Abstract
Background.
Individual measurements of inflammation have been utilized to assess adverse outcomes risk in older adults with varying degrees of success. This study was designed to identify biologically informed, aggregate measures of inflammation for optimal risk assessment and to inform further biological study of inflammatory pathways.
Methods.
In total, 15 nuclear factor-kappa B-mediated pathway markers of inflammation were first measured in baseline serum samples of 1,155 older participants in the InCHIANTI population. Of these, C-reactive protein, interleukin-1-receptor antagonist, interleukin-6, interleukin-18, and soluble tumor necrosis factor-α receptor-1 were independent predictors of 5-year mortality. These five inflammatory markers were measured in baseline serum samples of 5,600 Cardiovascular Health Study participants. A weighted summary score, the first principal component summary score, and an inflammation index score were developed from these five log-transformed inflammatory markers, and their prediction of 10-year all-cause mortality was evaluated in Cardiovascular Health Study and then validated in InCHIANTI.
Results.
The inflammation index score that included interleukin-6 and soluble tumor necrosis factor-α receptor-1 was the best predictor of 10-year all-cause mortality in Cardiovascular Health Study, after adjusting for age, sex, education, race, smoking, and body mass index (hazards ratio = 1.62; 95% CI: 1.54, 1.70) compared with all other single and combined measures. The inflammation index score was also the best predictor of mortality in the InCHIANTI validation study (hazards ratio 1.33; 95% CI: 1.17–1.52). Stratification by sex and CVD status further strengthened the association of inflammation index score with mortality.
Conclusion.
A simple additive index of serum interleukin-6 and soluble tumor necrosis factor-α receptor-1 best captures the effect of chronic inflammation on mortality in older adults among the 15 biomarkers measured.
Key Words: Inflammation, Cytokine, Mortality, Aging.
SERUM measures of inflammatory activation are among the most reliable markers of risk for adverse health outcomes in older persons (1). Interleukin-6 (IL-6) and other proinflammatory cytokines predict disability and mortality and have been correlated to many chronic diseases that are highly prevalent and frequently result in disability in older persons (1–3). Increasing evidence from basic biological studies suggest that inflammatory cytokines play a direct role in the development and clinical progression of chronic disease states, such as atherosclerosis, diabetes, and cancer, and the typical manifestations of aging, such as sarcopenia, anemia, and cognitive decline (4–9). Although the specific mechanisms that link inflammation to mortality and other adverse health outcomes have not been clarified, developing parsimonious and reliable measures of inflammation may be useful in clinical practice as risk assessment tools, as potential therapeutic targets, and to monitor clinical progression and effectiveness of interventions. In spite of this potential clinical utility, with the potential exception of C-reactive protein (CRP) in cardiovascular and inflammatory diseases (10), inflammatory markers have not yet been widely incorporated into clinical practice (11), partly because there is not yet a “gold standard” inflammatory measurement that best and most reliably predicts adverse outcomes in older adults. In fact, there have been few attempts to develop a comprehensive study of inflammatory markers for this purpose and to aggregate biologically informed measures to maximize predictive validity (11,12). As a result, most studies on the effect of proinflammatory state on health outcomes in older patients still consider cytokines separately.
Previous research has suggested that the upregulation of the nuclear factor-kappa B (NFkB)-mediated innate immune system plays a key role in the progressive rise of serum inflammatory markers with aging (13). Building on this biological knowledge, we hypothesized that a simple aggregate measure whose expression is influenced by NFkB activation would be independently predictive of mortality and other adverse outcomes than any single inflammatory marker, after adjusting for age, sex, body mass index (BMI), education, smoking, and CVD status. We sought to evaluate this hypothesis and identify the subset of 15 NFkB-related inflammatory markers that were most predictive of mortality for 10 years in two large longitudinal cohort populations of older adults. As exploratory analyses, we also evaluated (a) whether the inflammatory phenotype for predicting mortality differed according to sex and CVD status and (b) whether the impact of inflammation on mortality changed with age.
Materials and Methods
Participants
The Cardiovascular Health Study (CHS) is a prospective observational study of 5,888 men and women aged 65–100 at baseline who were recruited based on age- and sex-stratified samples from Medicare eligibility lists from four communities in the United States between 1989 and 1990. An additional cohort of 687 African Americans was added and followed longitudinally starting in 1992–1993 (14–16). Cytokines were measured from stored serum drawn at the baseline visit. Mortality data were ascertained at semiannual contacts and confirmed through intensive surveillance for 10 years (14).
InCHIANTI is a prospective population-based study of the factors that contribute to mobility decline in older Italian adults. The study sample (1,155 participants aged 65–102 years) was randomly selected using a multistage, stratified sampling method from two towns in the Chianti geographic area of Italy (Greve in Chianti and Bagno a Ripoli, Tuscany, Italy). The details of the data collection and sampling procedures have been described elsewhere (17). All participants gave written consent for study participation and the Italian National Research Council of Aging Ethical Committee approved the study. Baseline serum samples were utilized to measure inflammatory mediators as described later. Information on vital status during the follow-up was gathered from the Mortality General Registry maintained by the Tuscany region and the death certificates deposited at the Registry Office of the municipality of residence. In this study, mortality that occurred between 1998, the year of enrollment, and at the end of 2007 was considered. Frailty was determined in both populations using the method of Fried and colleagues (18). Demographic information describing the two populations and their differences are listed in Table 1.
Table 1.
Baseline Demographic Characteristics and Inflammatory Cytokine Measurements in the Two Study Cohorts
| Characteristics | CHS (n = 5,888) | InCHIANTI (n = 1,155) | p Values |
|---|---|---|---|
| Age, M (SD) | 72.8 (5.6) | 75.4 (7.6) | <.0001 |
| Gender, no (%) | .56 | ||
| Male | 2,495 (42.4) | 500 (43.3) | |
| Female | 3,393 (57.6) | 655 (56.7) | |
| Race, no (%) | NA | ||
| Caucasian | 4,925 (83.6) | 1,155 (100.0) | |
| African American | 924 (15.7) | NA | |
| Other | 39 (0.7) | NA | |
| BMI, M (SD) | 26.7(4.7) | 27.2 (4.1) | <.0001 |
| CVD, no (%) | 1,517 (25.7) | 125 (10.8) | <.0001 |
| Frailty, no (%) | .0004 | ||
| Not frail | 2,465 (46.5) | 555 (48.1) | |
| Intermediate | 2,475 (46.6) | 486 (42.1) | |
| Frail | 368 (6.9) | 114 (9.9) | |
| DM, no (%) | 953 (16.4) | 125 (10.8) | <.0001 |
| Smoking, no (%) | <.0001 | ||
| Never | 2,736 (46.5) | 687 (59.5) | |
| Former | 2,444 (41.5) | 307 (26.6) | |
| Current | 700 (11.9) | 161 (13.9) | |
| Inflammatory measures, median (Q1, Q3) (SD) | |||
| IL-6 (pg/mL) | 1.7 (1.2, 2.6) (1.9) | 3.0(2.1, 4.1) (2.5) | |
| sTNFR1 (pg/mL) | 1431.0 (1182.0, 1759.9) (620.5) | 1361.2 (1101.4, 1745.8) (747.3) | |
| CRP (µg/mL) | 2.5 (1.3, 4.4) (6.9) | 2.8 (1.3, 5.8) (7.9) | |
| IL-18 (pg/mL) | 245.5 (184.9, 326.7) (146.9) | 383.3 (301.8, 484.3) (155.9) | |
| IL-1RA (pg/mL) | 209.5 (152.1, 307.3) (267.7) | 131.1 (95.4, 182.9) (113.6) | |
Note: BMI = body mass index; CHS = Cardiovascular Health Study; CRP = C-reactive protein; DM = diabetes mellitus; IL-6 = interleukin-6; IL-18 = interleukin-18; IL-1RA = IL-1-receptor antagonist; M = mean; NA = not applicable; Q1 = first quartile; Q3 = third quartile; SD = standard deviation; sTNFR1 = soluble tumor necrosis factor-α receptor-1.
Overview of Study Design
Methods for measuring serum inflammatory markers and analytical methodology are detailed later. We first considered measures of 15 NFkB-related inflammatory markers from the baseline visit of the InCHIANTI study including CRP, IL-1-beta (IL-1β), IL-1-receptor antagonist (IL-1RA), IL-6, soluble IL-6 receptor (sIL-6R), IL-8, IL-10, IL-12, IL-15, IL-18, tumor necrosis factor-alpha (TNF-α), soluble TNF receptors 1 and 2 (sTNFR1 and sTNFR2), monocyte chemoattractant protein-1, and interferon-γ. In the initial analyses performed in the InCHIANTI population, five measurements were identified as predictive of 5-year mortality. These markers were then measured in the much larger CHS population, and the 10-year mortality risk was estimated first based on each individual marker and then based on the three aggregate measures of inflammation as described later. These analyses were adjusted for age, sex, BMI, education, smoking, and CVD status to determine the independent predictive value of the individual and aggregate measures. Finally, the aggregate measures developed using the CHS variables were then independently validated in the InCHIANTI population by comparing the predicted and observed 10-year mortality risk.
Cytokine Measurements
For the five inflammatory mediator assays used in the aggregate model development, stored serum from baseline study visits (1989–1990 for original CHS cohort, 1992–1993 for African American CHS subset, and 1998 for InCHIANTI) were utilized. High-sensitivity CRP was measured in 1991 for initial CHS cohort and in 1994 for African American subset using a validated in-house high-sensitivity assay, with intra- and interassay coefficients of variation at 3.0% and 6.0%, respectively, with detection limit of 0.007mg/L and assay range of 0.08–9.0mg/L (19). IL-6 was measured by high-sensitivity ELISA (Quantikine HS Human IL-6 Immunoassay, R&D Systems, Minneapolis, MN; analytical coefficient of variation 6.3%) in 1991 and 1994 as with CRP. IL-1RA and IL-18 were measured by the Mesoscale chemiluminiscent multiplex system, and sTNFR1 was measured in a Mesoscale chemiluminiscent singleplex assay in 2011. Human 2-plex kits (a prototype sandwich immunoassay for human IL-18, IL-1RA) were purchased from Mesoscale Discovery Technology (MSD; Gaithersburg, MD). SULFO-TAG human IL-18 detection antibody and biotinylated human IL-1RA detection antibody were used for the assay. MSD plates were measured on the MSD Sector Imager 2400 plate reader. The raw data were measured as electrochemiluminescence signal (light) detected by photo detectors and analyzed using the Discovery Workbench 3.0 software (MSD). A four-parameter logistic fit curve was generated for each analyte using the standards and the concentration of each sample was calculated. The kit’s sensitivity is 0.6 pg/mL for IL-18 and 1.2 pg/mL for IL-1RA. For sTNFR1 measurement, commercially available MSD MULTI-SPOT 96-well human sTNFR1 ultrasensitive plates and detection antibody were purchased, and the assays were carried out as per the manufacturer’s protocol after a 1:10 sample dilution. Plates were analyzed as described earlier. The kit’s sensitivity is 0.48 pg/mL. CHS core laboratory procedures have been previously described (20).
For the InCHIANTI population, 15 NFkB-related inflammatory markers were measured in stored serum taken from the baseline visit as listed earlier. High-sensitivity CRP was measured by ELISA and colorimetric competitive immunoassay that uses purified protein and polyclonal anti-CRP antibodies (Calbiochem, San Diego, CA). Interleukins IL-1RA, IL-1β, TNF-α, sTNFR1 and R2, IL-6, and IL-6RA were measured by ELISA using commercial kits (BIOSOURCE international, Camarillo, CA). Serum IL-18 was measured using highly sensitive quantitative sandwich assays (Quantikine HS, R&D systems). Assay precisions and detectable limits for these measures were reported previously (12,19,21). IL-15 was measured using a chemiluminescent immunoassay (Human IL-15 Chemiluminescent Immunoassay, R&D Systems, Minneapolis, MN; analytical coefficient of variation 6.3%). Monocyte chemoattractant protein-1, IL-8, IL-10, IL-12, and interferon-γ were measured by Bio-Plex Cytokine 7-Plex Panel (Biorad Laboratories, Hercules, CA) according to manufacturer’s instructions. Intra- and Interassay coefficient of variation for monocyte chemoattractant protein-1 was 9% and 7%; for IL-8, 9% and 4%; for IL-10, 5% and 6%; for IL-12, 6% and 6%; and for interferon-γ, 15% and 9%.
Statistical Methods
The cytokines were log transformed to reduce the skewness in their distributions. Cox proportional hazards models, with age and sex adjustment, were used to select the cytokines significantly associated with 5-year mortality in InCHIANTI (p < 005 for cytokine). Three methods were used for aggregating multiple cytokines into a single summary measure. First, a weighted summary score (WSS) for each individual was determined by simply summing that person’s cytokines standardized values. Second, a principal component summary score (PCS) was developed using the summation of weighted cytokines, where the weights are the loadings for the first principal component of the correlation matrix of log-transformed cytokines, obtained by principal component analysis (22). Third, for the inflammatory index score (IIS) development, an L1-penalty approach, also known as lasso, was employed to select the cytokines most predictive of mortality. The penalty parameter was chosen to minimize the 10-fold cross-validation error. The “glmnet” package in R (23) was used to implement this approach, which selected age, log(IL-6) and log(sTNFR1) as the most powerful predictors of mortality. The resulting model coefficients for log(IL-6) and log(sTNFR1) were used as weights to obtain the IIS.
In CHS, we also explored whether the inflammatory index most predictive of mortality varied according to sex, CVD status, race, and age. Sex and CVD status modified the effect of IIS on mortality. Therefore, we fitted separate models for 10-year all-cause mortality with IL-6 and sTNFR1 as predictors, within each of the four strata defined by sex and CVD status (yes/no), adjusting for age, race, education, BMI, and smoking. This provided four different IIS indices according to sex and CVD status. This stratified inflammatory index will be denoted as IIS*.
The mortality risk prediction ability of each inflammatory index, adjusted for age, sex, race, education, BMI, smoking, and CVD status was assessed quantitatively and qualitatively in different ways: magnitude of standardized hazard ratio (HR), HR relative to age, and Kaplan–Meier curves for tertiles of aggregate inflammatory indices. We also assessed the aggregate indices in terms of their ability to predict mortality in the validation study, InCHIANTI, using the aforementioned metrics. In addition, we compared the bootstrap cross-validated concordance probability at different times for the three indices (WSS, PCS, and IIS) in the CHS cohort (24) Concordance probability is the probability that the participant who died at an earlier time has a large value of model predicted risk compared with another participant who died at a later time. It denotes the degree of concordance between actual outcome times and predicted outcome risks. Concordance probability is mathematically related to the area under the ROC curve, which varies over time for survival data (25). Finally, we also evaluated the predictive ability of the different indices (26) in the InCHIANTI cohort. We used the entire 10-year follow-up, as well as greater than 5-year follow-up, data in InCHIANTI for validation. Because the 5-year follow-up data in InCHIANTI was used to select cytokines for measurement in CHS, the nonoverlapping more than 5-year follow-up data in InCHIANTI was selected for validation to avoid circularity. Weights from CHS for all three inflammatory indices were utilized in these validation assessments. All computations were done using R version 2.15.0 (27).
Results
The InCHIANTI cohort was older and more frail than CHS (Table 1; p < .0001[18]). Sex distributions were similar. All InCHIANTI participants were Caucasian, whereas CHS had 84% Caucasian participants. The distributions of the five cytokines used in the aggregate analyses were different between the two cohorts as expected since the InCHIANTI population was an average older and was more frail than the CHS population.
All inflammatory markers were standardized and examined after adjusting for age, sex, education, BMI, smoking, CVD status, and race using Cox proportional hazards models in order that the HR of different predictors may be compared (race was not included in the models in InCHIANTI because there was no race variation). Of the 15 NFkB-mediated inflammatory markers measured from baseline serum samples in the InCHIANTI sample, CRP, IL-1RA, IL-6, IL-18, and sTNFR1, were found to be significant independent predictors of 5-year mortality, after adjusting for age, sex, BMI, education, smoking, and CVD status. These five markers were therefore chosen to be measured in serum samples collected at baseline visit in CHS.
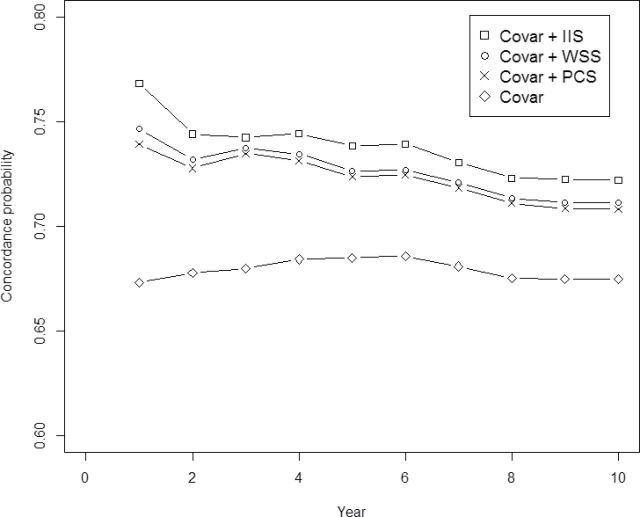
The HR for mortality for 10 years for individual inflammatory markers and the aggregated indices are shown in Table 2. Although all five inflammatory markers significantly predicted mortality, log IL-6 and log sTNFR1 were the most predictive among the five. They were also significant single-marker predictors of 1-, 2-, and 10-year mortality among the five measured inflammatory markers in both populations. The two aggregate measures that included all five cytokines (WSS, PCS) performed about the same as the individual IL-6 and sTNFR1 measurements. Table 3 displays the weights used for these cytokines. The IIS, which is equal to log (IL-6) + 2 log (sTNFR1), performed better than the individual cytokines and better than the WSS and PCS scores in terms of HR of 10-year mortality per standard deviation (SD) increase in inflammatory measure and in terms of concordance probability for mortality prediction. As the IIS increased by 1 unit, the HR of 10-year mortality increased by 62% (Table 2). IIS also had the best discriminatory power among all the inflammatory indices as evidenced by the uniformly largest concordance probability (ranging from 0.78 to 0.72) for mortality prediction for the 10-year period (Figure 1).
Table 2.
Mortality Risk of Inflammatory Phenotype in 1-, 2-, and 10-Year Cardiovascular Health Study (CHS) Cohort
| 10-Year CHS | 1-Year CHS | 2-Year CHS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | HR | 95% CI | HR | 95% CI | |||
| Log(IL-6) | 1.42 | 1.36 | 1.49 | 1.87 | 1.56 | 2.24 | 1.72 | 1.52 | 1.95 |
| Log(sTNFR1) | 1.46 | 1.39 | 1.53 | 1.98 | 1.69 | 2.32 | 1.72 | 1.52 | 1.94 |
| Log(CRP) | 1.25 | 1.19 | 1.31 | 1.63 | 1.33 | 1.98 | 1.59 | 1.39 | 1.82 |
| Log(IL-18) | 1.10 | 1.05 | 1.15 | 1.26 | 1.02 | 1.56 | 1.25 | 1.08 | 1.44 |
| Log(IL-1RA) | 1.21 | 1.15 | 1.26 | 1.44 | 1.19 | 1.73 | 1.29 | 1.13 | 1.48 |
| Age | 1.80 | 1.72 | 1.87 | 1.53 | 1.27 | 1.83 | 1.62 | 1.43 | 1.83 |
| WSS | 1.47 | 1.41 | 1.54 | 2.14 | 1.77 | 2.58 | 1.88 | 1.65 | 2.15 |
| PCS | 1.44 | 1.37 | 1.50 | 2.04 | 1.69 | 2.47 | 1.85 | 1.62 | 2.11 |
| IIS | 1.62 | 1.54 | 1.70 | 2.45 | 2.02 | 2.96 | 2.06 | 1.80 | 2.36 |
| IIS* | 1.88 | 1.77 | 2.00 | 3.21 | 2.50 | 4.11 | 2.58 | 2.16 | 3.08 |
Notes: CRP = C-reactive protein; HR = hazard ratio; IIS = inflammation index score; IIS* = stratified inflammatory index; IL-6 = interleukin-6; IL-18 = interleukin-18; IL-1RA = IL-1-receptor antagonist; PCS = principal components summary score; sTNFR1 = soluble tumor necrosis factor-α receptor-1; WSS = weighted summary score.
HR is the change in hazard of mortality per 1 standard deviation (SD) increase in the value of variables.
All analyses were adjusted for age, CVD, gender, education, smoke, and body mass index (BMI; except for age-only model), and the IIS* model was only adjusted for age, education, smoking, and BMI.
IIS and IIS* was calculated as follows:
IIS = 1/3 log(IL-6) + 2/3 log(sTNFR1), for all individuals.
IIS* = 1/3 log(IL-6) + 2/3 log(sTNFR1), if female and non-CVD.
2/5 log(IL-6) + 3/5 log(sTNFR1), if male and non-CVD,
1/4 log(IL-6) + 3/4 log(sTNFR1), if female and CVD,
1/3 log(IL-6) + 2/3 log(sTNFR1), if male and CVD.
Table 3.
Weights for Combining Individual Cytokines for the Inflammatory Indices
| Variable | WSS | PCS | IIS |
|---|---|---|---|
| Log(IL-6) | 0.13 | 0.5 | 1.0 |
| Log(sTNFR1) | 0.45 | 0.47 | 2.0 |
| Log(CRP) | 0.05 | 0.48 | 0 |
| Log(IL-18) | 0.22 | 0.31 | 0 |
| Log(IL-1RA) | 0.15 | 0.44 | 0 |
Notes: CRP = C-reactive protein; IIS = inflammation index score; IL-6 = interleukin-6; IL-18 = interleukin-18; IL-1RA = IL-1-receptor antagonist; sTNFR1 = soluble tumor necrosis factor-α receptor-1; PCS = principal components summary score; WSS = weighted summary score.
Figure 1.
Concordance probability for predicting mortality for a 10-year period for different regression models in the training sample (Cardiovascular Health Study, CHS). Concordance is the probability that the participant who died at an earlier time has a large value of model predicted risk compared with another participant who died at a later time. It denotes the degree of concordance between actual outcome times and predicted outcome risks. “Covar” refers to the model with age, sex, race, education, body mass index (BMI), smoking, and CVD status. Principal components summary score (PCS), weighted summary score (WSS), and inflammation index score (IIS) are the aggregate inflammatory measures.
Age was a much stronger predictor of mortality in InCHIANTI than it was in CHS (Table 4), possibly because of a wider age range in InCHIANTI. After adjusting for age, sex, education, BMI, and smoking, all the inflammatory measures, the individual cytokines and the aggregate indices, were not as strongly predictive of 10-year mortality in the InCHIANTI population compared with the CHS. The aggregate indices that included all five cytokines were not more predictive than the individual cytokines. The IIS again was the most predictive measure of 10-year mortality, showing better mortality prediction ability than WSS and PCS, and also better than any single cytokine measured in this study in terms of HR (Table 4).
Table 4.
Validation of the Mortality Risk of Inflammatory Phenotype in InCHIANTI for Total 10 Years and for Years 6–10
| 10-Year Validation in InCHIANTI | 6–10 Year Validation in InCHIANTI | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | HR | 95% CI | ||
| Log(IL-6) | 1.21 | 1.07 | 1.36 | 1.18 | 1.01 | 1.39 |
| Log(sTNFR1) | 1.30 | 1.13 | 1.49 | 1.25 | 1.05 | 1.48 |
| Log(CRP) | 1.22 | 1.08 | 1.39 | 1.16 | 0.99 | 1.37 |
| Log(IL-18) | 1.07 | 0.94 | 1.22 | 1.07 | 0.90 | 1.27 |
| Log(IL-1RA) | 1.20 | 1.05 | 1.38 | 1.10 | 0.93 | 1.31 |
| Age | 2.87 | 2.53 | 3.26 | 2.61 | 2.23 | 3.04 |
| WSS | 1.20 | 1.10 | 1.31 | 1.17 | 1.04 | 1.31 |
| PCS | 1.29 | 1.14 | 1.45 | 1.23 | 1.07 | 1.41 |
| IIS | 1.33 | 1.17 | 1.52 | 1.29 | 1.09 | 1.53 |
| IIS* | 1.42 | 1.21 | 1.67 | 1.37 | 1.12 | 1.69 |
Notes: BMI = body mass index; CI = confidence interval; CRP = C-reactive protein; HR = hazards ratio; IIS = inflammation index score; IIS* = stratified inflammatory index; IL-6 = interleukin-6; IL-18 = interleukin-18; IL-1RA = IL-1-receptor antagonist; PCS = principal components summary score; sTNFR1 = soluble tumor necrosis factor-α receptor-1; WSS = weighted summary score.
HR is the change in hazard of mortality per 1 standard deviation (SD) increase in the value of the variable.
All models are adjusted for age, CVD, sex, education, smoking, and BMI, except for the age-only model. The model with IIS* included adjustments for age, education, smoking and BMI because IIS* was obtained from sex and CVD stratification.
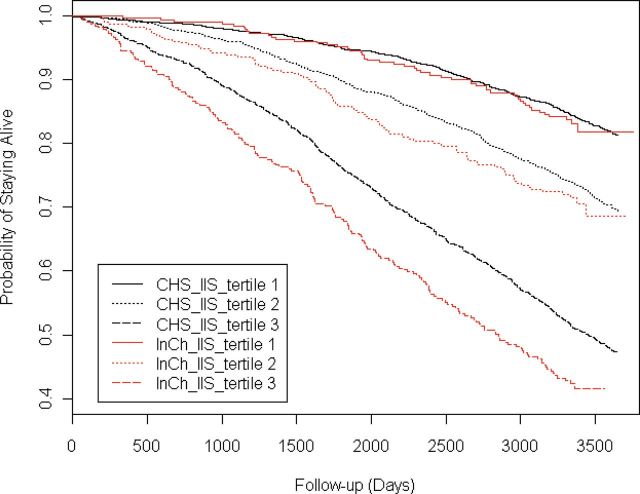
The comparisons of the survival rates among the tertiles of the IIS are shown in Figure 2. The Kaplan–Meier survival curves exhibited similar patterns in both the CHS training sample and the InCHIANTI validation sample. Specifically, the survival probability was strongly associated with the levels of the IIS, with the lowest tertiles of the inflammatory index associated with greatest survival, and the highest tertile associated with the worst survival (log-rank test p value < .001).
Figure 2.
Kaplan–Meier survival curves according to tertiles of estimated inflammatory index in the training (Cardiovascular Health Study, CHS) and validation (InCHIANTI) samples. Tertile 1 has the lowest inflammation index score (IIS) values, tertile 2 has intermediate, and tertile 3 has the highest IIS values. p value for log-rank tests of the differences across tertiles was <.001 for both cohorts.
In CHS, we also explored whether the linear combination of log(IL-6) and log(sTNFR1) that is most predictive of mortality varied according to sex, CVD status, race, and age. Sex and CVD status modified the effect of IIS on mortality. Therefore, we fitted separate models for 10-year all-cause mortality with IL-6 and sTNFR1 as predictors, within each of the four strata defined by sex and CVD status (yes/no), adjusting for age, race, education, BMI, and smoking. This provided four different IIS indices stratified by sex and CVD status, denoted as IIS*. IIS* was found to be a more powerful predictor of mortality than the unstratified index IIS (Table 2).
Finally, to determine if there were any differences in mortality prediction of IIS based on age group, we performed an age-stratified analysis. The test for Age × IIS interaction is statistically significant in CHS but not in InCHIANTI. This could be either real or likely due to a smaller sample size in InCHIANTI. We also identified a higher odds ratio for mortality in 65- to 69- and 70- to 80-year old groups in CHS compared with the oldest group of participants (Table 5).
Table 5.
Mortality effect of the inflammatory index score (IIS) across different age strata
| cohort | Age | HR | lower CI | upper CI | |
|---|---|---|---|---|---|
| CHS | (65, 70) | 1814 (35%) | 1.69 | 1.54 | 1.87 |
| (70, 80) | 2724 (53%) | 1.67 | 1.57 | 1.78 | |
| 80+ | 638 (19%) | 1.53 | 1.39 | 1.68 | |
| InCHIANTI | (65, 70) | 279 (28%) | 1.65 | 1.03 | 2.66 |
| (70, 80) | 471 (48%) | 1.46 | 1.20 | 1.76 | |
| 80+ | 240 (24%) | 1.54 | 1.34 | 1.76 | |
Note: The likelihood ratio test for age interaction was significant in the CHS (p = 0.04), but not in InCHIANTI (p = .88).
Discussion
Although numerous studies have linked inflammatory cytokines to adverse outcomes in older adults, there have been few, if any, attempts to comprehensively study a full array of cytokines in aging populations to determine which, if any single or combination of measures, are best at predicting mortality or other adverse outcomes (1). In this study, we selected potentially informative cytokine markers based on “a priori theory” and measured 15 NFkB-related inflammatory cytokines. Using these data, we developed three aggregate models and demonstrated that a simple index that combined sTNFR1 and IL-6 was the most robust cytokine predictor of 10-year mortality in two populations of older adults. Of the two cytokines comprising the index, IL-6 has long been known to be an important predictor of adverse outcomes and mortality in populations of older adults (28). In fact, IL-6 has been called the “cytokine for gerontologists” for nearly 20 years (29). sTNFR1 has been less studied in populations of older adults. However, this marker has been demonstrated to have a powerful predictive value for adverse outcomes in population-based studies of older adults with acute and chronic disease states. For example, higher serum levels of this protein are associated with the progression of mild cognitive impairment to Alzheimer’s disease and with coronary artery and renal disease in older adults (30,31). Elevated plasma levels of sTNFR1 are associated with increased mortality in study participants with acute lung injury (32). In addition, TNF pathway stimulation mediates cell cycle arrest compared with stimulation of the IL-1 pathway (33). Polymorphic variation in the TNFR1 gene modulates cytokine production through NFkB family subunit interactions, further supporting its key role in inflammatory and apoptotic signaling and chronic disease states (33,34). Indeed, both IL-6 and TNFR1 molecules play key gateway functions for inflammatory signal amplification and propagating signals that influence cellular senescence and apoptosis (35,36). Hence, chronic, low-grade signaling by either of these molecules would be expected to propagate inflammatory signals and modulate tissues toward a more vulnerable and less viable state.
IL-1RA is a surrogate marker for IL-1β, which along with IL-18 is a potent early activator of NFkB-driven inflammatory pathways. Together, these two proinflammatory cytokines provide an important link between infection and injury and in the activation of downstream inflammatory signaling (37). Indeed, they appear to be important in inflammasome activation and in transmitting oxidative stress–related signaling into inflammatory pathway activation via damaged mitochondria (37). These two cytokines, although predictive of mortality, are not as robust in prediction of mortality as are log IL-6 and log sTNFR1. This may be in part because they are early proinflammatory signaling molecules and do not act in pathways that have the same downstream tissue impact of IL-6 and sTNFR1. TNF-α, the ligand for sTNFR1 that has been occasionally associated with mortality other cohorts, was not found to be among the most predictive of 10-year mortality in the initial 15 cytokines screened in the InCHIANTI population. This result is consistent with several prior studies, which usually show stronger associations with more chronic markers of inflammation such as IL-6 and CRP (1).
The IIS was found to be a significantly stronger predictor of mortality in younger rather than older adults in CHS, and the individual cytokines and the aggregate measures were on the whole not as predictive of mortality in InCHIANTI as in CHS population, after adjusting for age, sex, education, BMI, and smoking. InCHIANTI participants were in general older and more frail than the CHS population at baseline (16,18). In an exploratory analysis, we found that the IIS that was optimally predictive of mortality varied according to sex and CVD status. Correspondingly, we derived a stratified inflammatory index score (IIS*), which was found to be the strongest predictor of mortality among all the inflammatory markers considered. In another exploratory analysis, age-stratified results suggested that the proinflammatory state may be a more important indicator of global health status for the younger subset of older adults than it is for the oldest subset, which may have led to the less robust mortality finding in InCHIANTI. We might speculate that the relative importance of inflammation is attenuated compared with other biological processes as people age. This deserves further study. Although the strength of the impact of inflammation appears to decrease with advancing age, our findings and other studies of very old adults still suggest that inflammatory pathway activation is still an important predictor of mortality well into the eighth and ninth decade of life (38,39).
It is important to note that findings on the changes in the relationship between inflammatory processes and mortality according to age, sex, and CVD status are exploratory. To move these results toward clinical risk prediction, they would need to be studied as part of a prespecified analytical plan for training and validation in larger populations of older adults.
Strengths of this study include large numbers of older participants, the targeting of cytokine measurement based on biologically relevant NFkB pathways, number of cytokines measured, high-quality mortality data, and validation in an independent study. There were some limitations as well. Measurements of some of the initial group of 15 cytokines were run in a multiplex format that may not be as accurate as those from single ELISA measurements (38). In addition, some of the measurements were made up to 10 years after the baseline blood collection, which may or may not have led to some deterioration of measured inflammatory proteins. Although this variability may have affected the screening processes and some of the measurement of the 15 cytokines initially screened in InCHIANTI population, the singleplex or duplex assays used to measure the top five inflammatory mediators are more robust and hence are likely to be reproducible. Although we considered a large number of inflammatory markers, we did not consider all the known ones. Therefore, it is possible that there may be more powerful predictors of mortality that we did not study. Despite these limitations, our study is the first to suggest that an aggregate measure of IL-6 and sTNFR1 is a powerful predictor of mortality for 10 years in community-dwelling older adults. Given that IL-6 and sTNFR1 predict mortality for 10 years and that they are both are key regulators of inflammation, apoptosis, and cellular senescence, our results support the need for further etiological studies regarding the role that these biologically active cytokines play in disease propagation and increased vulnerability to mortality. In addition, the results of our stratified analyses regarding risk assessment suggest that further development of these measures for clinical risk assessment tools might warranted.
Funding
This research was supported by National Institute of Aging (NIA; R01 AG027236) and the Johns Hopkins Older Americans Independence Center (P30 AG021334), by National Heart, Lung, and Blood Institute (NHLBI) contracts N01-HC-85239, N01-HC-85079 through N01-HC-85086; N01-HC- 35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, and NHLBI grant HL080295, with additional contribution from National Institute of Neurological Disorders and Stroke (NINDS) and AG-023629, AG-15928, AG-20098, and AG-027058 from the NIA. See also http://www.chs-nhlbi.org/pi.htm. The InCHIANTI study baseline (1998–2000) was supported by the Italian Ministry of Health and the U.S. National Institute on Aging, National Institutes of Health (Contracts 263 MD 9164 and 263 MD 821336). The InCHIANTI Follow-up (2001–2003) was funded by the U.S. National Institute on Aging (Contracts N.1-AG-1–1 and N.1-AG-1–2111).
Acknowledgments
Dr. Varadhan is a Brookdale Leadership in Aging Fellow at the Johns Hopkins University. Dr. Thomas Gerds of University of Copenhagen is acknowledged for providing statistical consultation on the estimation of concordance probability.
References
- 1. Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev. 2011; 10: 319–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cesari M, Penninx BW, Pahor M, et al. Inflammatory markers and physical performance in older persons: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004; 59: 242–248 [DOI] [PubMed] [Google Scholar]
- 3. Tracy RP. Inflammation in cardiovascular disease: cart, horse or both–revisited. Arterioscler Thromb Vasc Biol. 2002; 22: 1514–1515 [DOI] [PubMed] [Google Scholar]
- 4. Huber SA, Sakkinen P, Conze D, Hardin N, Tracy R. Interleukin-6 exacerbates early atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 1999; 19: 2364–2367 [DOI] [PubMed] [Google Scholar]
- 5. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005; 352: 1685–1695 [DOI] [PubMed] [Google Scholar]
- 6. Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993; 259: 87–91 [DOI] [PubMed] [Google Scholar]
- 7. Schmidt MI, Duncan BB, Sharrett AR, et al. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): a cohort study. Lancet. 1999; 353: 1649–1652 [DOI] [PubMed] [Google Scholar]
- 8. Davalos AR, Coppe JP, Campisi J, Desprez PY. Senescent cells as a source of inflammatory factors for tumor progression. Cancer Metastasis Rev. 2010; 29: 273–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Puntmann VO, Taylor PC, Mayr M. Coupling vascular and myocardial inflammatory injury into a common phenotype of cardiovascular dysfunction: systemic inflammation and aging—A mini-review. Gerontology. 2011; 57: 295–303 [DOI] [PubMed] [Google Scholar]
- 10. Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003; 107: 499–511 [DOI] [PubMed] [Google Scholar]
- 11. Jenny NS, Yanez ND, Psaty BM, Kuller LH, Hirsch CH, Tracy RP. Inflammation biomarkers and near-term death in older men. Am J Epidemiol. 2007; 165: 684–695 [DOI] [PubMed] [Google Scholar]
- 12. Bandeen-Roche K, Walston JD, Huang Y, Semba RD, Ferrucci L. Measuring systemic inflammatory regulation in older adults: evidence and utility. Rejuvenation Res. 2009; 12: 403–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hanada T, Yoshimura A. Regulation of cytokine signaling and inflammation. Cytokine Growth Factor Rev. 2002; 13: 413–421 [DOI] [PubMed] [Google Scholar]
- 14. Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995; 5: 278–285 [DOI] [PubMed] [Google Scholar]
- 15. Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991; 1: 263–276 [DOI] [PubMed] [Google Scholar]
- 16. Fried LP. The epidemiology of frailty: the scope of the problem. Perry HM, Morley JE, Coe RM, eds. Aging, Musculoskeletal Disorders and Care of the Frail Elderly. New York: Springer; 1993: 3–16 [Google Scholar]
- 17. Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000; 48: 1618–1625 [DOI] [PubMed] [Google Scholar]
- 18. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001; 56: M146–M156 [DOI] [PubMed] [Google Scholar]
- 19. Macy EM, Hayes TE, Tracy RP. Variability in the measurement of C-reactive protein in healthy subjects: implications for reference intervals and epidemiological applications. Clin Chem. 1997; 43: 52–58 [PubMed] [Google Scholar]
- 20. Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem. 1995; 41: 264–270 [PubMed] [Google Scholar]
- 21. Ferrucci L, Corsi A, Lauretani F, et al. The origins of age-related proinflammatory state. Blood. 2005; 105: 2294–2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jolliffe IT. Principal Component Analysis. 2nd ed. New York: Springer; 2004. [Google Scholar]
- 23. Friedman J, Hastie T, Tibshirani R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J Stat Softw. 2010; 33: 1–22 [PMC free article] [PubMed] [Google Scholar]
- 24. Gerds TA, Kattan MW, Schumacher M, Yu C. Estimating a time-dependent concordance index for survival prediction models with covariate dependent censoring. Statistics in Medicine. 2012; Published online in Wiley Online Library; [DOI] [PubMed] [Google Scholar]
- 25. Heagerty PJ, Zheng Y. Survival model predictive accuracy and ROC curves. Biometrics. 2005; 61: 92–105 [DOI] [PubMed] [Google Scholar]
- 26. Harrell FE, Jr,, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996; 15: 361–387 [DOI] [PubMed] [Google Scholar]
- 27. R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBM 3-900051-07-0. 2012; http://www.R-project.org/.
- 28. Wassel CL, Barrett-Connor E, Laughlin GA. Association of circulating C-reactive protein and interleukin-6 with longevity into the 80s and 90s: the Rancho Bernardo Study. J Clin Endocrinol Metab. 2010; 95: 4748–4755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ershler WB. Interleukin-6: a cytokine for gerontologists. J Am Geriatr Soc. 1993; 41: 176–181 [DOI] [PubMed] [Google Scholar]
- 30. Diniz BS, Teixeira AL, Ojopi EB, et al. Higher serum sTNFR1 level predicts conversion from mild cognitive impairment to Alzheimer’s disease. J Alzheimers Dis. 2010; 22: 1305–1311 [DOI] [PubMed] [Google Scholar]
- 31. Safranow K, Dziedziejko V, Rzeuski R, et al. Plasma concentrations of TNF-alpha and its soluble receptors sTNFR1 and sTNFR2 in patients with coronary artery disease. Tissue Antigens. 2009; 74: 386–392 [DOI] [PubMed] [Google Scholar]
- 32. Parsons PE, Matthay MA, Ware LB, Eisner MD. National Heart, Lung, Blood Institute Acute Respiratory Distress Syndrome Clinical Trials Network. Elevated plasma levels of soluble TNF receptors are associated with morbidity and mortality in patients with acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2005; 288: L426–L431 [DOI] [PubMed] [Google Scholar]
- 33. Churchman SM, Church LD, Savic S, et al. A novel TNFRSF1A splice mutation associated with increased nuclear factor kappaB (NF-kappaB) transcription factor activation in patients with tumour necrosis factor receptor associated periodic syndrome (TRAPS). Ann Rheum Dis. 2008; 67: 1589–1595 [DOI] [PubMed] [Google Scholar]
- 34. Wijsman CA, Maier AB, de Craen AJ, van den Biggelaar AH, Westendorp RG. An unopposed proinflammatory response is beneficial for survival in the oldest old. Results of the Leiden 85-plus Study. J Gerontol A Biol Sci Med Sci. 2011; 66: 393–399 [DOI] [PubMed] [Google Scholar]
- 35. Tsirpanlis G. Cellular senescence and inflammation: a noteworthy link. Blood Purif. 2009; 28: 12–14 [DOI] [PubMed] [Google Scholar]
- 36. Través PG, López-Fontal R, Cuadrado I, et al. Critical role of the death receptor pathway in the antitumoral effects induced by hispanolone derivatives. Oncogene. 2013; 32: 259–268 [DOI] [PubMed] [Google Scholar]
- 37. Sorbara MT, Girardin SE. Mitochondrial ROS fuel the inflammasome. Cell Res. 2011; 21: 558–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Leng SX, McElhaney JE, Walston JD, Xie D, Fedarko NS, Kuchel GA. ELISA and multiplex technologies for cytokine measurement in inflammation and aging research. J Gerontol A Biol Sci Med Sci. 2008; 63: 879–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jylhä M, Paavilainen P, Lehtimäki T, et al. Interleukin-1 receptor antagonist, interleukin-6, and C-reactive protein as predictors of mortality in nonagenarians: the vitality 90+ study. J Gerontol A Biol Sci Med Sci. 2007; 62: 1016–1021 [DOI] [PubMed] [Google Scholar]