Abstract
Background. Vaccine development has largely focused on the ability of vaccines to reduce disease in individual hosts, with less attention to assessing the vaccine's effects on transmission between hosts. Current acellular vaccines against Bordetella pertussis are effective in preventing severe disease but have little effect on less severe coughing illness that can mediate transmission.
Methods. Using mice that are natural host's of Bordetella bronchiseptica, we determined the effects of vaccination on shedding and transmission of this pathogen.
Results. Vaccination with heat-killed whole-cell B. bronchiseptica or B. pertussis inhibited shedding of B. bronchiseptica. Differences in neutrophil and B-cell recruitment distinguished sham-vaccine from whole-cell–vaccine responses and correlated with shedding output. Both B and T cells were essential for vaccine-induced control of shedding. Adoptive transfer of antibodies was able to limit shedding, while depletion of CD4+ T cells led to increased shedding in vaccinated mice. Finally, whole-cell vaccination was able to prevent transmission, but an acellular vaccine that effectively controls disease failed to control shedding and transmission.
Conclusions. Our results highlight discrepancies between whole-cell and acellular vaccination that could contribute to the increased incidence of B. pertussis infection since the transition to the use of acellular vaccination.
Keywords: Bordetella, Vaccination, Transmission
The incidence of whooping cough, once a common and deadly childhood disease, was greatly reduced following the introduction of whole-cell vaccines in the late 1940s [1]. However, concern about their side effects led to a transition to acellular vaccines in the 1980s [2]. Subsequently, the incidence of whooping cough has increased to levels 50-fold higher than the all-time low in the United States, in 1976 [3]. In many countries, even those with high vaccine coverage, B. pertussis continues to spread and cause periodic epidemics [4–9], raising questions about the effects of vaccines on the spread of disease.
Acellular vaccines provide protection against the most severe forms of whooping cough but are less effective against B. pertussis infections associated with milder forms of coughing illness [1, 4]. Consequently, there is debate about whether acellular vaccines induce the most effective type of immune response to protect vaccinated individuals and prevent the spread of disease [10]. Both whole-cell and acellular vaccines are able to generate high levels of antibodies towards the bacterial components present in each. But only the whole-cell vaccine efficiently activates T-helper type 1 (Th1) cells that generate an effective interferon γ (IFN-γ) response that is important in the control and clearance of B. pertussis infection [11, 12]. Acellular vaccination creates a largely Th2 and Th17 response that is less effective in animal models, potentially explaining the increased incidence coinciding with the switch to acellular vaccines [10, 12]. To date, these analyses have been limited to studies within individuals. However, the observed differences in response would also be expected to affect the inflammatory response that could contribute to symptoms such as coughing and sneezing, the primary mechanisms of transmission of B. pertussis. Therefore, the different effects of the 2 vaccines could also contribute to the observed increase in incidence by affecting the transmission of B. pertussis, although these effects have not been measured experimentally.
Detailed molecular studies of Bordetella pathogenesis have been performed in the mouse model, because of its simplicity and reproducibility, and findings have been consistent with those from the limited work done in humans and other animals [1, 13]. In many cases, Bordetella bronchiseptica, a closely related subspecies of B. pertussis that naturally infects a wide range of mammalian hosts, including humans and mice, has been used as a model system to study the infectious process [1, 14]. Since B. bronchiseptica naturally infects mice, both interactions between bacterial factors and host immune functions can be probed to the molecular level during the infectious process. These infection models have focused on the interactions between bacteria and an experimentally inoculated host, largely avoiding the defining characteristic of infectious disease: transmission. To overcome this limitation, we have recently developed a transmission system in mice, in which we have demonstrated the importance of innate immune activity regulated by Toll-like receptor 4 (TLR4) in limiting the transmission of B. bronchiseptica [15]. Defects in TLR4 led to an increase in both the infectiousness of the infected individual (ie, shedding) and the susceptibility of the host (ie, colonization of susceptible mice) in this model. Importantly, this work has provided an experimental system in which transmission can be studied experimentally, allowing direct measurement of the effects of vaccines on various aspects that contribute to transmission.
In this study, we examine the effects of vaccination on transmission and examine the immune mechanisms involved in these effects. Consistent with expectations, whole-cell vaccines (involving either B. pertussis or B. bronchiseptica) induce an immune response that limits shedding and blocks transmission from inoculated index cases. Both memory CD4+ T cells and antibodies are implicated in the control of shedding. Whole-cell vaccination was effective in limiting the shedding and infectivity of B. bronchiseptica, while acellular vaccination was less effective. Together these results suggest that the resurgence of B. pertussis could be due to 2 deficiencies of the acellular vaccines: failure to protect the vaccinated individual from infection, only blunting the severity of disease, and failure to prevent the transmission of B. pertussis. The different effects of vaccines on individual and herd immunity should be important considerations for the next generation of B. pertussis vaccines.
MATERIALS AND METHODS
Ethics Statement
This study was performed in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee at The Pennsylvania State University (number 40029). All animals were anesthetized using isoflurane or euthanized using carbon dioxide inhalation, to minimize animal suffering.
Mice
C57/B6, C3/HeJ, MµMT−/−, and TCRβδ−/− mice aged 4–6 weeks were obtained from The Jackson Laboratories and maintained in our specific-pathogen-free facility.
Vaccination
Liquid cultures were grown to 1 × 109 colony-forming units (CFUs)/mL. Bacteria were heat killed at 65°C for 30 minutes. Vaccinated mice were intraperitoneally injected with sham vaccine (phosphate-buffered saline [PBS]), 2 × 108 heat-killed bacteria in 200 µL of PBS (referred to hereafter as whole-cell vaccine), or Adacel (referred to hereafter as acellular vaccine; Sanofi Pasteur) at one-fifth the human dose on days 0 and 14. Challenge occurred on day 35 after initial vaccination.
Bacterial Strains
B. bronchiseptica strain RB50 [16] and B. pertussis strain 536 [17] were maintained on Bordet-Gengou agar with 10% defibrinated sheep blood (Hema Resources) and 20 µg/mL streptomycin (Sigma-Aldrich). Liquid cultures were grown in Stainer-Scholte broth at 37°C to mid-log phase.
Inoculation
Inocula were prepared from mid-log phase liquid cultures. Cultures were diluted to 3 × 104 CFUs/mL. Mice were anesthetized with 5% isoflurane in oxygen, and a 5-µL droplet was placed on nares for infection.
Flow Cytometry
A total of 10 mL of PBS was used to perfuse systemically. Nasal bones were excised and placed in 1 mL of Dulbecco's modified Eagle's medium (5% fetal bovine serum [FBS] plus 1 mg/mL collagenase D [Roche]). Samples were incubated for 45 minutes at 37°C and passed through a 70-µm mesh screen to form a single-cell suspension. Samples were resuspended in 200:1 Fc Block (BD Biosciences) in PBS plus 2% FBS and incubated on ice for 20 minutes. Cells were incubated in the following antibodies in PBS plus 2% FBS: anti-CD45:V500 (BD Bioscience), anti-CD11b:Horizon V450 (BD Bioscience), anti-Ly6G: APC-Cy7 (BD Bioscience), anti-CD11c:Fitc (BD Bioscience), Anti-F480:PE-Cy7 (E Bioscience) Anti I-Ad:PE (E Bioscience), anti-CD3:APC (E Bioscience), anti-CD19:PerCP (BioLegend).
Quantification of Bacteria
Shedding of bacteria was determined by swabbing the external nares with a Dacron-tipped swab (VWR) for 15 seconds. Swabs were placed into 1 mL of PBS and vortexed, whereas tissues were harvested and manually homogenized in 1 mL of PBS before being cultured on Bordet-Gengou agar.
Adoptive Transfer
For adoptive transfer, C57/B6 mice were vaccinated, and serum was collected on day 28 after death from vaccinated or naive mice. A total of 200 µL of vaccine-induced serum or naive serum was injected intraperitoneally into MµMT−/− mice directly before challenge with B. bronchiseptica.
Depletion of CD4+ T Cells
CD4+ T cells were depleted by intraperitoneal injection of 0.5 mg of monoclonal antibody from the GK1.5 hybridoma [18] 24 hours before inoculation and every other day thereafter until completion of the time course (day 21).
Antibody Detection
For detection of B. bronchiseptica–specific antibodies, mice were euthanized, and 100 µL of PBS was washed through the nasal cavity several times and then analyzed by enzyme-linked immunosorbent assay, using secondary antibodies for mouse immunoglobulin.
Transmission Studies
To determine transmission, a vaccinated single mouse (index) was inoculated and placed in a cage containing 3 susceptible animals. To monitor transmission, susceptible mice were swabbed every other day for detection of shedding until completion of the time course. Observed shedding was counted as a transmission event on the day detected and was confirmed by dissection and culture of the nasal cavity tissue on day 21 (limit of detection, 5 CFUs).
Statistical Analysis
Time-course analysis was analyzed by fit to a generalized linear model (GLM) to determine the effect of treatment over the time course. The effect at individual time points was determined by analysis of variance, using a Tukey simultaneous test for significance. Minitab, version 16, was used for all analyses.
RESULTS
Whole-Cell Vaccination Reduces Shedding
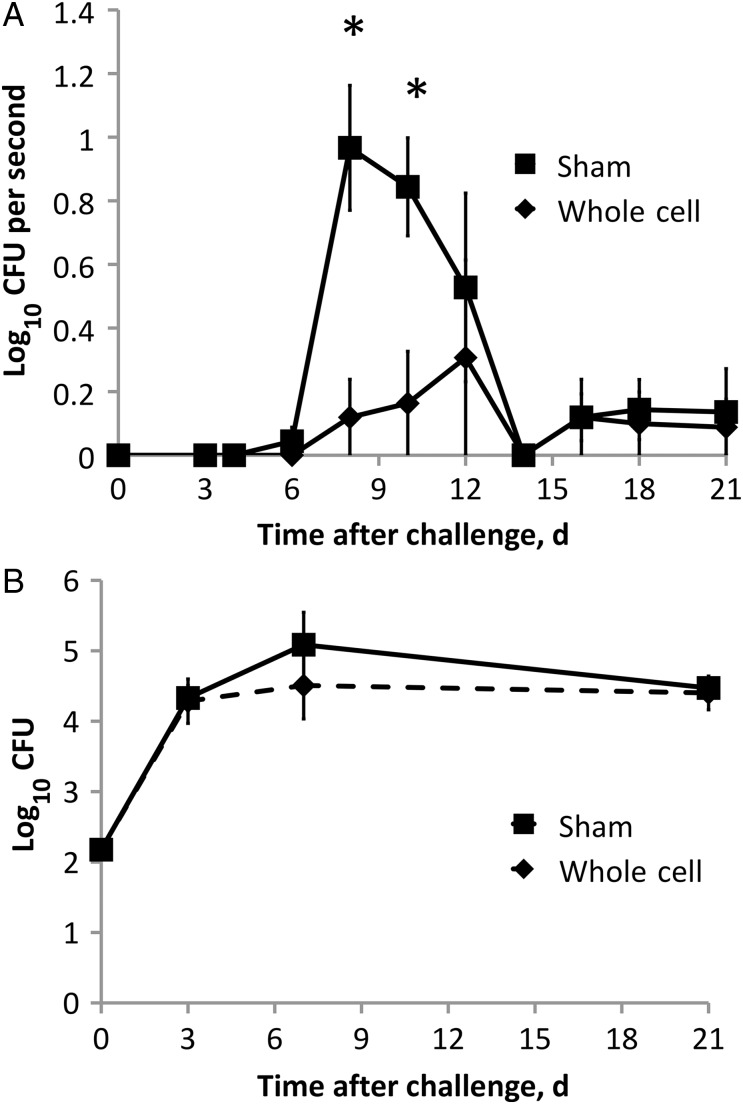
To determine whether vaccination affects shedding of bacteria from the nares, groups of C57/B6 mice were vaccinated with whole-cell B. bronchiseptica or sham vaccine. Thirty-five days after initial vaccination, mice were challenged with 150 bacteria in 5 µL of PBS. External nares were swabbed to quantify shedding periodically over a 3-week period after challenge. Sham-vaccinated mice shed 10 to 1000 bacteria at each time point sampled between days 6 and 14, with numbers peaking on day 8. Whole-cell vaccination decreased bacterial shedding by >90% (P ≤ .01) during the peak period of shedding and throughout the time course (GLM; F(10,66) = 4.49; P < .001; Figure 1A). To determine whether the decrease in shedding was due to reduced bacterial colonization, groups of similarly treated mice were euthanized on days 3, 7, and 21. Surprisingly, the bacterial burdens in the nares of whole-cell–vaccinated and sham-vaccinated mice were indistinguishable (Figure 1B). Together these data suggest that vaccination affects shedding by some mechanism other than reducing bacterial numbers.
Figure 1.
Vaccination affects Bordetella bronchiseptica shedding but not colonization. A, Groups of 4 whole-cell (♦) or sham-vaccinated (▪) mice were challenged, and shedding was detected by swabbing the external nares for 15 seconds throughout the infectious course. B, Nasal cavity colonization was quantified on days 3, 7, and 21 after challenge. Symbols represent the mean colony-forming units (CFUs) + 1 (± standard error of the mean). *P ≤ .05.
Immune Cell Recruitment Correlates With Shedding Output
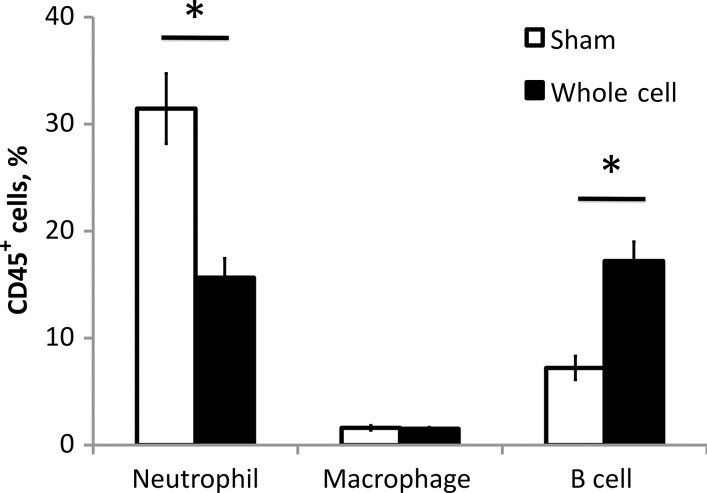
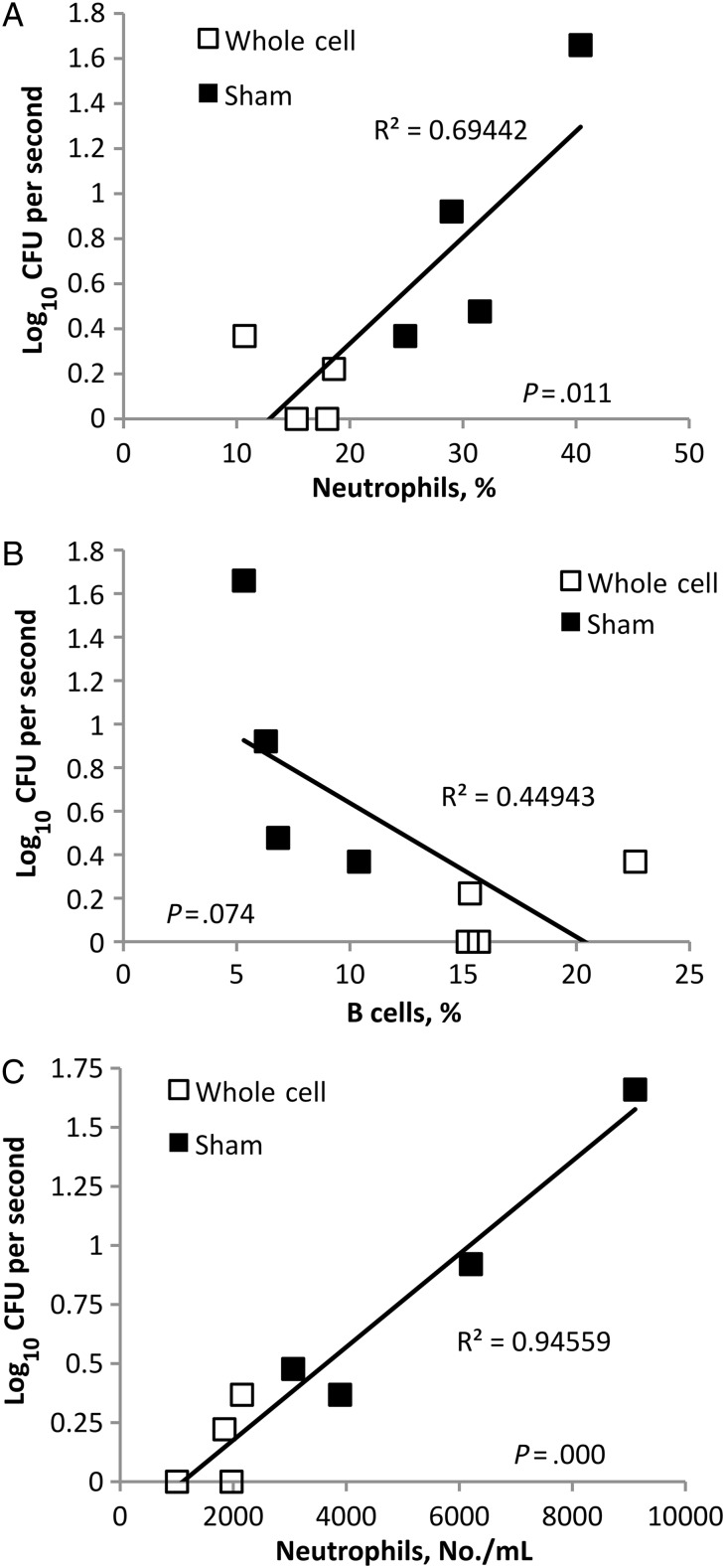
The immune response can affect symptoms that are known to contribute to shedding and transmission, such as coughing and sneezing. Vaccination, which can lead to rapid activation of immune components at the site of infection, could affect shedding in multiple different ways. To understand the effects of vaccination on the nasal cavity leukocyte response, nasal cavities of B. bronchiseptica inoculated sham- or whole-cell–vaccinated C57/B6 mice were analyzed at the peak of shedding, on day 7, by flow cytometry. Significant changes in the total leukocyte population were observed in whole-cell–vaccinated mice as compared to sham-vaccinated mice. Significant increases in the percentage of B cells (P ≤.01) and decreases in the percentage of neutrophils (P ≤ .01) were observed in the nasal cavity of whole-cell–vaccinated mice (Figure 2). To determine whether these changes correlate with the shedding output of mice, shedding from each mouse was determined before it was euthanized. Increases in numbers of B cells within the nasal cavity correlated with the decrease in shedding observed in vaccinated animals (P = .074), whereas increased neutrophil recruitment correlated to increased shedding output (P < .05; Figure 3). These results suggest that vaccination alters the recruitment of immune cells, making the environment less conducive to shedding of B. bronchiseptica.
Figure 2.
Vaccination alters the recruitment of B cells and neutrophils to the nasal cavity during Bordetella bronchiseptica infection. Four whole-cell or sham-vaccinated mice were assayed 7 days after challenge with B. bronchiseptica. Neutrophil, macrophage, and B-cell numbers in the nasal cavity were quantified by flow cytometry. Bars represent mean ± standard error. *P ≤ .05.
Figure 3.
Recruitment of B cells and neutrophils correlates with shedding output during Bordetella bronchiseptica infection. Groups of 4 whole-cell B. bronchiseptica–vaccinated (□) or sham-vaccinated (▪) mice were swabbed on day 7 after inoculation, and bacteria shed was plotted as a function of nasal cavity neutrophil (A) or B cell (B) percentages. C, Shedding is plotted as a function of the number of neutrophils per milliliter of blood. Abbreviation: CFU, colony-forming unit.
Vaccines Induce Adaptive Immunity That Limits Shedding
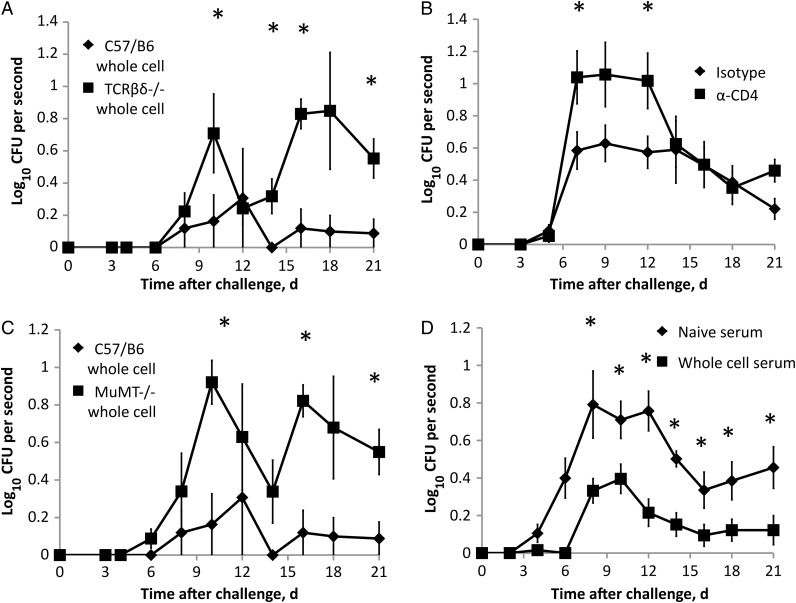
The immune memory generated by vaccination is mediated by B and T cells, which are important in directing an immune response that ultimately controls and clears the infection, but the distinct roles of B and T cells during shedding are not defined. Sham-vaccinated mice shed larger numbers of bacteria earlier in infection, but those numbers decreased to near the limit of detection on day 14. On the basis of their role in regulating inflammation, we hypothesized that memory CD4+ T cells play an important role in this reduction in shedding over time and in the vaccine-induced reduction in shedding. To determine whether T cells are involved in the effect whole-cell vaccination has on shedding, we compared wild-type (C57/B6) mice and T cell-deficient (TCRβδ−/−) mice. As before, vaccination of C57/B6 mice was effective at reducing shedding throughout the time course (Figure 4A). However, vaccination did not reduce shedding in TCRβδ−/− mice throughout the time course (GLM; F(10,66) = 3.48; P = .001). Whole-cell vaccination of TCRβδ−/− mice failed to limit shedding even after day 14, indicating that T cells are important in the control of shedding in naive animals. To look specifically at the function of CD4+ T cells, whole-cell vaccinated wild-type mice were administered an isotype control or an anti-CD4 antibody before challenge and throughout the time course. Depletion of CD4+ T cells in whole-cell–vaccinated mice increased shedding, as compared to mice given an isotype control (GLM; F(7,112) = 1.88; P = .079; Figure 4B). Depletion of CD4+ T in whole-cell-vaccinated mice resulted in shedding similar to those of sham-vaccinated mice (Figure 1A).
Figure 4.
Adaptive immune components limit shedding of Bordetella bronchiseptica during infection. A, Groups of 4 whole-cell–vaccinated C57/B6 (♦) or TCRβδ−/− (▪) mice were inoculated, and shedding was detected throughout the infectious course. B, Depletion of CD4+ T cells from groups of 5 whole-cell–vaccinated C57/B6 mice. C, Groups of 4 whole-cell–vaccinated C57/B6 (♦) or MµMT−/− (▪) mice were inoculated, and shedding was detected throughout the infectious course. D, Adoptive transfer of whole-cell–induced serum (▪) or naive serum (♦) to groups of 8 MµMT−/− mice; shedding was detected throughout the infectious course. Symbols represent the mean colony-forming units (CFUs) + 1 (± standard error of the mean) of B. bronchiseptica. *P ≤ .05.
To evaluate the effects of B cells recruited to the site of infection on the control of shedding, mice lacking B cells (MuMT−/−) received whole-cell vaccine and were compared to C57/B6 whole-cell–vaccinated mice. Vaccinated mice lacking B cells shed higher numbers of bacteria than wild-type–vaccinated mice from day 9 through at least day 21.
(GLM; F(10,66) = 2.90; P = .005; Figure 4C). The whole-cell vaccine induced an increase in anti–B. bronchiseptica immunoglobulin antibody titers within the nasal cavity of vaccinated mice, compared with sham vaccine (Supplementary Figure 1). To determine whether antibodies alone were sufficient to control shedding, antibodies induced by whole-cell vaccine were adoptively transferred into naive MuMT−/− mice before challenge. Adoptive transfer of vaccine-induced immune serum decreased shedding throughout the time course, compared with adoptively transferred naive serum (GLM; F(10,162) = 2.16; P = .022; Figure 4D), indicating that antibodies play an important role in the control of shedding. Together these results indicate that altered host response guided by antibodies and memory CD4+ T cells can limit the successful shedding of B. bronchiseptica.
Acellular Vaccination Fails to Inhibit Shedding
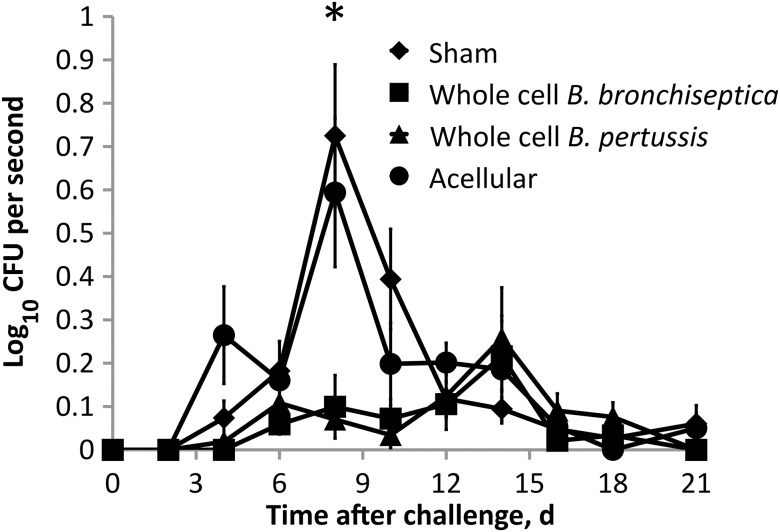
Although most of the world still uses whole-cell vaccines, they are no longer used in countries such as the United States, which now exclusively uses acellular vaccines that are known to induce different immunity than whole-cell vaccines. To experimentally test the effects of an acellular vaccine on shedding, we used a commercially available B. pertussis acellular vaccine, Adacel. Four of the 5 antigens in Adacel are highly conserved and expressed in both B. pertussis and B. bronchiseptica (Pertacin, Fim 2 and 3, and filamentous hemagglutinin). Previous studies show that Adacel is effective at controlling disease caused by B. bronchiseptica [19] and induces anti–B. bronchiseptica antibodies (Supplementary Figure 2). Mice were vaccinated and boosted as before with either sham, whole-cell B. bronchiseptica vaccine, whole-cell B. pertussis vaccine, or the acellular Adacel vaccine and challenged 35 days after the initial vaccination. Sham-vaccinated mice shed 10 to 1000 bacteria at each time point sampled between days 6 and 14 (Figure 5), as reported above (Figure 1). Vaccination with whole-cell B. pertussis or whole-cell B. bronchiseptica reduced shedding by >90% on day 8 (P ≤ .05). In contrast, acellular vaccination did not significantly affect shedding in C57/B6 mice (Figure 5) or C3/HeJ mice (Supplementary Figure 3). These findings highlight important differences in vaccines that could dramatically affect transmission rates.
Figure 5.
Acellular vaccination is ineffective at inhibiting shedding during Bordetella bronchiseptica infection. Groups of 4 mice vaccinated with whole-cell B. bronchiseptica, whole-cell B. pertussis, acellular vaccine (Adacel), or phosphate-buffered saline (sham) were challenged, and shedding of B. bronchiseptica was detected throughout the infectious course. Symbols represent the mean colony-forming units (CFUs) +1 of B. bronchiseptica (± standard error of the mean).*P ≤ .05.
Whole-Cell Vaccination Controls Transmission
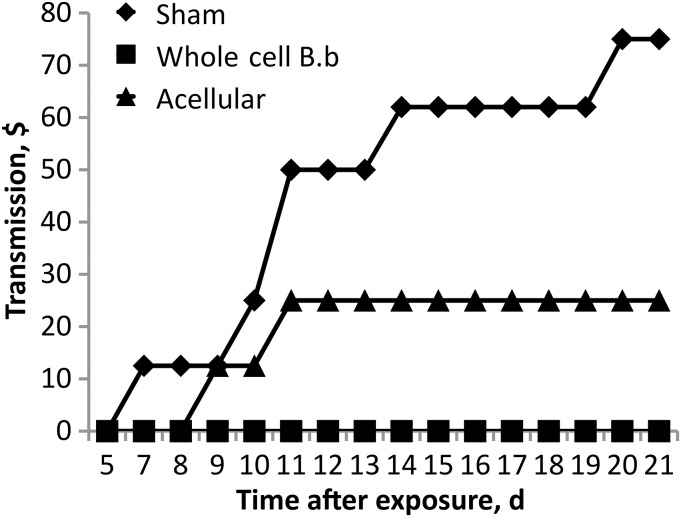
Based on the discrepancy between the effects of vaccines on shedding, their effects on transmission were determined using our recently developed transmission model in C3/HeJ (TLR4-deficient) mice [20]. Sham-, whole-cell–, or acellular-vaccinated mice were inoculated with B. bronchiseptica (index mice) and placed in cages with naive, unvaccinated mice (susceptible mice). Susceptible mice were then monitored via swabbing to determine when transmission events occurred. Sham-vaccinated mice readily transmitted to 6 of 8 exposed cage mates within 21 days, with the majority of transmissions occurring before day 14 (Figure 6). Vaccination of index mice with whole-cell B. bronchiseptica vaccine was effective at inhibiting transmission to secondary mice, as 0 of 8 exposed secondary mice became colonized. Vaccination with acellular Adacel, however, was less effective at controlling transmission to secondary mice, with 3 of 8 exposed mice becoming colonized relatively early in the time course (Figure 6). Together the results demonstrate that vaccination can substantially affect transmission to exposed animals. Furthermore, these results identify potentially important differences between whole-cell and acellular vaccines in an experimental model that could be used to develop vaccines that induce both individual and herd immunity by preventing transmission.
Figure 6.
Whole-cell vaccination but not acellular vaccination is effective at controlling transmission to susceptible mice. Percent transmission of Bordetella bronchiseptica to susceptible mice after cohousing is compared for mice previously vaccinated with whole-cell vaccine, acellular vaccine, or phosphate-buffered saline (sham). Transmission events were determined by observation of shedding from susceptible mice.
DISCUSSION
The rapid decline in whooping cough clinical cases after the introduction of the whole-cell vaccine suggested that herd immunity played some role in its decline. However, it has been suggested that whole-cell and acellular pertussis vaccines do not provide herd immunity, as cases increased significantly after vaccine scares in countries such as the United Kingdom [21]. Recent clinical observations conversely point to short-lived immunity created by the acellular vaccine as having drastic effects on the rates of the disease [22, 23]. Clinical studies conducted in cohorts from Australia during the transition to acellular vaccines determined that receiving an initial whole-cell vaccine had significant effect on reducing the incidence of pertussis, compared with receiving only the acellular vaccine [24]. Furthermore, recent clinical data have suggested that adolescent boosting effects the rate of whooping cough hospitalizations in infants, although the study did not distinguish between adolescents initially vaccinated with whole-cell or acellular vaccines [25]. These findings suggest that whole-cell vaccines provide improved priming and longer immunity that ultimately affects the incidence of whooping cough cases. Here we suggest an experimental mechanism in which current vaccines prevent disease but are less effective than their whole-cell counterparts at reducing transmission rates.
Ultimately, control of whooping cough is dependent on understanding the transmission mechanisms of Bordetella in naive and immune populations. Currently, both human and baboon experimental models lack the powerful immunological tools of the mouse model and therefore cannot similarly probe the immunological mechanisms involved in the effects of vaccination of transmission [26]. Understanding these mechanisms is necessary before there can be rational design of improved vaccines and therapeutic interventions to block transmission.
Although whole-cell vaccination did not reduce bacterial numbers, it prevented transmission of B. bronchiseptica, decreasing both the intensity and duration of shedding from infected animals. The adaptive immune response generated by whole-cell vaccination altered the recruitment of specific immune cell types to the site of infection. Reduced shedding from whole-cell vaccinated mice corresponded with an influx of B cells to the site of infection. Shedding intensity increases correlated with increased neutrophil infiltrate; vaccination reduced neutrophil numbers and shedding of bacteria. B cells were required for vaccine-induced reduction of shedding, and adoptive transfer of whole-cell induced antibodies was sufficient to reduce shedding. Cell depletion of CD4+ T cells demonstrated that these cells that control recruitment and inflammation are also an important component in the control of shedding. We were surprised to determine that an acellular vaccine previously found to affect pathology and colonization of the lungs was ineffective at inhibiting shedding and transmission. This finding has important implications and could partly explain the recent rise in the incidence of whooping cough cases.
Adaptive immunity plays an important role in controlling shedding past day 14 in unvaccinated animals (Figure 1). The type of immune responses induced by different vaccines affect the outcome of infection. A Th1 response is characteristic of whole-cell vaccination [27]. IFN-γ secretion during a Th1 response can lead to the activation of phagocytic cells clearing infection of B. pertussis [28]. Acellular vaccination, however, induces a Th2 response characterized by interleukin 4 secretion and a strong antibody response [29]. The largely Th2 response generated by acellular vaccines has been hypothesized to lower efficiency, compared with the largely Th1 whole-cell vaccines [10]. On the basis of the important role of this cell type in our mouse model, we hypothesize that the whole-cell vaccination–induced Th1 response is also responsible for the control of shedding. Results from a recent vaccine study in the baboon model similarly show that acellular whooping cough vaccines only blunt the severity of disease within individuals, failing to inhibit transmission [30]. Using this mouse model, future studies can determine what immune components whole-cell vaccines are activating to control transmission and why acellular vaccines are unable to. Similarly, new vaccine targets and adjuvants can be assessed for their effects on pathology, as well as on shedding and transmission.
Pathogens are known to manipulate the host response to benefit their fitness. Salmonella enterica serotype Typhimurium stimulates an inflammation response to compete with other host microbiota and enhance its transmission [31]. Immune regulation by either the host [32] or the pathogen [33] can have important effects on shedding. Memory CD4+ cells have been shown to reduce neutrophil infiltration during persistent S. Typhimurium infection, in which CD4+ T cell exhaustion can trigger release of large numbers of the pathogen in the feces of mice [34]. Our findings suggest that CD4+ T cells play an important role in controlling shedding, likely by their effects on the inflammatory response. Previous studies have shown that immune sera is sufficient for clearing B. bronchiseptica from the lower respiratory tract but not the upper respiratory tract of mice [35]. Here we observe an increase in B. bronchiseptica–specific antibodies within the nasal cavity of mice but little effect of those antibodies on colonization. Those antibodies, however, do have an effect on the shedding of B. bronchiseptica from the nares of mice, likely by helping to control inflammation [36, 37].
Cases of Bordetella-related coughing illnesses are increasing. There are several theories as to why this is occurring, from vaccine-driven antigenic shift to inefficient vaccine response. The data presented here support an alternative explanation: current vaccines do not effectively prevent transmission of Bordetella and thus fail to confer the full benefits of herd immunity in reducing clinical cases. Despite acellular vaccination being able to protect the majority of individuals from severe disease, increases in whooping cough cases coincide with the increased use of acellular vaccines beginning in the 1980s. It is possible that the effects of acellular vaccination could mask symptoms to allow infected individuals to act as unsuspecting reservoirs for potential spread to more-susceptible individuals. Importantly, full implementation of acellular-only vaccination began relatively recently, in the 1990s. Therefore, the proportion of the population that has only received the acellular vaccine will continue to rise for several years to come, raising the possibility that we may observe further increases in whooping cough cases.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgment. We thank Dr Andy Gunderson for providing α-CD4 neutralizing antibodies.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by the National Institutes of Health (grant GM083113 to E. T. H.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Mattoo S, Cherry JD. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin Microbiol Rev. 2005;18:326–82. doi: 10.1128/CMR.18.2.326-382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cherry JD. Pertussis: challenges today and for the future. PLoS Pathog. 2013;9:e1003418. doi: 10.1371/journal.ppat.1003418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen A. Public health. The pertussis paradox. Science. 2013;341:454–5. doi: 10.1126/science.341.6145.454. [DOI] [PubMed] [Google Scholar]
- 4.Crowcroft NS, Stein C, Duclos P, Birmingham M. How best to estimate the global burden of pertussis? Lancet Infect Dis. 2003;3:413–8. doi: 10.1016/s1473-3099(03)00669-8. [DOI] [PubMed] [Google Scholar]
- 5.Crowcroft NS, Booy R, Harrison T, et al. Severe and unrecognised: pertussis in UK infants. Arch Dis Child. 2003;88:802–6. doi: 10.1136/adc.88.9.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guris D, Strebel PM, Bardenheier B, et al. Changing epidemiology of pertussis in the United States: increasing reported incidence among adolescents and adults, 1990–1996. Clin Infect Dis. 1999;28:1230–7. doi: 10.1086/514776. [DOI] [PubMed] [Google Scholar]
- 7.de Greeff SC, de Melker HE, van Gageldonk PG, et al. Seroprevalence of pertussis in The Netherlands: evidence for increased circulation of Bordetella pertussis. PLoS One. 2010;5:e14183. doi: 10.1371/journal.pone.0014183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hellenbrand W, Beier D, Jensen E, et al. The epidemiology of pertussis in Germany: past and present. BMC Infect Dis. 2009;9:22. doi: 10.1186/1471-2334-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quinn HE, McIntyre PB. Pertussis epidemiology in Australia over the decade 1995–2005--trends by region and age group. Commun Dis Intell Q Rep. 2007;31:205–15. doi: 10.33321/cdi.2007.31.18. [DOI] [PubMed] [Google Scholar]
- 10.Higgs R, Higgins SC, Ross PJ, Mills KH. Immunity to the respiratory pathogen Bordetella pertussis. Mucosal Immunol. 2012;5:485–500. doi: 10.1038/mi.2012.54. [DOI] [PubMed] [Google Scholar]
- 11.Barbic J, Leef MF, Burns DL, Shahin RD. Role of gamma interferon in natural clearance of Bordetella pertussis infection. Infect Immun. 1997;65:4904–8. doi: 10.1128/iai.65.12.4904-4908.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryan M, Murphy G, Ryan E, et al. Distinct T-cell subtypes induced with whole cell and acellular pertussis vaccines in children. Immunology. 1998;93:1–10. doi: 10.1046/j.1365-2567.1998.00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warfel JM, Merkel TJ. Bordetella pertussis infection induces a mucosal IL-17 response and long-lived Th17 and Th1 immune memory cells in nonhuman primates. Mucosal Immunol. 2012;6:787–96. doi: 10.1038/mi.2012.117. [DOI] [PubMed] [Google Scholar]
- 14.de la Torre MJ, de la Fuente CG, de Alegria CR, Del Molino CP, Aguero J, Martinez-Martinez L. Recurrent respiratory infection caused by Bordetella bronchiseptica in an immunocompetent infant. Pediatr Infect Dis J. 2012;31:981–3. doi: 10.1097/INF.0b013e31825d2e84. [DOI] [PubMed] [Google Scholar]
- 15.Rolin O, Smallridge W, Henry M, Goodfield L, Place D, Harvill E. Toll-like receptor 4 limits transmission of Bordetella bronchiseptica. PLoS One. 2014;9:e85229. doi: 10.1371/journal.pone.0085229. doi:10.1371/journal.pone.0085229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cotter PA, Miller JF. BvgAS-mediated signal transduction: analysis of phase-locked regulatory mutants of Bordetella bronchiseptica in a rabbit model. Infect Immun. 1994;62:3381–90. doi: 10.1128/iai.62.8.3381-3390.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stibitz S, Yang MS. Subcellular localization and immunological detection of proteins encoded by the vir locus of Bordetella pertussis. J Bacteriol. 1991;173:4288–96. doi: 10.1128/jb.173.14.4288-4296.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dialynas DP, Wilde DB, Marrack P, et al. Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK1.5: expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen-reactivity. Immunol Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- 19.Goebel EM, Zhang X, Harvill ET. Bordetella pertussis infection or vaccination substantially protects mice against B. bronchiseptica infection. PLoS One. 2009;4:e6778. doi: 10.1371/journal.pone.0006778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rolin O, Muse SJ, Safi C, et al. Enzymatic modification of the lipid A by an ArnT protects B. bronchiseptica against cationic peptides and is required for transmission. Infect Immun. 2013;82:491–9. doi: 10.1128/IAI.01260-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gangarosa EJ, Galazka AM, Wolfe CR, et al. Impact of anti-vaccine movements on pertussis control: the untold story. Lancet. 1998;351:356–61. doi: 10.1016/s0140-6736(97)04334-1. [DOI] [PubMed] [Google Scholar]
- 22.Lavine J, Bjørnstad O, de Blasio B, Storsaeter J. Short-lived immunity against pertussis, age-specific routes of transmission, and the utility of a teenage booster vaccine. Vaccine. 2012;30:544–51. doi: 10.1016/j.vaccine.2011.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lavine J, Broutin H, Harvill E, Bjørnstad O. Imperfect vaccine-induced immunity and whooping cough transmission to infants. Vaccine. 2010;29:11–6. doi: 10.1016/j.vaccine.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheridan SL, Ware RS, Grimwood K, Lambert SB. Number and order of whole cell pertussis vaccines in infancy and disease protection. JAMA. 2012;308:454–6. doi: 10.1001/jama.2012.6364. [DOI] [PubMed] [Google Scholar]
- 25.Auger KA, Patrick SW, Davis MM. Infant hospitalizations for pertussis before and after Tdap recommendations for adolescents. Pediatrics. 2013;132:e1149–55. doi: 10.1542/peds.2013-1747. [DOI] [PubMed] [Google Scholar]
- 26.Warfel JM, Beren J, Merkel TJ. Airborne transmission of Bordetella pertussis. J Infect Dis. 2012;206:902–6. doi: 10.1093/infdis/jis443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ross PJ, Sutton CE, Higgins S, et al. Relative contribution of Th1 and Th17 cells in adaptive immunity to Bordetella pertussis: towards the rational design of an improved acellular pertussis vaccine. PLoS Pathog. 2013;9:e1003264. doi: 10.1371/journal.ppat.1003264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Torre D, Ferrario G, Bonetta G, Perversi L, Tambini R, Speranza F. Effects of recombinant human gamma interferon on intracellular survival of Bordetella pertussis in human phagocytic cells. FEMS Immunol Med Microbiol. 1994;9:183–8. doi: 10.1111/j.1574-695X.1994.tb00492.x. [DOI] [PubMed] [Google Scholar]
- 29.Ryan EJ, Nilsson L, Kjellman N, Gothefors L, Mills KH. Booster immunization of children with an acellular pertussis vaccine enhances Th2 cytokine production and serum IgE responses against pertussis toxin but not against common allergens. Clin Exp Immunol. 2000;121:193–200. doi: 10.1046/j.1365-2249.2000.01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Warfel JM, Zimmerman LI, Merkel TJ. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc Natl Acad Sci U S A. 2013;111:787–92. doi: 10.1073/pnas.1314688110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winter SE, Thiennimitr P, Winter MG, et al. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature. 2010;467:426–9. doi: 10.1038/nature09415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pathak A, Creppage K, Werner J, Cattadori I. Immune regulation of a chronic bacteria infection and consequences for pathogen transmission. BMC Microbiol. 2010;10:226. doi: 10.1186/1471-2180-10-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wickham M, Brown N, Boyle E, Coombes B, Finlay B. Virulence is positively selected by transmission success between mammalian hosts. Curr Biol. 2007;17:783–8. doi: 10.1016/j.cub.2007.03.067. [DOI] [PubMed] [Google Scholar]
- 34.Gopinath S, Hotson A, Johns J, Nolan G, Monack D. The systemic immune state of super-shedder mice is characterized by a unique neutrophil-dependent blunting of TH1 responses. PLoS Pathog. 2013;9:e1003408. doi: 10.1371/journal.ppat.1003408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kirimanjeswara GS, Mann PB, Harvill ET. Role of antibodies in immunity to Bordetella infections. Infect Immun. 2003;71:1719–24. doi: 10.1128/IAI.71.4.1719-1724.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russell MW, Reinholdt J, Kilian M. Anti-inflammatory activity of human IgA antibodies and their Fab alpha fragments: inhibition of IgG-mediated complement activation. Eur J Immunol. 1989;19:2243–9. doi: 10.1002/eji.1830191210. [DOI] [PubMed] [Google Scholar]
- 37.van der Neut Kolfschoten M, Schuurman J, Losen M, et al. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science. 2007;317:1554–7. doi: 10.1126/science.1144603. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.