To the Editor—We commend Yang et al [1] for their important work using serology to draw new epidemiological conclusions about the recent epidemic of the novel A(H7N9) influenza virus. By determining the levels of antibody titers against this virus among poultry workers and the general population of Zhejiang province, in which a relatively high number of confirmed infections of A(H7N9) virus occurred, they found that the former have higher hemagglutination inhibition (HI) titers against the A(H7N9) influenza virus, on average, compared to the latter. This is important evidence that subclinical human infections of A(H7N9) virus have occurred among poultry workers, and further supports the role of poultry in the A(H7N9) epidemic in China. Furthermore, finding low antibody titers among the general population confirms the absence of a major “silent” A(H7N9) epidemic.
The authors also reported that postinfection antibody titers appeared to be lower among the hospitalized A(H7N9) patients who died compared to those who survived, and speculated that therefore “the presence of antibodies may improve clinical outcome in infected patients.” However, using the data kindly provided by the authors in their Supplementary Table 1B, we were not able to find evidence that survival was associated with higher antibody titers among the cohort of patients in this study, after adjustment for the time from onset of symptoms to collection of sera. We fitted a nonlinear model using the nls function of R version 3.0.1, with the logarithm of the HI titer as the outcome variable and survival status and a sigmoidal function of time since symptom onset as the explanatory variables. The fitted model is shown against the raw data in Figure 1. There is no statistically significant difference between antibody titers for the fatal vs nonfatal cases (P = .49). After including age in the regression model as a confounder, the association between antibody titer and outcome remained nonsignificant (P = .57).
Figure 1.
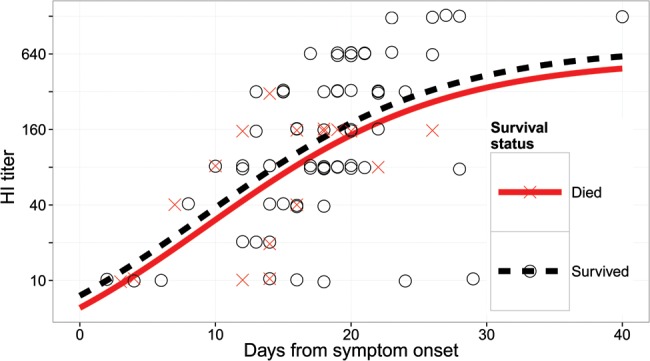
Antibody titers against influenza A(H7N9) virus measured by hemagglutination inhibition (HI) assay as given in Yang et al's Supplementary Table 1B [1], and the fitted model.The crosses correspond to patients with H7N9 who subsequently died, and the circles correspond to those who survived. The solid and dotted lines correspond to the fitted model for patients with H7N9 who died and survived, respectively.
Our observation should not be taken as evidence against the potential role of convalescent sera in treatment of influenza A(H7N9) or other severe influenza virus infections. Passive immunotherapy may still have an important role to play in the response to A(H7N9) and other novel influenza viruses [2], and we agree with the authors that studies on such treatment approaches are urgently needed [3].
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Financial support This work was supported by the Harvard Center for Communicable Disease Dynamics from the National Institute of General Medical Sciences (grant number U54 GM088558), and the Area of Excellence Scheme of the University Grants Committee of Hong Kong (grant number AoE/M-12/06).
Potential conflicts of interest. B. J. C. has received research funding from MedImmune Inc., and consults for Crucell NV. G. F. reports no potential conflicts.
Both authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Yang S, Chen Y, Cui D, et al. Avian-origin influenza A(H7N9) virus infection in influenza A(H7N9)–affected areas of China: a serological study. J Infect Dis. 2014;209:265–9. doi: 10.1093/infdis/jit430. [DOI] [PubMed] [Google Scholar]
- 2.Wu JT, Lee CK, Cowling BJ, Yuen KY. Logistical feasibility and potential benefits of a population-wide passive-immunotherapy program during an influenza pandemic. Proc Natl Acad Sci U S A. 2010;107:3269–74. doi: 10.1073/pnas.0911596107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hung IFN, To KKW, Lee C-K, et al. Hyperimmune iv immunoglobulin treatment: A multicenter double-blind randomized controlled trial for patients with severe 2009 influenza a(h1n1) infection. Chest J. 2013;144:464–73. doi: 10.1378/chest.12-2907. [DOI] [PubMed] [Google Scholar]


