Abstract
C-terminal tensin-like (cten, also known as tensin4, TNS4) is a member of the tensin family. Cten protein, like the other three tensin family members, localizes to focal adhesion sites but only shares sequence homology with other tensins at its C-terminal region, which contains the Src SH2 and PTB domains. Cten is abundantly expressed in normal prostate and placenta and is down-regulated in prostate cancer. However, overexpression of cten frequently associates with tumors derived from breast, colon, lung, stomach, skin and pancreas. A variety of cancer-associated growth factors and cytokines induce cten expression. Up-regulated cten promotes cell motility, prolongs epidermal growth factor receptor signaling, and enhances tumorigenicity. Emerging findings suggest that cten is a promising biomarker and therapeutic target for various cancers.
Keywords: cten, tensin, tumor promoter, tumor suppressor, focal adhesion
Introduction
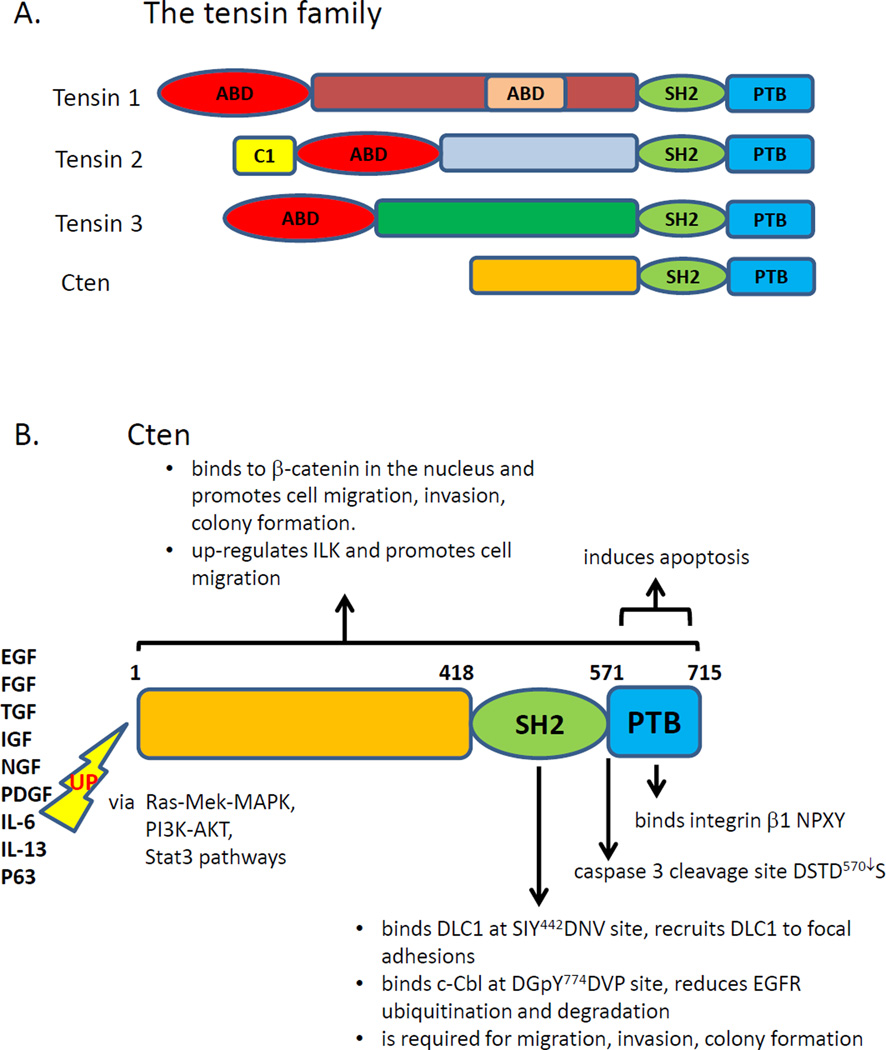
Cten (C-terminal tensin-like, aka tensin4, TNS4) was identified as a distant member of the tensin focal adhesion family (Lo and Lo, 2002). It is a much smaller protein compared to other tensins and only shares the SH2 (Src homology 2) and PTB (phosphotyrosine binding) domains found at the C-terminal ends of all other tensins (Lo, 2004) (figure 1). It was included in the tensin family due to the following reasons. (1) The tensin family is the only family which contains an SH2 domain immediately followed by a PTB domain. (2) The genomic structures encoding the SH2 and PTB regions of tensins are almost identical. (3) Cten like other tensins mainly localizes to focal adhesions. Many lines of evidence have demonstrated that cten’s critical roles in cell motility, apoptosis and growth factor receptor homeostasis may contribute to the development of various cancers.
Figure 1.
(A) Domain structures of tensins. ABD: actin-binding domain. C1: protein kinase C conserved regions. SH2: Src homology 2. PTB: phosphotyrosine binding. (B) Cten expression is induced by numerous growth factors and cytokines (listed in bold) through Ras-Mek-MAPK, PI3K-Akt, or Stat3 pathways. Up-regulated cten promotes cell migration, invasion and colony formation activities, which require the functional SH2 domain. The SH2 domain binds to DLC1 in a phosphorylation independent manner and recruits DLC1 to the focal adhesion site. The SH2 domain also interacts with tyrosine phosphorylated c-Cbl and reduces ligand-induced EGFR degradation. The PTB domain binds to β integrin NPXY sites. Caspase 3 cleaves cten between the SH2 and PTB domains. The resulting PTB domain fragment is able to promote apoptosis.
Structure
Human cten is a 715-residue polypeptide which contains two conserved domains: the SH2 domain and PTB domain (Lo and Lo, 2002)(figure 1). Both were originally identified as binding modules for phosphotyrosine-containing peptides. PTB domain binding specificity is conferred by residues N-terminal to the phosphotyrosine residue. It was soon discovered that many PTB domains bind to tyrosine residues regardless of their phosphorylation status. Cten’s PTB domain binds to the NPXY motif of the integrin β1 tail (Katz et al., 2007) and the assay conditions strongly suggested that this interaction does not require tyrosine phosphorylation. Together with studies on PTB domains of tensin1 and tensin2, it is believed that the interaction of integrin β tails with PTB domains of cten and other tensins is independent of tyrosine phosphorylation (Chen and Lo, 2003, Calderwood et al., 2003). In contrast to PTB domains, SH2 domains recognize an essential phosphotyrosine and adjacent C-terminal residues. Nonetheless, there are a few exceptions, including the SH2 domains of SLAM-associated protein (aka SAP, SH2D1A) and cten, in which the binding requires the tyrosine but regardless of its phosphorylation status. SH2 domains of cten and other tensins bind to the SIY442DNV site on DLC1 (Deleted in Liver Cancer 1) and phosphorylation of the tyrosine is not required (Liao et al., 2007, Dai et al., 2011). This interaction recruits DLC1, a tumor suppressor, to focal adhesions (Liao et al., 2007). The SH2 domain of cten still interacts with phosphotyrosine-containing proteins. For example, it binds to pY744DVPK site on c-Cbl and this interaction is critical in regulating homeostasis of EGFR (epidermal growth factor receptor)(Hong et al., 2013).
Expression, activation and turnover
Cten shows a relatively unique and restricted expression pattern in normal tissues. It is readily detected in normal prostate and placenta but is not detectable in other tissues by Northern blot assays (Lo and Lo, 2002). The tissue specific expression pattern has triggered the identification of CTEN promoter region. By promoter bashing, a 327-bp fragment around exon 1 was identified as the essential region of human CTEN promoter activity (Chen et al., 2013). It showed very strong promoter activities in human prostatic epithelial cell lines and significantly weaker activities in non-prostatic cells in reporter assays. When a Cre transgenic mouse line driven by this 327-bp human CTEN fragment was generated and crossed with R26R reporter mice, the β-galactosidase reporter signals were detected strongly in the prostate, brain, pancreas, lung and testis (Chen et al., 2013). These results suggest that the fragment does contain a functional promoter activity. However, it is not sufficient for regulating a very tight tissue specific expression pattern due to either missing critical regulatory elements and/or the discrepancy between human and mouse CTEN promoters. Interestingly, a p63 binding site within this 327-bp region was identified by the ChIP assay (Seo et al., 2012), indicating that CTEN is a target gene of p63. However, the biological relevance is currently unclear.
In human cancers, cten expression is reduced or absence in advanced prostate cancers (Chen et al., 2013, Lo and Lo, 2002, Li et al., 2010)(table 1). Despite of its low expression in the normal kidney, cten is further downregulated in kidney cancers (Martuszewska et al., 2009). Although it may not be expressed in other normal tissues, cten expression has been found to increase significantly in many types of cancer including thymoma, gastric, colorectal, breast, lung, skin, and pancreatic cancer (Liao et al., 2009, Sasaki et al., 2003b, Sasaki et al., 2003a, Katz et al., 2007, Albasri et al., 2011b, Albasri et al., 2011c, Al-Ghamdi et al., 2011, Sjoestroem et al., 2013, Li et al., 2010, Al-Ghamdi et al., 2013, Sakashita et al., 2008), suggesting that overexpression of cten in certain tissues may play a critical role in tumorigenesis.
Table 1.
Cten expression analyses in various cancers
| Cancer type |
Sample number |
Detection method |
Finding | Reference |
|---|---|---|---|---|
| Lung | 89 pairs | RT-PCR | Cten mRNA was correlated with tumor stages | (Sasaki et al., 2003a) |
| Lung | 96 samples | IHC | Cten protein was upregulated in cancer and was correlated with EGFR protein levels | (Hong et al., 2013) |
| Lung | 20 pairs | Array | Cten mRNA was upregulated in cancer but was not correlated with EGFR mRNA | (Hong et al., 2013) |
| Breast | 272 samples | IHC | Cten protein was correlated with EGFR, HER2, and estrogen receptor protein levels, lymph-node metastasis and tumor grades | (Katz et al., 2007) |
| Breast | 1409 samples | IHC | Cten protein was correlated with tumor size, lymph node stage, tumor grade, NPI, recurrence, distant metastasis, histology, and biomarkers including PR, N-cadherin, PIK3, AKT, but not EGFR or HER2. Cten positive patients had shorter breast cancer specific survival and shorter distant metastasis free interval. | (Albasri et al., 2011b) |
| Breast | 43 pairs | Array | Cten mRNA was upregulated in cancer but was not correlated with EGFR mRNA | (Hong et al., 2013) |
| Colon | 48 samples plus 24 pairs and 118 samples | QPCR IHC | 91% in 48 samples and 83% in 24 pairs showed cten mRNA upregulated (>4 folds). 76% in 118 samples showed cten protein up-regulated. | (Liao et al., 2009) |
| Colon | 462 samples | IHC | Cten protein was associated with advanced Dukes stage, poor prognosis, vascular invasion, distant metastasis | (Albasri et al., 2011a) |
| Colon | 24 pairs | Array | Cten mRNA was upregulated in cancer but was not correlated with EGFR mRNA | (Hong et al., 2013) |
| Thymoma | 45 samples | RT-PCR | Cten mRNA was correlated with tumor stages | (Sasaki et al., 2003b) |
| Pancreas | 44 samples | IHC | 70.45% showed upregulated | (Al-Ghamdi et al., 2013) |
| Pancreas | 45 pairs | Array | Cten mRNA was upregulated in cancer but was not correlated with EGFR mRNA | (Hong et al., 2013) |
| Stomach | 114 pairs | RT-PCR IHC | 72% showed cten mRNA up-regulated. Cten protein was correlated with histology, serosal invasion, lymph node metastasis, peritoneal dissemination, cancer-related death, and shorter survival after surgery | (Sakashita et al., 2008) |
| Melanoma | 562 samples | IHC | Cten protein was significantly higher in dysplastic nevi than normal nevi, and in primary melanoma than dysplastic nevi, but no difference between metastatic and primary melanoma. Cten protein correlated with AJCC stages and was significantly associated with a worse 5-year overall and disease-specific survival for primary melanoma patients. | (Sjoestroem et al., 2013) |
| Prostate | 72 tumor and 16 normal samples | IHC | Cten protein was inversely correlated with pathological Gleason scores of cancer | (Li et al., 2010) |
| Prostate | 6 normal, 31 cancer, 11 BPH samples 47 cancer vs 48 normal |
QPCR Array |
45% of cancer and 55% BPH samples showed cten mRNA down-regulated. Cten mRNA was significantly reduced in cancer. |
(Chen et al., 2013) |
| Kidney | 21 normal and 131 tumor samples | QPCR | Cten mRNA was significantly reduced in cancer | (Martuszewska et al., 2009) |
Cten expression is up-regulated by EGF, FGF2 (fibroblast growth factor 2), TGF-β (transforming growth factor beta), NGF (nerve growth factor), PDGF (platelet-derived growth factor), IGF-1 (insulin-like growth factor 1), IL-6 (interleukin 6) and IL-13 (interleukin 13) (Hung et al., 2013) mainly through the RAS-Raf-Mek, PI3K-Akt and Stat3 pathways activated by these cancer associated growth factors and cytokines (Hung et al., 2013, Barbieri et al., 2010, Al-Ghamdi et al., 2011). This may partially explain how cten levels increase dramatically in various cancers.
MicroRNAs (miRs) are a class of noncoding RNAs that post-transcriptionally regulate gene expression in cells. These 21–23 nucleotide RNAs match to sites in the mRNAs of protein-coding genes and negatively regulate the targeted gene expressions. Cten expression is potentially regulated by many miRs based on sequence alignment predictions. However, only cten down-regulations by miR-1, miR-26b, miR-124, and miR-335 are supported by microarray data (Lim et al., 2005, Gennarino et al., 2009, Baek et al., 2008, Tavazoie et al., 2008). Interestingly, these four miRs play roles in preventing tumor formation and are down-regulated in various cancers. Re-expression of these miRs were able to reverse tumorigenic properties of cancer cells (Nasser et al., 2008, Verghese et al., 2013, Zhang et al., 2013, Png et al., 2011).
Biological function
Cell motility
Studies have demonstrated that overexpression of cten promotes migration, whereas silencing of cten reduces migration of normal mammary epithelial cells (Katz et al., 2007) and prostate epithelial cells (Hung et al., 2013). Similar effects are observed in a variety of cancer cell lines (Liao et al., 2009, Albasri et al., 2009, Albasri et al., 2011b, Al-Ghamdi et al., 2011, Al-Ghamdi et al., 2013) and are believed to contribute to the tumorigenicities of these cancer cells. Several molecular mechanisms underlying ctenmediated cell migration have been established. (1) Cten PTB domain binds to the integrin β1 tail. As cten lacks the actin-binding domain found in other tensins, this interaction disrupts the tensin3 link between integrin and the actin cytoskeleton, leading to a condition that is more favorable for cell migration and invasion (Katz et al., 2007). (2) Cten up-regulates ILK (integrin linked kinase) expression which promotes cell migration in HCT116 cells (Albasri et al., 2011b). (3) Cten binds to DLC1, a RhoGAP containing tumor suppressor, through its SH2 domain, and as mentioned this binding is not reliant upon tyrosine phosphorylation. This interaction recruits DLC1 to focal adhesion sites and the focal adhesion localization, as well as its RhoGAP activity, is essential for DLC1’s tumor suppressor activity (Liao et al., 2007). Studies on other tensins suggest that the PTB domains could bind to a separate binding site on DLC1 (Chan et al., 2009). Interestingly, a recent study suggests that cten binds to DLC1 and keeps its RhoGAP inactivated, in turn, leading to higher RhoA activity which promotes cell migration through the ROCK pathway (Cao et al., 2012). Nonetheless, these mechanisms are likely to be cell context dependent and other mechanisms may exist in other cell types.
Apoptosis
Cten is cleaved mainly by caspase 3 at its DSTD570↓S site in normal prostate epithelial cells during apoptosis induced by staurosporine (Lo et al., 2005). One of the resulting fragments, polypeptide 571–715 containing only the PTB domain, is able to reduce cell growth by inducing apoptosis. However, this function is likely to be human cten specific, as the cleavage site is not conversed in other species.
Homeostasis of epidermal growth factor receptors
EGF treatment induces EGFR tyrosine phosphorylation and recruits c-Cbl as well as other signaling molecules binding to activated EGFR. c-Cbl is an E3 ubiquitin-protein ligase which participates in cell signaling and protein ubiquitination. Bound c-Cbl induces EGFR ubiquitination. The attachment of ubiquitin to EGFR leads to its removal from the plasma membrane and subsequent trafficking to the lysosome for degradation. This ligand-induced EGFR degradation is an important negative regulation to ensure the EGFR signaling turned on by ligands is switched off. It has been shown that the SH2 domain of cten binds to tyrosine phosphorylated c-Cbl and this interaction reduces the level of EGFR ubiquitination and degradation, and therefore prolongs EGFR signaling (Hong et al., 2013) which enhances tumorigenicities of cancer cells.
Tumorigenicity
In cancer cells, cten levels appear to correlate with their tumorigenicities as measured by cell invasion, anchorage-independent growth, colony formation, and xenograft assays (Liao et al., 2009, Albasri et al., 2009, Albasri et al., 2011b, Al-Ghamdi et al., 2011, Al-Ghamdi et al., 2013). However, cten does not contribute to tumorigenicity by promoting cell proliferation, since overexpression of cten has limited effects on cancer cell growth (Lo et al., 2005, Albasri et al., 2009, Al-Ghamdi et al., 2013). In addition to mechanisms involving cten-mediated cell migration and EGFR homeostasis mentioned above, cten interaction with β-catenin also contributes to enhancing tumorigenicity (Liao et al., 2009). β-catenin is found in adherent junctions, cytoplasm, and nucleus. In the nucleus, β-catenin binds to TCF/LEF forming a transcriptional complex that turns on gene expression. Cten is reported to interact with β-catenin only in the nucleus (Liao et al., 2009). Nuclear localization of cten is intriguing, but how cten translocates from focal adhesion to the nucleus and whether cten participates in gene expression regulation and/or other nuclear functions are currently unknown and warrant extensive investigation. Nonetheless, the tumorigenicity of cten in colon cancer cells apparently is dependent on β-catenin (Liao et al., 2009). Interestingly, overexpression of cten in colon cancer cells down-regulated E-cadherin protein but not mRNA levels (Albasri et al., 2009). Since Ecadherin binds to β-catenin at adherent junctions, and cten interacts with β-catenin in the nucleus, it is possible that up-regulated cten sequesters β-catenin away from E-cadherin and destabilizes adherent junctions, leading to E-cadherin degradation and epithelialmesenchymal transition. In addition, cten mediated tumorigenicity requires the functional SH2 domain, as cten SH2 dead mutant does not have the same effect (Hong et al., 2013).
Renal tubulogenesis
In a 2.5 Dimension tubulogenesis system developed by Kwon et al (Kwon et al., 2011), several morphological stages are defined, including multicellular apical protrusion, extension, followed by tubule initiation and tubule elongation. Cten was identified as a highly upregulated gene in the extension stage. Silencing of cten reduces the formation of extensions and tubules. Overexpression of cten increases cell invasion, whereas cten SH2 dead mutant locks the process at extension stage with increased active Stat3, suggesting a critical role of the cten-Stat3 pathway in renal epithelial tubulogenesis. It will be interesting and important to investigate the significance of cten in kidney development and function in animals.
Possible Medical and Industrial Applications
Cten expression profiles in various cancers and its biological functions indicate that cten actively participates in tumor development. On one hand, cten may act as a tumor suppressor in the prostate since it is downregulated in prostate cancer and its expression induces sensitivity to paclitaxel (Li et al., 2010). On the other hand, cten appears to promote tumorigenesis of a majority of cancers. Either way cten could be a diagnostic and/or prognostic cancer marker. In prostate cancers, cten protein levels are inversely correlated with Gleason scores of cancer (Li et al., 2010) (table 1). In invasive breast cancer, cten expression correlates with high EGFR and HER2 in one study, or PI3K/Akt in another, and is significantly associated with poor prognostic variables, including larger tumor size, higher histological grade, metastasis to lymph nodes, and poor Nottingham Prognostic Index (Katz et al., 2007, Albasri et al., 2011c). Overexpression of cten is associated with advanced Dukes’ stage, poor prognosis and distant metastasis in colorectal cancer as well (Albasri et al., 2011a). In gastric cancer, cten overexpression is associated with poor grade, metastasis, and poor prognosis (Sakashita et al., 2008). In primary melanoma, cten levels correlate with AJCC stages and high cten is associated with a worse survival for patients (Sjoestroem et al., 2013).
Although cten may be a prostate-specific tumor suppressor, its role as a tumor promoter in other cancers may be more useful for clinical applications. As mentioned above, cten can be a cancer diagnosis and/or prognosis biomarker. With no or very low expression levels in most normal tissues, the chance of false positive will be significantly lower than other markers. Similarly, the adverse effect in normal tissues when targeting cten will be very minimal. Together with the findings that cten regulates activated EGFR levels and is a downstream molecule of the Ras/Raf/Mek/MAPK pathway, targeting cten along with EGFR appears to be a promising approach for EGFR dysregulated cancer therapies.
Acknowledgments
The work in this laboratory is supported by the NIH/NCI (CA102537, CA151366) and the Gordon and Betty Moore foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Ghamdi S, Albasri A, Cachat J, Ibrahem S, Muhammad BA, Jackson D, Nateri AS, Kindle KB, Ilyas M. Cten is targeted by Kras signalling to regulate cell motility in the colon and pancreas. PLoS One. 2011;6:e20919. doi: 10.1371/journal.pone.0020919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ghamdi S, Cachat J, Albasri A, Ahmed M, Jackson D, Zaitoun A, Guppy N, Otto WR, Alison MR, Kindle KB, Ilyas M. C-terminal tensinlike gene functions as an oncogene and promotes cell motility in pancreatic cancer. Pancreas. 2013;42:135–140. doi: 10.1097/MPA.0b013e3182557ceb. [DOI] [PubMed] [Google Scholar]
- Albasri A, Al-Ghamdi S, Fadhil W, Aleskandarany M, Liao YC, Jackson D, Lobo DN, Lo SH, Kumari R, Durrant L, Watson S, Kindle KB, Ilyas M. Cten signals through integrin-linked kinase (ILK) and may promote metastasis in colorectal cancer. Oncogene. 2011a doi: 10.1038/onc.2011.26. [DOI] [PubMed] [Google Scholar]
- Albasri A, Al-Ghamdi S, Fadhil W, Aleskandarany M, Liao YC, Jackson D, Lobo DN, Lo SH, Kumari R, Durrant L, Watson S, Kindle KB, Ilyas M. Cten signals through integrin-linked kinase (ILK) and may promote metastasis in colorectal cancer. Oncogene. 2011b;30:2997–3002. doi: 10.1038/onc.2011.26. [DOI] [PubMed] [Google Scholar]
- Albasri A, Aleskandarany M, Benhasouna A, Powe DG, Ellis IO, Ilyas M, Green AR. CTEN (C-terminal tensin-like), a novel oncogene overexpressed in invasive breast carcinoma of poor prognosis. Breast Cancer Res Treat. 2011c;126:47–54. doi: 10.1007/s10549-010-0890-3. [DOI] [PubMed] [Google Scholar]
- Albasri A, Seth R, Jackson D, Benhasouna A, Crook S, Nateri AS, Chapman R, Ilyas M. C-terminal Tensin-like (CTEN) is an oncogene which alters cell motility possibly through repression of E-cadherin in colorectal cancer. J Pathol. 2009;218:57–65. doi: 10.1002/path.2508. [DOI] [PubMed] [Google Scholar]
- Baek D, Villen J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of microRNAs on protein output. Nature. 2008;455:64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri I, Pensa S, Pannellini T, Quaglino E, Maritano D, Demaria M, Voster A, Turkson J, Cavallo F, Watson CJ, Provero P, Musiani P, Poli V. Constitutively active Stat3 enhances neu-mediated migration and metastasis in mammary tumors via upregulation of Cten. Cancer Res. 2010;70:2558–2567. doi: 10.1158/0008-5472.CAN-09-2840. [DOI] [PubMed] [Google Scholar]
- Calderwood DA, Fujioka Y, de Pereda JM, Garcia-Alvarez B, Nakamoto T, Margolis B, McGlade CJ, Liddington RC, Ginsberg MH. Integrin beta cytoplasmic domain interactions with phosphotyrosine-binding domains: a structural prototype for diversity in integrin signaling. Proc Natl Acad Sci U S A. 2003;100:2272–2277. doi: 10.1073/pnas.262791999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Voss C, Zhao B, Kaneko T, Li SS. Differential regulation of the activity of deleted in liver cancer 1 (DLC1) by tensins controls cell migration and transformation. Proc Natl Acad Sci U S A. 2012;109:1455–1460. doi: 10.1073/pnas.1114368109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan LK, Ko FC, Ng IO, Yam JW. Deleted in liver cancer 1 (DLC1) utilizes a novel binding site for Tensin2 PTB domain interaction and is required for tumor-suppressive function. PLoS One. 2009;4:e5572. doi: 10.1371/journal.pone.0005572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Lo SH. Regulation of tensin-promoted cell migration by its focal adhesion-binding and Src Homology 2 domains. Biochem J. 2003;370:1039–1045. doi: 10.1042/BJ20021308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen NT, Kuwabara Y, Conley C, Liao YC, Hong SY, Chen M, Shih YP, Chen HW, Hsieh F, Lo SH. Phylogenetic analysis, expression patterns, and transcriptional regulation of human CTEN gene. Gene. 2013;520:90–97. doi: 10.1016/j.gene.2013.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai K, Liao S, Zhang J, Zhang X, Tu X. Solution structure of tensin2 SH2 domain and its phosphotyrosine-independent interaction with DLC-1. PLoS One. 2011;6:e21965. doi: 10.1371/journal.pone.0021965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennarino VA, Sardiello M, Avellino R, Meola N, Maselli V, Anand S, Cutillo L, Ballabio A, Banfi S. MicroRNA target prediction by expression analysis of host genes. Genome Res. 2009;19:481–490. doi: 10.1101/gr.084129.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SY, Shih YP, Li T, Carraway KL, 3rd, Lo SH. CTEN prolongs signaling by EGFR through reducing its ligand-induced degradation. Cancer Res. 2013;73:5266–5276. doi: 10.1158/0008-5472.CAN-12-4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung SY, Shih YP, Chen M, Lo SH. Up-Regulated Cten by FGF2 Contributes to FGF2-Mediated Cell Migration. Mol Carcinog. 2013 doi: 10.1002/mc.22034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz M, Amit I, Citri A, Shay T, Carvalho S, Lavi S, Milanezi F, Lyass L, Amariglio N, Jacob-Hirsch J, Ben-Chetrit N, Tarcic G, Lindzen M, Avraham R, Liao YC, Trusk P, Lyass A, Rechavi G, Spector NL, Lo SH, Schmitt F, Bacus SS, Yarden Y. A reciprocal tensin-3-cten switch mediates EGF-driven mammary cell migration. Nat Cell Biol. 2007;9:961–969. doi: 10.1038/ncb1622. [DOI] [PubMed] [Google Scholar]
- Kwon SH, Nedvetsky PI, Mostov KE. Transcriptional profiling identifies TNS4 function in epithelial tubulogenesis. Curr Biol. 2011;21:161–166. doi: 10.1016/j.cub.2010.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Mizokami A, Izumi K, Narimoto K, Shima T, Zhang J, Dai J, Keller ET, Namiki M. CTEN/tensin 4 expression induces sensitivity to paclitaxel in prostate cancer. Prostate. 2010;70:48–60. doi: 10.1002/pros.21037. [DOI] [PubMed] [Google Scholar]
- Liao YC, Chen NT, Shih YP, Dong Y, Lo SH. Up-regulation of C-terminal tensin-like molecule promotes the tumorigenicity of colon cancer through beta-catenin. Cancer Res. 2009;69:4563–4566. doi: 10.1158/0008-5472.CAN-09-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao YC, Si L, Devere White RW, Lo SH. The phosphotyrosine-independent interaction of DLC-1 and the SH2 domain of cten regulates focal adhesion localization and growth suppression activity of DLC-1. J Cell Biol. 2007;176:43–49. doi: 10.1083/jcb.200608015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- Lo SH. Tensin. Int J Biochem Cell Biol. 2004;36:31–34. doi: 10.1016/s1357-2725(03)00171-7. [DOI] [PubMed] [Google Scholar]
- Lo SH, Lo TB. Cten, a COOH-terminal tensin-like protein with prostate restricted expression, is down-regulated in prostate cancer. Cancer Res. 2002;62:4217–4221. [PubMed] [Google Scholar]
- Lo SS, Lo SH, Lo SH. Cleavage of cten by caspase-3 during apoptosis. Oncogene. 2005;24:4311–4314. doi: 10.1038/sj.onc.1208571. [DOI] [PubMed] [Google Scholar]
- Martuszewska D, Ljungberg B, Johansson M, Landberg G, Oslakovic C, Dahlback B, Hafizi S. Tensin3 is a negative regulator of cell migration and all four Tensin family members are downregulated in human kidney cancer. PLoS One. 2009;4:e4350. doi: 10.1371/journal.pone.0004350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasser MW, Datta J, Nuovo G, Kutay H, Motiwala T, Majumder S, Wang B, Suster S, Jacob ST, Ghoshal K. Down-regulation of micro-RNA-1 (miR-1) in lung cancer. Suppression of tumorigenic property of lung cancer cells and their sensitization to doxorubicin-induced apoptosis by miR-1. J Biol Chem. 2008;283:33394–33405. doi: 10.1074/jbc.M804788200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Png KJ, Yoshida M, Zhang XH, Shu W, Lee H, Rimner A, Chan TA, Comen E, Andrade VP, Kim SW, King TA, Hudis CA, Norton L, Hicks J, Massague J, Tavazoie SF. MicroRNA-335 inhibits tumor reinitiation and is silenced through genetic and epigenetic mechanisms in human breast cancer. Genes Dev. 2011;25:226–231. doi: 10.1101/gad.1974211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakashita K, Mimori K, Tanaka F, Kamohara Y, Inoue H, Sawada T, Hirakawa K, Mori M. Prognostic relevance of Tensin4 expression in human gastric cancer. Ann Surg Oncol. 2008;15:2606–2613. doi: 10.1245/s10434-008-9989-8. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Moriyama S, Mizuno K, Yukiue H, Konishi A, Yano M, Kaji M, Fukai I, Kiriyama M, Yamakawa Y, Fujii Y. Cten mRNA expression was correlated with tumor progression in lung cancers. Lung Cancer. 2003a;40:151–155. doi: 10.1016/s0169-5002(03)00037-0. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Yukiue H, Kobayashi Y, Fukai I, Fujii Y. Cten mRNA expression is correlated with tumor progression in thymoma. Tumour Biol. 2003b;24:271–274. doi: 10.1159/000076141. [DOI] [PubMed] [Google Scholar]
- Seo EY, Lee DH, Lee Y, Cho KH, Eun HC, Chung JH. Microarray analysis reveals increased expression of DeltaNp63alpha in seborrhoeic keratosis. Br J Dermatol. 2012;166:337–342. doi: 10.1111/j.1365-2133.2011.10665.x. [DOI] [PubMed] [Google Scholar]
- Sjoestroem C, Khosravi S, Zhang G, Martinka M, Li G. C-terminal tensin-like protein is a novel prognostic marker for primary melanoma patients. PLoS One. 2013;8:e80492. doi: 10.1371/journal.pone.0080492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavazoie SF, Alarcon C, Oskarsson T, Padua D, Wang Q, Bos PD, Gerald WL, Massague J. Endogenous human microRNAs that suppress breast cancer metastasis. Nature. 2008;451:147–152. doi: 10.1038/nature06487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese ET, Drury R, Green CA, Holliday DL, Lu X, Nash C, Speirs V, Thorne JL, Thygesen HH, Zougman A, Hull MA, Hanby AM, Hughes TA. MiR-26b is down-regulated in carcinoma-associated fibroblasts from ER-positive breast cancers leading to enhanced cell migration and invasion. J Pathol. 2013;231:388–399. doi: 10.1002/path.4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Lu Y, Yue X, Li H, Luo X, Wang Y, Wang K, Wan J. MiR-124 suppresses growth of human colorectal cancer by inhibiting STAT3. PLoS One. 2013;8:e70300. doi: 10.1371/journal.pone.0070300. [DOI] [PMC free article] [PubMed] [Google Scholar]