriconazole levels to be higher and the N-oxide level to be lower in the 2C19 *2/*2 patients. This difference was statistically significant for the difference between voriconazole *1/*1 and *2/*2 when tallied by serum (P = .0029) and by patient (P = .027). Small numbers of *2/*2 patients decreased the power of the statistical tests to detect a difference, if the difference was real, for the N-oxide. The 4-OH metabolite levels appear slightly higher in 3 *2/*2 patients, but the small numbers and high variability prevent drawing any conclusions.
Table 2.
CYP Genotype and Serum Concentration, Averaged by Serum
| Genotype | cDNA | Voriconazole |
Voriconazole N-oxide |
4-hydroxyvoriconazole |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2C19 Genotype | Number of Patients | No. Serums | Mean Level | SE | Pts | Sera | Mean | SE | Pts | Sera | Mean | SE | |
| *1/*1 | 63 | 200 | 2.468 | 0.17 | 39 | 106 | 2.54 | 0.15 | 39 | 106 | 0.0734 | 0.0073 | |
| *1/*2 | 681 G/G > A/G | 18 | 63 | 3.071 | 0.33 | 12 | 28 | 2.16 | 0.16 | 12 | 28 | 0.0672 | 0.0144 |
| *2/*2 | 681 G/G > A/A | 4 | 11 | 4.33 | 0.59 | 3 | 5 | 1.66 | 0.33 | 3 | 5 | 0.0970 | 0.0506 |
| *1/*3 | 636 G/G > A/G, 99 C/C > C/T, 1251 A/A > A/C | 1 | 1 | 0.89 | 1 | 1 | 2.31 | 1 | 1 | 0.0570 | |||
| *1/*9 | 431 G/G > G/A | 1 | 22 | 1.85 | 0.23 | 1 | 17 | 2.38 | 0.14 | 1 | 17 | 0.0372 | 0.0071 |
| *1/*11 | 449 G/G > A/G, 99 C/C > C/T, 990 C/C > C/T | 1 | 1 | 4.87 | 0.14 | 1 | 1 | 2.50 | nd | 1 | 1 | 0.0180 | |
| *1/*15a | 55 A/A > A/C, 991 A/A > G/G, 1251 A/A > A/C | 1 | 4 | 0.14 | 0.14 | 1 | 4 | 1.09 | 0.49 | 1 | 4 | 0.0438 | 0.0131 |
| Newb | 217 C/C > C/T (Arg > Cys) | 1 | 9 | 1.037 | 0.32 | ||||||||
| Newb | 276 G/G > G/C (Glu > Gly) | 2 | 5 | 4.054 | 0.43 | 2 | 2 | 0.63 | 0.14 | 2 | 2 | 0.0700 | 0.0040 |
| Total | 92 | 316 | 60 | 164 | 60 | 164 | |||||||
| Minus 806 promoter | |||||||||||||
| *1/*1 | 806 C/C | 45 | 130 | 2.89 | 0.22 | 28 | 70 | 1.96 | 0.14 | 28 | 70 | 0.0684 | 0.0088 |
| *1/*17 | 806 C/C > C/T | 29 | 92 | 2.28 | 0.21 | 13 | 31 | 2.62 | 0.24 | 13 | 31 | 0.0857 | 0.0158 |
| *17/*17 | 806 C/C > T/T | 4 | 13 | 3.645 | 0.82 | 3 | 9 | 1.57 | 0.18 | 3 | 9 | 0.0782 | 0.0220 |
| Total | 78 | 235 | 44 | 44 | |||||||||
| 2C9 genotype | |||||||||||||
| *1/*1 | 430 C/C | 56 | 200 | 2.57 | 0.18 | 46 | 138 | 2.40 | 0.12 | 46 | 138 | 0.0703 | 0.0060 |
| *1/*2 | 430 C/C > C/T | 3 | 4 | 3.28 | 1.40 | 3 | 4 | 2.32 | 0.61 | 3 | 4 | 0.1305 | 0.0837 |
| *2/*2 | 430 C/C > T/T | 1 | 1 | 2.39 | 1 | 1 | 1.75 | 1 | 1 | 0.0100 | |||
| *1/*3 | 1075 A/A > A/C | 6 | 13 | 2.47 | 0.59 | 6 | 10 | 1.67 | 0.27 | 6 | 10 | 0.0387 | 0.0181 |
| Total | 66 | 218 | 56 | 56 | |||||||||
Abbreviation: SE, standard error.
a 55 A/A > A/C = I19L, 991 A/A > G/G = I331V, 1251 A/A > A/C = G417G.
b Patients were heterozygous for a nonsynonymous base change, a combination not previously published.
Figure 3.
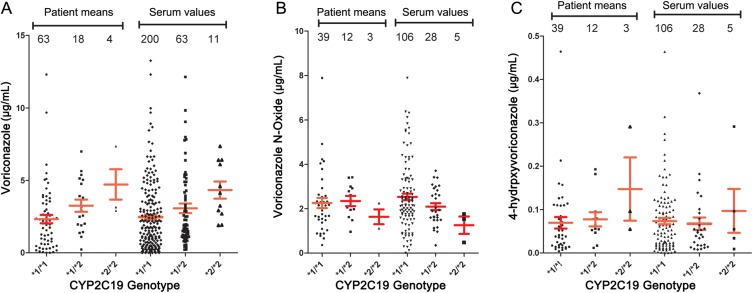
Serum concentrations of (A) voriconazole, (B) voriconazole N-oxide, and (C) 4-hydroxyvoriconazole by CYP2C19 *1/*1, *1/*2, and *2/*2 genotypes, tallied by average of all sera and by patient averages. Means and standard errors are shown. Numbers of values in each genotype are shown.
As seen in Table 1, there were 2 patients with the single nucleotide polymorphism (SNP) 276 G/C, coding for Glu > Gly and 1 with the SNP 217G/T, coding for Arg > Cys. Both SNPs are listed in the National Center for Biotechnology Information database (http://www.ncbi.nlm.nih.gov/projects/SNP/snp_ref.cgi?geneId=1557), but functional significance is unknown. The 2 patients with the 276 G/G > G/C genotype appeared to have higher voriconazole levels (P = .03) and lower N-oxide levels, but the numbers of patients and samples were too small to permit drawing conclusions. There were only single patients in the other described genotypes of CYP219. The single patient with the *1/*15 genotype had lower blood levels but was an outpatient taking only 2.62 mg kg twice daily by mouth. Results of sequencing the promoter region for the accelerated metabolism genotype 2C19*17 are also shown in Table 1. There was no effect of the 2C19*17 genotype, considering it with or without the presence in the same patient with the CYP2C19*1/*2 genotype (slow metabolizer haplotype).
Genotype CYP2C9
We found no effect of the 2C9 genotype on voriconazole levels. As shown in Table 2, there was only 1 CYP 2C9*2/*2 homozygote and nothing to suggest an effect of CYP2C9 *1/*2 on voriconazole metabolism. There were no CYP2C9 *3/*3 patients. However, there were 6 CYP2C9 *1/*3 patients, and their 10 sera had somewhat lower 4-hydroxyvoriconazole and perhaps lower N-oxide levels compared with 138 sera from 46 CYP2C9 *1/*1 patients (4-hydroxyvoriconazole: 0.0387 vs 0.0703, P = .038; N-oxide: 1.67 vs 2.40, P = .07). Significance of this observation is unknown.
Toxicity
Hepatotoxicity
Hepatotoxicity leading to discontinuation of voriconazole occurred in 6 patients (6.3%; Table 3). Liver function tests were normal prior to voriconazole and began to become abnormal within 7 days of starting voriconazole except in 1 patient. In case 2, alkaline phosphatase began to rise on day 7 of a current course following a recent 8-day course, shown as day 15 in Table 3. He and 1 other patient had received voriconazole on a prior hospitalization. Case 1 had received multiple courses in the past; she developed a rapid rise in alanine aminotransferase (ALT) to 1054 µ/mL after a 3-day course. She was rechallenged, and after only 2 days of treatment had an ALT rise to 464. No other patient was rechallenged with voriconazole, though 2 received fluconazole, 1 posaconazole, and 1 both drugs without hepatotoxicity. Abnormalities accelerated rapidly in all 6 patients, with the ALT reaching a peak within 4 days of the first abnormality and falling rapidly to normal after drug discontinuation. Voriconazole and N-oxide trough levels were considered to be in the usual range (Table 3).
Table 3.
Voriconazole Hepatotoxicity
| Case Number | Underlying Disease | Sex | Age, y | Voriconazole Treatment |
Liver Function Test |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day of Abnormality |
Maximum Abnormality |
Voriconazole Treatment |
Trough Level |
|||||||||||
| (start day) | (peak day) | AST | ALT | AP | Bili | Dose mg/12 h | Length (days) | Day | Voriconazole (μg/mL) | N-oxide (μg/mL) | ||||
| 1 | AML, allo HSCT 1st episode | F | 18 | 3 | 6 | 441 | 1054 | 144 | 3.1 | 200 po | 7 | 7 | 0 | ND |
| AML, allo HSCT 2nd episode | 18 | 2 | 5 | 125 | 464 | 191 | 0.5 | 150 po | 5 | 6 | 0.1 | 0.88 | ||
| 2 | CLL | M | 60 | 15 | 16 | 124 | 137 | 769 | 1 | 200 po | 19 | 11 | 2.58 | ND |
| 3 | Metastatic melanoma | F | 53 | 3 | 4 | 349 | 241 | 99 | 0.5 | 228 IV | 3 | 2 3 |
4.81 3.16 |
0.872 1.875 |
| 4 | Multiple myeloma | M | 55 | 2 | 6 | 270 | 506 | 71 | 1.7 | 270 IV | 4 | 2 | 2.73 | 0.49 |
| 5 | Cryptococcosis | M | 48 | 7 | 9 | 159 | 210 | 251 | 0.3 | 450 po | 8 | 7 | 2.96 | ND |
| 6 | Hodgkin disease | M | 47 | 2 | 3 | 126 | 176 | 876 | 1.6 | 200 po | 16 | 7 14 |
0.36 0.9 |
0.12 1.94 |
Abbreviations: ALT, alanine aminotransferase; AML, acute myelogenous leukemia; AP, alkaline phosphatase; bili, total bilirubin; AST, aspartate aminotransferase; CLL, chronic lymphocytic leukemia; HSCT, hematopoietic stem cell transplant; IV, intravenous; ND, not determined; po, per oral administration.
Visual Hallucinations
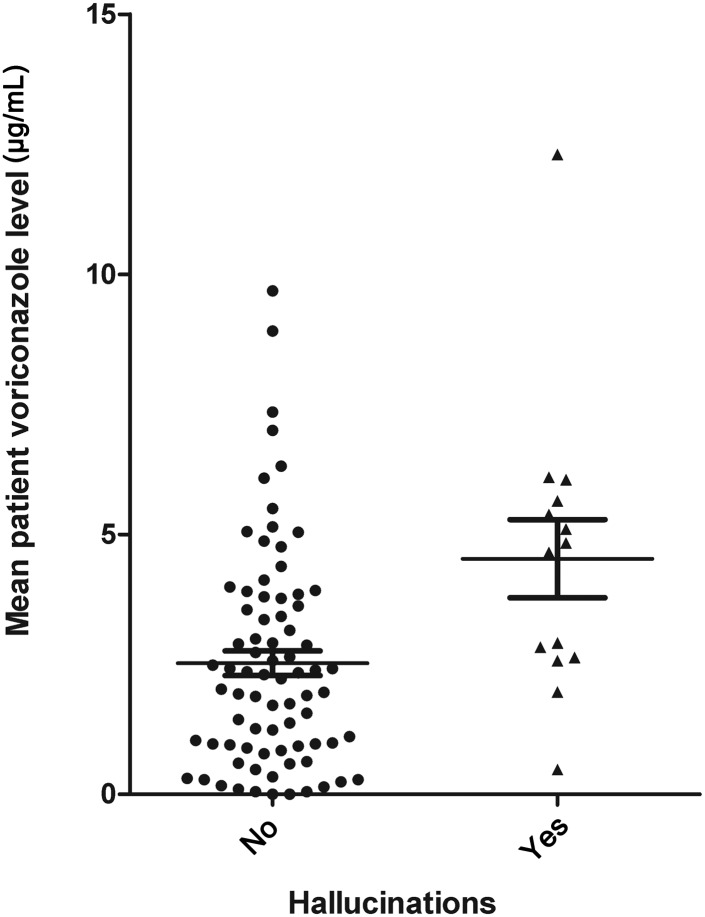
Visual hallucinations occurred in 16 of 95 (16.8%) patients, accompanied by auditory hallucinations in 5, all within the first week. Fifteen were receiving intravenous voriconazole at the time of the hallucinations and 1 was receiving oral drug. Hallucinations led to drug discontinuation in 10 patients and dose lowering in 6. Hallucinations resolved promptly following both strategies. Trough voriconazole levels were measured during the first week of treatment in 14 of the 16 patients (31 levels) with hallucinations. Taking the mean level for each patient, the range was 0.48–12.3 μg/mL with an average of 4.53 μg/mL, a significantly higher mean than the average for the 78 patients without hallucinations (2.52; P = .004), but overlap was extensive (Figure 4). Metabolite levels were measured during the first week in 9 patients with hallucinations (14 levels) vs 52 without hallucinations, but no differences were found (data not shown).
Figure 4.
Average serum voriconazole concentrations during the first week of treatment in 14 patients having hallucinations that week compared with averages of patients who did not have hallucinations.
Visual Changes and Photosensitivity
Seventeen patients (17.7%) had visual changes, that is, blurred vision, a sense of brighter light, or a sense of more intense colors. Ten patients (10.5%) had photosensitivity. Voriconazole levels were measured in all 10 and metabolites in 9 photosensitive patients. Blood concentrations of voriconazole and the N-oxide were not higher in patients with visual changes or photosensitivity (data not shown). This was true even when patients only in outpatient status were included in the photosensitivity analysis. An unexplained finding was that the concentrations of the 4-hydroxymetabolite were lower in the 10 photosensitive patients than in the 52 nonphotosensitive patients who had the metabolite measured (0.0354 vs 0.0750 μg/mL; P = .003).
Other Side Effects
One patient had the drug discontinued for mild confusion and decreased ability to concentrate. One patient from North Carolina developed a keratoacanthoma, a low-grade malignant tumor, on his forehead. The lesion was excised and did not recur. Single patients had rash, insomnia, or myalgia.
DISCUSSION
Side Effects
The incidence of hepatotoxicity in our patients (6.3%) was in the range reported previously (6.5%) [11]. There was no correlation between hepatotoxicity and genotype or drug levels. Rechallenge resulted in prompt return of hepatitis in 1 patient, but other azoles were tolerated in 4 of the other 5 patients. Visual disturbances, such as light sensitivity, altered color perception, or blurring, occurred in 17.7% of our patients compared with a reported incidence of 8 of 46 (17%) normal volunteers [12]. Photosensitivity was noted in 10.5% of patients and could be distinguished from graft-versus-host disease rash by the distribution in sun-exposed areas [13]. While patients were in the study, most remained in the Washington, DC, area while being followed in the outpatient clinic. Our experience may not apply to patients with more intense sun exposure. Patients taking long-term voriconazole have developed skin cancers in sun-exposed skin [14]. In our group, 1 patient developed a keratoacanthoma in the 6 months after he had completed a year of voriconazole. During the last 6 months of voriconazole, he had extensive sun exposure while working much of the time outdoors in North Carolina. This was in accord with the reported conditions for this complication: prolonged voriconazole use and intense sun exposure [14]. Hallucinations occurred in 16.8%, continuing the same percentage we had reported in the first 72 patients of this study [15] and also similar to a report from Switzerland (4 of 26, or 15%) [16]. Encephalopathy from voriconazole was not thought to have occurred during our study, though it has been reported in 5 patients and considered correlated to higher voriconazole levels [1, 17]. We did not detect any cases of periostitis [18].
Voriconazole Metabolism
One obvious finding was the large range of voriconazole and metabolite levels at the doses given our patients (Figure 1). Similar variability in voriconazole serum concentrations has been reported in hematology patients [19]. Drug interactions are an obvious possibility in patients receiving multiple other agents, but the only interaction we recognized, with phenytoin, has been documented in a study of volunteers [12]. The effects of food on oral absorption, age, and minor drug–drug interactions can be seen in normal volunteers [10] but may disappear in the heterogeneity of patient responses. This variability also might have interfered with our ability to detect autoinduction; however, we failed to find that prolonged therapy autoinduced metabolism and reduced voriconazole levels. We did find that higher voriconazole blood levels resulted in reduced metabolism as measured by the ratios of metabolites to voriconazole (Supplementary Figure 1).
We were unable to confirm that a blood level predicted hepatotoxicity [20] or any other toxicity. Although patients with hallucinations had higher average levels, 10 of the 14 patients who had levels measured during the period of hallucinations had average levels below the proposed cutoff of 5.5 μg/mL [1]. Of the 16 with hallucinations, treatment was begun intravenously in 15, starting with 2 loading doses of 6 mg/kg that were given 12 hours apart. Because initial therapy was oral in 51 patients (Table 1), our incidence of hallucinations may be lower than in patient populations where initial therapy is intravenous.
Presence of both the N-oxide and 4-hydroxy metabolites of voriconazole have been found in blood of normal volunteers and patients, as has the dihydroxymetabolite, which we did not measure [4, 5]. CYP3A4 has low affinity for voriconazole but is considered to be the main pathway for hydroxylation. Hydroxylated voriconazole is rapidly cleared, with only low plasma levels of hydroxy- and dihydroxyvoriconazole [5]. The main metabolite in blood of our patients was the N-oxide, in part due to its slow clearance [3]. The N-oxide has only minimal antifungal activity [3] and did not correlate with toxicity in our patients. CYP2C19 is a major cytochrome involved in N-oxidation and is the most important cytochrome determining voriconazole plasma levels in normal volunteers [20]. Poor metabolizers with a CYP2C19*2 or 2C19*3 genotype are known to have a slower plasma clearance. In Europeans, frequency of the 2C19*2 allele has been reported to be 0.15 and frequency of the 2C19*3 allele to be 0.004, both values are close to the 0.15 and 0.005 in our population [3] (Table 2). The effect of these alleles was only seen in the 4 CYP*2/*2 patients, and this effect was relatively small, raising the mean voriconazole level by 75% and the 4-hydroxy level by 33% while decreasing the N-oxide level by 35%. The rapid metabolizer genotype, 2C9*17, has been reported to decrease voriconazole levels [4, 21] but was not correlated with lower levels in our patients. We did find 1 or more undetectable voriconazole levels in 10 of 94 patients (10.6%) but considered the cause to be failure to dose oral drug by weight, as is done for intravenous voriconazole. We found only an equivocal effect of the CYP2C9 genotype, similar to other observations [3].
Use of therapeutic drug monitoring to improve voriconazole efficacy remains a possibility but was not included in our study [1, 2, 17]. Because most voriconazole usage in our institution is empirical or prophylactic, impact of low blood levels on efficacy cannot be assessed with confidence.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://cid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Financial support. This work was supported in part by the Ministry of Education, Science, Sports and Culture of Japan and by the Intramural Program of the National Institute of Allergy and Infectious Diseases. This project also was funded in part by the National Cancer Institute, National Institutes of Health, under contract HHSN261200800001E.
Potential conflict of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Pascual A, Calandra T, Bolay S, Buclin T, Bille J, Marchetti O. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin Infect Dis. 2008;46:201–11. doi: 10.1086/524669. [DOI] [PubMed] [Google Scholar]
- 2.Dolton MJ, Ray JE, Chen SC, Ng K, Pont LG, McLachlan AJ. Multicenter study of voriconazole pharmacokinetics and therapeutic drug monitoring. Antimicrob Agents Chemother. 2012;56:4793–9. doi: 10.1128/AAC.00626-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mikus G, Scholz IM, Weiss J. Pharmacogenomics of the triazole antifungal agent voriconazole. Pharmacogenomics. 2011;12:861–72. doi: 10.2217/pgs.11.18. [DOI] [PubMed] [Google Scholar]
- 4.Hassan A, Burhenne J, Riedel KD, et al. Modulators of very low voriconazole concentrations in routine therapeutic drug monitoring. Ther Drug Monit. 2011;33:86–93. doi: 10.1097/FTD.0b013e31820530cd. [DOI] [PubMed] [Google Scholar]
- 5.Scholz I, Oberwittler H, Riedel KD, et al. Pharmacokinetics, metabolism and bioavailability of the triazole antifungal agent voriconazole in relation to CYP2C19 genotype. Br J Clin Pharmacol. 2009;68:906–15. doi: 10.1111/j.1365-2125.2009.03534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blaisdell J, Jorge-Nebert LF, Coulter S, et al. Discovery of new potentially defective alleles of human CYP2C9. Pharmacogenetics. 2004;14:527–37. doi: 10.1097/01.fpc.0000114759.08559.51. [DOI] [PubMed] [Google Scholar]
- 7.Murayama N, Imai N, Nakane T, Shimizu M, Yamazaki H. Roles of CYP3A4 and CYP2C19 in methylhydroxylated and N-oxidized metabolite formation from voriconazole, a new anti-fungal agent, in human liver microsomes. Biochem Pharmacol. 2007;73:2020–6. doi: 10.1016/j.bcp.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Perea S, Pennick GJ, Modak A, et al. Comparison of high-performance liquid chromatographic and microbiological methods for determination of voriconazole levels in plasma. Antimicrob Agents Chemother. 2000;44:1209–13. doi: 10.1128/aac.44.5.1209-1213.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS for mixed models. 2nd edn. Cary, NC, USA: SAS Institute Inc; 2006. [Google Scholar]
- 10.Theuretzbacher U, Ihle F, Derendorf H. Pharmacokinetic/pharmacodynamic profile of voriconazole. Clin Pharmacokinet. 2006;45:649–63. doi: 10.2165/00003088-200645070-00002. [DOI] [PubMed] [Google Scholar]
- 11.den Hollander JG, van Arkel C, Rijnders BJ, Lugtenburg PJ, de Marie S, Levin MD. Incidence of voriconazole hepatotoxicity during intravenous and oral treatment for invasive fungal infections. J Antimicrob Chemother. 2006;57:1248–50. doi: 10.1093/jac/dkl108. [DOI] [PubMed] [Google Scholar]
- 12.Purkins L, Wood N, Ghahramani P, Love ER, Eve MD, Fielding A. Coadministration of voriconazole and phenytoin: pharmacokinetic interaction, safety, and toleration. Br J Clin Pharmacol. 2003;56(Suppl 1):37–44. doi: 10.1046/j.1365-2125.2003.01997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel AR, Turner ML, Baird K, et al. Voriconazole-induced phototoxicity masquerading as chronic graft-versus-host disease of the skin in allogeneic hematopoietic cell transplant recipients. Biol Blood Marrow Transplant. 2009;15:370–6. doi: 10.1016/j.bbmt.2008.12.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vadnerkar A, Nguyen MH, Mitsani D, et al. Voriconazole exposure and geographic location are independent risk factors for squamous cell carcinoma of the skin among lung transplant recipients. J Heart Lung Transplant. 2010;29:1240–4. doi: 10.1016/j.healun.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 15.Zonios DI, Gea-Banacloche J, Childs R, Bennett JE. Hallucinations during voriconazole therapy. Clin Infect Dis. 2008;47:e7–10. doi: 10.1086/588844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imhof A, Schaer DJ, Schanz U, Schwarz U. Neurological adverse events to voriconazole: evidence for therapeutic drug monitoring. Swiss Med Wkly. 2006;136:739–42. doi: 10.4414/smw.2006.11547. [DOI] [PubMed] [Google Scholar]
- 17.Pascual A, Csajka C, Buclin T, et al. Challenging recommended oral and intravenous voriconazole doses for improved efficacy and safety: population pharmacokinetics-based analysis of adult patients with invasive fungal infections. Clin Infect Dis. 2012;55:381–90. doi: 10.1093/cid/cis437. [DOI] [PubMed] [Google Scholar]
- 18.Wang TF, Wang T, Altman R, et al. Periostitis secondary to prolonged voriconazole therapy in lung transplant recipients. Am J Transplant. 2009;9:2845–50. doi: 10.1111/j.1600-6143.2009.02837.x. [DOI] [PubMed] [Google Scholar]
- 19.Trifilio S, Ortiz R, Pennick G, et al. Voriconazole therapeutic drug monitoring in allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2005;35:509–13. doi: 10.1038/sj.bmt.1704828. [DOI] [PubMed] [Google Scholar]
- 20.Weiss J, Ten Hoevel MM, Burhenne J, et al. CYP2C19 genotype is a major factor contributing to the highly variable pharmacokinetics of voriconazole. J Clin Pharmacol. 2009;49:196–204. doi: 10.1177/0091270008327537. [DOI] [PubMed] [Google Scholar]
- 21.Wang G, Lei HP, Li Z, et al. The CYP2C19 ultra-rapid metabolizer genotype influences the pharmacokinetics of voriconazole in healthy male volunteers. Eur J Clin Pharmacol. 2009;65:281–5. doi: 10.1007/s00228-008-0574-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.