Abstract
No vaccines are available for human use for any parasitic infections, including the helminthic disease schistosomiasis. Sm-p80, the large subunit of Schistosoma mansoni calpain, is a leading antigen candidate for a schistosomiasis vaccine. Prophylactic and antifecundity efficacies of Sm-p80 have been tested using a variety of vaccine approaches in both rodent and nonhuman primate models. However, the therapeutic efficacy of a Sm-p80–based vaccine had not been determined. In this study, we evaluated the therapeutic efficacy of Sm-p80 by using 2 different strategies and 3 Sm-p80–based vaccine formulations in baboons. Vaccine formulations were able to decrease established adult worms by 10%–36%, reduce retention of eggs in tissues by 10%–57%, and decrease egg excretion in feces by 13%–33%, compared with control formulations. Marked differences were observed in B and T cell immune correlates between vaccinated and control animals. This is the first report of killing of established adult schistosome worms by a vaccine. In addition to distinct prophylactic efficacy of Sm-p80, this study adds to the evidence that Sm-p80 is a potentially important antigen with both substantial prophylactic and therapeutic efficacies. These data reinforce that Sm-p80 should be moved forward along the path toward human clinical trials.
Keywords: Schistosomiasis, Schistosoma mansoni, Sm-p80, calpain, therapeutic vaccine, nonhuman primate, baboon
Schistosomiasis is of immense public health concern to an estimated 1 billion people in 74 countries: 200 million to 400 million people are currently infected with Schistosoma mansoni, and an additional 779 million individuals are at potential risk for infection [1–6]. Control of the disease is suboptimal, despite significant efforts to eradicate the intermediate snail host, improvements in sanitation infrastructure, and mass administration of praziquantel [7–12]. Currently, there is no vaccine available for human use to prevent or treat schistosomiasis. A vaccine that protects against S. mansoni infection, reduces egg-induced pathology, lessens transmission, and aids in eliminating established schistosome adult worms would be considered a significant milestone in the control and eventual eradication of this major neglected tropical disease. The discovery of Schistosoma mansoni calpain has led to the understanding of the important role of this antigenic protein in surface membrane renewal/recycling, a phenomenon by which schistosomes evade the host immune response [13–15]. Neutralizing immune evasion mechanisms of the worms by vaccination with calpain is therefore a logical target for a potentially efficacious schistosome vaccine. To this effect, the large subunit of calpain, Sm-p80, is now a leading vaccine candidate for immune prophylaxis of S. mansoni, Schistosoma japonicum, and possibly for Schistosoma haematobium infections [16–21]. However, the therapeutic efficacy of an Sm-p80–based vaccine has not yet been fully assessed.
In this study, using a total of 16 baboons, we evaluated the therapeutic efficacy of Sm-p80 via 2 vaccination strategies that included the recombinant protein formulated in adjuvant and a DNA prime/protein boost. The following 3 Sm-p80–based vaccine formulations were used: rSm-p80 plus glucopyranosyl lipid adjuvant (GLA), DNA prime with Sm-p80–VR1020 followed by boost with rSm-p80 in alum, and a prime-boost approach using Sm-p80–VR1020 and rSm-p80 with CpG oligonucleotides. In some of the pilot/exploratory studies, to mimic the natural conditions we used baboons with prevailing intestinal infections with the whipworm, Trichuris, and, in addition, developed a chronic schistosome infection in baboons by repeated challenge with the infectious cercariae (ie, trickle infection). In all of the vaccination experiments, baboons were allowed to develop chronic infection, which was confirmed by the detection of eggs in feces. Once the egg output trend in feces was established, the baboons were vaccinated. Sm-p80–based vaccine formulations reduced the established adult worm burden by 10%–36%, markedly reduced the number of eggs in tissues (ie, liver and intestine) by 10%–57%, and decreased egg excretion in feces by up to 33%, compared with control formulations. This is the first ever report documenting the immune system–mediated killing of established adult schistosome worms by a vaccine in chronically infected animals.
MATERIALS AND METHODS
Animals and Parasites
Male and female baboons (Papio anubis) aged 4.0–13.41 years were obtained from the University of Oklahoma Health Sciences Center (OUHSC) baboon breeding colony and housed in Association for Assessment and Accreditation of Laboratory Animal Care International–accredited facilities at the OUHSC. Animals were prescreened for intestinal and blood parasites and for antibodies that were cross-reactive to Sm-p80. Four baboons with prevailing Trichuris infection but no cross-reactive antibodies to Sm-p80 were also used in this study. The remaining 12 baboons were negative for both the parasites and cross-reactive antibodies. S. mansoni–infected snails (Biomphalaria glabrata) were acquired from the Schistosomiasis Resource Center, Biomedical Research Institute (Rockville, MD). This study was approved by the institutional animal care and use committee of OUHSC.
Preparation of Vaccine Formulations
DNA immunogen was prepared as described previously [21]. Briefly, the full-length coding sequence of Sm-p80 was subcloned into BamHI/BglII sites of VR1020 (Vical, San Diego, CA). For DNA vaccination, Sm-p80–VR1020 plasmid was isolated via a conventional alkaline lysis method and purified on Sepharose CL4B columns. Protein immunogen (recombinant Sm-p80) was generated as described earlier [17]. Briefly, the full-length coding sequence of Sm-p80 was cloned into a pCold II vector (GenScript, Piscataway, NJ), and protein was expressed in Escherichia coli strain BL21 (DE3). The expressed protein was purified via Ni-nitrilotriacetic acid-agarose, followed by a Sephadex G-150 column. Endotoxin levels in both DNA and protein samples were analyzed with a Limulus amebocyte lysate assay (Charles River Laboratories International, Wilmington, MA).
Parasite Challenge, Baboon Vaccinations, and Worm and Egg Burden Determination
The complete schedule of challenge and vaccine formulations and their administration frequencies, as well as the time of baboon necropsies, is outlined in Table 1. Briefly, baboons from each group were infected with 1000 cercariae of S. mansoni to develop the chronic infection. The adjuvants used were GLA oil-in-water emulsion (GLA-SE; a Toll-like receptor 4 [TLR4] agonist–based formulation), alum, and CpG ODN (a TLR9 agonist). The vaccines were injected intramuscularly in the quadriceps. Necropsies and determination of percentage protection were performed as described previously [17, 21].
Table 1.
Immunization Protocol Used to Determine the Therapeutic Efficacy of Sm-p80 Using Recombinant Protein and Prime Boost Approaches for Schistosoma mansoni in Baboons
| Group | First Infection (Week 0), Inoculum |
First Immunization, Formulation (Time) | Second Immunization, Formulation (Time) | Second Infection, Inoculum (Time) | Third Immunization, Formulation (Time) | Necropsy Time |
|---|---|---|---|---|---|---|
| GLA-SE control | 1000 S. mansoni cercariae | GLA-SE (50 µg)(week 16) | GLA-SE (50 µg) (week 20) | … | GLA-SE (50 µg) (week 24) | Week 28 |
| rSm-p80 + GLA-SE | 1000 S. mansoni cercariae | rSm-p80 (250 µg) + GLA-SE (50 µg) (week 16) | rSm-p80 (250 µg) + GLA-SE (50 µg) (week 20) | … | rSm-p80 (250 µg) + GLA-SE (50 µg) (week 24) | Week 28 |
| VR1020 + alum control | 1000 S. mansoni cercariae | VR1020 (500 µg) (week 7) | Alum (1250 µg) (week 11) | … | Alum (1250 µg) (week 15) | Week 22 |
| Sm-p80–VR1020 + rSm-p80 + alum | 1000 S. mansoni cercariae | Sm-p80–VR1020 (500 µg) (week 7) | rSm-p80 (250 µg) +alum (1250 µg) (week 11) | … | rSm-p80 (250 µg) + alum (1250 µg) (week 15) | Week 22 |
| VR1020 + CpG-ODN control | Prevailing Trichuris infection + 1000 S. mansoni cercariae | VR1020 (500 µg) (week 21) | Control CpG-ODN (250 µg) (week 25) | 1000 S. mansoni cercariae and Trichuris (week 27) | Control CpG-ODN (250 µg) (week 31) | Week 38 |
| Sm-p80-VR1020 + rSm-p80 + CpG-ODN10104 | Prevailing Trichuris infection + 1000 S. mansoni cercariae | Sm-p80–VR1020 (500 µg) (week 21) | rSm-p80 (250 µg) + CpG-ODN-10104 (250 µg) (week 25) | 1000 S. mansoni cercariae and Trichuris (week 27) | rSm-p80 (250 µg) + CpG-ODN-10104 (250 µg) (week 31) | Week 38 |
Abbreviation: GLA, glucopyranosyl lipid adjuvant.
Daily Fecal Egg Output Processing and Monitoring
The fecal samples were collected starting 6–8 weeks after infection from each baboon in the control (GLA-SE) and vaccinated (Sm-p80–GLA-SE) groups. The interval of collection was every 3–5 days until the animals were euthanized. A 3–5g fecal specimen was emulsified in 25 mL of 10% formalin. After the emulsion was filtered and centrifuged, it was resuspended in 10 mL of water with the addition of 3 mL of ethyl acetate. After vigorous shaking and centrifugation, the pellet was resuspended in saline. The number of eggs present at each time point was determined, and the total number was calculated for each group for that time point. The mean value corresponding to each group was then used to calculate the percentage reduction in egg production [22].
Collection of Blood Specimens and Peripheral Blood Mononuclear Cell (PBMC) Isolation
Blood specimens were collected just before challenge infection and at the time of every immunization. The collected sera were investigated with an enzyme-linked immunosorbent assay. PBMCs from the blood were isolated using the Histopaque 1077 system (Sigma-Aldrich, St. Louis, MO).
Baboon Sera Antibody Assays
Ninety six–well plates were coated with 1.2 µg/well of recombinant Sm-p80 and incubated overnight at 4°C. Serum samples from each individual animal were used to determine the antibody titers for total immunoglobulin G (IgG), IgG subtypes (IgG1, IgG2, IgG3, and IgG4), immunoglobulin M (IgM), and immunoglobulin A (IgA) as described previously [17, 21, 23]. All of the samples were assayed in triplicate. The curve of antibody titer was drawn as a function of collection time. The highest titer with time point was selected and results were expressed as mean values ± SD.
RNA Extraction, Complementary DNA (cDNA) Synthesis, and Reverse-Transcription Polymerase Chain Reaction (RT-PCR)
PBMCs, splenocytes, and lymph node cells (isolated from the mesenteries) were cultured in the presence or absence of rSm-p80 antigen. The cultures were maintained at 37°C in 5% CO2 for 24 hours. Total RNA was extracted from cells via the TRIzol method (Invitrogen, Carlsbad, CA). Reverse-transcription reactions for the first-strand cDNA synthesis were performed as described previously [21, 24]. Expression levels of different cytokines (interleukin 1α, interleukin 1β, interleukin 2 [IL-2], interleukin 4 [IL-4], interleukin 5, interleukin 6, interleukin 8 [IL-8], interleukin 10 [IL-10], interleukin 12α [IL-12α], interleukin 12β [IL-12β], interleukin 13 [IL-13], interleukin 17 [IL-17], interleukin 18, interleukin 21 [IL-21], interleukin 22 [IL-22], interleukin 23, tumor necrosis factor α [TNF-α], interferon γ [IFN-γ], transforming growth factor β1, transforming growth factor β2 [TGF-β2] and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were determined via Stepone plus real-time PCR system. The relative cytokine messenger RNA (mRNA) expression was calculated, by means of DataAssist, version 3.0, by comparing the differences in the message levels of the control group with those of the respective experimental group after standardization using GAPDH.
Enzyme-Linked Immunosorbent Spot (ELISPOT) Assay
An ELISPOT assay was used to estimate the frequencies of cells secreting IFN-γ, IL-4, and IL-17 following in vitro stimulation with rSm-p80. Briefly, PBMCs from each baboon were seeded (1× 105–2 × 105 cells/100 µL/well) into anti-IFN-γ–, anti-IL-4–, or anti-IL-17–coated 96-well plates (U-cyTech, the Netherlands). The cells were stimulated with 0.5 µg of ConA, 1.2 µg of ovalbumin or 1.2 µg of rSm-p80 and incubated at 37°C with 5% CO2 for 48 hours, allowing for production and capture of released cytokines. A total of 100 µL of biotinylated detection antibody was loaded to each well, and wells were incubated overnight at 4°C. The next day, streptavidin–horseradish peroxidase was added; after 1 hour of incubation at 37°C, freshly prepared AEC (3-amino-9-ethylcarbazole) substrate solution was added, and wells were incubated for 20–30 minutes at room temperature to develop the spots. The reaction was terminated by washing with distilled water. All assays were run in triplicate. The spot-forming units (SFUs) representing single cells were counted using an ELISPOT Bioreader 5000 assay (ImmunoBioSystem, The Colony, TX). Antigen-specific SFUs were calculated in 1 million cells for each group.
Flow Cytometry Analysis
To obtain T-helper type 1 (Th1), Th2, and Th17 cell profiles, PBMCs were seeded into 24-well plates (2 × 106 cells/well). The cells were incubated with or without rSm-p80 (12 μg/mL) overnight; GolgiPlug (1:1000 dilution) was added in the last 10 hours of the incubation period. Cells maintained in phorbol 12-myristate 13-acetate (100 ng/mL) and ionomycin (1 µg/mL) were processed as positive controls. Direct staining was performed to identify CD4+ T cells, using PerCP-Cy5.5–conjugated mouse anti-human CD4. Intracellular staining was performed to detect cells secreting IFN-γ, IL-4, and IL-17, using APC-mouse anti-human IL-4, PE-mouse anti-human IFN-γ, and FITC-mouse anti-human IL-17. All immunological reagents and antibodies were obtained from BD Biosciences (San Diego, CA). Data were collected using CellQuest Pro Software (BD Biosciences, San Diego, CA) and analyzed via FlowJo software (Tree Star, Ashland, OR).
Statistical Analyses
The statistical significance of differences between the 2 groups was calculated via t tests, using SPSS and GraphPad Prism 5. P values obtained by these methods were considered statistically significant if they were <.05.
RESULTS
Reduction in Worm and Egg Burdens Following Therapeutic Administration of Sm-p80–Based Vaccine
The chronic baboon model of intestinal schistosomiasis was established and confirmed by steady egg excretion in feces before the administration of vaccine. The therapeutic vaccinations were administered as outlined in Table 1. Three different vaccination strategies were performed: (1) immunization with rSm-p80 protein formulated in GLA-SE adjuvant, (2) priming with Sm-p80 plasmid DNA and boosting with rSm-p80 protein formulated in alum adjuvant, and (3) priming with Sm-p80 plasmid DNA and boosting with rSm-p80 protein formulated in CpG-ODN adjuvant. The protective effect of Sm-p80 therapeutic vaccine was determined (Table 2). The infected baboons immunized with rSm-p80 formulated in GLA-SE showed 36.06% worm reduction, 53.81% tissue egg reduction, and 33.02% fecal egg reduction, compared with control (Table 2). DNA prime-protein boost approach (Sm-p80–VR1020 followed by rSm-p80 plus alum) showed 10.05% protection, 10.17% decrease in tissue egg load, and only a 15.25% reduction in fecal egg expulsion, compared with control (Table 2). Use of another prime-boost approach (Sm-p80–VR1020 prime and boost with rSm-p80 plus CpG ODNs) resulted in a moderate reduction in worm burden (23.18%), a robust decrease in tissue egg load (57.41%), and a low key egg excretion (13.11%), compared with control (Table 2).
Table 2.
Therapeutic Efficacy of Sm-p80 Using Recombinant Protein and Prime Boost Approaches for Schistosoma mansoni in Baboons
| Group, Baboon | Individual-Level Findings, Mean ± SE |
Group-Level Findings, Mean ± SE |
Percentage Reduction From Controla |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Age, y | Worm Burden, No. | Tissue Egg Burden, Eggs/g | Fecal Egg Burden, Eggs/g | Worm Burden, Worms, No. | Tissue Egg Burden, Eggs/g | Fecal Egg Burden, Eggs/g | Worm Burden | Tissue Egg Burden | Fecal Egg Burden | |
| GLA-SE control | 556.50 ± 72.06 | 6345.01 ± 679.40 | 101.11 ± 5.62 | … | … | … | |||||
| IS84 | F | 13.41 | 415 | 5363.42 ± 1714.28 | 133.75 ± 12.47 | ||||||
| IN61 | F | 4.64 | 727 | 6163.22 ± 1657.71 | 92.21 ± 10.22 | ||||||
| BA02 | M | 9.59 | 462 | 8316.97 ± 3677.79 | 54.95 ± 6.46 | ||||||
| ST32 | M | 8.70 | 622 | 5536.44 ± 2376.92 | 123.51 ± 10.57 | ||||||
| rSm-p80 + GLA-SE | 356.00 ± 39.23 | 2930.70 ± 241.43 | 67.72 ± 3.57 | 36.06 | 53.81 | 33.02 | |||||
| ZO00 | F | 10.99 | 262 | 3054.32 ± 326.94 | 63.42 ± 6.00 | ||||||
| PO96 | M | 9.79 | 336 | 2341.31 ± 837.72 | 59.77 ± 7.01 | ||||||
| ED59 | M | 8.90 | 376 | 3501.46 ± 1187.86 | 69.25 ± 8.16 | ||||||
| AN63 | F | 13.30 | 450 | 2825.72 ± 407.90 | 78.46 ± 7.14 | ||||||
| VR1020 + alum control | 881.00 ± 2.00 | 2509.17 ± 585.83 | 27.32 ± 9.11 | … | … | … | |||||
| ES45 | F | 5.60 | 883 | 3095.00 ± 1665.00 | 23.15 ± 9.40 | ||||||
| KI11 | M | 4.50 | 879 | 1923.33 ± 743.33 | 31.48 ± 17.59 | ||||||
| Sm-p80–VR1020 + rSm-p80 + alum | 792.50 ± 56.50 | 2254.04 ± 848.63 | 23.15 ± 7.94 | 10.05 | 10.17 | 15.25 | |||||
| TO81 | M | 4.34 | 736 | 3102.67 ± 989.33 | 10.19 ± 6.07 | ||||||
| FO45 | M | 4.48 | 849 | 1405.42 ± 972.08 | 36.11 ± 10.52 | ||||||
| VR1020 + CpG- ODN control | 729.00 ± 64.00 | 3440.00 ± 1462.31 | 28.24 ± 7.43 | … | … | … | |||||
| RA75 | M | 4.00 | 665 | 3893.33 ± 2811.22 | 27.78 ± 9.75 | ||||||
| CY62 | F | 4.60 | 793 | 2986.67 ± 1193.17 | 28.70 ± 13.45 | ||||||
| Sm-p80–VR1020 + rSm-p80 + CpG-ODN10104 | 560.00 ± 108.00 | 1465.00 ± 345.70 | 24.54 ± 3.61 | 23.18 | 57.41 | 13.11 | |||||
| ZU03 | F | 5.90 | 452 | 1013.33 ± 421.48 | 24.07 ± 8.07 | ||||||
| LU04 | M | 5.40 | 668 | 1916.67 ± 516.33 | 25.00 ± 0.00 | ||||||
Abbreviations: GLA, glucopyranosyl lipid adjuvant; SE, standard error.
a P ≤ .05 for worm burden, ≤.01 for tissue egg burden, and <.0001 for fecal egg burden.
Daily Fecal Egg Output Trend in Experimental Baboons Before and After Vaccination With Recombinant Protein Formulation
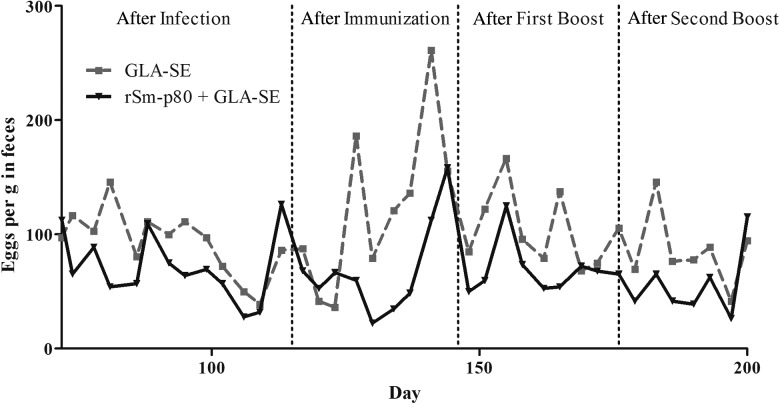
Daily fecal egg output was monitored in the Sm-p80–GLA-SE groups every 3–5 days. A clear trend was observed and egg output in feces decreased significantly following vaccinations (Figure 1).
Figure 1.
Daily fecal egg output trend in chronically infected baboons before and after vaccination with Sm-p80 formulated with a Toll-like receptor 4 agonist–based emulsion, glucopyranosyl lipid adjuvant (GLA)–SE. The fecal samples were collected starting 6–8 weeks after infection from each baboon belonging to adjuvant-only control (GLA-SE) or vaccinated (Sm-p80–GLA-SE) groups. The numbers of eggs in feces were monitored every 3–5 days, starting 72 days after infection and continuing until the animals were euthanized.
Antibody Responses to Sm-p80 in Vaccinated Baboons
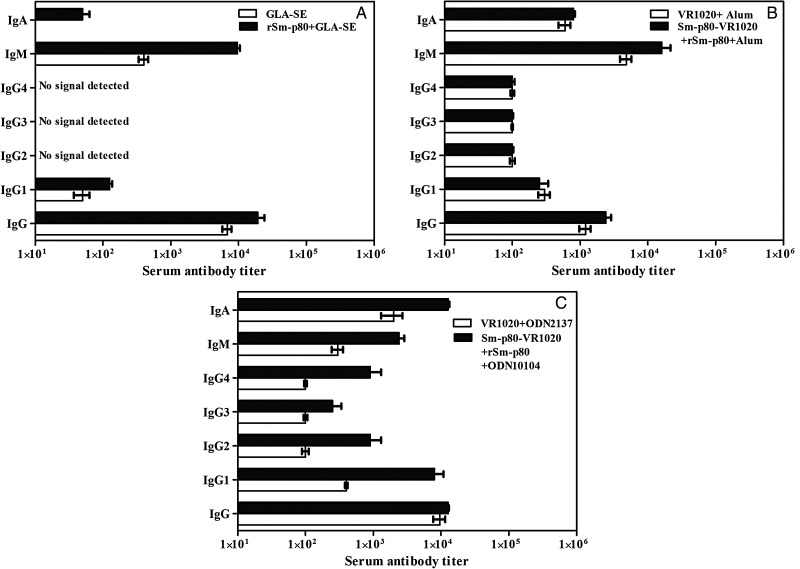
The peak antibody titers recorded (at weeks 24 and 28) in the sera of animals immunized with rSm-p80 formulated in GLA-SE are shown in Figure 2A; for the DNA prime-protein boost approach Sm-p80–VR1020 followed by rSm-p80 plus alum (peak titer, weeks 15 and 22), values are shown in Figure 2B; and for Sm-p80–VR1020 prime and boost with rSm-p80 plus CpG ODNs (peak titer, weeks 20 and 24), values are shown in Figure 2C. For all 3 approaches, robust total IgG titers were detected (Figure 2). IgG2, IgG3, and IgG4 signals were not detected in any of the rSm-p80/GLA-SE groups. For recipients of DNA prime-protein boost formulated in ODN, IgG2 and IgG4 signals were detected, whereas for recipients of DNA prime-protein boost formulated in alum, no differences were found in IgG2, IgG,3 and IgG4 titers between the control and experimental groups. The IgM titer peaked ahead of the second immunization for all the 3 approaches. A distinctly high IgA titer was detected in recipients of DNA prime-protein boost formulated in ODN (Figure 2C). By contrast, lower levels of IgA were recorded in recipients of rSm-p80 formulated in GLA-SE (Figure 2A), and a moderate IgA titer was observed in groups that were primed with DNA and boosted with rSm-p80 formulated in alum (Figure 2B).
Figure 2.
Titers of anti-Sm-p80 antibodies in immunized baboons. Enzyme-linked immunosorbent assays were performed with sera obtained from each baboon (every 4 weeks) in their respective control and experimental groups. The peak antibody titers for total immunoglobulin G (IgG), IgG1, IgG2, IgG3, IgG4, immunoglobulin M (IgM), and immunoglobulin A (IgA) in control and vaccinated groups are shown for each of the 3 vaccination strategies: recombinant protein vaccination using rSm-p80 plus glucopyranosyl lipid adjuvant (GLA)–SE (A), DNA prime boost protein with Sm-p80–VR1020 and rSm-p80 in alum (B), and similar prime and boost approach using Sm-p80–VR1020 and rSm-p80 with CpG ODNs (C). The values represent the mean (±SD) of 3 experiments.
Detection of IFN-γ–, IL-4–, and IL-17–Secreting Cells With rSm-p80 Stimulation, Using ELISPOT
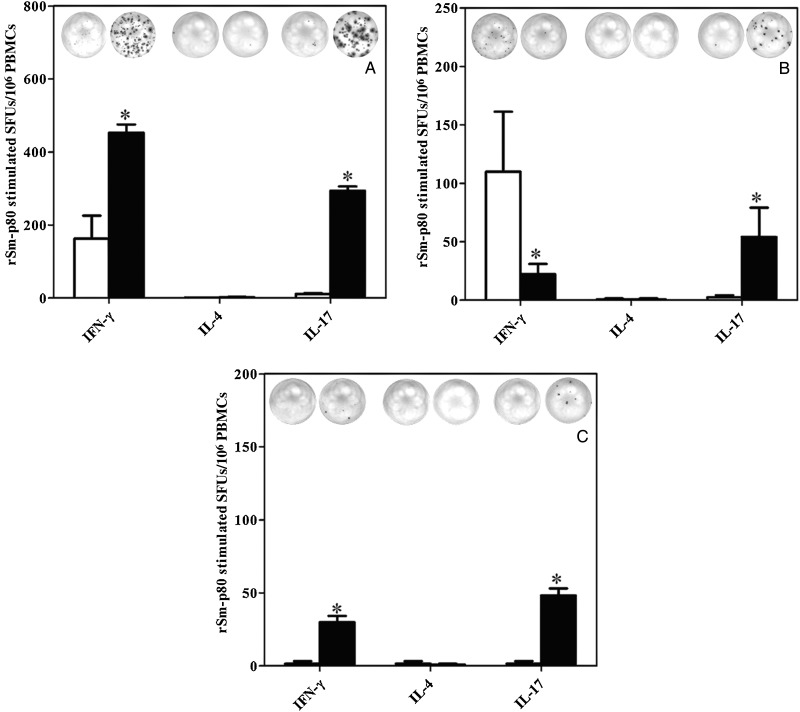
rSm-p80–stimulated SFUs for IFN-γ among recipients of protein formulated in GLA-SE and of DNA prime-protein boost formulated in ODN were 2.78-fold and 17.96-fold higher, respectively, than those for their associated control groups (Figure 3A and 3C). In contrast, rSm-p80–stimulated SFUs for IFN-γ among recipients of DNA prime protein boost formulated in alum were 4.89-fold less than those for their respective control group. For IL-4, very little to no stimulation was recorded for all 3 vaccination approaches (Figure 3A–C). However, for IL-17, appreciable increases in SFUs (range, 21–29-fold) were recorded in experimental groups of all of the 3 vaccination strategies, compared with findings for their respective control groups (Figure 3A–C).
Figure 3.
Detection of interferon γ (IFN-γ), interleukin 4 (IL-4), and interleukin 17 (IL-17) secretory cells in peripheral blood mononuclear cells (PBMCs). IFN-γ–, IL-4–, and IL-17–secreting cells were assayed by an enzyme-linked immunosorbent spot assay. Spot-forming units (SFUs) were calculated in 1 million cells. The SFUs for cells obtained from the control group are shown as unfilled bars, and filled bars represent rSm-p80–stimulated cells from the experimental group. Results for different vaccination strategies are as follows: recombinant protein vaccination using rSm-p80 plus glucopyranosyl lipid adjuvant (GLA)–SE (A), DNA prime followed by protein boost with Sm-p80–VR1020 and rSm-p80 in alum (B), and similar prime boost approach using Sm-p80–VR1020 and rSm-p80 with CpG ODNs (C).
Assessment of Cytokine mRNA Profiles
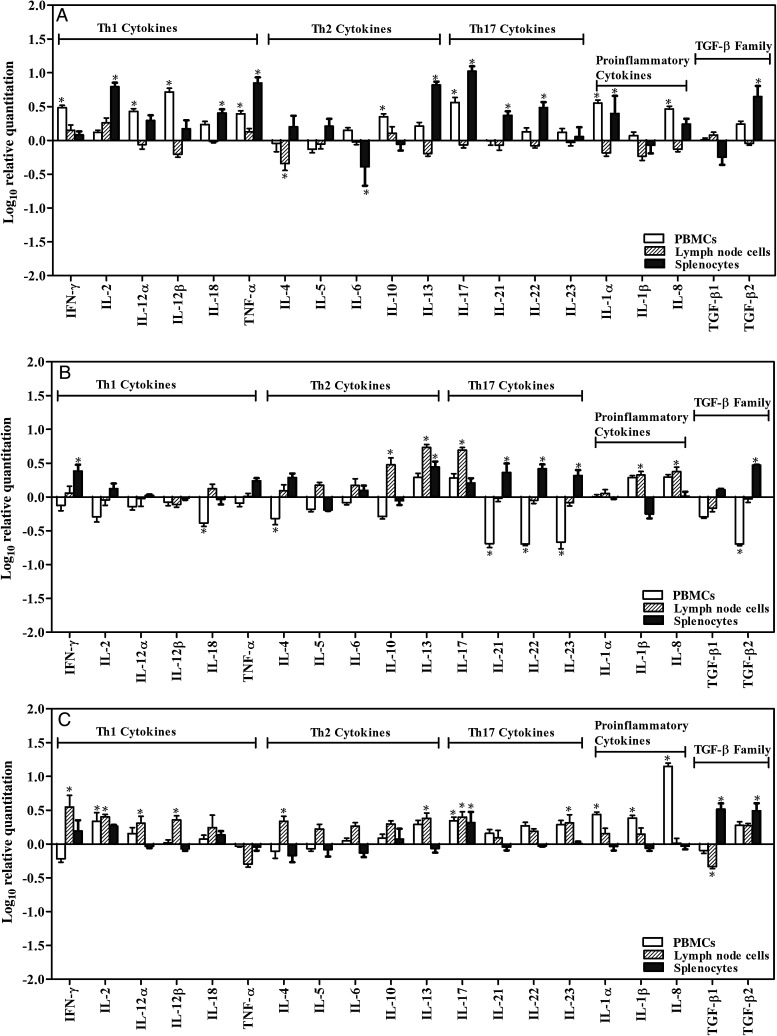
A large number of cytokines categorized into several subgroups were studied, and relative differences in their expression levels were recorded in PBMCs, splenocytes, and lymph node cells following stimulation in vitro with rSm-p80 for all 3 vaccination approaches. For rSm-p80 formulated in GLA-SE (Figure 4A), the major Th1 cytokines recorded were IFN-γ, IL-2, IL-12α, IL-12β, and TNF-α, and the Th17 cytokines included IL-17, IL-21, and IL-22; all of these cytokines were upregulated in PBMCs and/or spleen cells. However, for DNA prime-protein boost formulated in alum (Figure 4B), Th17 cytokine downregulation was observed in PBMCs, but upregulation of Th17 cytokine was observed in lymph node and spleen cells. For DNA prime-protein boost formulated in ODN, the expression of Th1 cytokines (IL-2, IL-12α, and IL-12β) was increased in PBMCs and lymph node cells. Th17 cytokines such as IL-17 or IL-23 was found to be upregulated in PBMCs and lymph node cells; some of the proinflammatory cytokines were also upregulated in PBMCs (Figure 4A–C). The synthesis of TGF-β2 increased in all of the spleen cells (Figure 4A–C). Furthermore, some Th2 cytokines, such as IL-10, were upregulated in lymph node cells (Figure 4B), whereas IL-13 was upregulated in spleen and lymph node cells (Figure 4A and 4B).
Figure 4.
Relative fold-changes of cytokine messenger RNA (mRNA) expression by reverse-transcription polymerase chain reaction. After 24-hour incubation with rSm-p80, RNA was extracted from stimulated pooled peripheral blood mononuclear cells (PBMCs), lymph node cells, and splenocytes. The relative ratio of cytokine mRNA expression was compared between control group and experimental group after 24-hour stimulation by rSm-p80. Analysis for different vaccination strategies are as follows: rSm-p80 plus glucopyranosyl lipid adjuvant (A), prime boost with Sm-p80-VR1020 and rSm-p80 in alum (B), and prime boost using Sm-p80-VR1020 and rSm-p80 with CpG ODNs (C). The relative cytokine mRNA expression was calculated after standardization using glyceraldehyde 3-phosphate dehydrogenase, through DataAssist, version 3.0. Abbreviations: IL-1α, interleukin 1α; IL-1β, interleukin 1β; IL-2, interleukin 2; IL-4, interleukin 4; IL-5, interleukin 5; IL-6, interleukin 6; IL-8, interleukin 8; IL-10, interleukin 10; IL-12α, interleukin 12α; IL-12β, interleukin 12β; IL-13, interleukin 13; IL-17, interleukin 17; IL-18, interleukin 18; IL-21, interleukin 21; IL-22, interleukin 22; IL-23, interleukin 23; TNF-α, tumor necrosis factor α; IFN-γ, interferon γ; TGF-β1, transforming growth factor β1; TGF-β2, transforming growth factor β2.
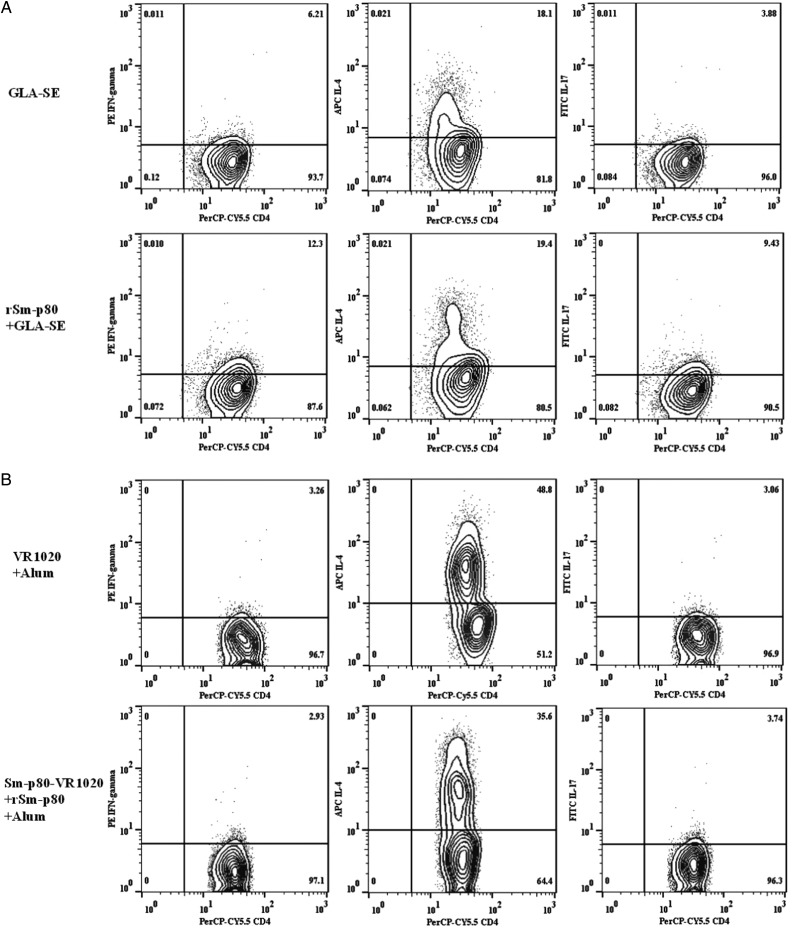
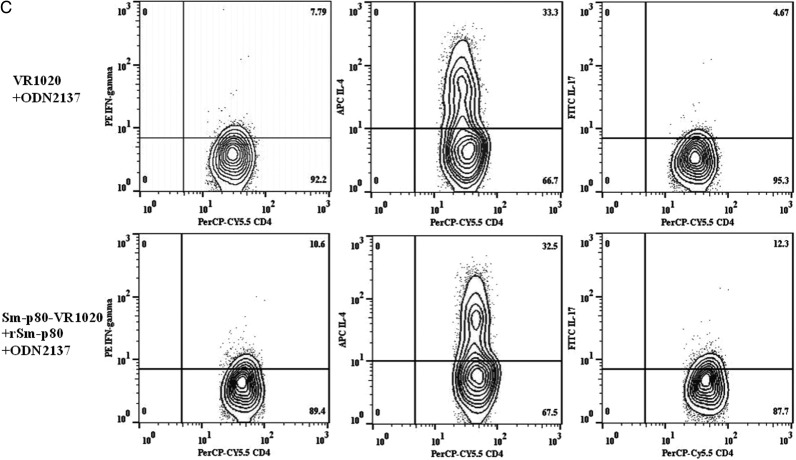
Identification of IFN-γ–, IL-4–, and IL-17–Producing Cell Populations via Flow Cytometry
The secretion of IFN-γ, IL-4, and IL-17 by CD3+CD4+ T cells was analyzed in PBMCs obtained from vaccinated and control baboons (Figure 5A–C). Analyses revealed 6.09% and 2.81% increases in the population of IFN-γ–secreting CD3+CD4+ T cells in recipients of protein formulated in GLA-SE and recipients of DNA prime-protein boost formulated in CpG-ODN, respectively, compared with findings for the control animals (Figure 5A and 5C). No clear change was found for recipients of DNA prime-protein boost formulated in alum (Figure 5B). On the contrary, the population of IL-4–secreting CD3+CD4+ T cells in baboons that received the DNA prime-protein boost formulated in alum was 13.2% less than that in the control group, which was consistent with the results obtained by real-time PCR for PBMCs. Furthermore, for the other 2 approaches, no significant changes in IL-4–secreting populations, compared with their respective control groups, were detected (Figure 5A and 5C). The populations of IL-17–secreting CD3+CD4+ T cells in recipients of protein formulated in GLA-SE and recipients of DNA prime-protein boost formulated in CpG-ODN were 5.55% and 7.63% greater, respectively, than those in their respective control groups (Figure 5A and 5C). A minor increase of 0.68% in IL-17–secreting cells was demonstrated in recipients of DNA prime-protein boost formulated in alum, compared with controls (Figure 5B).
Figure 5.
Quantification of intracellular interferon-γ (IFN-γ), interleukin 4 (IL-4), and interleukin 17 (IL-17) secretory CD3+CD4+ T cells from rSm-p80–stimulated peripheral blood mononuclear cells. Initial gating was performed using the CD4 marker. A, The percentage of IFN-γ, IL-4, and IL-17 secretory CD3+CD4+ T cells in recipients of glucopyranosyl lipid adjuvant (GLA)–SE and rSm-p80. B, The percentage of IFN-γ, IL-4, and IL-17 secretory cells in recipients of prime boost with Sm-p80-VR1020 and rSm-p80 in alum. C, The percentage of IFN-γ, IL-4, and IL-17 secretory cells in recipients of the prime boost using Sm-p80-VR1020 and rSm-p80 with CpG ODNs groups.
DISCUSSION
On the basis of their studies in a self-curing rhesus macaque-schistosome model, Wilson et al [25] stated that clear evidence of anti-adult worm immunity would open a new avenue for the development of a schistosome vaccine, potentially by coupling an additional therapeutic target to the prophylactic formulation. However, this important immune therapeutic aspect has not been explored fully because most schistosome vaccine efficacy studies have historically been done using mouse models [16, 26–30]. In these models, after the development of infection and the laying of eggs (4–5 weeks later), the mice survive for only an additional 3–4 weeks because of rapid disease progression. This interval is too short to determine the therapeutic efficacy of a vaccination regimen. On the contrary, in baboons, who are natural hosts of schistosomes, infection yields a remarkably high rate of cercarial penetration, fast schistosomula migration from the skin to the lungs and from the lungs to the liver, and fast development of schistosomula into adult worms. Maturation of infecting larvae often exceeds 90% compared with <50% in mice [31, 32]. Significantly, baboons, like humans, develop an acute schistosomiasis syndrome and, more importantly, the chronic disease after exposure to cercariae [31–34].
Since there have been no literature reports about the vaccine-mediated killing of established schistosome worms, we first performed 2 pilot/exploratory studies (4 animals per group per study) to work out the establishment of the chronic infection model in baboons. We then selected an optimal vaccination strategy for detailed evaluation, using 8 baboons (4 in the control group, and 4 in the experimental group). The optimal vaccine strategy (rSm-p80 plus GLA-SE) was then exhaustively tested for antiworm, antipathology, and antifecundity efficacy. Additionally, to decipher the potential role of B- and T-cell immune correlates that may be involved in therapeutic targeting of adult worms, multiple immunological parameters were measured following vaccination.
Data presented here indicate that intricately balanced proinflammatory (Th17 and Th1) responses and, to a much smaller extent, antiinflammatory (Th2) types of immune responses were generated following vaccination with the Sm-p80 vaccine. Like previous studies dealing with the prophylactic efficacy of a Sm-p80–based vaccine, in which Th1- and Th17-type immune responses were indicative of vaccine-mediated protection [17, 21], it appears that similar Th1- and Th17-type responses generated by an Sm-p80–based vaccine are also helpful in overriding some of the prevailing nonprotective Th2 responses [35, 36] that are the hallmark in chronic schistosome infections, especially those involving mixed infection with the whipworm Trichuris [35–37]. These Sm-p80 vaccine–mediated immune responses were potent enough to kill significant numbers of established adult worms, and they reduced egg retention in tissues and expulsion in feces. Furthermore, it has been observed that previous or prevailing schistosome infections do not negatively affect the efficacy of radiation-attenuated cercarial vaccine, and the immune responses to adult worm antigens elicited by the adult worms do not compromise those to the radiation-attenuated vaccine that parallel the development of protection [38].
Therapeutic vaccines represent a viable option that is being explored in cancers, infectious diseases, parasitic infections, allergies, and other maladies [39–43]. If a schistosome vaccine has both immune therapeutic and prophylactic efficacy, as is the case with Sm-80–based vaccine, then this vaccine could greatly aid in decreasing the burden of schistosomiasis when implementing praziquantel-linked vaccination/control campaigns. With regard to schistosomiasis control, absolute reliance on the drug therapy approach alone is barely adequate in the short term because this strategy has had little bearing on the reduction of disease transmission, and there is always the inherent threat of drug resistance by the parasite [44–46]. A significant impact on the reduction in disease sequelae and transmission can only be accomplished through long-term protection via vaccination, coupled with drug treatment [19, 47, 48].
Praziquantel treatment influences the polarization of schistosome-specific cytokine responses that may be important in determining the susceptibility to reinfection [49]; vaccination with Sm-p80 vaccine following praziquantel treatment would be helpful in skewing immune responses toward those that are protective and will be detrimental to new invading cercariae, thus preventing reinfection. For optimal efficacy of praziquantel, an immune response against parasite antigens is required, and these antigens include a wide variety of proteins, including perhaps Sm-p80 [50]. A viable campaign against schistosomes may therefore involve mass drug and vaccine administration, a vaccine-deployment strategy that would require treating infected individuals with praziquantel and then vaccinating them with the schistosome vaccine.
To summarize the status of Sm-p80 vaccine, it is the sole schistosome vaccine candidate that has been tested for its prophylactic efficacy in different vaccine formulations and approaches (eg, DNA alone, recombinant protein, and prime boost) in 2 very different experimental animal models (mouse and baboon) of infection and disease and has shown pronounced protection both in mice and baboons at levels that could previously only be achieved with the irradiated cercarial vaccine [16, 21, 24]. Furthermore, as demonstrated in this study, Sm-p80 vaccine is the only schistosome vaccine that has been shown to have a significant therapeutic effect on adult worms. Cumulatively, the data to date on the Sm-p80 vaccine provide a clear proof of concept, using a nonhuman primate model of schistosomiasis, that a recombinant Sm-p80 protein formulation can be the basis for further preclinical vaccine development that leads to human clinical trials.
Notes
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH; grant R01AI071223 to A. A. S.); the NIH (grants P40RR012317, P40OD010431, and P40OD010988 to G L. W. and R. F. W. on behalf of the baboon facility at the University of Oklahoma Health Sciences Center); the Small Business Innovation Research Program, NIH (grant 1R43AI103983 to D. C. and A. A. S); and the Bill and Melinda Gates Foundation (grant OPP1055855 to S. G. R. and D.C. on behalf of the Infectious Disease Research Institute).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006;368:1106–18. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 2.Gryseels B. Schistosomiasis. Infect Dis Clin North Am. 2012;26:383–97. doi: 10.1016/j.idc.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Hotez PJ, Kamath A. Neglected tropical diseases in sub-Saharan Africa: review of their prevalence, distribution, and disease burden. PLoS Negl Trop Dis. 2009;3:e412. doi: 10.1371/journal.pntd.0000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rollinson D, Knopp S, Levitz S, et al. Time to set the agenda for schistosomiasis elimination. Acta Trop. 2013;128:423–40. doi: 10.1016/j.actatropica.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 5.Ross AG, Bartley PB, Sleigh AC, et al. Schistosomiasis. N Engl J Med. 2002;346:1212–20. doi: 10.1056/NEJMra012396. [DOI] [PubMed] [Google Scholar]
- 6.Beaumier CM, Gillespie PM, Hotez PJ, Bottazzi ME. New vaccines for neglected parasitic diseases and dengue. Transl Res. 2013;162:144–55. doi: 10.1016/j.trsl.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 7.King CH. Parasites and poverty: the case of schistosomiasis. Acta Trop. 2010;113:95–104. doi: 10.1016/j.actatropica.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.King CH, Olbrych SK, Soon M, Singer ME, Carter J, Colley DG. Utility of repeated praziquantel dosing in the treatment of schistosomiasis in high-risk communities in Africa: a systematic review. PLoS Negl Trop Dis. 2011;5:e1321. doi: 10.1371/journal.pntd.0001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lustigman S, Prichard RK, Gazzinelli A, et al. A research agenda for helminth diseases of humans: the problem of helminthiases. PLoS Negl Trop Dis. 2012;6:e1582. doi: 10.1371/journal.pntd.0001582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parker M, Allen T. Does mass drug administration for the integrated treatment of neglected tropical diseases really work? Assessing evidence for the control of schistosomiasis and soil-transmitted helminths in Uganda. Health Res Policy Syst. 2011;9:3. doi: 10.1186/1478-4505-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prichard RK, Basanez MG, Boatin BA, et al. A research agenda for helminth diseases of humans: intervention for control and elimination. PLoS Negl Trop Dis. 2012;6:e1549. doi: 10.1371/journal.pntd.0001549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terer CC, Bustinduy AL, Magtanong RV, et al. Evaluation of the health-related quality of life of children in Schistosoma haematobium-endemic communities in Kenya: a cross-sectional study. PLoS Negl Trop Dis. 2013;7:e2106. doi: 10.1371/journal.pntd.0002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karcz SR, Podesta RB, Siddiqui AA, Dekaban GA, Strejan GH, Clarke MW. Molecular cloning and sequence analysis of a calcium-activated neutral protease (calpain) from Schistosoma mansoni. Mol Biochem Parasitol. 1991;49:333–6. doi: 10.1016/0166-6851(91)90078-k. [DOI] [PubMed] [Google Scholar]
- 14.Siddiqui AA, Zhou Y, Podesta RB, et al. Characterization of Ca(2+)-dependent neutral protease (calpain) from human blood flukes, Schistosoma mansoni. Biochim Biophys Acta. 1993;1181:37–44. doi: 10.1016/0925-4439(93)90087-h. [DOI] [PubMed] [Google Scholar]
- 15.Silva EE, Clarke MW, Podesta RB. Characterization of a C3 receptor on the envelope of Schistosoma mansoni. J Immunol. 1993;151:7057–66. [PubMed] [Google Scholar]
- 16.Ahmad G, Zhang W, Torben W, et al. Prime-boost and recombinant protein vaccination strategies using Sm-p80 protects against Schistosoma mansoni infection in the mouse model to levels previously attainable only by the irradiated cercarial vaccine. Parasitol Res. 2009;105:1767–77. doi: 10.1007/s00436-009-1646-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmad G, Zhang W, Torben W, et al. Protective and antifecundity effects of Sm-p80-based DNA vaccine formulation against Schistosoma mansoni in a nonhuman primate model. Vaccine. 2009;27:2830–7. doi: 10.1016/j.vaccine.2009.02.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohta N, Kumagai T, Maruyama H, Yoshida A, He Y, Zhang R. Research on calpain of Schistosoma japonicum as a vaccine candidate. Parasitol Int. 2004;53:175–81. doi: 10.1016/j.parint.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Siddiqui AA, Siddiqui BA, Ganley-Leal L. Schistosomiasis vaccines. Hum Vaccin. 2011;7:1192–7. doi: 10.4161/hv.7.11.17017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang R, Yoshida A, Kumagai T, et al. Vaccination with calpain induces a Th1-biased protective immune response against Schistosoma japonicum. Infect Immun. 2001;69:386–91. doi: 10.1128/IAI.69.1.386-391.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang W, Ahmad G, Torben W, et al. Sm-p80-based DNA vaccine provides baboons with levels of protection against Schistosoma mansoni infection comparable to those achieved by the irradiated cercarial vaccine. J Infect Dis. 2010;201:1105–12. doi: 10.1086/651147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Long EG, Tsin AT, Robinson BA. Comparison of the FeKal CON-Trate system with the formalin-ethyl acetate technique for detection of intestinal parasites. J Clin Microbiol. 1985;22:210–1. doi: 10.1128/jcm.22.2.210-211.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmad G, Torben W, Zhang W, Wyatt M, Siddiqui AA. Sm-p80-based DNA vaccine formulation induces potent protective immunity against Schistosoma mansoni. Parasite Immunol. 2009;31:156–61. doi: 10.1111/j.1365-3024.2008.01091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmad G, Zhang W, Torben W, et al. Preclinical prophylactic efficacy testing of sm-p80-based vaccine in a nonhuman primate model of Schistosoma mansoni infection and immunoglobulin G and E responses to Sm-p80 in human serum samples from an area where schistosomiasis is endemic. J Infect Dis. 2011;204:1437–49. doi: 10.1093/infdis/jir545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson RA, Langermans JA, Van Dam GJ, et al. Elimination of Schistosoma mansoni adult worms by rhesus macaques: basis for a therapeutic vaccine? PLoS Negl Trop Dis. 2008;2:e290. doi: 10.1371/journal.pntd.0000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Da'dara AA, Skelly PJ, Walker CM, Harn DA. A DNA-prime/protein-boost vaccination regimen enhances Th2 immune responses but not protection following Schistosoma mansoni infection. Parasite Immunol. 2003;25:429–37. doi: 10.1111/j.1365-3024.2003.00651.x. [DOI] [PubMed] [Google Scholar]
- 27.Bickle QD, James ER. Resistance against Schistosoma mansoni induced by immunization of mice with cryopreserved schistosomula. Trans R Soc Trop Med Hyg. 1978;72:677–8. doi: 10.1016/0035-9203(78)90043-3. [DOI] [PubMed] [Google Scholar]
- 28.Cook RM, Carvalho-Queiroz C, Wilding G, LoVerde PT. Nucleic acid vaccination with Schistosoma mansoni antioxidant enzyme cytosolic superoxide dismutase and the structural protein filamin confers protection against the adult worm stage. Infect Immun. 2004;72:6112–24. doi: 10.1128/IAI.72.10.6112-6124.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El Ridi R, Tallima H. Schistosoma mansoni ex vivo lung-stage larvae excretory-secretory antigens as vaccine candidates against schistosomiasis. Vaccine. 2009;27:666–73. doi: 10.1016/j.vaccine.2008.11.039. [DOI] [PubMed] [Google Scholar]
- 30.Pearson MS, Pickering DA, McSorley HJ, et al. Enhanced protective efficacy of a chimeric form of the schistosomiasis vaccine antigen Sm-TSP-2. PLoS Negl Trop Dis. 2012;6:e1564. doi: 10.1371/journal.pntd.0001564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nyindo M, Farah IO. The baboon as a non-human primate model of human schistosome infection. Parasitol Today. 1999;15:478–82. doi: 10.1016/s0169-4758(99)01569-0. [DOI] [PubMed] [Google Scholar]
- 32.Siddiqui AA, Ahmad G, Damian RT, Kennedy RC. Experimental vaccines in animal models for schistosomiasis. Parasitol Res. 2008;102:825–33. doi: 10.1007/s00436-008-0887-6. [DOI] [PubMed] [Google Scholar]
- 33.Damian RT, de la Rosa MA, Murfin DJ, Rawlings CA, Weina PJ, Xue YP. Further development of the baboon as a model for acute schistosomiasis. Mem Inst Oswaldo Cruz. 1992;87(Suppl 4):261–9. doi: 10.1590/s0074-02761992000800041. [DOI] [PubMed] [Google Scholar]
- 34.Kariuki TM, Farah IO. Resistance to re-infection after exposure to normal and attenuated schistosome parasites in the baboon model. Parasite Immunol. 2005;27:281–8. doi: 10.1111/j.1365-3024.2005.00783.x. [DOI] [PubMed] [Google Scholar]
- 35.Fairfax K, Nascimento M, Huang SC, Everts B, Pearce EJ. Th2 responses in schistosomiasis. Semin Immunopathol. 2012;34:863–71. doi: 10.1007/s00281-012-0354-4. [DOI] [PubMed] [Google Scholar]
- 36.Schramm G, Haas H. Th2 immune response against Schistosoma mansoni infection. Microbes Infect. 2010;12:881–8. doi: 10.1016/j.micinf.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 37.Klementowicz JE, Travis MA, Grencis RK. Trichuris muris: a model of gastrointestinal parasite infection. Semin Immunopathol. 2012;34:815–28. doi: 10.1007/s00281-012-0348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kariuki TM, Van Dam GJ, Deelder AM, et al. Previous or ongoing schistosome infections do not compromise the efficacy of the attenuated cercaria vaccine. Infect Immun. 2006;74:3979–86. doi: 10.1128/IAI.01657-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garcia F, Leon A, Gatell JM, Plana M, Gallart T. Therapeutic vaccines against HIV infection. Hum Vaccin Immunother. 2012;8:569–81. doi: 10.4161/hv.19555. [DOI] [PubMed] [Google Scholar]
- 40.Guo C, Manjili MH, Subjeck JR, Sarkar D, Fisher PB, Wang XY. Therapeutic cancer vaccines: past, present, and future. Adv Cancer Res. 2013;119:421–75. doi: 10.1016/B978-0-12-407190-2.00007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prabowo SA, Groschel MI, Schmidt ED, et al. Targeting multidrug-resistant tuberculosis (MDR-TB) by therapeutic vaccines. Med Microbiol Immunol. 2013;202:95–104. doi: 10.1007/s00430-012-0278-6. [DOI] [PubMed] [Google Scholar]
- 42.Valenta R, Campana R, Marth K, van Hage M. Allergen-specific immunotherapy: from therapeutic vaccines to prophylactic approaches. J Intern Med. 2012;272:144–57. doi: 10.1111/j.1365-2796.2012.02556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zimmermann S, Dalpke A, Heeg K. CpG oligonucleotides as adjuvant in therapeutic vaccines against parasitic infections. Int J Med Microbiol. 2008;298:39–44. doi: 10.1016/j.ijmm.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 44.Doenhoff MJ, Cioli D, Utzinger J. Praziquantel: mechanisms of action, resistance and new derivatives for schistosomiasis. Curr Opin Infect Dis. 2008;21:659–67. doi: 10.1097/QCO.0b013e328318978f. [DOI] [PubMed] [Google Scholar]
- 45.Doenhoff MJ, Hagan P, Cioli D, et al. Praziquantel: its use in control of schistosomiasis in sub-Saharan Africa and current research needs. Parasitology. 2009;136:1825–35. doi: 10.1017/S0031182009000493. [DOI] [PubMed] [Google Scholar]
- 46.Wang W, Wang L, Liang YS. Susceptibility or resistance of praziquantel in human schistosomiasis: a review. Parasitol Res. 2012;111:1871–7. doi: 10.1007/s00436-012-3151-z. [DOI] [PubMed] [Google Scholar]
- 47.Bergquist NR, Leonardo LR, Mitchell GF. Vaccine-linked chemotherapy: can schistosomiasis control benefit from an integrated approach? Trends Parasitol. 2005;21:112–7. doi: 10.1016/j.pt.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 48.Bergquist R, Utzinger J, McManus DP. Trick or treat: the role of vaccines in integrated schistosomiasis control. PLoS Negl Trop Dis. 2008;2:e244. doi: 10.1371/journal.pntd.0000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bourke CD, Nausch N, Rujeni N, et al. Integrated analysis of innate, Th1, Th2, Th17, and regulatory cytokines identifies changes in immune polarisation following treatment of human schistosomiasis. J Infect Dis. 2013;208:159–69. doi: 10.1093/infdis/jis524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soonawala D, Geerts JW, de Mos M, Yazdanbakhsh M, Visser LG. The immune response to schistosome antigens in formerly infected travelers. Am J Trop Med Hyg. 2011;84:43–7. doi: 10.4269/ajtmh.2011.10-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]